Abstract
Smallpox vaccine based on live, replicating vaccinia virus (VACV) is associated with several potentially serious and deadly complications. Consequently, a new generation of vaccine based on non-replicating Modified vaccinia virus Ankara (MVA) has been under clinical development. MVA seems to induce good immune responses in blood tests, but it is impossible to test its efficacy in vivo in human. One of the serious complications of the replicating vaccine is eczema vaccinatum (EV) occurring in individuals with atopic dermatitis (AD), thus excluding them from all preventive vaccination schemes. In this study, we first characterized and compared development of eczema vaccinatum in different mouse strains. Nc/Nga, Balb/c and C57Bl/6J mice were epicutaneously sensitized with ovalbumin (OVA) or saline control to induce signs of atopic dermatitis and subsequently trans-dermally (t.d.) immunized with VACV strain Western Reserve (WR). Large primary lesions occurred in both mock- and OVA-sensitized Nc/Nga mice, while they remained small in Balb/c and C57Bl/6J mice. Satellite lesions developed in both mock- and OVA-sensitized Nc/Nga and in OVA-sensitized Balb/c mice with the rate 40–50%. Presence of mastocytes and eosinophils was the highest in Nc/Nga mice. Consequently, we have chosen Nc/Nga mice as a model of AD/EV and tested efficacy of MVA and Dryvax vaccinations against a lethal intra-nasal (i.n.) challenge with WR, the surrogate of smallpox. Inoculation of MVA intra-muscularly (i.m.) or t.d. resulted in no lesions, while inoculation of Dryvax t.d. yielded large primary and many satellite lesions similar to WR. Eighty three and 92% of mice vaccinated with a single dose of MVA i.m. or t.d., respectively, survived a lethal i.n. challenge with WR without any serious illness, while all Dryvax-vaccinated animals survived. This is the first formal prove of protective immunity against a lethal poxvirus challenge induced by vaccination with MVA in an atopic organism.
Introduction
Vaccinia virus (VACV) from the Poxvirus family has been used as a live vaccine against smallpox. A worldwide vaccination campaign using various strains of VACV led to the eradication of this life-threatening disease. Consequently, vaccination of the general population has been stopped. At the beginning of this century due to concerns about the smallpox misuse in a bioterrorist attack, vaccination against smallpox was re-introduced [1]. Original stocks of VACV strain Dryvax as well as second generation tissue culture-grown stocks (ACAM2000) have been used [2]. New, highly attenuated vaccine based on non-replicating Modified vaccinia virus Ankara (MVA; IMVAMUNE) is under clinical development and seems to induce good immune responses in blood tests and in animal models [3], [4], [5], [6], [7]. It is impossible, though, to test its efficacy in vivo in human.
Vaccination against smallpox induces good response of both cellular and humoral immunity, but it may be associated with several post-vaccination complications together with a substantial risk of spread of VACV among the contacts of vaccinees [8], [9], [10]. The most severe complications include progressive vaccinia, post-vaccination encephalitis, eczema vaccinatum and a recently described myopericarditis [8]. In this article we focused on eczema vaccinatum (EV). It occurs namely in individuals with a history of atopic dermatitis or eczema (AD), and it is caused by a dissemination of VACV in the skin apart from the vaccination site, after a self-inoculation or through a contact with a vaccinee. During the worldwide vaccination campaign, EV incidence among primary vaccinees was approximately 10–40 per million [11], while the lethality was around 1–6% [12].
Atopic dermatitis is an increasingly common inflammatory skin disease that is genetically determined but the environmental and neuropsychological factors contribute to the development of the disease also [13]. Individuals with AD develop itchy skin lesions on distinct parts of the body and roughly 70–80% of them have elevated serum IgE levels [13]. According to one hypothesis, AD is primarily caused by abnormalities of skin innate immunity, resulting in the infiltration with skin-homing Th2 cells during the acute phase and in complex inflammatory responses. Second hypothesis considers as a primary defect the immune dysregulation towards Th2 immune responses together with IgE-mediated sensitization [12], [14], [15].
Currently, there is a variety of mouse models of AD. They include models with spontaneous manifestation of AD, genetically engineered mice, models with skin lesions induced by sensitization with an antigen, and models with severe combined immunodeficiencies [16].
In our studies, we compared the effects of wild-type VACV strain Western Reserve (WR) inoculation in the skin of three different mouse strains. Namely, we used Japanese Nc/Nga mice, a spontaneously mutated inbred strain that reveals many characteristics similar to human AD. About 50% of mice develop spontaneous skin lesions in non-SPF conditions, suggesting that the epigenetic or environmental factors play a role also [17]. The mice were shown to have elevated serum levels of IgE, histopathological changes of the skin (thickening of dermis and epidermis, infiltration with eosinophils and mononuclears) and Th2-dominant immune responses [18]. Further, we used Balb/c and C57Bl/6 mice. Both strains were described to develop AD when sensitized epicutaneously with ovalbumin using tape-stripping [19], [20]. In general, Balb/c mice reveal Th2-shifted immune responses, while C57Bl/6 mice reveal Th1-dominant immune responses; accordingly, the individual mouse strains are more sensitive or resistant to intracellular pathogens, respectively [21].
For studies of eczema vaccinatum, both Nc/Nga and Balb/c mice sensitized with various allergens have been used [22], [23], [24], but genetically modified mice derived from C57Bl/6 strain are also commonly used to study responses to VACV. Nevertheless, different sensitization and inoculation protocols used by individual research groups make the results difficult to compare. In this paper, we show for the first time a direct comparison of all three mouse strains as an AD/EV model. Our comparison showed that Nc/Nga mice developed the condition most similar to human EV, namely forming satellite pox lesions distant from the site of WR inoculation and highest WR titer in the inoculation site. Therefore we used Nc/Nga mice as a model atopic organism to test the ability of attenuated non-replicating MVA in comparison with the replicating Dryvax to mount a protective response against the intra-nasal infection with a lethal dose of wild-type vaccinia virus strain WR, the surrogate of smallpox [25]. In this work, we clearly show that despite of the defects in skin immunity, atopic Nc/Nga mice are able to mount an effective protective immunity against the lethal i.n. challenge with VACV strain WR. To our knowledge, this is the first formal report proving efficiency of MVA immunization against a lethal poxvirus challenge in an atopic organism.
Materials and Methods
1. Ethics statement
This work was carried out in accordance with European regulations for transport, housing and care of laboratory animals (Directive 2010/63/EU on the protection of animals used for scientific purposes). The animals were housed in housing facilities accredited by the Ministry of Agriculture of the Czech Republic and monitored daily. All the infectious experiments were performed in the Biosafety Level 2 laboratory with negative pressure and using HEPA-filtered animal boxes. Full details of the animal experiments, including the mortality aspects, were approved by the Experimental Animal Use Committee of the 1st Medical Faculty of Charles University and the Ministry of Education of the Czech Republic (experimental protocols No. GA UK 100307 – 208/06, 0021620806 MSM, P302/10/0083 – 298/09). These protocols also addressed the cases of unexpected mortality. All individuals working with infected animals were vaccinated with the standard smallpox vaccine in the past.
2. Animals and Viruses
We used male and female mice of three strains: Nc/Nga (kind gift of Riken BioResource center, Japan) and C57Bl/6J bred and reared in specific pathogen-free (SPF) conditions in The Center for Experimental Biomodels at the 1st Medical Faculty, Charles University, and Balb/c purchased from Harlan. Faculty facility ensures standard SPF conditions (HEPA-filtered air, sterilized beddings and food with all manipulations in laminar flow hoods). The animals bred in the faculty facility were routinely checked by a veterinary service, while the animals purchased from Harlan were delivered with a Health Monitoring Report. They were acclimatized in conventional housing for 1 week before the start of sensitization at the age of 6–8 weeks. The animals were assigned to cages and or/experimental groups based on their sex and litter (individual littermates were split into different experimental groups) with maximum of 5 mice/cage. The weight of Nc/Nga mice was 18–20 g at the start of sensitization, and 24–30 and 26–32 g, females and males respectively, at the start of immunization and prior to the lethal challenge. Balb/c and C57Bl/6J mice weighed generally 2 g less.
VACV strain Western Reserve (WR), Dryvax and Modified vaccinia virus Ankara (MVA) were used. MVA [26] and a plaque isolate of Dryvax (D50; [27], [28], [29]) were kindly provided by Dr. Nemeckova. VACV strains WR and Dryvax were propagated in BSC-40 cells as described previously [29], [30], while MVA was propagated in primary chicken embryonal fibroblasts (CEFs; kindly provided by Dr. Geryk from the Czech Academy of Sciences). Dryvax and WR were purified by a sucrose gradient sedimentation [31], MVA by a centrifugation through a cushion of 45% sucrose. Virus titers were determined by serial dilutions and plaque assays in BSC-40 cells (Dryvax, WR) [29], [30] or in CEFs using immunodetection of plaques with a rabbit polyclonal antiserum against vaccinia virus (MVA); [32].
3. Epicutaneous (EC) sensitization and VACV infection
All the experimental manipulations with mice were performed in anesthesia with avertin (2,2,2,tribromoethanol in tertial amylalcohol, 1 mg/ml) administered intra-peritoneally (i.p.; 12–16 µl/g of weight). Mice in experiments were monitored daily and at the specified time points, they were sacrificed humanely under terminal anesthesia with diethyl ether and exsanguination from the jugular vein. In protective experiments, the basic aim was to assess the effect of the prior immunization on the survival of atopic animals; therefore, the lethality was expected in control non-immune mice, while the survival was anticipated in immune ones. The criteria for humane endpoint defined in the study protocol P302/10/0083 – 298/09 were weight loss under 25% and decreased body temperature. All efforts were made to minimize the suffering of the mice during manipulations with them.
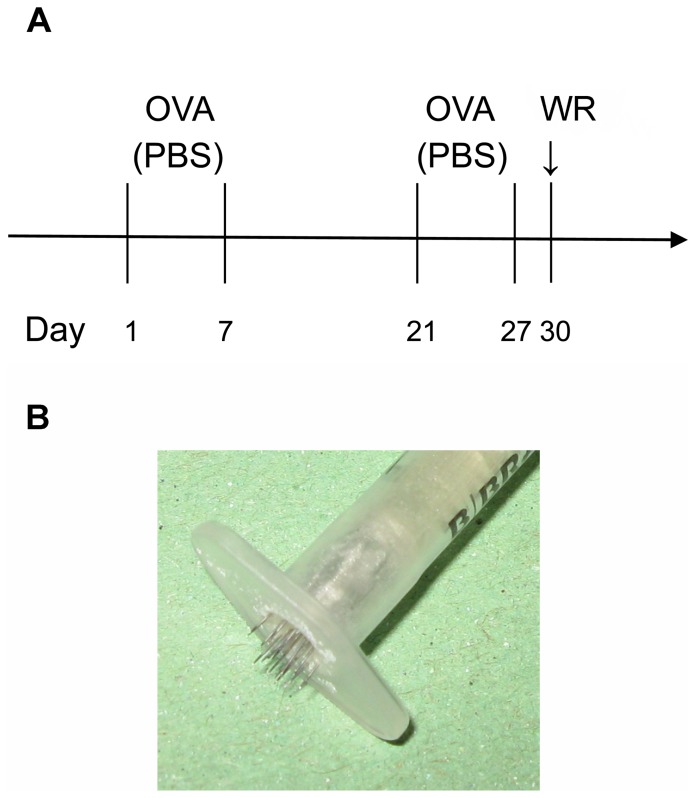
For experiments studying development of AD and EV, mice of all strains were anesthetized with avertin, and sensitized epicutaneously with ovalbumin (OVA; 100 µl of 1 mg/ml in PBS) or PBS only on the right side of the back using tape-stripping (TS) according to scheme in Fig. 1A [19], [20]. Three days after the end of second sensitization, the mice were anesthetized with avertin, mock-inoculated at the site of sensitization with OVA in PBS (final concentration 0.5 mg/ml) or inoculated with 1×107 PFU of VACV strain WR with OVA in PBS using a vaccination stamp (trans-dermal (t.d.) inoculation). The inocula were then covered for 7 days with an impermeable bandage and the mice were humanely sacrificed 7 or 14 days after virus inoculation. For experiments studying protective effect of MVA and Dryvax immunizations, the mice were sensitized with PBS and immunized with 108 PFU of MVA t.d. or intra-muscularly (i.m.) or with 107 PFU of Dryvax t.d. (Fig. 1A) under anesthesia with avertin. Three weeks after the immunization, the anesthetized mice were intra-nasally (i.n.) challenged with a lethal dose of VACV strain WR, 5×106 PFU in 10 µl of PBS. The infected animals were monitored and clinically scored daily as described above. All control non-immune and three immunized mice died spontaneously before meeting the humane endpoint criteria, while the surviving animals were sacrificed humanely 2 weeks after the challenge. The mice were post-mortem dissected and the weights of spleen, lungs, liver and kidneys were determined. The exact cause of death was not further explored. No additional adjustments to the approved protocol were made during the original experiments presented in this study.
Figure 1. Epicutaneous sensitization and WR inoculation protocol.
(A) Two cycles of sensitization with OVA or control PBS by tape-stripping and inoculation of WR using a vaccination stamp. (B) Vaccination stamp.
The vaccination stamp (Fig. 1B) was prepared using 20 acupuncture needles (thickness 0.16 mm; HuangQiu Acupuncture Medical Appliance Co. Ltd, Suzhou, China) inserted in the outer part of a syringe, forming a circle with a diameter of 5 mm and protruding over the rim 1.5 mm. The depth of the transdermal puncture was typically 0.5–1 mm.
4. Titer of WR in the skin lesions
The skin lesions of infected animals were frozen, and then homogenized in ice-cold DMEM (in a volume corresponding to 4x weight of the skin lesion). After 2 cycles of freezing and thawing, the samples were sonicated and centrifuged and the supernatant was used for determination of virus titer using serial dilutions and plaque assays in BSC-40 cells [29], [30]. Similarly, WR titers were determined in lungs, liver, kidney and spleen of Nc/Nga mice that succumbed to lethal i.n. infection with WR.
5. Histology and histochemistry
Skin biopsies were taken 7 days after virus inoculation, fixed with 4% paraformaldehyde in PBS and embedded in paraffin. Deparaffinized sections were stained with hematoxylin and eosin and analyzed by light microscopy using camera Olympus C-5050 mounted on Olympus microscope BX 51 (Olympus Optical, Hamburg, Germany) and software Olympus DP-SOFT (Software Imaging Systems, Münster, Germany); original magnification 100x, 200x and 400x.
Mast cells were determined in multiple 4-µm skin sections using staining with 0.05% Toluidine blue in distilled water and quantified in 5–10 high power fields (original magnification 200x) for each mouse, the mean of which was used for further calculations and statistical evaluations.
6. Immunohistochemistry
WR proteins were detected using a primary rabbit anti-VACV antibody (ViroStat, Inc., Portland, Maine), dilution 1∶200, and secondary peroxidase-labelled polymer conjugated to goat anti-rabbit immunoglobulins, dilution 1∶200 (EnVision + System; Dako), DAB was used as a chromogenic substrate. The sections were then counterstained with hematoxylin.
7. Determination of serum IgG1 and IgG2a antibodies specific for VACV and of total serum IgE antibodies
VACV-specific antibodies IgG2a and IgG1 were determined using sandwich Elisa performed in 96 well plates (Nunc, MaxiSorp) coated with 1×107 PFU/well of purified, UV-inactivated VACV strain WR (Advanced Biotechnologies and our own preparation [33]). Sera collected 14 days after VACV inoculation were diluted 1∶1,000 and 1∶5,000 for IgG1 and 10-times more for IgG2a determinations. In protective experiments, sera were collected 3 weeks after immunization and used in dilutions 1∶50–1∶25,000 for IgG2a and 1∶50–1∶2,000 for IgG1, and 14 days after a lethal challenge with VACV strain WR in surviving Nc/Nga mice in dilutions 1∶100,000–∶200,000 for IgG2a and 1∶10,000–1∶20,000 for IgG1. Consequently, the plates were incubated overnight in 4°C. Bound IgG2a and IgG1 were detected using biotinylated rat anti-mouse antibodies (0.5 and 1 µg/ml for IgG2a and IgG1, respectively; BD Pharmingen), streptavidin coupled to HRP (0.5 µg/ml; Immunotech) and TMB (Amresco). The reaction was stopped with 1 M H2SO4 and optical density was measured at 450 nm using a plate reader Victor 1420-012 (PerkinElmer). Levels of IgG1 and IgG2a are expressed as relative values calculated from a calibration curve of a standard. This standard was prepared from infected Nc/Nga mouse serum and was added to each Elisa test in several dilutions defined for each isotype. As the amount of individual IgG1 and IgG2a in the standard is unknown, relative levels of IgG1 cannot be compared to the levels of IgG2a in the tested samples. Total serum IgE antibodies were determined similarly as specific IgG's, using rat anti-mouse IgE antibody (2 µg/ml in PBS) for coating and biotinylated rat anti-mouse antibody (2 µg/ml; BD Pharmingen) for detection. Purified mouse IgE standard (BD Pharmingen) was used for the calibration curve.
8. Characterization of the neutralization capacity of mice sera
The total neutralizing capacities of mice sera were determined by a plaque reduction neutralization test. Sera of infected and uninfected mice (9 µl) were incubated with 1 µl of DMEM containing 8.75×103 PFU of VACV strain WR (total volume 10 µl; final virus concentration 8.75×105 PFU/ml) for 12 h in 37°C. The resulting virus titers were then determined using serial dilutions and plaque assays as described in section 4. The original amount of input WR in the dilution used for determination of the number of plaques was 723 PFU/well. Efficiency of neutralization capacity was expressed as percentage of resulting plaque numbers in each group compared to the numbers in mock-inoculated Nc/Nga mice.
9. Statistical analysis
Data were analyzed by one-way ANOVA global test followed by LSD post-hoc comparison tests using Statistica software.
Results
1. Induction of atopic dermatitis and eczema vaccinatum
At the onset of experiments, we determined the conditions for development of atopic dermatitis in three different mouse strains: in spontaneously atopic Nc/Nga mice, in Th2-skewed Balb/c and in Th1-skewed C57Bl/6 mice. In SPF environment, Nc/Nga mice did not spontaneously develop any macroscopic signs of AD and even in conventional housing, the incidence of macroscopic dermatitis-like lesions was very low. Therefore, we started to epicutaneously sensitize the mice with OVA and tape-stripping. The skin lesions in Nc/Nga mice developed already after the first sensitization, being most severe after the second one. Inclusion of a third cycle of sensitization led to a slight decline in the severity of lesions; therefore two cycles of sensitization were used in the following experiments. Interestingly, dry, scaly lesions and itchy behavior were evident predominantly in male Nc/Nga mice. In contrast, Balb/c or C57Bl/6 mice did not develop any macroscopic lesions during the sensitization with OVA. We also tested dust mites and household fungi in Nc/Nga mice, getting similar results as with OVA application (data not shown). For VACV inoculation, we used a specifically developed vaccination stamp (Fig. 1B). This stamp provides a uniform size and depth of VACV inoculation, the resulting lesions can be better quantified, and satellite lesions can be clearly distinguished.
1.1. Histological analysis of skin samples
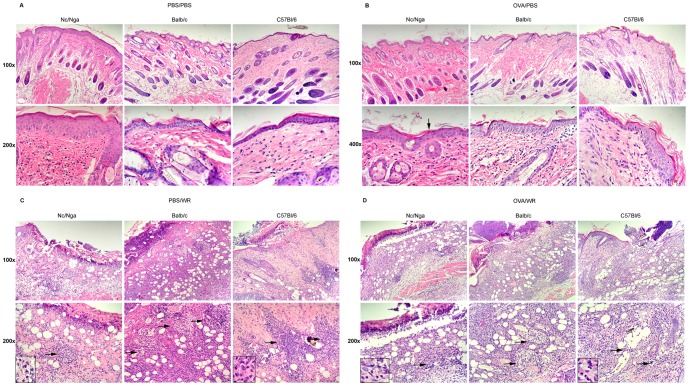
First, we analyzed histological features of the skin of mock- and OVA-sensitized mice after a mock or WR inoculation in hematoxylin-eosin-stained skin sections. Among the mock-inoculated animals (Fig. 2A, B), both mock- and OVA-sensitized Nc/Nga mice displayed most characteristic features of AD, particularly a mild inflammation, thickening of epidermis and dermis all over the skin sections, proliferation of connective tissue and parakeratosis. Balb/c and C57Bl/6 mice also exhibited thickening of epidermis and dermis but only in some regions of the skin sections; a minimal inflammation was more accentuated in OVA-sensitized animals. In comparison with Nc/Nga mice, the AD-like skin changes were generally milder in Balb/c and C57BL/6 mice, being present mostly in OVA-sensitized animals.
Figure 2. Histological features of the skin of Nc/Nga, Balb/c and C57Bl/6 mice 7 days after mock or WR inoculation by vaccination stamp.
Mock- (A, C) and OVA-sensitized (B, D) mice were mock-inoculated with OVA in PBS (A, B) or inoculated with 107 PFU of a purified stock of WR with OVA in PBS (C, D). Hematoxylin-eosin stained skin sections. Original magnification: (A, B) upper panel 100x, lower panel 400x; (C, D) upper panel 100x, lower panel 200x with inserts 400x. (A) Nc/Nga - Mild mixed predominantly mononuclear dermal inflammatory infiltrate. Thickening of epidermis, increased thickness of dermis due to proliferation of the connective tissue. Balb/c - Mild fibrosis and no or minimal mixed inflammation in the dermis. C57Bl/6 - Minimal mixed dermal inflammatory infiltrate in the skin, focal thickening of epidermis. (B) Nc/Nga – Very mild mixed dermal inflammatory infiltrate. Thickening of epidermis and parakeratosis (↓), increased thickness of dermis. Balb/c - Thickening of epidermis, mild fibrosis and mild mixed dermal inflammation. C57Bl/6 - Focal thickening of epidermis, very mild mixed dermal inflammatory infiltrate. (C, D) WR-inoculated mice. In all strains - mixed dermal inflammatory infiltrate with an epidermal erosion/ulcer, covered by a crust. Florid necrotizing folliculitis with high incidence of chromatin debris →, eosinophils found in Nc/Nga, Balb/c and C57Bl/6 mice in about 5, 1 and 3%, respectively – shown in the inserts. Representative results of skin sections of 3-5 animals.
The inflammatory changes in the skin of WR-inoculated Nc/Nga, Balb/c and C57Bl/6 mice were qualitatively similar in all strains studied regardless of saline/OVA sensitization (Fig. 2C, D). The skin displayed a necrotizing folliculitis, a high incidence of the apoptotic debris, and a mixed inflammatory infiltrate (polymorphonuclear and mononuclear cells) in dermis. Occasionally, eosinophils, important mediators of allergic responses, were observed in sections of all three mouse strains, constituting about 5, 1 and 3% of inflammatory elements in Nc/Nga, Balb/c and C57Bl/6 mice, respectively (inserts in Fig. 2C, D). The site of inoculation was ulcerated, often covered with a crust. The size of the lesions and skin injury was generally greater in Nc/Nga mice, as characterized by deep ulcerations. In contrast in Balb/c and C57Bl/6 mice, the activity of the inflammation was greater, while the ulcerations were more superficial.
In addition to eosinophils, allergic responses are mediated also by mast cells. Therefore, we have determined number of mast cells in sections parallel to hematoxylin-eosin stained sections before and after WR inoculation using Toluidine blue staining (Table 1). In Nc/Nga mice, the number of mast cells was significantly higher than in Balb/c and C57Bl/6 mice and did not seem to be affected by sensitization with OVA or by WR 7 days p.i. On the other hand, there was a decrease in numbers of mast cells associated with the OVA sensitization in both Nc/Nga and Balb/c mice at the day 0.
Table 1. Quantification of mast cells in Toluidine blue-stained skin sections from mock- and OVA-sensitized mice at the day of inoculation and 7 days after mock- or WR-inoculations.
| Number of mast cells | ||||||
| Day of inoculation | 7 days post infection | |||||
| PBS | OVA | PBS/PBS | OVA/PBS | PBS/WR | OVA/WR | |
| Nc/Nga | 23.45±1.41 | 18.65±2.15 | 16.10±3.50 | 21.78±7.88 | 21.63±3.17 | 22.28±4.45 |
| Balb/c | 12.50±1.01*** | 8.03±0.67*** | 7.31±1.24x | 6.07±0.72** a | 11.13±1.21** | 10.99±2.03* a |
| C57Bl/6 | ND | ND | 8.15±3.55 | 6.53±0.47** | 9.23±3.35** | 13.56±1.93* |
Data represent mean +/− S.E.M.; n = 2–5 mice in each group. ND, not determined. Statistically significant decrease in the number of mast cells compared to analogously sensitized Nc/Nga mice,
P<0.1,
*P<0.05,
**P<0.01;
***P<0.001. Statistically significant decrease in the number of mast cells in OVA-sensitized Balb/c mice compared to mock-sensitized Nc/Nga mice.
P<0.05.
Altogether, the histological analyses revealed that skin samples of both mock- and OVA-sensitized Nc/Nga mice displayed the most characteristic features of AD of all mouse strains and treatments. In contrast, only the skin samples of OVA-sensitized Balb/c and C57Bl/6 mice revealed signs of AD that were generally milder than in Nc/Nga mice. The inflammatory changes in the skin after inoculation of WR were qualitatively similar in all strains and treatments, while infiltration with eosinophils and mastocytes, elements typical for allergic responses, was the highest in Nc/Nga mice regardless of sensitization.
1.2. WR spread in the skin
Eczema vaccinatum is characterized by an uncontrolled replication and spread of vaccinia virus throughout the skin apart from the site of inoculation. Therefore, the size of the primary lesions, virus yields and development of satellite lesions can serve as quantitative indicators of WR spread and development of EV. As shown in Fig. 3A, the size of lesions developed in Nc/Nga mice was significantly bigger than in Balb/c and C57Bl/6 mice in both mock- and OVA-sensitized groups. Then, we determined the yields of WR achieved in the inoculation sites of all three mouse strains 7 days p.i. (Fig. 3B), with WR yields in mock-sensitized Nc/Nga mice being about 3 logs higher than in Balb/c and about 1.5 log higher than in C57Bl/6 mice. We have also quantified virus growth in the lesions of Nc/Nga and Balb/c mice in a time course experiment (Fig. 3C). At all time points, virus yields were found higher in Nc/Nga mice, confirming that control of WR replication and clearance were impaired in comparison to Balb/c mice. Nevertheless, the differences among individual strains and treatments presented in Figs. 3B and 3C were not found statistically significant due to a considerable variability.
Figure 3. Primary and secondary lesions in the skin of Nc/Nga, Balb/c and C57Bl/6 mice after WR inoculation by vaccination stamp.
Mock- and OVA-sensitized mice were inoculated with 107 PFU of a purified stock of WR with OVA in PBS, sacrificed at indicated days p.i, and skin biopsies were taken. (A) Size of primary lesions 7 days p.i. Data represent mean +/− S.E.M. *P<0.05, **P<0.01. Results of 2 experiments with n = 6–9 mice in each group. (B) WR titers (PFU/ml) in the site of WR inoculation 7 days p.i.; median values are indicated. No statistically significant differences. Results of 1–2 independent experiments. (C) Time course of WR growth. Data represent mean +/− S.E.M.; n = 3–12 mice in each group. No statistically significant differences. Results of 3 and 2 independent experiments in Nc/Nga mice and Balb/c mice, respectively. (D) Site of primary inoculation (upper panels) and a satellite lesion (lower panels) in mock-sensitized, WR-inoculated Nc/Nga mice 7 days p.i. Immunohistochemical detection of WR (right), control hematoxylin-eosin staining (left); original magnification 200x. Positive IHC signal (brown) overlaps with the sites of increased apoptosis (→).Representative results of 3 skin sections.
Further, we quantified the number of satellite lesions, characterizing WR spread in the skin of infected animals. We found the occurrence of satellite lesions very frequently in all Nc/Nga and in OVA-sensitized Balb/c mice (Table 2). In all mice included in this table, the presence of virus in the secondary lesions was proved either by IHC (Fig. 3D) or by virus titration. IHC sections were also used to analyze the presence of apoptotic debris in parallel to hematoxylin-eosin stained skin sections, confirming that apoptosis occurred mostly in cells that stained positive also for WR (Fig. 3D).
Table 2. Percentage (%) of Nc/Nga, Balb/c and C57Bl/6 mice with 1 or more satellite lesions 7 days p.i. with WR.
| Percentage of mice with satellite lesions | ||||
| PBS/WR | OVA/WR | |||
| % | n | % | n | |
| Nc/Nga | 44 | 9 | 56 | 9 |
| Balb/c | 0 | 10 | 40 | 10 |
| C57Bl/6 | 0 | 6 | 0 | 6 |
n = number of mice in each group.
In summary, these results indicate that Nc/Nga mice, both mock- and OVA-sensitized, revealed the largest primary lesions, the highest virus yields and occurrence of eosinophils and mastocytes in primary lesions and the highest occurrence of satellite lesions of all three strains and treatments. Thus, Nc/Nga mice seem to represent a better model of AD/EV than OVA-sensitized Balb/c or C57Bl/6 mice.
1.3. Levels of total IgE and specific antibodies against WR
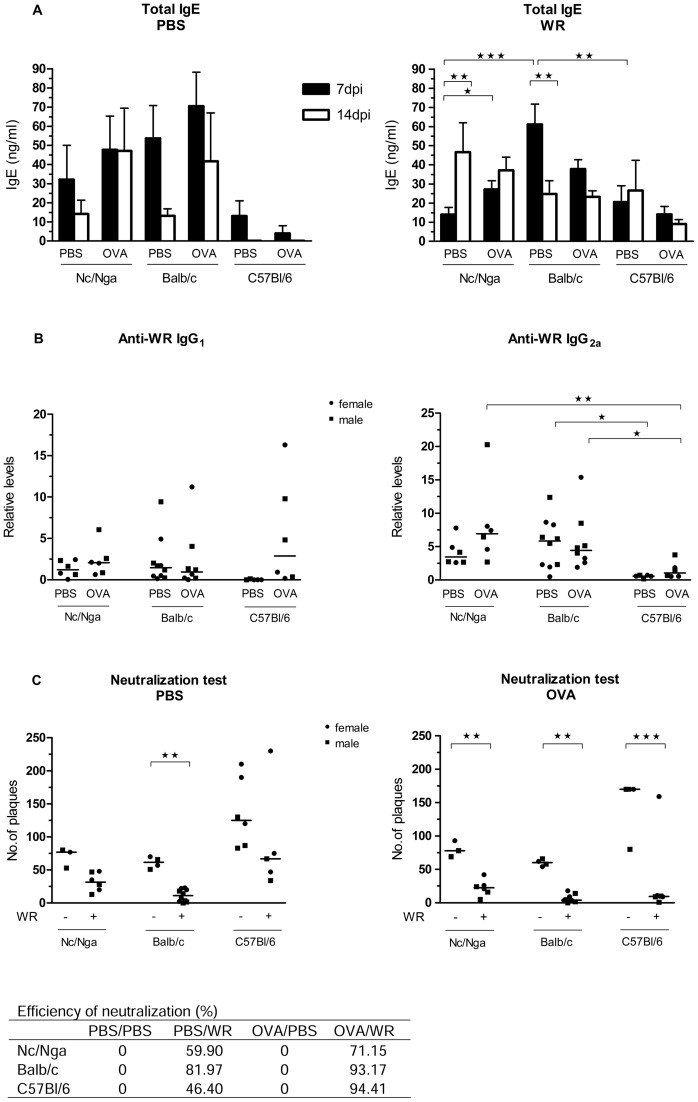
In order to further characterize allergic and specific immune responses in the three strains, we determined levels of total IgE antibodies (Fig. 4A) and levels of WR-specific antibodies IgG1 and IgG2a as isotypes typical for Th2- and Th1-skewed responses, respectively (Fig. 4B). Between 7 and 14 days p.i., total IgE decreased in all groups of mock-infected animals, except of OVA-sensitized Nc/Nga mice in which the levels of total IgE remained comparably high at both time points. In WR-inoculated animals, a statistically significant increase in the levels of total IgE was found between 7 and 14 days p.i. in mock-sensitized Nc/Nga mice and a slight, statistically not significant increase in OVA-sensitized Nc/Nga mice again. In contrast in Balb/c mice, total IgE levels decreased in time in both groups, while in C57Bl/6 total IgE levels were low and the changes insignificant. These results suggest that IgE levels increase in time in response to OVA and/or WR in Nc/Nga mice, while they decrease in time in the other strains and treatments.
Figure 4. Immune responses after WR inoculation.
Mock- and OVA-sensitized Nc/Nga, Balb/c and C57Bl/6 mice were mock-inoculated with OVA in PBS or inoculated with 107 PFU of a purified stock of WR with OVA in PBS, sacrificed 7 and 14 days later, and the levels of antibodies were determined by ELISA. (A) Levels of total serum IgE 7 and 14 days after inoculation expressed as mean +/− S.E.M. Results of 1–4 independent experiments. (B) Relative levels of WR-specific antibodies 14 days after inoculation were determined by ELISA (dilution 1∶1,000); median values are indicated. Results of 2 independent experiments. (C) Neutralization capacity of naive (-WR) and immune (+WR) mice sera 14 days after inoculation expressed as number of plaques detected after a 12-h incubation of the input virus with the individual sera; see Materials and Methods section 8 for experimental details. Median values of the resulting numbers of plaques are indicated in the graphs; the table represents efficiency of neutralization capacity in percentage, where mock-inoculated mice represent 0% of neutralization. Results of 2 independent experiments for each mouse strain. *P<0.05, **P<0.01, ***P<0.001.
The production of WR-specific antibodies IgG1 and IgG2a was found very low, almost undetectable 7 days after WR-inoculation in all 3 mouse strains studied (data not shown). Fourteen days p.i., the levels of WR-specific antibodies were already detectable, with higher levels found in Nc/Nga and Balb/c strains (Fig. 4B). In C57Bl/6 mice, levels of specific IgG1 and IgG2a were very low, with the exception of IgG1 levels in OVA-sensitized animals. Yet, the relative levels of IgG1 cannot be compared to the levels of IgG2a in the tested samples as the amount of individual IgG1 and IgG2a in the standard is unknown. When comparing levels of individual IgG isotypes among the three mouse strains, C57Bl/6 mice can serve as an established control for Th1-dominant responses. However at this time interval, our data do not confirm the higher IgG1 levels typical for Th2-skewed responses in Nc/Nga and Balb/c mice as it is reported elsewhere.
We have further characterized expression of IL-17A and IFNγ in the skin after WR inoculation in Nc/Nga and Balb/c mice using real-time RT-PCR. IL-17A was previously reported to inhibit immigration of NK and other cell types into the site of infection in AD skin [22] and to promote WR replication [23], while IFNγ reveals immunomodulatory and antiviral properties ([34]). The results summarized in S1 Figure indicate that both cytokines were increased 5 days after WR inoculation in both mouse strains.
1.4. Serum neutralization capacity
The efficiency of humoral immunity can be assessed by determination of total neutralization capacity of the immune sera. Therefore, the sera of mock- and WR-inoculated Nc/Nga, Balb/c and C57Bl/6 mice were used for plaque reduction assays. The control, non-immune sera decreased the input virus about 10-times in all strains, with sera of C57Bl/6 revealing somewhat lower neutralizing capacity. This result reflects the action of complement, as no heat inactivation was performed. Seven days p.i., no sera of WR-inoculated mice studied were able to neutralize WR (data not shown), correlating with the minimal production of WR-specific antibodies 7 days p.i. described above. Fourteen days p.i., sera of all three WR-inoculated mouse strains already revealed a capacity to neutralize WR (Fig. 4C), with the differences being significant in all OVA-sensitized animals and in mock-sensitized Balb/c mice. Importantly, neutralization capacity of WR-inoculated Nc/Nga mouse sera was lower than the one of WR-inoculated Balb/c mouse sera (See table included in Fig.4C).
In summary, neutralization tests indicated a clear difference between immune and non-immune sera, with Nc/Nga mice revealing lower or delayed specific neutralization capacity than Balb/c and OVA-sensitized C57Bl/6 mice.
2. Protective effect of MVA and Dryvax immunizations in atopic Nc/Nga mice
Based on the above described results, we have decided to use the atopic Nc/Nga mice, the model of AD susceptible to develop EV after inoculation of replicating WR, to test the efficiency and safety of immunization with a non-replicating vaccinia virus MVA against a lethal challenge with the intra-nasal administration of WR, the surrogate of smallpox. For comparison, we also immunized the mice with the commonly used Dryvax, the original smallpox vaccine associated with various post-vaccination complications.
2.1. Immunization with MVA and Dryvax
The mock-sensitized Nc/Nga mice were mock-immunized with PBS or immunized with Dryvax and MVA t.d. using the vaccination stamp or with MVA i.m. Seven days after the immunization, no lesions were visible at the sites of t.d. administration of MVA, while there were relatively large skin lesions at the sites of t.d. administration of Dryvax; the diameter of the lesions caused by Dryvax was comparable to the lesions produced by WR described above (Fig. 3A). Occasionally, satellite lesions were found in Dryvax-inoculated, mostly male mice, confirming again the risk of VACV spread in the atopic organism. Administration of MVA i.m. did not cause any apparent macroscopic changes. Dryvax was not administered i.m., since it is a lytic virus and the route of administration used in human is t.d. only. Thus, vaccination with MVA seems to be safe, without any signs of spread of the virus.
2.2. Specific antibodies against VACV and serum neutralization capacity
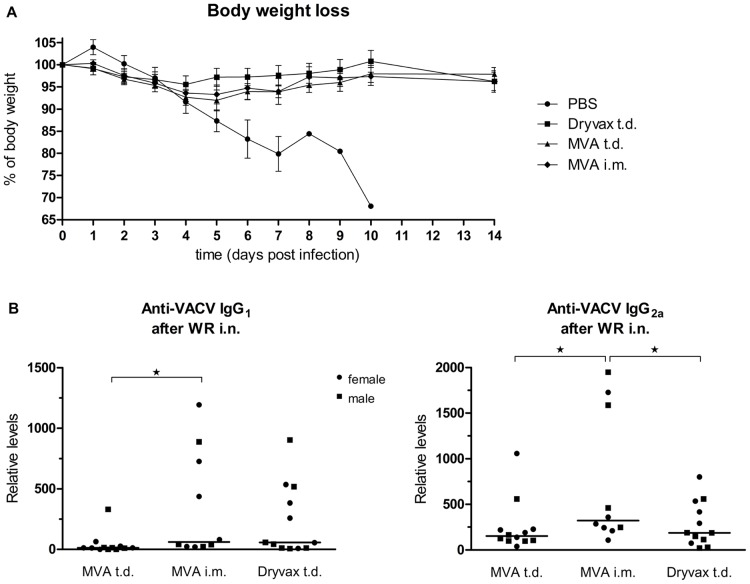
In order to characterize the immune responses to the individual immunization protocols, the levels of VACV-specific IgG1 and IgG2a and the neutralization capacity were determined three weeks after the immunization, just before exposure to the lethal dose of WR. As shown in Fig. 5A, immunization with Dryvax resulted in fair amounts of antibodies of both isotypes, while MVA administered t.d. yielded non-detectable or very low amounts of both isotypes. MVA administered i.m. resulted in low levels of VACV-specific IgG1 and in somewhat higher levels of IgG2a. Additionally performed neutralization tests (Fig. 5B) inversely correlated with the levels of IgG2a antibodies. In summary, after MVA-immunization t.d., the animals revealed lower humoral responses compared to MVA i.m. and Dryvax-immunized ones.
Figure 5. Immune responses of atopic Nc/Nga mice immunized with MVA and Dryvax.
Mock-sensitized Nc/Nga mice were inoculated using a vaccination stamp trans-dermally (t.d.) or intra-muscularly (i.m.) with 108 PFU of a purified stock of MVA or t.d. with 107 PFU of a purified stock of Dryvax, and sacrificed 3 weeks later. (A) Levels of total serum IgG1 and IgG2a antibodies specific to VACV were determined by ELISA (dilution 1∶1,000); median values are indicated. Results of 2 independent experiments. (B) Neutralization capacity of mice sera expressed as number of plaques detected after a 12-h incubation of the input virus with the individual sera; see Materials and Methods section 6 for experimental details. Median values of the resulting numbers of plaques are indicated. Results of 1–2 independent experiments. *P<0.05, **P<0.01.
2.3. Lethal intranasal challenge with VACV strain WR
In order to test the protective effect of immunization with MVA and Dryvax in atopic animals in vivo, the animals were challenged with a lethal i.n. dose of WR, the surrogate of smallpox. In spite of the variation in immune responses raised by the two virus strains and different ways of immunization, results of two independent experiments clearly demonstrated that a vast majority of MVA-immunized mice as well as all Dryvax-immunized mice were able to survive a lethal dose of VACV strain WR administered i.n. (Table 3). The surviving animals did not display any changes in behavior or moving, any significant weight losses (Fig. 6A) or signs of a serious illness. In contrast, all the mock-immunized controls (with PBS administered t.d.) succumbed to the infection. They displayed initially a bristled hair, later an enlargement of abdomen and difficult breathing; nevertheless they died spontaneously before meeting criteria for the humane endpoint defined in the study protocol. The post-mortem dissection revealed inflation of the gut, saddle macroscopic changes in color of lungs (somewhat hemorrhagic) and very small, exhausted spleens. WR titers were determined at the day of death in mock-immunized mice. The virus was commonly found in lungs, with titers ranging from 101 to 107 PFU/organ. In some mice, the virus was found also in liver, spleen and kidneys, with the highest virus yields usually in lungs at day 5 p.i., occasionally in liver. In MVA-immunized mice that succumbed to the infection, no virus was found in any organ. The exact cause of death was not further explored. In the surviving, previously immunized animals, virus titers were not determined when they were sacrificed humanely 14 days after the lethal infection as the virus is never detected at this time interval. When sacrificed, they displayed pale, pink lungs and the increased size of spleen.
Table 3. Survival of atopic Nc/Nga mice immunized with MVA or Dryvax after a lethal intranasal challenge with VACV strain WR.
| Survival of immunized mice | ||||
| PBS | MVA | MVA | Dryvax | |
| t.d. | t.d. | i.m. | t.d. | |
| Survival | 0/17 | 12/13* | 10/12* | 12/12 |
| Survival (%) | 0 | 92 | 83 | 100 |
| Mean day of death ± S.D. | 6±0.5 | 6 | 10 | - |
| Splean ± S.D. (mg) | 52±5† | 132±10 | 118±14 | 109±5 |
| Lungs ± S.D. (mg) | 145±12‡ | 168±6 | 171±5 | 186±9 |
Mean day of death and weights of spleens and lungs at death or at sacrification 14 days after the challenge are indicated. SD, standard deviation;
*, dead mice were males;
, weight of normal spleens of age-matched mice is 70–80 mg;
, weight of normal lungs of age-matched mice is 140–170 mg. Results of 2 independent experiments performed for each virus strain together with the PBS controls.
Figure 6. Survival of atopic Nc/Nga mice immunized with MVA or Dryvax after a lethal intranasal challenge with VACV strain WR.
(A) Body weight changes of mock-immunized (PBS), MVA- or Dryvax-immunized Nc/Nga mice after lethal i.n. challenge with WR. Results are expressed as mean +/− S.E.M. At day 10, all mock-immunized mice were spontaneously dead. Results of 2 independent experiments performed for each virus strain together with the PBS controls. (B) Levels of total serum IgG1 and IgG2a antibodies specific to VACV determined 14 days after a lethal challenge with VACV strain WR determined by ELISA in sera dilutions 1∶10,000–1∶20,000 and 1∶100,000–1∶200,000 for IgG1 and IgG2a, respectively, and expressed as relative values; median values are indicated. Results of 2 independent experiments.
Additionally, we have determined levels of VACV-specific IgG1 and IgG2a antibodies in the surviving animals. As summarized in Fig. 6B no significant differences in the levels of either IgG1 or IgG2a antibodies were found, with the levels of both isotypes being about 10–100-times higher than those determined just before the i.n. challlenge. This increase might suggest that the challenge virus replicated in the organism but its replication was controlled by specific immune responses of Nc/Nga mice.
In summary, immunization of atopic Nc/Nga mice with a single dose of MVA administered either t.d. or. i.m., resulted in protection of most animals against a lethal poxvirus challenge without any serious illness.
Discussion
In this work, we compared characteristics of atopic dermatitis and immune responses of three different mouse strains, Nc/Nga, Balb/c and C57Bl/6, towards inoculation of VACV strain WR into the skin, concluding that Nc/Nga mice are the most suitable model of eczema vaccinatum. Consequently, we used these mice to prove the ability of the non-replicating MVA to induce a protective immunity against a lethal i.n. challenge with VACV strain WR, the surrogate of smallpox. To our knowledge, this is the first report proving efficiency of MVA immunization against a lethal poxvirus infection in vivo in an atopic organism.
Based on our results, the skin changes of Nc/Nga mice meet most characteristics of an atopic skin. The histological analysis showed that the pathological changes were similar in both mock- and OVA-sensitized skins of Nc/Nga mice, while they were less pronounced in Balb/c and C57Bl/6 mice, mostly after the sensitization with OVA. The authors of this EC sensitization protocol, Spergel and Jin et al. [19], [20], reported development of skin allergic inflammation after TS and an epicutaneous sensitization with OVA in Balb/c mice, and also Scott et al. [24] reported development of allergic inflammation with eosinofilia and CD4+ cells infiltration in skin of Balb/c mice after EC application of OVA together with TS. In Nc/Nga mice, eczematous lesions were reported to be effectively induced also by other allergens. The group of Kawakami et al. [22], [35] induced allergic inflammation in Nc/Nga mice using dust mites and Staphylococcal enterotoxin B. Accordingly, we have tested dust mites and household fungi with results similar to OVA treatment (data not shown).
After inoculation of WR, both mock- and OVA-sensitized Nc/Nga mice developed large skin lesions together with satellite lesions that could be easily distinguished from the site of inoculum. The primary skin lesions in other strains and groups were much smaller and satellite lesions could be found only in OVA-sensitized Balb/c mice. In Nc/Nga mice, use of lower doses of WR and Dryvax was also tested, resulting in even larger primary lesions and higher occurrence of secondary lesions, regardless of the addition of OVA into the virus inoculum (unpublished results). Indeed, this outcome is in agreement with previous observations in human vaccinees [36]. Development of satellite lesions were observed also by others, but the presence of VACV was not proven [23], [24] or their occurence was low [22].
In human, AD is often associated with elevated IgE production, with total serum IgE being increased in about 80% of AD (extrinsic AD; [13]). In Nc/Nga mice kept in conventional housing, serum IgE levels were found increased compared to those kept in SPF conditions [18], [37], [38], but IgE production was described to be dispensable for atopic changes in the skin of Nc/Nga mice [20], [39]. Accordingly, we observed the atopic changes of the skin histologically, but we found relatively low levels of IgE in mock-sensitized, mock-inoculated Nc/Nga mice and also relatively low levels of WR-specific IgG1, described to be elevated in epicutaneously sensitized and WR-infected Balb/c mice [24]. Our results of IFNγ expression (S1 Figure) are compatible with those of Yagi et al. [39] who demonstrated that AD pathology in Nc/Nga was IgE/Th2-independent and that the eczematous symptoms were caused by the IFNγ-favored environment. Also, Mammesier et al. [40] proposed that the allergic inflammatory response would not be due to a Th1/Th2 imbalance, but rather to a Treg deficiency.
Interestingly, in Nc/Nga mice, total serum IgE levels were increasing between 7 and 14 days post infection with WR (with the statistical significance only in mock-sensitized Nc/Nga mice). We suggest that this IgE increase in Nc/Nga mice might be due to constantly high amounts of mast cells in the skin of these mice that might lead to the release of mediators like heparin and histamin. Heparin can activate interferon-inducible dsRNA-activated protein kinase R, PKR [41], which is involved in IgE class switching and plays a role in IgE production [42]. It was reported, that VACV inhibits PKR activation through the E3L protein [43], [44], but in atopic Nc/Nga mice, this inhibition might be overcome by heparin; thus PKR can be still activated and contribute to IgE increase.
Histological and immunohistochemical analyses further revealed the presence of chromatin debris at the sites of VACV inoculation, indicating a DNA fragmentation during the ongoing apoptosis. These results thus suggest that VACV inoculation into the skin might result in apoptosis rather than necrosis of infected cells. It has been previously established that VACV, typically considered as a lytic virus, causes apoptosis of various immune cell types (DC, macrophages, B-cells; [33], [45], [46]. Further, we have recently demonstrated, that VACV strain WR caused either apoptosis or necrosis in different epithelial cell lines and that vaccination strains Dryvax and Praha caused apoptosis in two epithelial cell lines tested [29]. Further, different recombinants of VACV strain WR caused apoptosis in human fibroblast cell line NIH 3T3 (unpublished results). The type of cell death and presence of various PAMP's and DAMP's [47] are critical for the type of immune responses raised, and thus for better understanding to the post-vaccination complications. It is therefore important to closely characterize the type of cell death induced by VACV in vivo in the individual cell types.
The specific immunity of Nc/Nga, Balb/c and C57Bl/6 mice towards VACV strain WR was characterized by production of WR-specific IgG1 and IgG2a and by the capacity of mouse sera to neutralize the virus infectivity. The antibody responses were detectable only 14 days p.i. and they were relatively weak in all strains, as the peak levels are expected only later. Additionally, C57Bl/6 mice were reported to express the IgG2c isotype instead of IgG2a [48]. On the other hand, neutralization tests indicated a clear difference between immune and non-immune animals, with Nc/Nga mice revealing lower or delayed specific neutralization capacity than Balb/c mice.
The above discussed results showed that Nc/Nga mice inoculated with VACV strain WR fulfill characteristics of a model of eczema vaccinatum independently of OVA sensitization, suggesting that they are the model of choice for such studies. In contrast, in Balb/c and C57Bl/6J mice, sensitization with OVA was necessary. Nevertheless, Balb/c mice sensitized with OVA and other allergens have been successfully used in studies of EV [23], [24].
MVA, attenuated VACV that does not replicate in mammals, seems to be a safe option for inducing antipoxviral immunity, especially in atopics that cannot be vaccinated with replicating VACV. Previously, MVA effect was demonstrated in atopic, OVA-sensitized Balb/c mice, in which MVA immunization prevented the appearance of lesions after a subsequent inoculation of WR into the skin [49]. MVA also seems to induce good immune responses in blood tests in human atopics [50], but the efficacy cannot be tested in vivo. Therefore, we used the atopic Nc/Nga mice for testing efficiency and safety of MVA immunization against a lethal poxviral challenge and compared it with Dryvax, the old smallpox vaccine associated with post-vaccination complications. We have used immunization with a single dose of t.d. or i.m. administered MVA and a single dose of t.d. administered Dryvax. The immunization with Dryvax protected 100% of mice, but it should be emphasized that these mice revealed relatively large skin lesions and formation of satellite lesions after the virus inoculation, further confirming an increased risk of development of eczema vaccinatum in AD individuals. On the other hand, immunization with non-replicating MVA that is safe even for atopics and that did not lead to development of any lesions detectable one week after immunization, led to a substantial, but incomplete survival of the immunized animals. The number of MVA-immunized animals that succumbed to the lethal challenge with WR was too low to indicate any significant differences between the t.d. and i.m. immunizations. However it appears that most animals that probably did not develop any detectable levels of antibodies after a single dose of MVA were still able to successfully defeat the lethal challenge with VACV strain WR. These results suggest that either the production of specific antibodies amplified quickly to sufficient levels after the second encounter with VACV or that the control of virus spread was mediated mostly by cellular immunity. Nevertheless both wild-type, replicating strains of VACV and MVA were previously shown to induce apoptosis of dendritic cells and thus affect their antigen-presenting function as well as other immune responses [46], [51], [52]. In any case, regardless of the exact mechanisms involved, our results prove the usefulness and efficiency of the immunization with MVA in an atopic organism. Probably, this efficiency could be further improved if a protocol consisting of several consecutive doses of MVA was used.
For obvious reasons, the mounting of protective responses against smallpox cannot be tested in human, but the re-introduction of vaccination of the general population still has been considered [53]. Our work thus supports usefulness and efficacy of MVA-based vaccines against lethal poxviral infection also in an atopic organism.
Supporting Information
Expression of IL-17A and IFNγ cytokines in the skin after WR inoculation. Skin biopsies were taken from mock- and OVA-sensitized Nc/Nga and Balb/c mice 5 days after mock-inoculation with OVA in PBS (PBS) or after inoculation with 107 PFU of a purified stock of WR with OVA in PBS (WR). Expression of individual cytokines in the skin was determined by real-time RT-PCR and standardized to GAPDH (expressed as ratio cytokine/GAPDH ×105). Data represent mean +/− S.E.M.; number of animals in Nc/Nga and Balb/c groups: 3 and 4, respectively. *P<0.05, **P<0.01, *** P<0.001. S1 Figure Methods: Determination of cytokine expression by real-time RT-PCR. Biopsies of skin lesions were stored in RNAlater RNA Stabilization Reagent (Qiagene) or Allprotect Tissue Reagent (Qiagene). RNA was isolated after homogenization of the skin samples in RNA Blue (Top-Bio, Czech Republic) using manufacturer's protocol. Purified RNA was resuspended in RNase-free water with the addition of RNase inhibitor RiboLock (Fermentas). RNA concentration and purity were determined by measuring the absorbance at 260 and 280 nm, respectively, using UV spectrophotometer BioPhotometer (Eppendorf AG). RNA was treated with RNase-free DNase (Fermentas) in the presence of RiboLock and quantification of RNA of interest was performed by Power SYBR Green RNA-to-CT 1-Step Kit (Applied Biosystems) using Applied Biosystems 7300 Real-time PCR System according to manufacturer's protocol. Quantification of each RNA was performed in duplicate together with GAPDH using 50 ng of RNA in 20 µl reactions and 40 cycles. Specific primers for individual genes were described previously or newly designed using Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/): Mu GAPDH cDNA forward 5′–CGGTGCTGAGTATGTCGTGGA–3′, reverse 5′–GGCAGAAGGGGCGGAGATGA–3′ [54]; Mu IL-17A cDNA forward 5′–GGACTCTCCACCGCAATGAA–3′, reverse 5′–TTTCCCTCCGCATTGACACA–3′; Mu IFNγ cDNA forward 5′–TGGCATAGATGTGGAAGAAAAGAG–3′, reverse 5′–TGCAGGATTTTCATGTCACCA–3′ [55].
(TIF)
Acknowledgments
We are grateful to the Riken BioResource center Japan for kindly providing Nc/Nga mice, to Dr. Nemeckova for providing MVA and Dryvax, to Dr. Geryk for providing primary chicken embryonal fibroblasts, to Dr. Chanova for help with determination of mastocytes and to Dr. Belacek and M. Komarc for statistical analysis of the data. We thank Drs. Fucikova and Prokesova for helpful discussions. The work of J.K. was performed in partial fulfillment of the requirements for Ph.D. degree at the 1st Medical Faculty, Charles University. The work of J.E. and B.O. was performed in partial fulfillment of the requirements for M.Sc. degree at the Faculty of Science, Charles University.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by Grant Agency of Charles University – project No. 100307 (http://www.cuni.cz/UK-1673.html) (JK); Charles University – project No. PRVOUK-P24/LF1/3 (http://www.cuni.cz/UK-4157.html) (ZM); and European Regional Development Fund - project “BIOCEV – Biotechnology and Biomedicine Centre of the Academy of Sciences and Charles University in Vestec” (CZ.1.05/1.1.00/02.0109) ((http://www.biocev.eu/en/about/project-in-numbers/) (ZM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Artenstein AW (2008) New generation smallpox vaccines: a review of preclinical and clinical data. Rev Med Virol 18:217–231. [DOI] [PubMed] [Google Scholar]
- 2. Nalca A, Zumbrun EE (2010) ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther 4:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frey SE, Newman FK, Kennedy JS, Sobek V, Ennis FA, et al. (2007) Clinical and immunologic responses to multiple doses of IMVAMUNE (Modified Vaccinia Ankara) followed by Dryvax challenge. Vaccine 25:8562–8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von Krempelhuber A, Vollmar J, Pokorny R, Rapp P, Wulff N, et al. (2010) A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine 28:1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stittelaar KJ, van Amerongen G, Kondova I, Kuiken T, van Lavieren RF, et al. (2005) Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol 79:7845–7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Townsend MB, Keckler MS, Patel N, Davies DH, Felgner P, et al. (2012) Humoral Immunity to Smallpox Vaccines and Monkeypox virus Challenge; Proteomic Assessment and Clinical Correlations. J Virol doi:101128/JVI02089-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davies DH, Wyatt LS, Newman FK, Earl PL, Chun S, et al. (2008) Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus ankara is comparable to that of Dryvax. J Virol 82:652–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arness MK, Eckart RE, Love SS, Atwood JE, Wells TS, et al. (2004) Myopericarditis following smallpox vaccination. Am J Epidemiol 160:642–651. [DOI] [PubMed] [Google Scholar]
- 9. Marcinak J, Vora S, Weber S, Thomson K, Garcia-Houchins S, et al. (2007) Household transmission of vaccinia virus from contact with a military smallpox vaccine–Illinois and Indiana, 2007. MMWR Morb Mortal Wkly Rep 56:478–481. [PubMed] [Google Scholar]
- 10. Wertheimer ER, Olive DS, Brundage JF, Clark LL (2012) Contact transmission of vaccinia virus from smallpox vaccinees in the United States, 2003-2011. Vaccine 30:985–988. [DOI] [PubMed] [Google Scholar]
- 11. Neff JM, Lane JM, Fulginiti VA, Henderson DA (2002) Contact vaccinia–transmission of vaccinia from smallpox vaccination. Jama 288:1901–1905. [DOI] [PubMed] [Google Scholar]
- 12. Becker Y (2003) Vaccinia virus pathogenicity in atopic dermatitis is caused by allergen-induced immune response that prevents the antiviral cellular and humoral immunity. Virus Genes 27:269–282. [DOI] [PubMed] [Google Scholar]
- 13. Leung DY, Bieber T (2003) Atopic dermatitis. Lancet 361:151–160. [DOI] [PubMed] [Google Scholar]
- 14. De Benedetto A, Agnihothri R, McGirt LY, Bankova LG, Beck LA (2009) Atopic dermatitis: a disease caused by innate immune defects? J Invest Dermatol 129:14–30. [DOI] [PubMed] [Google Scholar]
- 15. Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y (2009) Mast cells in atopic dermatitis. Curr Opin Immunol 21:666–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gutermuth J, Ollert M, Ring J, Behrendt H, Jakob T (2004) Mouse models of atopic eczema critically evaluated. Int Arch Allergy Immunol 135:262–276. [DOI] [PubMed] [Google Scholar]
- 17. Shiohara T, Hayakawa J, Mizukawa Y (2004) Animal models for atopic dermatitis: are they relevant to human disease? J Dermatol Sci 36:1–9. [DOI] [PubMed] [Google Scholar]
- 18. Vestergaard C, Yoneyama H, Matsushima K (2000) The NC/Nga mouse: a model for atopic dermatitis. Mol Med Today 6:209–210. [DOI] [PubMed] [Google Scholar]
- 19. Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, et al. (1998) Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest 101:1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin H, He R, Oyoshi M, Geha RS (2009) Animal models of atopic dermatitis. J Invest Dermatol 129:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A (2004) Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22:460–466. [DOI] [PubMed] [Google Scholar]
- 22. Kawakami Y, Tomimori Y, Yumoto K, Hasegawa S, Ando T, et al. (2009) Inhibition of NK cell activity by IL-17 allows vaccinia virus to induce severe skin lesions in a mouse model of eczema vaccinatum. J Exp Med 206:1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oyoshi MK, Elkhal A, Kumar L, Scott JE, Koduru S, et al. (2009) Vaccinia virus inoculation in sites of allergic skin inflammation elicits a vigorous cutaneous IL-17 response. Proc Natl Acad Sci U S A 106:14954–14959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scott JE, ElKhal A, Freyschmidt EJ, MacArthur DH, McDonald D, et al. (2007) Impaired immune response to vaccinia virus inoculated at the site of cutaneous allergic inflammation. J Allergy Clin Immunol 120:1382–1388. [DOI] [PubMed] [Google Scholar]
- 25. Meseda CA, Garcia AD, Kumar A, Mayer AE, Manischewitz J, et al. (2005) Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model. Virology 339:164–175. [DOI] [PubMed] [Google Scholar]
- 26. Nemeckova S, Hainz P, Otahal P, Gabriel P, Sroller V, et al. (2001) Early gene expression of vaccinia virus strains replicating (Praha) and non-replicating (modified vaccinia virus strain Ankara, MVA) in mammalian cells. Acta Virol 45:243–247. [PubMed] [Google Scholar]
- 27. Kutinova L, Ludvikova V, Simonova V, Otavova M, Krystofova J, et al. (1995) Search for optimal parent for recombinant vaccinia virus vaccines. Study of three vaccinia virus vaccinal strains and several virus lines derived from them. Vaccine 13:487–493. [DOI] [PubMed] [Google Scholar]
- 28. Kutinova L, Ludvikova V, Krystofova J, Otavova M, Simonova V, et al. (1996) Influence of the parental virus strain on the virulence and immunogenicity of recombinant vaccinia viruses expressing HBV preS2-S protein or VZV glycoprotein I. Vaccine 14:1045–1052. [DOI] [PubMed] [Google Scholar]
- 29. Liskova J, Knitlova J, Honner R, Melkova Z (2011) Apoptosis and necrosis in vaccinia virus-infected HeLa G and BSC-40 cells. Virus Res 160:40–50. [DOI] [PubMed] [Google Scholar]
- 30. Kalbacova M, Spisakova M, Liskova J, Melkova Z (2008) Lytic infection with vaccinia virus activates caspases in a Bcl-2-inhibitable manner. Virus Res 135:53–63. [DOI] [PubMed] [Google Scholar]
- 31. Joklik WK (1962) The purification fo four strains of poxvirus. Virology 18:9–18. [DOI] [PubMed] [Google Scholar]
- 32. Barchichat S, Katz E (2002) Immunization of rabbits with a modified vaccinia Ankara recombinant virus bearing the HIV envelope antigen on its outer membrane. Virus Res 90:243–251. [DOI] [PubMed] [Google Scholar]
- 33. Humlova Z, Vokurka M, Esteban M, Melkova Z (2002) Vaccinia virus induces apoptosis of infected macrophages. J Gen Virol 83:2821–2832. [DOI] [PubMed] [Google Scholar]
- 34. Combadiere B, Boissonnas A, Carcelain G, Lefranc E, Samri A, et al. (2004) Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans. J Exp Med 199:1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kawakami Y, Yumoto K, Kawakami T (2007) An improved mouse model of atopic dermatitis and suppression of skin lesions by an inhibitor of Tec family kinases. Allergol Int 56:403–409. [DOI] [PubMed] [Google Scholar]
- 36. Frey SE, Couch RB, Tacket CO, Treanor JJ, Wolff M, et al. (2002) Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med 346:1265–1274. [DOI] [PubMed] [Google Scholar]
- 37. Matsumoto M, Ra C, Kawamoto K, Sato H, Itakura A, et al. (1999) IgE hyperproduction through enhanced tyrosine phosphorylation of Janus kinase 3 in NC/Nga mice, a model for human atopic dermatitis. J Immunol 162:1056–1063. [PubMed] [Google Scholar]
- 38. Suto H, Matsuda H, Mitsuishi K, Hira K, Uchida T, et al. (1999) NC/Nga mice: a mouse model for atopic dermatitis. Int Arch Allergy Immunol 120 Suppl 170–75. [DOI] [PubMed] [Google Scholar]
- 39. Yagi R, Nagai H, Iigo Y, Akimoto T, Arai T, et al. (2002) Development of atopic dermatitis-like skin lesions in STAT6-deficient NC/Nga mice. J Immunol 168:2020–2027. [DOI] [PubMed] [Google Scholar]
- 40. Mamessier E, Botturi K, Vervloet D, Magnan A (2005) [T regulatory lymphocytes, atopy and asthma: a new concept in three dimensions]. Rev Mal Respir 22:305–311. [DOI] [PubMed] [Google Scholar]
- 41. Anderson E, Pierre-Louis WS, Wong CJ, Lary JW, Cole JL (2011) Heparin activates PKR by inducing dimerization. J Mol Biol 413:973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rager KJ, Langland JO, Jacobs BL, Proud D, Marsh DG, et al. (1998) Activation of antiviral protein kinase leads to immunoglobulin E class switching in human B cells. J Virol 72:1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davies MV, Chang HW, Jacobs BL, Kaufman RJ (1993) The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J Virol 67:1688–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Williams BR (1999) PKR; a sentinel kinase for cellular stress. Oncogene 18:6112–6120. [DOI] [PubMed] [Google Scholar]
- 45. Baixeras E, Cebrian A, Albar JP, Salas J, Martinez AC, et al. (1998) Vaccinia virus-induced apoptosis in immature B lymphocytes: role of cellular Bcl-2. Virus Res 58:107–113. [DOI] [PubMed] [Google Scholar]
- 46. Engelmayer J, Larsson M, Subklewe M, Chahroudi A, Cox WI, et al. (1999) Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol 163:6762–6768. [PubMed] [Google Scholar]
- 47. Haga IR, Bowie AG (2005) Evasion of innate immunity by vaccinia virus. Parasitology 130 Suppl: S11–25 [DOI] [PubMed] [Google Scholar]
- 48. Martin RM, Brady JL, Lew AM (1998) The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods 212:187–192. [DOI] [PubMed] [Google Scholar]
- 49. Oyoshi MK, Ramesh N, Geha RS (2011) Vaccinia Ig ameliorates eczema vaccinatum in a murine model of atopic dermatitis. J Invest Dermatol 132:1299–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. von Sonnenburg F, Perona P, Darsow U, Ring J, von Krempelhuber A, et al. (2014) Safety and immunogenicity of modified vaccinia Ankara as a smallpox vaccine in people with atopic dermatitis. Vaccine 32:5696–5702. [DOI] [PubMed] [Google Scholar]
- 51. Guzman E, Cubillos-Zapata C, Cottingham MG, Gilbert SC, Prentice H, et al. (2012) Modified vaccinia virus Ankara-based vaccine vectors induce apoptosis in dendritic cells draining from the skin via both the extrinsic and intrinsic caspase pathways, preventing efficient antigen presentation. J Virol 86:5452–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perdiguero B, Gomez CE, Najera JL, Sorzano CO, Delaloye J, et al. (2012) Deletion of the viral anti-apoptotic gene F1L in the HIV/AIDS vaccine candidate MVA-C enhances immune responses against HIV-1 antigens. PLoS One 7:e48524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reed JL, Scott DE, Bray M (2012) Eczema vaccinatum. Clin Infect Dis 54:832–840. [DOI] [PubMed] [Google Scholar]
- 54. Stahlberg A, Hakansson J, Xian X, Semb H, Kubista M. (2004) Properties of the reverse transcription reaction in mRNA quantification. Clin Chem 50:509–515. [DOI] [PubMed] [Google Scholar]
- 55. Miljkovic Z, Momcilovic M, Miljkovic D, Mostarica-Stojkovic M (2008) Methylprednisolone inhibits IFN-gamma and IL-17 expression and production by cells infiltrating central nervous system in experimental autoimmune encephalomyelitis. J Neuroinflammation 6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of IL-17A and IFNγ cytokines in the skin after WR inoculation. Skin biopsies were taken from mock- and OVA-sensitized Nc/Nga and Balb/c mice 5 days after mock-inoculation with OVA in PBS (PBS) or after inoculation with 107 PFU of a purified stock of WR with OVA in PBS (WR). Expression of individual cytokines in the skin was determined by real-time RT-PCR and standardized to GAPDH (expressed as ratio cytokine/GAPDH ×105). Data represent mean +/− S.E.M.; number of animals in Nc/Nga and Balb/c groups: 3 and 4, respectively. *P<0.05, **P<0.01, *** P<0.001. S1 Figure Methods: Determination of cytokine expression by real-time RT-PCR. Biopsies of skin lesions were stored in RNAlater RNA Stabilization Reagent (Qiagene) or Allprotect Tissue Reagent (Qiagene). RNA was isolated after homogenization of the skin samples in RNA Blue (Top-Bio, Czech Republic) using manufacturer's protocol. Purified RNA was resuspended in RNase-free water with the addition of RNase inhibitor RiboLock (Fermentas). RNA concentration and purity were determined by measuring the absorbance at 260 and 280 nm, respectively, using UV spectrophotometer BioPhotometer (Eppendorf AG). RNA was treated with RNase-free DNase (Fermentas) in the presence of RiboLock and quantification of RNA of interest was performed by Power SYBR Green RNA-to-CT 1-Step Kit (Applied Biosystems) using Applied Biosystems 7300 Real-time PCR System according to manufacturer's protocol. Quantification of each RNA was performed in duplicate together with GAPDH using 50 ng of RNA in 20 µl reactions and 40 cycles. Specific primers for individual genes were described previously or newly designed using Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/): Mu GAPDH cDNA forward 5′–CGGTGCTGAGTATGTCGTGGA–3′, reverse 5′–GGCAGAAGGGGCGGAGATGA–3′ [54]; Mu IL-17A cDNA forward 5′–GGACTCTCCACCGCAATGAA–3′, reverse 5′–TTTCCCTCCGCATTGACACA–3′; Mu IFNγ cDNA forward 5′–TGGCATAGATGTGGAAGAAAAGAG–3′, reverse 5′–TGCAGGATTTTCATGTCACCA–3′ [55].
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.