Abstract
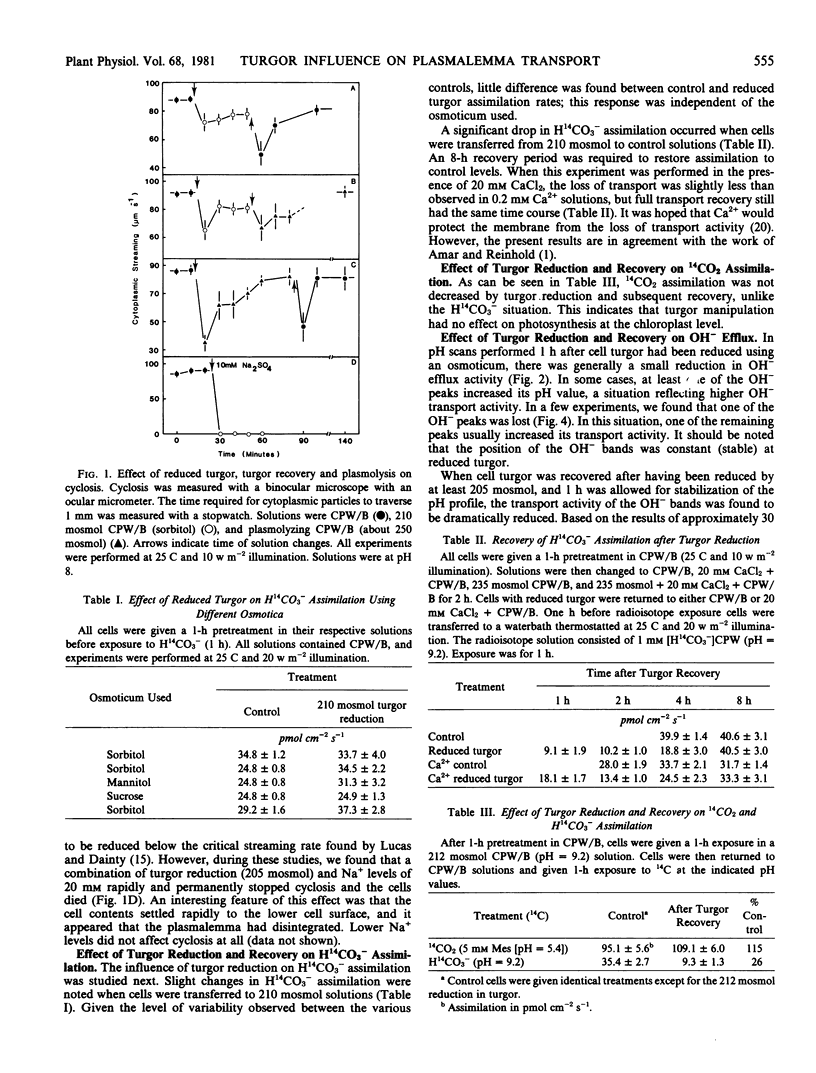
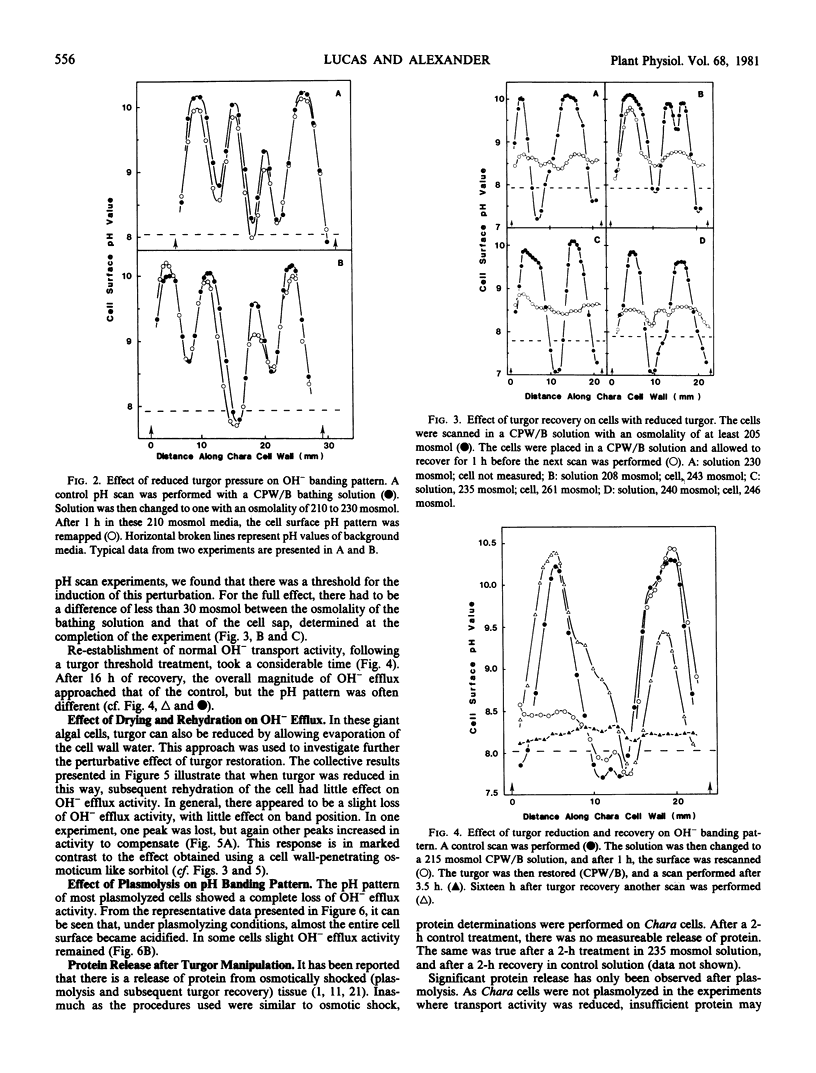
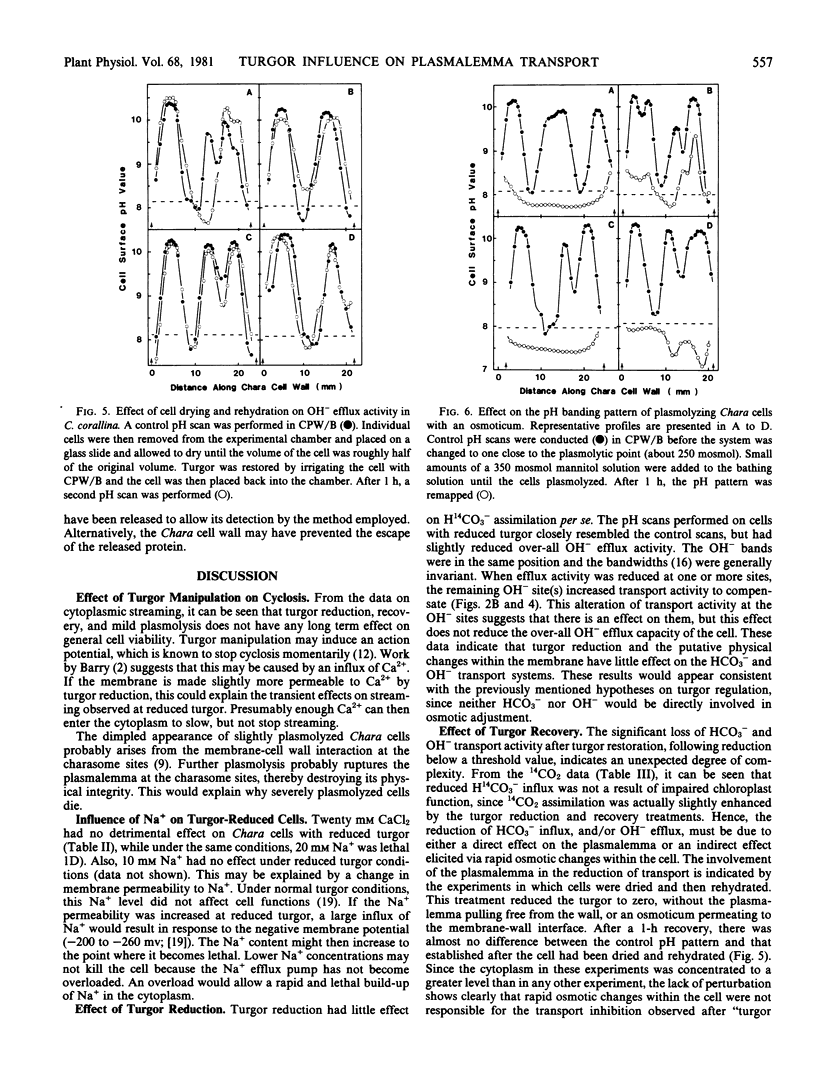
A modified version of the osmotic shock technique was used to investigate HCO3− and OH− transport in the alga Chara corallina. Cell turgor was brought close to zero and then restored. When turgor was reduced to near the plasmolytic point using an osmoticum, little effect was observed on H14CO3− assimilation and OH− transport. However, when turgor was recovered in these cells, there was a large reduction in HCO3− and OH− transport activity. In contrast, when cells were air-dried to zero turgor, and rewetted to restore turgor, no significant effect on OH− transport was observed.
The effect of plasmolysis was also studied. When Chara cells were plasmolyzed, in most cases, all OH− transport activity was lost and acidification was observed along the cell surface.
None of the above treatments had any long term effect on cyclosis. These results are discussed with respect to present concepts of turgorinduced changes in membrane functions.
Full text
PDF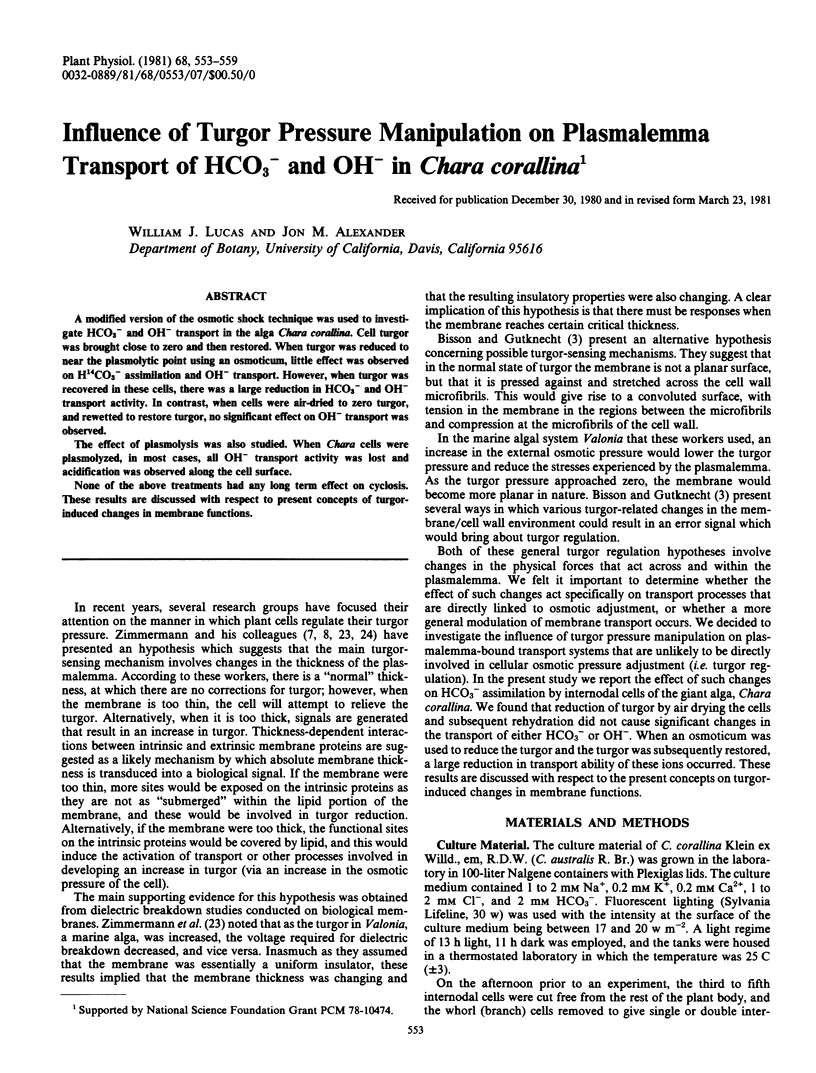



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar L., Reinhold L. Loss of membrane transport ability in leaf cells and release of protein as a result of osmotic shock. Plant Physiol. 1973 Apr;51(4):620–625. doi: 10.1104/pp.51.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry W. H. Coupling of excitation and cessation of cyclois in Nitella: role of divalent cations. J Cell Physiol. 1968 Dec;72(3):153–160. doi: 10.1002/jcp.1040720303. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Briskin D. P., Leonard R. T. Ion Transport in Isolated Protoplasts from Tobacco Suspension Cells: III. Membrane Potential. Plant Physiol. 1979 Dec;64(6):959–962. doi: 10.1104/pp.64.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster H. G. Turgor pressure sensing in plant cell membranes. Plant Physiol. 1976 Nov;58(5):636–643. doi: 10.1104/pp.58.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas W. J., Dainty J. HCO(3) Influx Across the Plasmalemma of Chara corallina: Divalent Cation Requirement. Plant Physiol. 1977 Dec;60(6):862–867. doi: 10.1104/pp.60.6.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas W. J., Dainty J. Spatial distribution of functional OH- carriers along a characean internodal cell: determined by the effect of cytochalasin B on H14CO3- assimilation. J Membr Biol. 1977 Apr 7;32(1-2):75–92. doi: 10.1007/BF01905210. [DOI] [PubMed] [Google Scholar]
- Lucas W. J., Ferrier J. M., Dainty J. Plasmalemma transport of OH- in Chara corallina: dynamics of activation and deactivation. J Membr Biol. 1977 Apr 7;32(1-2):49–73. doi: 10.1007/BF01905209. [DOI] [PubMed] [Google Scholar]
- Lucas W. J. HCO(3) Influx across the Plasmalemma of Chara corallina: Physiological and Biophysical Influence of 10 mm K. Plant Physiol. 1978 Apr;61(4):487–493. doi: 10.1104/pp.61.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Nichoalds G. E., Oxender D. L. Cellular localization of leucine-binding protein from Escherichia coli. Science. 1968 Jul 12;161(3837):182–183. doi: 10.1126/science.161.3837.182. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Racusen R. H., Kinnersley A. M., Galston A. W. Osmotically induced changes in electrical properties of plant protoplast membranes. Science. 1977 Oct 28;198(4315):405–407. doi: 10.1126/science.198.4315.405. [DOI] [PubMed] [Google Scholar]


