Abstract
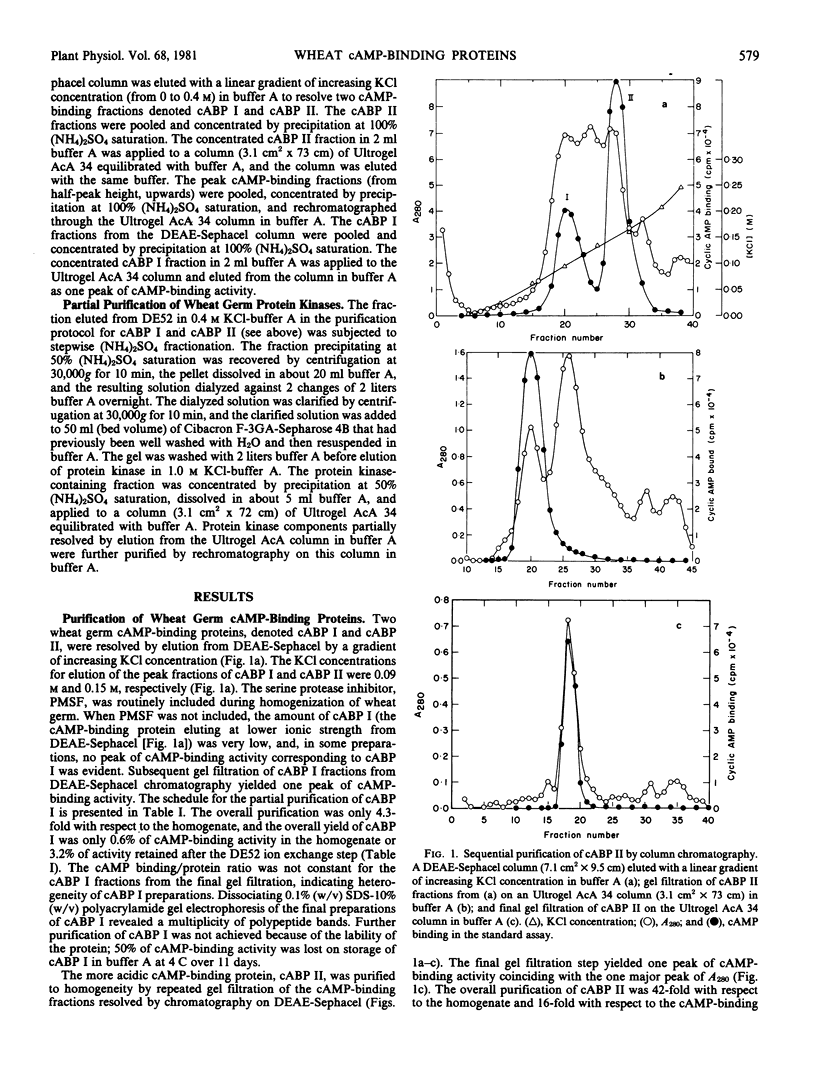
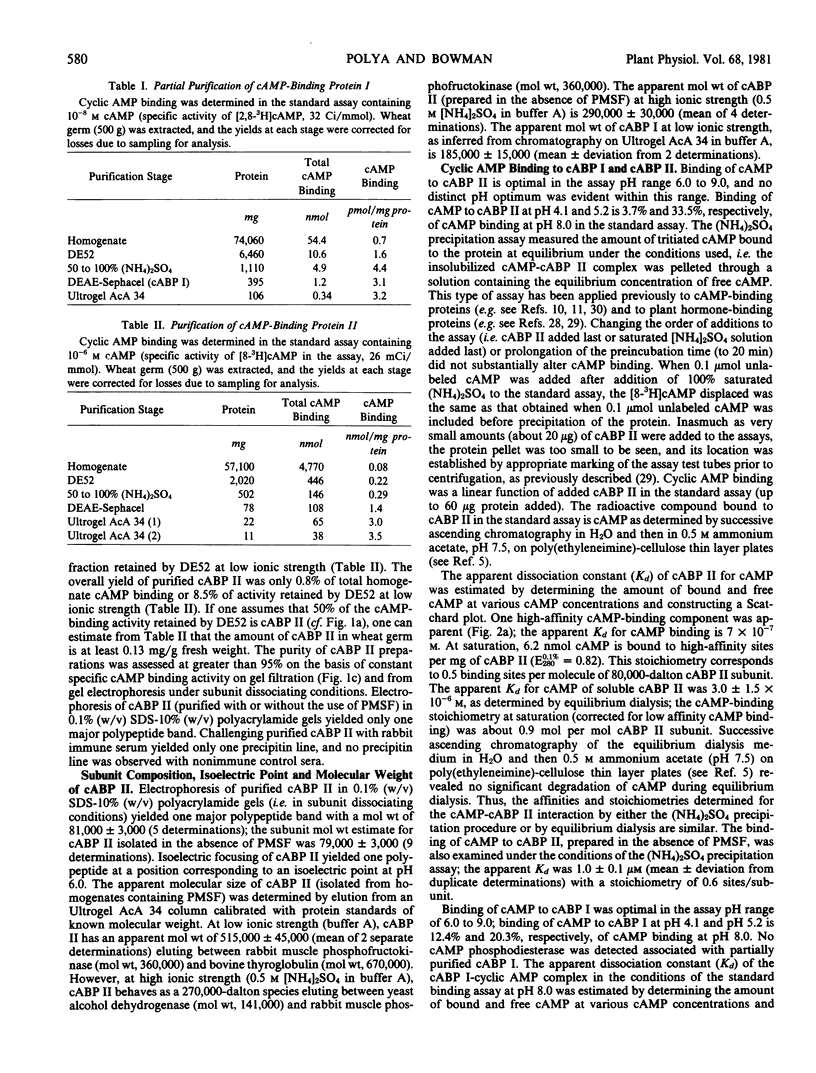
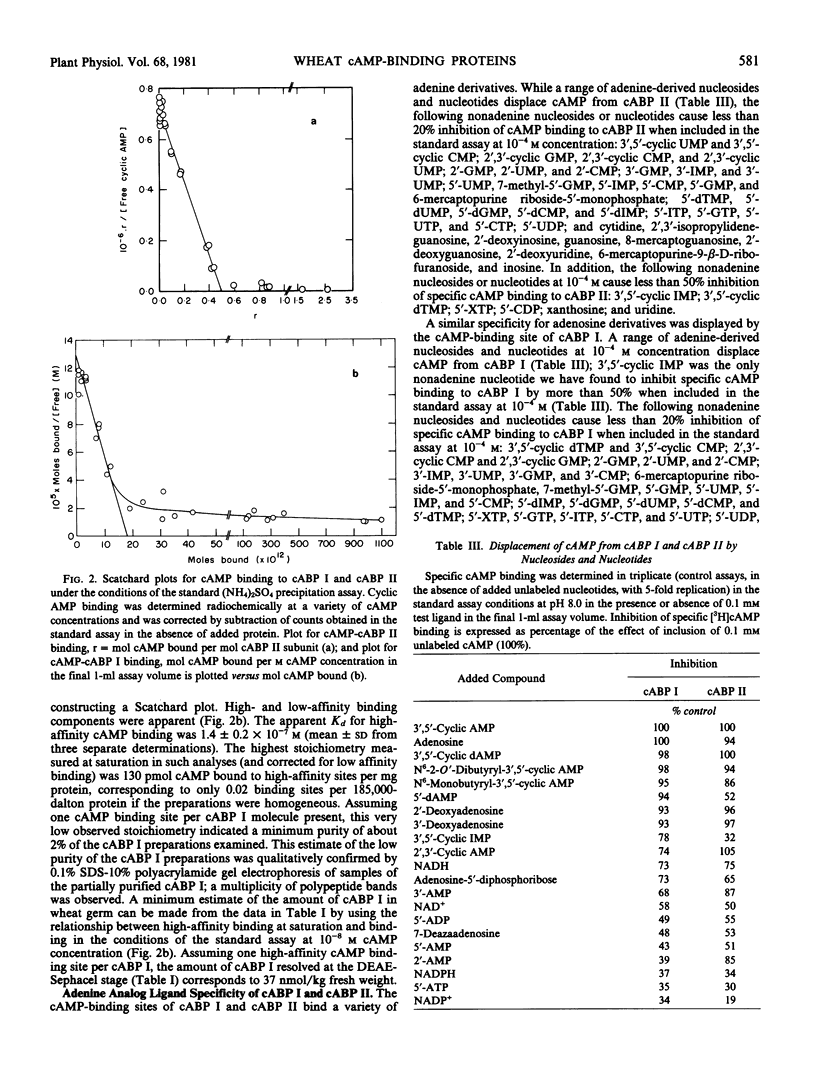
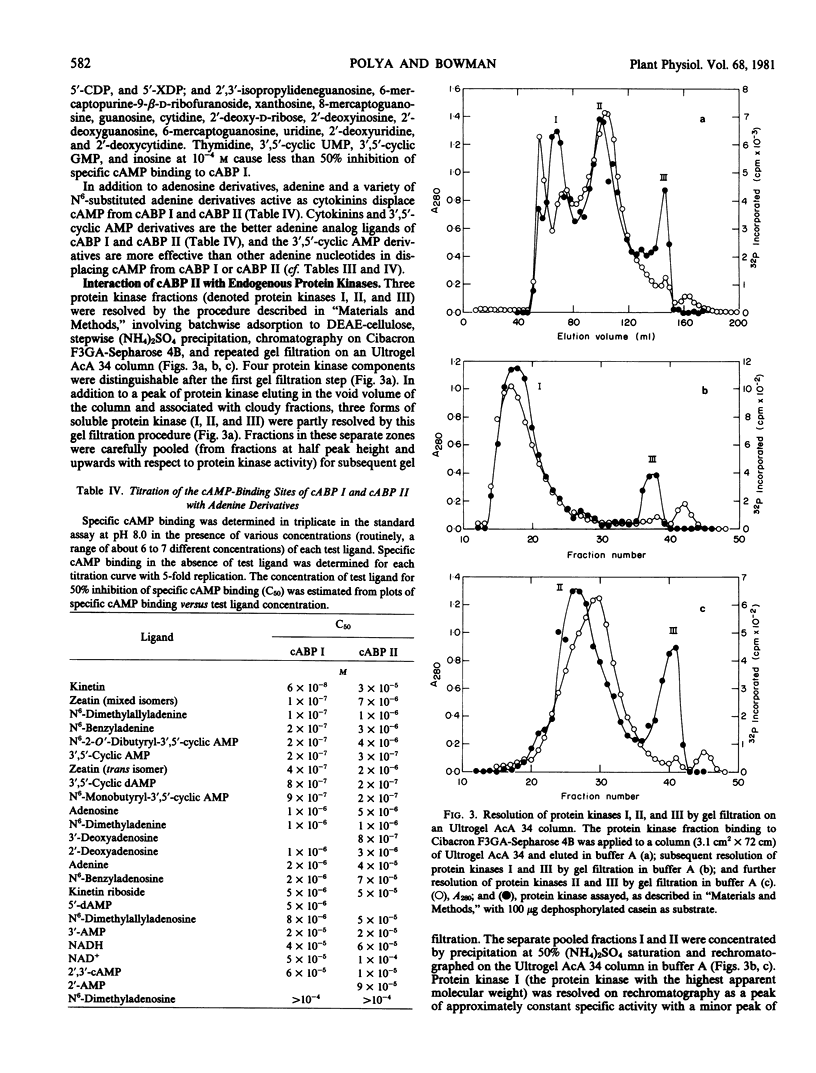
A high affinity cAMP-binding protein (cABP II) was purified to homogeneity from wheat germ. The apparent molecular weight of cABP II, as determined from gel exclusion chromatography, is 5.2 × 105 (at low ionic strength) and 2.8 × 105 (at high ionic strength). One polypeptide subunit (molecular weight, 80,000) was resolved by polyacrylamide gel electrophoresis of cABP II under subunit dissociating conditions. The purification protocol employed resolves cABP II from a distinct, less acidic cAMP-binding protein (cABP I). The Kd values for cAMP are about 10−6 molar and 10−7 molar for cABP II and cABP I, respectively. The cAMP-binding sites of cABP I and cABP II have a marked adenine-analog specificity, binding adenine, adenosine, adenine-derived nucleosides and nucleotides and a variety of adenine derivatives having cytokinin activity. While cABP II is phosphorylated in reactions catalyzed by endogenous protein kinases, there is no evidence for modulation of these cABP II-protein kinase interactions by cAMP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton A. R., Polya G. M. Adenosine 3':5'-cyclic monophosphate in higher plants: Isolation and characterization of adenosine 3':5'-cyclic monophosphate from Kalanchoe and Agave. Biochem J. 1977 Jul 1;165(1):27–32. doi: 10.1042/bj1650027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Polya G. M. Cyclic adenosine 3':5'-monophosphate in axenic rye grass endosperm cell cultures. Plant Physiol. 1978 May;61(5):718–722. doi: 10.1104/pp.61.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Polya G. M. Higher-plant cyclic nucleotide phosphodiesterases. Resolution, partial purification and properties of three phosphodiesterases from potato tuber. Biochem J. 1975 Aug;149(2):329–339. doi: 10.1042/bj1490329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bideon G. M. Purification and characterization of a cyclic nucleotide-regulated 5'-nucleotidase from potatoe. Biochim Biophys Acta. 1975 Apr 19;384(2):443–457. doi: 10.1016/0005-2744(75)90045-5. [DOI] [PubMed] [Google Scholar]
- Brewin N. J., Northcote D. H. Partial purification of a cyclic AMP phosphodiesterase from soybean callus. Isolation of a non-dialysable inhibitor. Biochim Biophys Acta. 1973 Aug 17;320(1):104–122. doi: 10.1016/0304-4165(73)90171-2. [DOI] [PubMed] [Google Scholar]
- Brownlee A. G., Phillips D. R., Polya G. M. Purification and characterization of two high-affinity (adenosine 3',5'-monophosphate)-binding proteins from yeast. Identification as multiple forms of glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1980 Aug;109(1):39–49. doi: 10.1111/j.1432-1033.1980.tb04765.x. [DOI] [PubMed] [Google Scholar]
- Brownlee A. G., Polya G. M. The ligand specificity of the (adenosine 3',5'-monophosphate)-binding site of yeast glyceraldehyde-3-phosphate dehydrogenase. Interaction with adenosine derivatives and pharmacologically-active compounds. Eur J Biochem. 1980 Aug;109(1):51–59. doi: 10.1111/j.1432-1033.1980.tb04766.x. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of cyclic-AMP-dependent protein kinase in the regulation of glycogen metabolism in mammalian skeletal muscle. Curr Top Cell Regul. 1978;14:117–196. doi: 10.1016/b978-0-12-152814-0.50008-3. [DOI] [PubMed] [Google Scholar]
- Giannattasio M., Carratu G., Tucci G. F. Presence of a cyclic-AMP-binding protein in Jerusalem artichoke rhizome tissues. FEBS Lett. 1974 Dec 15;49(2):249–253. doi: 10.1016/0014-5793(74)80523-5. [DOI] [PubMed] [Google Scholar]
- Heyns W., De Moor P. A 3(17)beta-hydroxysteroid dehydrogenase in raterythrocytes. Conversion of 5alpha-dihydrotestosterone into 5alpha-androstane-3beta,17beta-diol and purification of the enzyme by affinity chromatography. Biochim Biophys Acta. 1974 Jul 17;358(1):1–13. doi: 10.1016/0005-2744(74)90251-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanzani G. A., Giannattasio M., Manzocchi L. A., Bollini R., Soffientini A. N., Macchia V. The influence of cyclic GMP on polypeptide synthesis in a cell-free system derived from wheat embryos. Biochem Biophys Res Commun. 1974 May 7;58(1):172–177. doi: 10.1016/0006-291x(74)90907-3. [DOI] [PubMed] [Google Scholar]
- Lin P. P. Cyclic nucleotides in higher plants? Adv Cyclic Nucleotide Res. 1974;4(0):439–460. [PubMed] [Google Scholar]
- Lin P. P., Varner J. E. Cyclic nucleotide phosphodiesterase in pea seedlings. Biochim Biophys Acta. 1972 Aug 28;276(2):454–474. doi: 10.1016/0005-2744(72)91007-8. [DOI] [PubMed] [Google Scholar]
- Morrison J. F. Chromatographic separation of nucleoside phosphates on diethylaminoethylcellulose paper. Anal Biochem. 1968 Jul;24(1):106–111. doi: 10.1016/0003-2697(68)90064-x. [DOI] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polya G. M., Bowman J. A. Ligand Specificity of a High Affinity Cytokinin-binding Protein. Plant Physiol. 1979 Sep;64(3):387–392. doi: 10.1104/pp.64.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polya G. M. Regulation of a plant 5'(3')-ribonucleotide phosphohydrolase by cyclic nucleotides and pyrimidine, purine, and cytokinin ribosides. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1299–1303. doi: 10.1073/pnas.71.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M., Rangel-Aldao R., Erlichman J. Soluble cyclic AMP-dependent protein kinases: review of the enzyme isolated from bovine cardiac muscle. Curr Top Cell Regul. 1977;12:39–74. [PubMed] [Google Scholar]
- Schendel P. F., Wells R. D. The synthesis and purification of (gamma-32P)-adenosine triphosphate with high specific activity. J Biol Chem. 1973 Dec 10;248(23):8319–8321. [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]


