Abstract
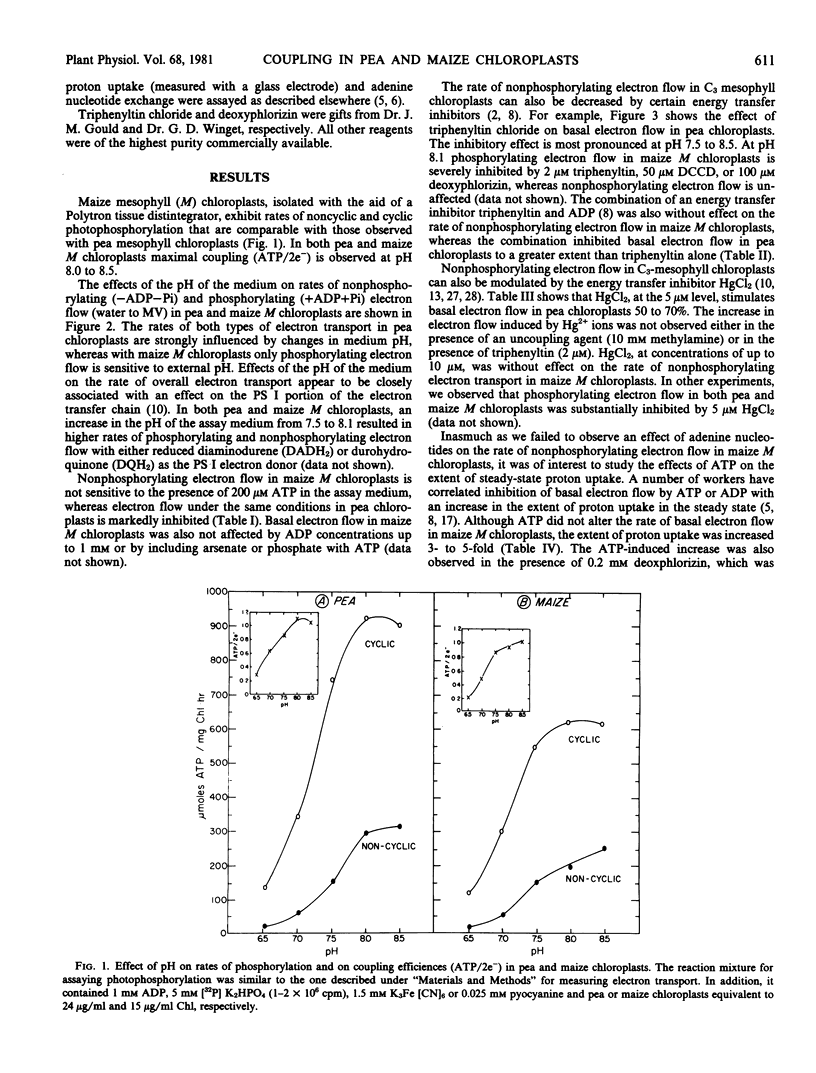
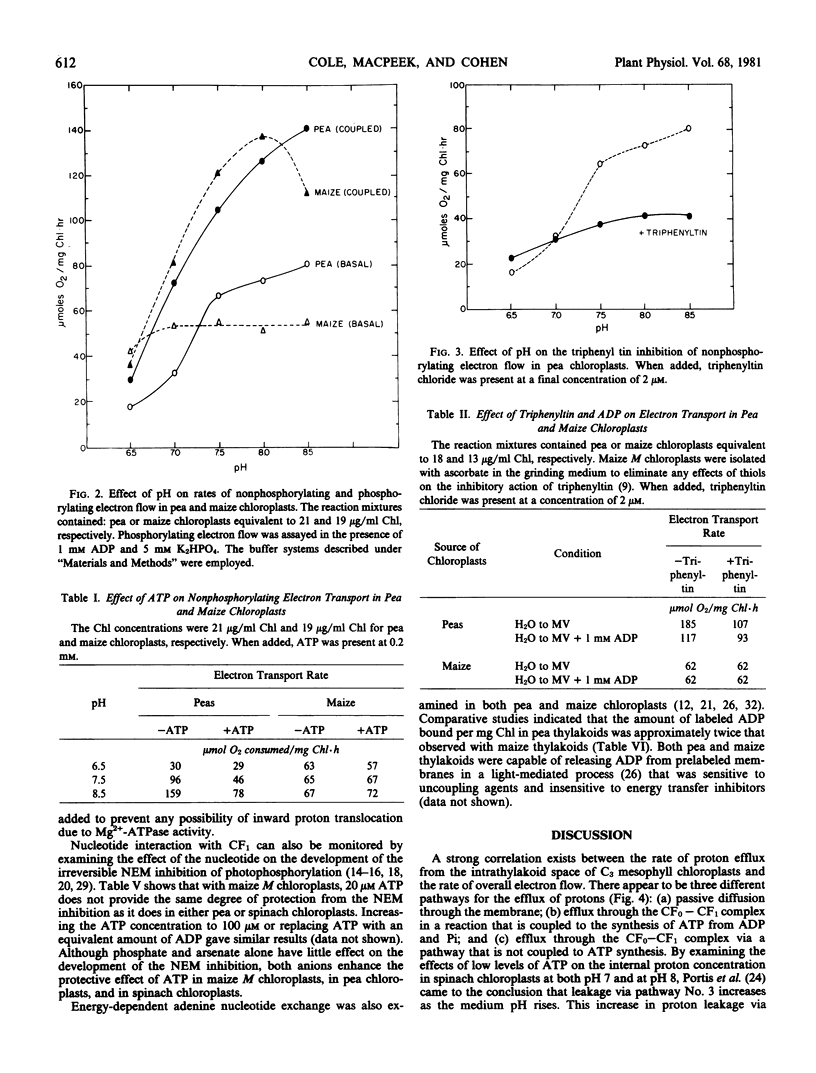
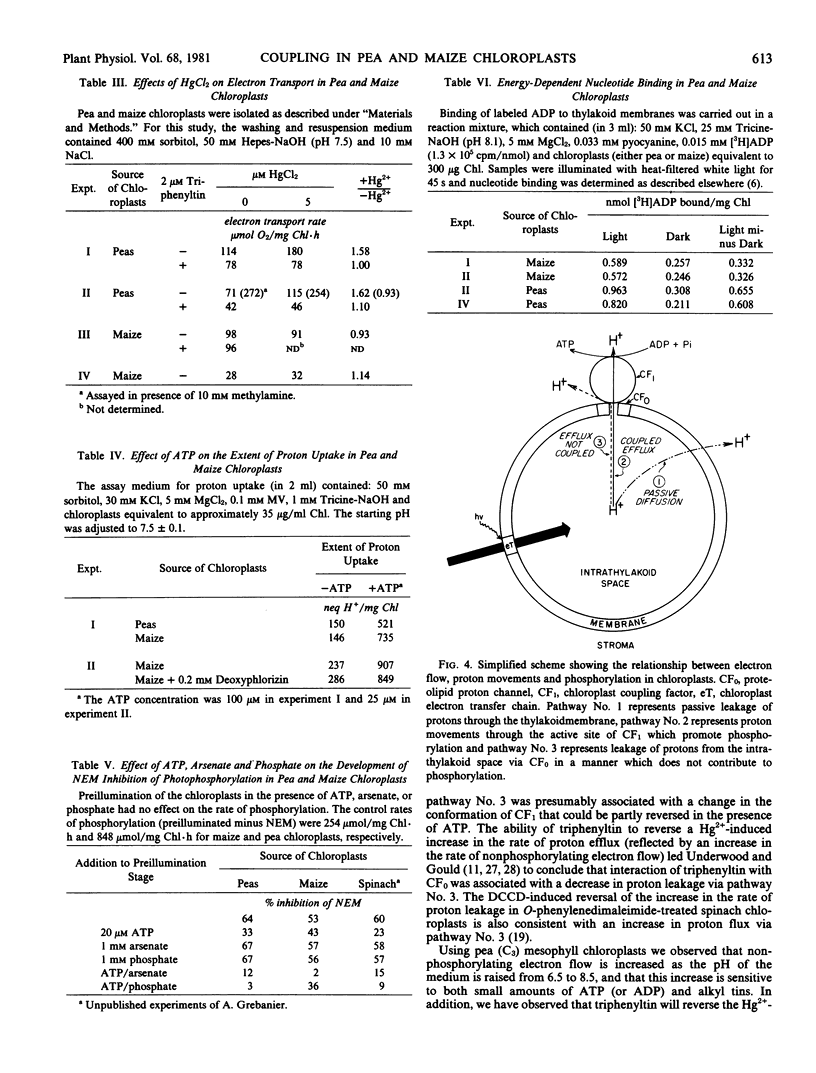
The rate of nonphosphorylating electron transport (in the absence of ADP and inorganic phosphate) in well-coupled (ATP/2e− = 0.9-1.1) maize mesophyll chloroplasts is not modulated by external pH (6.5-8.5), low levels of ADP or ATP, or energy transfer inhibitors, e.g. triphenyltin and Hg2+ ions. In contrast nonphosphorylating electron flow in pea chloroplasts is sensitive to alterations in medium pH, and to the presence of adenine nucleotides and energy transfer inhibitors in the assay medium. Although ATP is without effect on the rate of basal electron transport in maize chloroplasts, steady-state proton uptake is stimulated 3- to 5-fold by low levels of ATP. These results suggest that differences may exist in the manner in which the coupling factor complex controls proton efflux from the intrathylakoid space in C3 and C4 mesophyll chloroplasts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. R., Leech R. M. Development of Photosystem I and Photosystem II Activities in Leaves of Light-grown Maize (Zea mays). Plant Physiol. 1977 Oct;60(4):640–644. doi: 10.1104/pp.60.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg M. W., Whitmarsh J., Rieck C. E., Cohen W. S. Inhibition of photosynthetic electron transport by diphenyl ether herbicides. Plant Physiol. 1980 Jan;65(1):47–50. doi: 10.1104/pp.65.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen W. S., Macpeek W. A. A proposed mechanism for the stimulatory effect of bicarbonate ions on ATP synthesis in isolated chloroplasts. Plant Physiol. 1980 Aug;66(2):242–245. doi: 10.1104/pp.66.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J. M. Inhibition by triphenyltin chloride of a tightly-bound membrane component involved in photophosphorylation. Eur J Biochem. 1976 Mar 1;62(3):567–575. doi: 10.1111/j.1432-1033.1976.tb10191.x. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Izawa S. Studies on the energy coupling sites of photophosphorylation. I. Separation of site I and site II by partial reactions of the chloroplast electron transport chain. Biochim Biophys Acta. 1973 Aug 31;314(2):211–223. doi: 10.1016/0005-2728(73)90136-9. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Underwood C. Hg2+-induced turnover of the chloroplast ATP synthetase complex in the absence of ADP and phosphate. FEBS Lett. 1978 Nov 15;95(2):197–201. doi: 10.1016/0014-5793(78)80992-2. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Slater E. D. Tightly bound nucleotides of the energy-transducing ATPase of chloroplasts and their role in photophosphorylation. Biochim Biophys Acta. 1975 May 15;387(2):335–348. doi: 10.1016/0005-2728(75)90114-0. [DOI] [PubMed] [Google Scholar]
- Magnusson R. P., McCarty R. E. Light-induced exchange of nucleotides into coupling factor 1 in spinach chloroplast thylakoids. J Biol Chem. 1976 Dec 10;251(23):7417–7422. [PubMed] [Google Scholar]
- McCarty R. E., Fuhrman J. S., Tsuchiya Y. Effects of adenine nucleotides on hydrogen-ion transport in chloroplasts. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2522–2526. doi: 10.1073/pnas.68.10.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty R. E., Pittman P. R., Tsuchiya Y. Light-dependent inhibition of photophosphorylation by N-ethylmaleimide. J Biol Chem. 1972 May 25;247(10):3048–3051. [PubMed] [Google Scholar]
- McCarty R. E., Portis A. R., Jr A simple, quantitative approach to the coupling of photophosphorylation to electron flow in terms of proton fluxes. Biochemistry. 1976 Nov 16;15(23):5110–5114. doi: 10.1021/bi00668a025. [DOI] [PubMed] [Google Scholar]
- Nelson N. Structure and function of chloroplast ATPase. Biochim Biophys Acta. 1976 Nov 30;456(3-4):314–338. doi: 10.1016/0304-4173(76)90003-3. [DOI] [PubMed] [Google Scholar]
- Portis A. R., Jr, Magnusson R. P., McCarty R. E. Conformational changes in coupling factor 1 may control the rate of electron flow in spinach chloroplasts. Biochem Biophys Res Commun. 1975 Jan 2;64(3):877–884. doi: 10.1016/0006-291x(75)90129-1. [DOI] [PubMed] [Google Scholar]
- Portis A. R., Jr, McCarty R. E. Effects of adenine nucleotides and of photophosphorylation on H+ uptake and the magnitude of the H+ gradient in illuminated chloroplasts. J Biol Chem. 1974 Oct 10;249(19):6250–6254. [PubMed] [Google Scholar]
- Portis A. R., Jr, McCarty R. E. Quantitative relationships between phosphorylation, electron flow, and internal hydrogen ion concentrations in spinach chloroplasts. J Biol Chem. 1976 Mar 25;251(6):1610–1617. [PubMed] [Google Scholar]
- Strotmann H., Bickel S., Huchzermeyer B. Energy-dependent release of adenine nucleotides tightly bound to chloroplast coupling factor CF1. FEBS Lett. 1976 Jan 15;61(2):194–198. doi: 10.1016/0014-5793(76)81036-8. [DOI] [PubMed] [Google Scholar]
- Underwood C., Gould J. M. Modulation of proton efflux from chloroplasts in the light by external pH. Arch Biochem Biophys. 1980 Oct 1;204(1):241–246. doi: 10.1016/0003-9861(80)90029-6. [DOI] [PubMed] [Google Scholar]
- Underwood C., Gould J. M. Proton efflux through the chloroplast ATP synthase (CF0 . CF1) in the presence of sulfhydryl-modifying agents. Biochim Biophys Acta. 1980 Feb 8;589(2):287–298. doi: 10.1016/0005-2728(80)90045-6. [DOI] [PubMed] [Google Scholar]
- Weiss M. A., McCarty R. E. Cross-linking within a subunit of coupling factor 1 increases the proton permeability of spinach chloroplast thylakoids. J Biol Chem. 1977 Nov 25;252(22):8007–8012. [PubMed] [Google Scholar]



