Abstract
Background
Legionella pneumophila is an intracellular bacterial pathogen that invades and replicates within alveolar macrophages through injection of ∼300 effector proteins by its Dot/Icm type IV translocation apparatus. The bona fide F-box protein, AnkB, is a nutritional virulence effector that triggers macrophages to generate a surplus of amino acids, which is essential for intravacuolar proliferation. Therefore, the ankB mutant represents a novel genetic tool to determine the transcriptional response of human monocyte-derived macrophages (hMDMs) to actively replicating L. pneumophila.
Methodology/Principal Findings
Here, we utilized total human gene microarrays to determine the global transcriptional response of hMDMs to infection by wild type or the ankB mutant of L. pneumophila. The transcriptomes of hMDMs infected with either actively proliferating wild type or non-replicative ankB mutant bacteria were remarkably similar. The transcriptome of infected hMDMs was predominated by up-regulation of inflammatory pathways (IL-10 anti-inflammatory, interferon signaling and amphoterin signaling), anti-apoptosis, and down-regulation of protein synthesis pathways. In addition, L. pneumophila modulated diverse metabolic pathways, particularly those associated with bio-active lipid metabolism, and SLC amino acid transporters expression.
Conclusion/Significance
Taken together, the hMDM transcriptional response to L. pneumophila is independent of intra-vacuolar replication of the bacteria and primarily involves modulation of the immune response and metabolic as well as nutritional pathways.
Introduction
Legionella pneumophila is found ubiquitously in the aquatic environment and shares an intimate intracellular relationship with many species of amoeba and ciliates [1], [2], [3]. L. pneumophila, the causative agent of Legionnaires' disease, invades and replicates in human alveolar macrophages [4], [5]. When L. pneumophila invades amoeba or human macrophages, it evades the default endosomal-lysosomal degradation pathway and remodels its phagosome into a specialized ER-derived vacuole via intercepting ER-to-golgi vesicular trafficking [2], [3], [5], [6]. This is achieved by the translocation of ∼300 effector proteins via the Dot/Icm type IVB secretion system [5], [7], [8], [9]. These effectors modulate a myriad of eukaryotic processes including host signaling, vesicular trafficking, protein synthesis, apoptosis, prenylation, ubiquitination, and proteasomal degradation [2], [6], [10], [11], [12], [13]. Surprisingly, very few of these effectors are essential for intracellular replication of L. pneumophila [5], [14], suggesting specific requirements for different effectors in different environmental hosts.
The AnkB translocated effector is one of very few effectors essential for proliferation of L. pneumophila strain AA100/130B within the two evolutionarily-distant hosts, mammalian and protozoan cells, and for intrapulmonary bacterial proliferation and manifestation of pulmonary disease in the mouse model [15], [16], [17], [18], [19]. In addition, AnkB in L. pneumophila strain Paris contributes to intravacuolar proliferation in the THP-1 human macrophage cell line and in A549 human lung epithelial cells and is needed for lung colonization of A/J mice, albeit at a less extent than in strain AA100 [20]. In contrast, AnkB is dispensable for intravacuolar replication of L. pneumophila strain Philadelphia-derived Lp02 in macrophages [21], suggesting that a compensatory genetic repertoire exists in this strain that overcomes the loss of AnkB. AnkB is a non-canonical F-box protein that interacts with the host SCF1 ubiquitin ligase on the LCV membrane [22] and functions as a platform for the docking of Lys48-linked polyubiquitinated proteins to the Legionella-containing vacuolar (LCV) membrane [18], [20]. The AnkB-assembled Lys48-linked polyubiquitinated proteins are degraded by the host proteasome machinery, which generates higher levels of cellular amino acids [19]. These amino acids are used by L. pneumophila to feed the tri-carboxylic acid (TCA) cycle to generate ATP and secondary metabolites to power intra-vacuolar replication of L. pneumophila [19], [23], [24]. Since the only defect of the ankB mutant is its inability to import sufficient levels of host amino acids and is localized within an ER-derived LCV that evades lysosomal fusion similar to the wild type strain [15], the ankB mutant is a useful genetic tool to probe the global human macrophage responses to actively replicating L. pneumophila.
The global transcriptional profile of L. pneumophila-resistant bone marrow-derived C57BL/6J and the congenic L. pneumophila-susceptible BcA75 murine macrophages in response to L. pneumophila infection revealed striking host modulation of gene expression [25], [26]. C57BL/6J mouse macrophages are inherently resistant to L. pneumophila because the inflammasome is activated through Naip5-dependent sensing of bacterial flagellin [27], [28], [29], [30], [31]. In contrast, A/J mice have an altered Naip5 allele that renders this mouse strain sensitive to L. pneumophila infection. The C57BL/6J congenic mouse strain BcA75 harbors the A/J Naip5 allele [25]. The transcriptional profile of bone marrow-derived macrophages (bMDMs) isolated from C57BL/6J or BcA75 mice in response to L. pneumophila infection are very similar, indicating that the mouse macrophage transcriptional response is independent of inflammasome activation [25]. Further transcriptome studies using C57BL/6J bMDMs, revealed induction of a novel innate immune response termed the ‘effector triggered response’ (ETR) [26] that is dependent on five L. pneumophila effectors that directly block host protein translation [32], [33], [34], [35], [36]. In addition to this finding, recent work has demonstrated that infected cells exhibit a frustrated MAP kinase response due to effector-dependent host protein synthesis inhibition, but inhibition of pro-inflammatory cytokine translation is overcome in these cells by a MyD88-dependent mechanism [37]. Additional work demonstrated the global transcriptional response of the human macrophage-like U937 cell line to a low dose challenge of L. pneumophila and found up-regulation of anti-apoptotic genes controlled by the transcriptional regulator NF-κB [38]. However, genome wide global transcriptome analysis of primary human monocyte-derived macrophages (hMDMs), the mammalian host cell for L. pneumophila, has not been evaluated. Analyzing the global transcriptome response of hMDMs to L. pneumophila infection is important to determine the cellular responses in the human host and would allow us to compare the findings to the model system of bMDMs of the mouse animal model.
Here we show the global transcriptional response of hMDMs infected with wild type L. pneumophila and its isogenic ankB mutant. The ankB mutant was selected for this study because it fails to replicate intracellularly, but is enclosed within an ER-derived vacuole that evades lysosomal fusion and translocate its full repertoire of effectors, similar to the wild type strain. It is a unique nutritional mutant that resides within a replicative vacuole but simply lacks sufficient levels of amino acids to power its proliferation. Interestingly, the hMDM transcriptional response to L. pneumophila, involves modulation of several immunological pathways, protein synthesis pathways, metabolic pathways and amino acid transporters. Importantly, the global response of hMDMs to infection by L. pneumophila is independent of intracellular bacterial replication.
Materials and Methods
Ethics statement
Human monocyte-derived macrophages were obtained from healthy donors who had given written consent for their use, and this work was approved by the University of Louisville Institutional Review Board.
Bacterial strains and cell cultures
L. pneumophila strain AA100/130b (ATCC BAA-74) and the isogenic ankB mutant were grown on BCYE agar plates for 3 days at 37°C prior to use for infection of macrophages [15]. Human monocyte-derived macrophages (hMDMs) were isolated from healthy donors as described previously IRB [18]. Briefly, monocytes were isolated from whole blood and allowed to adhere to low adherence cell culture plates for 3 days in RPMI 1640 supplemented with 20% FBS at 37°C and 5% CO2. The monocytes were then counted and resuspended RPMI 1640 supplemented with 10% FBS and plated at a density of 3×106 cells per well of a 6 well cell culture plate and incubated for a further 2 days. The cell culture media was then replaced with RPMI 1640 supplemented with 5% FBS for one day, and then with RPMI 1640 supplemented with 1% FBS for one day. The mature hMDMs were then used for infection by L. pneumophila.
Isolation of RNA from L. pneumophila infected hMDMs
The hMDM monolayers plated at density of 3×106 cells per well in a 6 well plate were either uninfected or infected in triplicate with either the wild type L. pneumophila or the ankB mutant at an MOI of 20 for 1 h and then treated for 1 h with gentamicin to kill remaining extracellular bacteria and incubated at 37°C and 5% CO2. The infections were allowed to proceed for a total of 8 h prior to isolation of total RNA. Total RNA from the hMDM monolayers was isolated from 3 individual wells for each condition (uninfected, wild type or ankB infected) using the Qiagen RNeasy Mini kit (Qiagen) according to the manufacturer's instructions. A total of 3 biological replicates were performed. Quality of the purified RNA from each sample was confirmed by using a Bioanalyzer (Agilent) prior to use for microarray analysis.
Microarray sample preparation and analysis
Total RNA was amplified from each sample and labeled following the Affymetrix (Santa Clara, CA) standard protocol for whole transcript expression analysis followed by hybridization to individual Affymetrix Human Gene 1.0 ST arrays for each sample. The arrays were processed following the manufacturer recommended wash and stain protocol on an Affymetrix FS-450 fluidics station and scanned on an Affymetrix GeneChip 7G scanner using Command Console 3.1. The resulting.cel files were imported into Partek Genomics Suite 6.6 and transcripts were normalized on a gene level using RMA as normalization and background correction method. Contrasts in a 1-way ANOVA were set up to compare the treatments of interest. The data was then analyzed using Metacore Pathway software (Thomson Reuters) with a threshold set to set to 0 and a p-value of 0.15. Microarray data was submitted to the Gene Expression Omnibus (GEO) repository and can be accessed through accession number GSE61535. This data set also contains additional microarray data of hMDMs infected with a type II secretion mutant of L. pneumophila.
Results and Discussion
Global transcriptional response of hMDMs to infection by wild type and an ankB mutant of L. pneumophila
We utilized the ankB mutant, that resides within an ER-derived LCV, similar to the wild type strain [15], as a genetic tool to determine if the transcriptional response of hMDMs to infection by L. pneumophila was dependent on intra-vacuolar proliferation. In order to examine the global transcriptional response of hMDMs to infection by either wild type or ankB mutant L. pneumophila, RNA from uninfected or infected hMDMs was isolated at 8 h post-infection and examined by microarray analysis. Interestingly, even though the ankB mutant fails to proliferate in hMDMs, the overall global transcriptional response of hMDMs to the mutant strain was similar to infection by the wild type strain (S1 Table). This result is likely due to Dot/Icm translocation-dependent activation of the global transcription factor NF- κB by L. pneumophila infection of human macrophages, which drives major changes in macrophage gene transcription [15], [28], [29], [30], [38], [39], [40], [41], [42], [43]. In addition, the ‘effector triggered response’ in C57BL/6J bMDMs, which is mediated by 5 translocated effectors that inhibit protein synthesis [26], are shared by the wild type strain and the ankB mutant. Therefore, analyses were focused on the shared transcriptional response of hMDMs to wild type and ankB mutant infection. The top 40 genes that showed the greatest differential expression in response to wild type or the ankB mutant are shown in Table 1. The up-regulated genes primarily encode mediators of the immune response such as IL23A, IL10 and TNF (Table 1), many of which but not all, have been shown previously to be up-regulated in C57BL/6J bMDMs infection by L. pneumophila Lp02 [25], [26]. The most down-regulated genes encode proteins with diverse cellular functions (Table 1). APOBEC3A was the most down-regulated genes in hMDMs infected with L. pneumophila. This gene encodes a cytidine deaminase that plays a key role in restriction of retroviruses by converting cytidine bases in viral DNA to uridine [44]. It is not currently known if APOBEC3A can affect DNA of intracellular bacterial pathogens, but it appears L. pneumophila actively protects itself from this host enzyme through down-regulation of its transcription. GAPT, the second most down-regulated gene in hMDMs infected with L. pneumophila binds to Grb2 and plays a role in B-cell activation and maintenance [45]. To facilitate the analysis of the microarray data, MetaCore Pathway Enrichment analyses were performed.
Table 1. The top 40 up- and down-regulated genes in hMDMs infected by either wild type or an isogenic ankB L. pneumophila mutant strain compared to uninfected hMDMs at 8 h post-infection.
| Upregulated | Downregulated | ||||
| Gene | wild type | ankB | Gene | wild type | ankB |
| RANBP3L | 24.9558 | 26.77 | APOBEC3A | −2.1301 | −2.73152 |
| EGR1 | 20.546 | 24.0411 | GAPT | −2.03252 | −2.7872 |
| NR4A2 | 17.8068 | 18.0274 | ACAA1 | −1.68363 | −1.1802 |
| CCL20 | 12.9572 | 13.7933 | CD177 | −1.61745 | −1.56168 |
| RHCG | 12.7492 | 15.4015 | MIR186 | −1.61347 | −1.5868 |
| PMAIP1 | 11.8732 | 12.7965 | PSG3 | −1.57598 | 1.05404 |
| IL23A | 9.56236 | 12.0511 | MSL3L2 | −1.57256 | −1.37738 |
| GADD45B | 8.87087 | 10.1934 | LRRC25 | −1.57178 | −1.88843 |
| HES1 | 8.83049 | 10.3995 | CLEC4A | −1.5458 | −1.75417 |
| LIF | 8.79145 | 10.6728 | EFTUD1 | −1.53079 | −1.38747 |
| FOS | 8.7718 | 8.89375 | SNORD49A | −1.52728 | −1.37486 |
| CSF2 | 8.40756 | 10.9112 | INSIG2 | −1.50978 | −1.34251 |
| IL10 | 7.94675 | 8.5155 | LOC100133315 | −1.50807 | −1.19079 |
| CXCL2 | 7.55389 | 8.69098 | C5orf20 | −1.47832 | −1.60939 |
| IL12B | 7.53365 | 10.1927 | CMKLR1 | −1.47262 | −1.74118 |
| PTX3 | 7.49085 | 9.93165 | SNORD5 | −1.46528 | −1.4449 |
| IL6 | 7.41431 | 7.90096 | CD209 | −1.46377 | −1.05034 |
| TNF | 6.88512 | 7.34775 | C5orf44 | −1.45662 | −1.43777 |
| E2F7 | 6.83706 | 9.88067 | LOC221442 | −1.45215 | −1.4062 |
| IL20 | 6.72872 | 9.20687 | ZNF814 | −1.45034 | −1.44363 |
| CLCF1 | 6.67757 | 7.83769 | CLCN4 | −1.44066 | −1.44317 |
| LOC440896 | 6.5948 | 7.38741 | RAB42 | −1.43825 | −1.36904 |
| ZFP36 | 6.59423 | 8.02248 | C8orf44 | −1.43825 | −1.44839 |
| MIR155 | 5.77859 | 5.98857 | SUMO1P3 | −1.43729 | −1.04598 |
| GADD45A | 5.51276 | 7.40294 | RASA4 | −1.43024 | −1.74162 |
| KLF4 | 4.73673 | 5.55325 | MIR142 | −1.42423 | −1.76193 |
| CCL4 | 4.73258 | 5.05042 | ZNF573 | −1.42362 | −1.2825 |
| HIVEP2 | 4.64881 | 5.47726 | AKR1C3 | −1.42287 | −1.38282 |
| TNFSF9 | 4.61437 | 5.90509 | FCGR3A | −1.41971 | −1.44462 |
| FOSB | 4.53659 | 4.5787 | CLEC6A | −1.41775 | −1.48098 |
| NFKBIZ | 4.52984 | 6.05722 | ZNF846 | −1.41227 | −1.13047 |
| DENND4A | 4.46864 | 5.57978 | ZNF594 | −1.40638 | −1.70581 |
| NIPAL4 | 4.39544 | 5.99992 | CDKN3 | −1.40499 | −1.39675 |
| TFPI2 | 4.3389 | 4.61199 | LYPLAL1 | −1.40468 | −1.38222 |
| GEM | 4.29366 | 5.00829 | SNORD42B | −1.40304 | −1.61795 |
| IFNG | 4.21353 | 2.68894 | FPR3 | −1.40273 | −1.73894 |
| ZBTB10 | 4.17859 | 4.82323 | C5orf54 | −1.40168 | −1.88889 |
| OVOS | 4.17333 | 4.11822 | TRIM16 | −1.3968 | −1.37838 |
| CXCL3 | 3.99019 | 4.59161 | LOC344887 | −1.3964 | −1.44194 |
| GPR109B | 3.9855 | 5.21438 | SNORD96A | −1.3957 | −1.36989 |
Numbers indicate fold change.
Intra-vacuolar L. pneumophila triggers transcription of multiple immunologic pathways in hMDMs
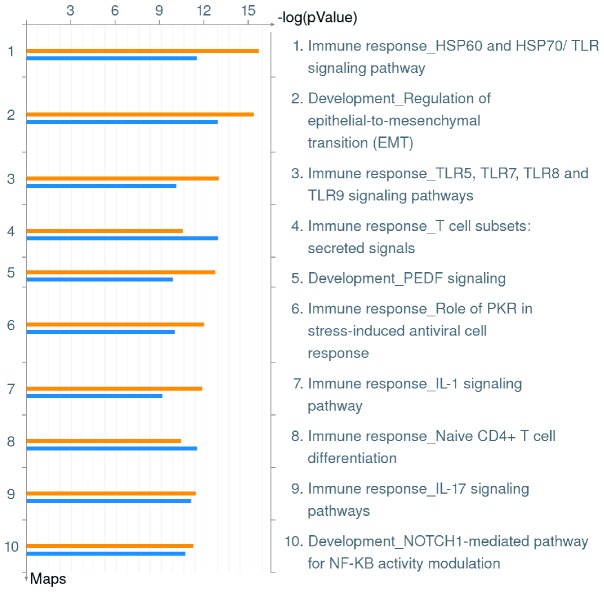
MetaCore enrichment analysis of the microarray data revealed that several key immunological response pathways are the most significantly up-regulated during infection by the wild type strain or the ankB mutant of L. pneumophila. The top scoring Pathway Maps for both wild type and ankB mutant infected hMDMs are shown in Fig. 1 and individual genes affected by L. pneumophila infection in each of these Pathway Maps can be seen in S2 Table. The top three Pathway Maps include ‘Immune response HSP60 and HSP70/TLR’ (p value wild type 1.886−16, ankB 3.530−12), ‘Development_Regulation of epithelial-to-mesenchymal transition (EMT) signaling pathway’ (p value wild type 4.154−16, ankB 1.290−13) and ‘Immune response TLR5, TLR7, TLR8 and TLR9 signaling pathways’ (p value wild type 1.082−13, ankB 9.093−11) (Fig. 1, S2 Table). This indicates L. pneumophila instigates a robust innate immune response in infected hMDMs and this response is independent of intra-vacuolar proliferation.
Figure 1. Top 10 Pathway Maps up-regulated in hMDMs upon infection by L. pneumophila.
Metacore analysis of triplicate microarrays of wild type strain and ankB mutant-infected hMDMs at 8 h post-infection showing the top 10 Pathway Maps. The upper orange bar of each column represents wild type-infected hMDMs while the lower blue bar represents the ankB mutant-infected hMDMs.
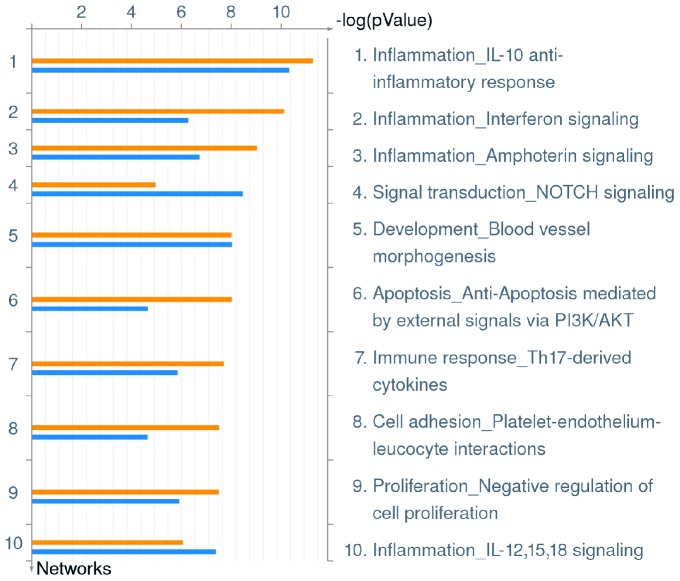
A clearer overall impact of L. pneumophila infection on the hMDM global transcriptional response can be observed through enrichment of Process Networks, which combine the manually collated Pathway Maps and Gene Ontology (GO) processes to demonstrate major cellular processes. Genes in each of these process networks affected by L. pneumophila are listed in S2 Table. The data revealed that infection of hMDMs with either wild type or ankB mutant L. pneumophila induces a broad transcriptional response of genes involved in innate immunity. Five of the top ten Process Networks identified were associated with inflammation and the immune response (IL-10 anti-inflammatory response, interferon signaling, amphoterin signaling, IL-12,15,18 signaling and immune response to Th17-derived cytokines) (Fig. 2, S2 Table). L. pneumophila infection stimulates both pro-inflammatory Th1 and anti-inflammatory Th2 cytokine responses. Studies have shown that L. pneumophila stimulates the production of many pro-inflammatory cytokines including IL-1α, IL-1β, IL-6, IL-8, TNF-α, IFN-γ, IL-12, IL-17 and IL-18 by mouse bMDMs, human macrophage cell lines, murine models of L. pneumophila infection and in patients with Legionnaires' disease [28], [29], [30], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55]. In addition, L. pneumophila stimulates the production of IL-10, the chief mediator of the Th2 response, which suppresses production of Th1 cytokines such as IFN-γ, promoting an anti-inflammatory response and allowing L. pneumophila to proliferate within hMDMs, primary human alveolar macrophages, U937 human macrophage cell line and mouse bMDMs [56], [57], [58], [59]. Therefore, it appears L. pneumophila endeavors to strike a balance between Th1 and Th2 responses and our microarray data clearly demonstrate that L. pneumophila modulates both pro- and anti-inflammatory pathways in hMDMs, and these host modulations are independent of intra-vacuolar proliferation.
Figure 2. Top 10 Process Networks up-regulated in hMDMs upon infection by L. pneumophila.
Metacore analysis of triplicate microarrays of wild type strain and ankB mutant-infected hMDMs at 8 h post-infection showing the top 10 Process Networks. The upper orange bar of each column represents wild type-infected hMDMs while the lower blue bar represents the ankB mutant-infected hMDMs.
Two Process Networks involved in cellular differentiation were also upregulated in hMDMs during infection by wild type or ankB mutant bacteria. The NOTCH signaling Process Network, which plays a key role in cell-cell communication during differentiation (p value wild type 1.238−04, ankB 3.857−09) and the Process Network defining blood vessel morphogenesis (p value wild type 3.503−07, ankB 1.044−08) were both up-regulated during wild type or ankB mutant infection (Fig. 2, S2 Table). Currently, little is known regarding these processes in relation to L. pneumophila infection.
L. pneumophila employs a multi-faceted approach to modulate host cell apoptosis, delaying death of the infected cell, enabling time for the bacteria to proliferate [39], [60], [61], [62], [63]. This is partially achieved through translocation of several effectors including SdhA, SidF, LegK1 and LnaB [64], [65], [66], [67] that either directly or indirectly modulates the apoptosis pathway. L. pneumophila also induces NF-κB dependent transcription of anti-apoptotic genes [38], [39] and this is at least dependent on the LegK1 effector, which directly phosphorylates the NF-κB inhibitor IκBα, promoting its proteasomal degradation [66], and on five effectors that block host cell protein synthesis that ultimately promote NF-κB dependent transcriptional activity [26]. The PI3K/Akt signaling pathway plays a crucial role in maintenance of eukaryotic cell survival through control of major cellular processes including glucose metabolism, protein synthesis, anti-apoptosis and phagocytosis [68]. Our microarray data show that the Process Network defining anti-apoptosis mediated by external signals via PI3K/Akt (p value wild type 1.075−08, ankB 2.859−05) is up-regulated in hMDMs infected with wild type or ankB mutant L. pneumophila (Fig. 2, S2 Table). Activation of the PI3K/Akt pathway is important in the phagocytosis of L. pneumophila by macrophages [69]. Therefore, our data implicate that upon phagocytosis, L. pneumophila reprograms diverse pathways in hMDMs to block apoptosis through activation of the PI3K/Akt pathway, in addition to the activity of various translocated effectors that promote NF-κB activity which prolongs cell survival [26], [39], [66], protecting the intracellular niche for L. pneumophila.
Infection of hMDMs with wild type or ankB mutant L. pneumophila also impacted genes involved in platelet-endothelium-leucocyte interactions (p value wild type 3.547−08, ankB 2.894−05) and regulation of cell proliferation (p value wild type 3.650−08, ankB 1.474−06) (Fig. 2, S2 Table). Infection of mouse bMDMs by the L. pneumophila Lp02 strain also induced genes associated with cell proliferation [25]. However currently, there are little data available to indicate the importance of these pathways in relation to L. pneumophila infection. Taken together, L. pneumophila infection of hMDMs up-regulates transcription of multiple immune responses, anti-apoptosis, cellular differentiation, proliferation and adhesion processes. This robust global transcriptional response in hMDMs is similar to that observed in infected bMDMs and U937 cells despite differences in genetic susceptibility and mammalian species [25], [26], [38]. Importantly, these global L. pneumophila-triggered host modulations are independent of intra-vacuolar proliferation of the bacteria.
Repression of transcription of protein synthesis pathways in hMDMs independent of intra-vacuolar proliferation of L. pneumophila
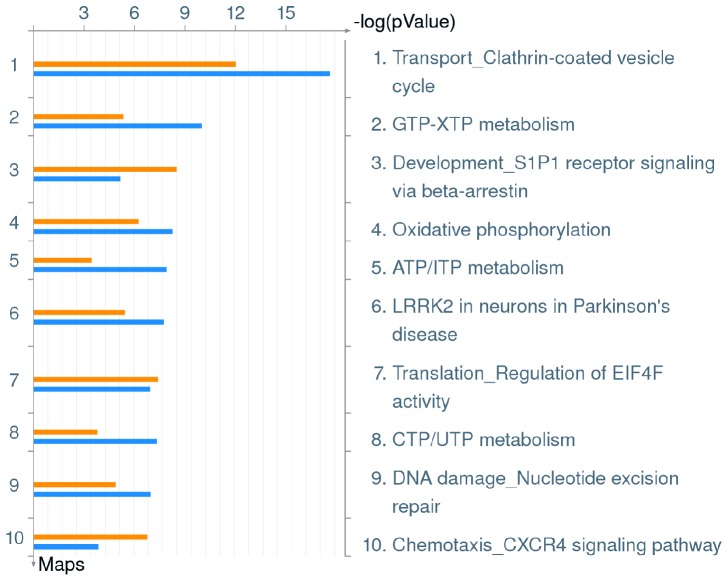
In addition to analyses of cellular pathways up-regulated in hMDMs upon infection by L. pneumophila, down-regulated pathways were analyzed. Initial enrichment analysis for Pathway Maps revealed multiple host pathways that are down-regulated upon infection by both the wild type strain and the ankB mutant. These included clathrin-coated vesicle cycle, multiple nucleotide metabolism pathways, oxidative phosphorylation, EIF4F protein translation, S1P1 receptor signaling, LRRK2 signaling, CXCR4 signaling and nucleotide excision repair (Fig. 3, S3 Table). It is clear L. pneumophila infection of hMDMs down-regulates many pathways that have broad effects on the host cell. For example LRRK2 interacts with a wide range of signaling proteins including the MAPK family, and can modulate GTPase-activating and GTPase-exchange factors that affect vesicular trafficking and autophagy and altering this pathway may provide benefit for the pathogen [70]. Genes affected by L. pneumophila in these pathways are listed in S3 Table.
Figure 3. Top 10 Pathway Maps down-regulated in hMDMs upon infection by L. pneumophila.
Metacore analysis of triplicate microarrays of wild type strain and ankB mutant-infected hMDMs at 8 h post-infection showing the top 10 Pathway Maps. The upper orange bar of each column represents wild type-infected hMDMs while the lower blue bar represents the ankB mutant-infected hMDMs.
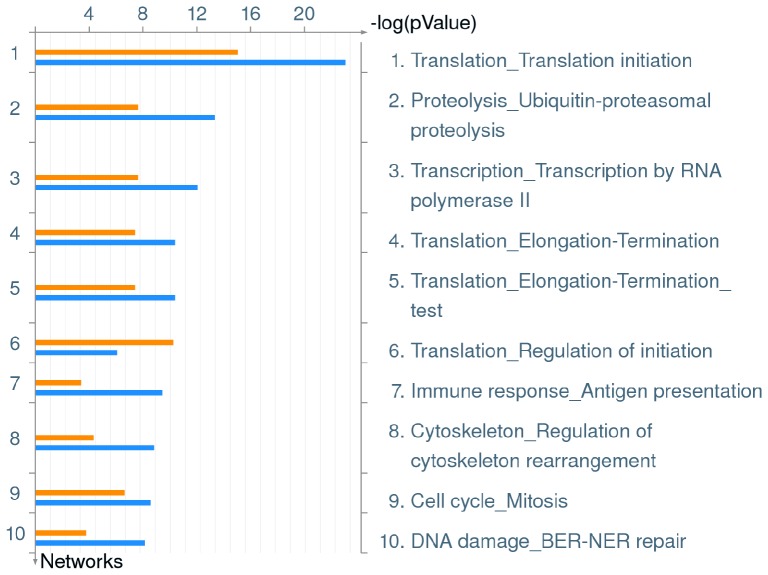
To develop a broad understanding of the effect of L. pneumophila infection in hMDMs in terms of down-regulated pathways, enrichment of down-regulated Process Networks was performed. Strikingly, L. pneumophila infection causes a broad repression of genes involved in protein translation in hMDMs, with 3 out of top 10 scoring Process Networks involved in protein translation initiation and termination (Fig. 4, S3 Table). L. pneumophila infection directly affects networks defining ‘Protein Translation Initiation’ (p value wild type 1.157−15, ankB 9.261−24) ‘Regulation of Initiation’ (p value wild type 9.097−11, ankB 1.527−06 and also ‘Elongation – Termination’ (p value wild type 6.960−08, ankB 6.962−11), indicating a significant and diverse impact on overall host protein translation (Fig. 4, S3 Table). Interestingly, infection of hMDMs with either wild type or ankB mutant L. pneumophila also negatively impacted the network defining ‘Transcription by RNA polymerase II’ (p value wild type 4.222−08, ankB 1.369−12) (Fig. 4, S3 Table). Genes directly impacted by L. pneumophila in these Process Networks can be seen in S3 Table. L. pneumophila is known to reduce protein translation in the host cell through the action of at least five translocated effectors [32], [33], [34], [35], [36]. It is possible that blocking host cell protein synthesis and transcription is beneficial for L. pneumophila through increasing cytosolic availability of amino acids in addition to promoting proteasomal degradation [19]. L. pneumophila relies heavily on amino acids as its primary source of carbon and energy and actively promotes increased host cytosolic amino acid concentrations through AnkB-dependent proteasomal degradation [19]. Similar to the wild type strain, the ankB mutant also reduced transcription of the protein synthesis pathways in hMDMs, but this mutant fails to replicate. This suggests any increase in bioavailability of amino acids due to reduced host protein translation plays only a minor role for L. pneumophila in terms of raising the levels of amino acids in hMDMs above the threshold needed for intra-vacuolar proliferation. L. pneumophila encodes five effectors that directly block host protein translation [32], [33], [34], [35], [36], and in non-permissive bMDMs derived from C57BL/6J mice leads to induction of the ‘effector-triggered response (ETR)’ characterized by increased transcription of IL23a and Gem [26]. In addition, L. pneumophila-dependent inhibition of host translation generates a frustrated MAP kinase response where many genes are transcribed but not translated, however a subset of pro-inflammatory cytokines including Il-1α and Il-1β can bypass this effect in a MyD88-dependent manner [37]. Transcription of both IL23a and Gem were amongst the top 40 highest expressed genes in hMDMs infected with L. pneumophila (Table 1), indicating the ETR phenotype also occurs in primary human macrophages, regardless of the genetic susceptibility or mammalian species of the host cell. Taken together, it appears L. pneumophila combines inhibition of protein translation pathway transcription and a direct effector-dependent inhibition of protein synthesis that leads to elevated expression of innate immunological pathways against pathogens in hMDMs.
Figure 4. Top 10 Process Networks down-regulated in hMDMs upon infection by L. pneumophila.
Metacore analysis of triplicate microarrays of wild type strain and ankB mutant-infected hMDMs 8 h post-infection showing the top 10 Process Networks. The upper orange bar of each column represents wild type-infected hMDMs while the lower blue bar represents the ankB mutant-infected hMDMs.
Global transcriptional down-regulation of host pathways in L. pneumophila-infected hMDMs
In addition to major impacts on gene transcription and protein translation, L. pneumophila infection negatively impacted several other major cellular processes. The second highest scoring down-regulated Process Network identified in L pneumophila-infected hMDMs defines ‘Ubiquitin-proteasomal proteolysis’ with p values of 4.243−08 and 6.605−14 for wild type and ankB mutant infection, respectively (Fig. 4, S3 Table). L. pneumophila hijacks the ubiquitin-proteasome system through multiple mechanisms to promote successful intracellular infection [19], [20], [21], [71], [72], [73]. The effector, LubX, mimics the function of eukaryotic U-box domain proteins to ubiquitinate the host cell kinase CDC2-like kinase 1 [71], though the consequences of LubX-mediated ubiquitination are currently unclear, it appears to be important for bacterial replication in A/J mouse macrophages [71]. LubX also functions as a ‘meta-effector’ by promoting the ubiquitination and subsequent proteasomal degradation of the SidH effector [72]. L. pneumophila harbors several effector proteins that encode the eukaryotic F-box domain [74], [75]. F-box domain proteins play an important role in directing target proteins to the SCF1 ubiquitin ligase complex to promote their ubiquitination [75]. In L. pneumophila strain Philadelphia-derived Lp02, the F-box effector LegU1 promotes the ubiquitination of the host chaperone protein BAT3, a protein that plays an important role in regulation of ER stress [21], [76], [77]. Another F-box effector protein, AnkB interacts with the host SCF1 ubiquitin ligase in the LCV membrane [22] and functions as a platform for the docking of lys48-linked polyubiquitinated proteins to the LCV membrane [18], [20] that are subsequently degraded by the host proteasome machinery, generating higher levels of cellular amino acids that are used by L. pneumophila to feed the TCA cycle [19]. L. pneumophila infection of bMDMs derived from A/J mice also reduces formation of dendritic cell aggresome-like structures (DALIS) that are enriched in ubiquitinated proteins and is dependent on the Dot/Icm T4SS [73]. DALIS formation is believed to protect ubiquitinated proteins from proteasomal degradation and serve as a pool of antigens available for downstream processing in dendritic cells [78]. Currently, the significance of the inhibition of DALIS formation in A/J mouse bMDMs by L. pneumophila is unclear.
In addition to effector-dependent modulation of host ubiquitination pathways, recognition of L. pneumophila ‘signatures’ by macrophages results in ubiquitination and subsequent proteasomal degradation of mTOR regulators [79]. This results in an enhanced pro-inflammatory cytokine response in macrophages [79]. It is clear that L. pneumophila actively hijacks the host ubiquitin/proteasome system at the protein level, however transcription of genes involved in the Process Network ‘ubiquitin-proteasomal proteolysis’ are also repressed by L. pneumophila. This suggests that hMDMs endeavor to limit the ability of L. pneumophila to affect the ubiquitin-proteasomal pathway at the transcriptional level albeit with limited success.
The Process Network that defines ‘Antigen presentation’ was also down-regulated in hMDMs by L. pneumophila infection (p value wild type 8.228−04, ankB 6.411−10), suggesting that even though a robust immune transcriptional response is induced, presentation of antigens is reduced (Fig. 4, S3 Table). The Process Network which encompasses ‘Regulation of cytoskeleton rearrangement’ was significantly down-regulated in hMDMs infected with L. pneumophila (p value wild type 9.401−05, ankB 2.536−09), and the related Process Network ‘Cell Cycle – Mitosis’ that shares many genes as ‘Regulation of cytoskeleton rearrangement’ was similarly affected in hMDMs infected with L. pneumophila (p value wild type 4.495−07, ankB 4.911−09) (Fig. 4, S3 Table). Two L. pneumophila translocated effectors have been shown to directly modulate the host cell cytoskeleton. The VipA effector functions as an actin nucleator and localizes to endosomes during L. pneumophila infection and likely interferes with organelle trafficking in the host cell [80]. In contrast to VipA, the Ceg14 effector inhibits actin polymerization through an uncharacterized mechanism [81]. Interestingly, protein translation in eukaryotic cells is tightly linked to the cytoskeleton [82]. We found that genes involved in protein synthesis and the cytoskeleton are down-regulated in infected hMDMs. Therefore, it will be interesting to determine if effectors that modulate the cytoskeleton have downstream effects on host protein synthesis. Conversely, it is possible effectors that inhibit protein synthesis [32], [33], [34], [36] may also impact the host cytoskeleton.
Our data show that infection of hMDMs with L. pneumophila reduce transcription of genes that define the Process Network ‘DNA damage – BER-NER repair’ (p value wild type 3.552−04, ankB 1.250−08), suggesting intracellular infection of L. pneumophila reduces the ability of hMDMs to repair DNA damage (Fig. 4, S3 Table). Currently, how this applies to L. pneumophila infection is unclear.
Modulation of metabolic pathways in hMDMs by L. pneumophila
We have previously shown that infection of hMDMs by L. pneumophila triggers a rapid rise in intracellular amino acid concentrations above the threshold needed for L. pneumophila to utilize as a source of carbon and energy to power rapid bacterial growth [19]. Therefore, we examined which metabolic pathways in hMDMs are up- or down-regulated in response to infection by wild type or ankB mutant of L. pneumophila. The data show that infection of hMDMs by L. pneumophila triggers modulation of many metabolic pathways at the transcriptional level. The top 50 up- and down-regulated metabolic pathways are shown in S4 and S5 Tables and include broad alterations in lipid and amino acid metabolism and transport, as well as sugar metabolism (Figs. 5 and 6).
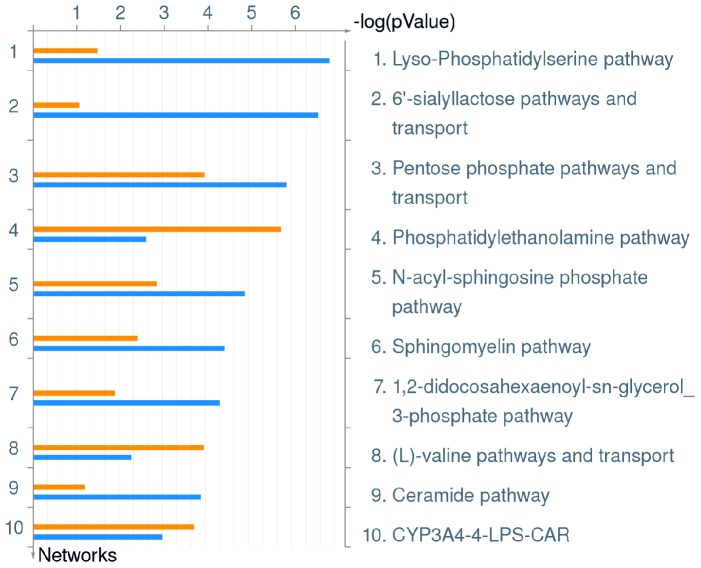
Figure 5. Top 10 Metabolic Pathways up-regulated in hMDMs upon infection by L. pneumophila.
Metacore analysis of triplicate microarrays of wild type strain and ankB mutant-infected hMDMs 8 h post-infection showing the top 10 Metabolic Pathways. The upper orange bar of each column represents wild type-infected hMDMs while the lower blue bar represents the ankB mutant-infected hMDMs.
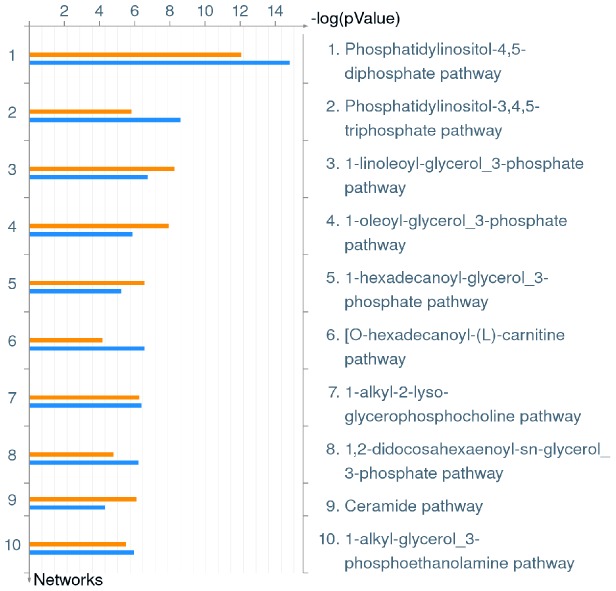
Figure 6. Top 10 Metabolic Pathways down-regulated in hMDMs upon infection by L. pneumophila.
Metacore analysis of triplicate microarrays of wild type strain and ankB mutant-infected hMDMs 8 h post-infection showing the top 10 Metabolic Pathways. The upper orange bar of each column represents wild type-infected hMDMs while the lower blue bar represents the ankB mutant-infected hMDMs.
Interestingly, changes in diverse lipid metabolic pathways were the most pronounced metabolic alterations observed in hMDMs infected with by the wild type strain or the ankB mutant. Membrane lipids including sphingolipids and phosphoinositides regulate diverse cellular processes such as apoptosis, immunoregulation and migration, through interaction with specific cellular proteins that trigger diverse signaling cascades [83], [84]. Local concentrations of specific lipids act as thresholds for triggering cellular events. Therefore, lipid biosynthesis, degradation and transport are tightly controlled processes [84]. Our data show that L. pneumophila infection of hMDMs causes significant alterations in these diverse lipid metabolic pathways independent of bacterial replication, which in turn likely results in stimulation or repression of many host immunological pathways as demonstrated above. A number of L. pneumophila effectors have been shown to affect lipid pathways. For example, the effectors LecE and LpdA affect phospholipid metabolism in the host cell, while LegS2 affects sphingolipid metabolism [85], [86]. Hijacking phosphoinositides is a key virulence strategy of L. pneumophila [87]. These lipids play a central role in diverse processes including membrane trafficking, cytoskeleton and signaling pathways [84]. The LCV membrane is enriched for phosphatidylinositol-4-phosphate and several effectors anchor to this lipid to modulate biogenesis of the LCV [87]. Interestingly, the top down-regulated metabolic pathway in hMDMs infected with the wild type strain or the ankB mutant was the phosphatidylinositol-4,5-diphosphate pathway (Fig. 6, S5 Table). Phosphatidylinositol-4,5-diphosphate is hydrolyzed to phosphatidylinositol-4-phosphate via the enzyme OCRL1, which is localized to the LCV membrane [88]. This indicates that even though L. pneumophila hijacks phosphoinositide lipids during intracellular infection to modulate biogenesis of the LCV, host pathways for their generation are down-regulated in hMDMs during infection by L. pneumophila, and this is independent of intra-vacuolar proliferation.
The top up-regulated metabolic pathway in hMDMs infected by the wild type strain or the ankB mutant was the lyso-phosphatidylserine pathway (Fig. 5, S4 Table). Lyso-phosphatidylserine is a bio-active lipid that is increasingly shown to play a key role in initiation of acute inflammation and its subsequent resolution [89]. To date, the role of this bio-active lipid in macrophages during infection by intracellular bacteria remains unknown.
Amino acid transporters
L. pneumophila has a strict requirement for amino acids that it satisfies by promoting elevated amino acid levels through proteasomal degradation of Lys48-linked polyubiquitinated proteins in the host cell cytosol [19]. Since L. pneumophila resides within a membrane bound compartment in its host cell, the bacteria must employ amino acid transporters to import cytosolic amino acids. The eukaryotic amino acid transporter SLC1A5, which transports a variety of neutral amino acids, is required for L. pneumophila replication in the Mono Mac 6 human macrophage cell line and has been identified via proteomics on LCVs isolated from RAW 264.7 mouse macrophages [90], [91]. The SLC7A5 and SLC3A2 amino acid transporters have also been identified by proteomic analysis to be present on LCVs isolated from RAW 264.7 mouse macrophages [91]. It is likely these transporters and others are recruited to the LCV to mediate the import of amino acids from the cytosolic milieu into the LCV lumen. Therefore, the microarray data were analyzed to determine if infection of hMDMs by L. pneumophila causes alterations in transcription of amino acid transporter genes. We observed that infection of hMDMs with L. pneumophila resulted in minor changes in transcription of amino acid transporters, with only 3 out of 45 genes showing statistically significant up- or down-regulation (Table 2). The cationic amino acid transporter SLC7A2 (CAT-2) was up-regulated 1.4 fold in L. pneumophila infected hMDMs and mediates the transport of arginine into macrophages [92], [93]. The mouse homolog of CAT-2, mCAT-2, is upregulated by Salmonella infection of mouse bMDMs and likely aids the import of arginine into the Salmonella-containing vacuole [94]. In addition, expression of SLC7A2 has been implicated in the ability of macrophages to mediate an innate immune response to Helicobacter pylori and Leishmania infection [93], [95], [96]. It is possible increased arginine transport in L. pneumophila infected hMDMs enhances the ability of the macrophage to clear infection by contributing to the production of nitric oxide by NOS2, or alternatively L. pneumophila hijacks this transporter to mediate arginine transport into the LCV. The glutamate transporter SLC1A2 (GLT1) was also induced 1.4 fold in hMDMs infected by L. pneumophila (Table 2). This transporter is primarily found in neural cells [97] but can be expressed in macrophages where it mediates uptake of glutamate [98], [99] and possibly hijacked by L. pneumophila to mediate transport of glutamate into the LCV during infection of hMDMs. SLC7A5 transports large neutral amino acids and has been implicated in the pathogenesis of Salmonella [100], and has been identified on the LCV by proteomics [91]. We observed a small but statistically significant 1.2 fold increase in SLC7A5 gene transcription in hMDMs infected with L. pneumophila (Table 2). It is likely L. pneumophila hijacks SLC7A5 to mediate import of amino acids into the LCV.
Table 2. Amino acid transporters up-regulated in hMDMs infected by L. pneumophila.
| Gene Name | p-value | Fold-Change | Transporter function |
| SLC7A2 | 0.00079 | 1.43096 | cationic L-amino acids |
| SLC1A2 | 0.000521 | 1.40055 | glutamate, aspartate |
| SLC7A5 | 0.013758 | 1.20244 | large neutral L-amino acids |
Although up-regulation of the SLC amino acid transporters by L. pneumophila is modest, it is important to note that our infection conditions result in infection of ∼20% of the hMDMs in the monolayers. Therefore, it is expected that most transcriptional modulations throughout this study are much more pronounced in the infected hMDMs in the monolayers. However, small changes in transcription of certain genes in the infected cells may not be detectable in the whole cell population. Taken together, L. pneumophila up-regulates transcription of various host transporters during infection, which can contribute to the ability of this pathogen to import amino acids and other host molecules from the host cytosol into the LCV lumen.
In conclusion, we have shown that infection of hMDMs by L. pneumophila triggers robust transcription of inflammatory and immunological pathways whilst transcription of protein synthesis pathways is repressed. Furthermore, transcription of metabolic pathways in hMDMs is significantly altered by L. pneumophila infection and in particular lipid metabolism. Importantly, this global host cell response is independent of intra-vacuolar bacterial proliferation and validates findings of other studies that used bMDMs and human macrophage cells lines. Taken together, it is clear that human macrophages alter their transcriptional landscape in response to infection by L. pneumophila in an endeavor to mount a successful immune response to the pathogen regardless if the bacterium is able to proliferate intracellularly. Finally, the microarray data presented will be a useful resource for the research community in understanding global and complex L. pneumophila-hMDM interactions.
Supporting Information
The total processed microarray data of hMDMs uninfected or infected with wild type or ankB mutant L. pneumophila at 8 h post-infection.
(XLSX)
Metacore enrichment analysis of hMDMs infected with wild type or ankB mutant L. pneumophila at 8 h post-infection showing up-regulated pathways.
(XLS)
Metacore enrichment analysis of hMDMs infected with wild type or ankB mutant L. pneumophila at 8 h post-infection showing down-regulated pathways.
(XLS)
Metacore enrichment analysis of hMDMs infected with wild type or ankB mutant L. pneumophila at 8 h post-infection showing up-regulated metabolic pathways.
(XLS)
Metacore enrichment analysis of hMDMs infected with wild type or ankB mutant L. pneumophila at 8 h post-infection showing down-regulated metabolic pathways.
(XLS)
Acknowledgments
Part of this work was performed with assistance of Sabine Waigel at the University of Louisville Genomics Facility.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files. Microarray data was submitted to the Gene Expression Omnibus (GEO) repository and can be accessed through accession number GSE61535.
Funding Statement
The YAK lab is supported by Public Health Service Awards R01AI069321 and R21AI107978 from the National Institute of Allergy and Infectious Diseases and by the Commonwealth of Kentucky Research Challenge Trust Fund. Part of this work was performed at the University of Louisville Genomics Facility, which is supported by the National Institutes of Health: 2P20GM103436-14, NIH: 1P30GM106396-01 and the J. G. Brown Cancer Center at the University of Louisville. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Franco IS, Shuman HA, Charpentier X (2009) The perplexing functions and surprising origins of Legionella pneumophila type IV secretion effectors. Cell Microbiol 11:1435–1443. [DOI] [PubMed] [Google Scholar]
- 2. Price CT, Richards AM, Von Dwingelo JE, Samara HA, Abu Kwaik Y (2014) Amoeba host-Legionella synchronization of amino acid auxotrophy and its role in bacterial adaptation and pathogenic evolution. Environ Microbiol 16:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richards AM, Von Dwingelo JE, Price CT, Abu Kwaik Y (2013) Cellular microbiology and molecular ecology of Legionella-amoeba interaction. Virulence 4:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luo ZQ (2012) Legionella secreted effectors and innate immune responses. Cell Microbiol 14:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Isberg RR, O'Connor TJ, Heidtman M (2009) The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol 7:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al-Quadan T, Price CT, Abu Kwaik Y (2012) Exploitation of evolutionarily conserved amoeba and mammalian processes by Legionella. Trends Microbiol 20:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Felipe KS, Glover RT, Charpentier X, Anderson OR, Reyes M, et al. (2008) Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog 4:e1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu W, Banga S, Tan Y, Zheng C, Stephenson R, et al. (2011) Comprehensive Identification of Protein Substrates of the Dot/Icm Type IV Transporter of Legionella pneumophila. PLoS One 6:e17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luo Z-Q (2011) Targeting one of its own: expanding roles of substrates of the Legionella pneumophila Dot/Icm type IV secretion system. Front Microbio 2 10.3389/fmicb.2011.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rolando M, Buchrieser C (2014) Legionella pneumophila type IV effectors hijack the transcription and translation machinery of the host cell. Trends Cell Biol [DOI] [PubMed] [Google Scholar]
- 11. Ivanov SS, Roy C (2013) Host lipidation: a mechanism for spatial regulation of Legionella effectors. Curr Top Microbiol Immunol 376:135–154. [DOI] [PubMed] [Google Scholar]
- 12. Hubber A, Kubori T, Nagai H (2013) Modulation of the ubiquitination machinery by Legionella. Curr Top Microbiol Immunol 376:227–247. [DOI] [PubMed] [Google Scholar]
- 13. Haneburger I, Hilbi H (2013) Phosphoinositide lipids and the Legionella pathogen vacuole. Curr Top Microbiol Immunol 376:155–173. [DOI] [PubMed] [Google Scholar]
- 14. O'Connor TJ, Adepoju Y, Boyd D, Isberg RR (2011) Minimization of the Legionella pneumophila genome reveals chromosomal regions involved in host range expansion. Proc Natl Acad Sci U S A 108:14733–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al-Khodor S, Price CT, Habyarimana F, Kalia A, Abu Kwaik Y (2008) A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol Microbiol 70:908–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Price CT, Jones SC, Amundson KE, Abu Kwaik Y (2010) Host-mediated post-translational prenylation of novel Dot/Icm-translocated effectors of Legionella pneumophila. Front Microbio 1 10.3389/fmicb.2010.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Price CT, Al-Quadan T, Santic M, Jones SC, Abu Kwaik Y (2010) Exploitation of conserved eukaryotic host cell farnesylation machinery by an F-box effector of Legionella pneumophila. J Exp Med 207:1713–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Price CT, Al-Khodor S, Al-Quadan T, Santic M, Habyarimana F, et al. (2009) Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa. PLoS Pathog 5:e1000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Price CT, Al-Quadan T, Santic M, Rosenshine I, Abu Kwaik Y (2011) Host proteasomal degradation generates amino acids essential for intracellular bacterial growth. Science 334:1553–1557. [DOI] [PubMed] [Google Scholar]
- 20. Lomma M, Dervins-Ravault D, Rolando M, Nora T, Newton HJ, et al. (2010) The Legionella pneumophila F-box protein Lpp2082 (AnkB) modulates ubiquitination of the host protein parvin B and promotes intracellular replication. Cell Microbiol 12:1272–1291. [DOI] [PubMed] [Google Scholar]
- 21. Ensminger AW, Isberg RR (2010) E3 ubiquitin ligase activity and targeting of BAT3 by multiple Legionella pneumophila translocated substrates. Infect Immun 78:3905–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bruckert WM, Price CT, Abu Kwaik Y (2014) Rapid nutritional remodeling of the host cell upon attachment of Legionella pneumophila. Infect Immun 82:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schunder E, Gillmaier N, Kutzner E, Herrmann V, Lautner M, et al. (2014) Amino Acid Uptake and Metabolism of Legionella pneumophila Hosted by Acanthamoeba castellanii. J Biol Chem [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eylert E, Herrmann V, Jules M, Gillmaier N, Lautner M, et al. (2010) Isotopologue profiling of Legionella pneumophila: role of serine and glucose as carbon substrates. J Biol Chem 285:22232–22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fortier A, Faucher SP, Diallo K, Gros P (2011) Global cellular changes induced by Legionella pneumophila infection of bone marrow-derived macrophages. Immunobiology 216:1274–1285. [DOI] [PubMed] [Google Scholar]
- 26. Fontana MF, Banga S, Barry KC, Shen X, Tan Y, et al. (2011) Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila. PLoS Pathog 7:e1001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diez E, Lee SH, Gauthier S, Yaraghi Z, Tremblay M, et al. (2003) Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat Genet 33:55–60. [DOI] [PubMed] [Google Scholar]
- 28. Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, et al. (2006) Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med 203:1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE (2006) Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog 2:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, et al. (2006) The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol 7:318–325. [DOI] [PubMed] [Google Scholar]
- 31. Akhter A, Caution K, Abu Khweek A, Tazi M, Abdulrahman BA, et al. (2012) Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 37:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCusker KT, Braaten BA, Cho MW, Low DA (1991) Legionella pneumophila inhibits protein synthesis in Chinese hamster ovary cells. Infect Immun 59:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Belyi Y, Niggeweg R, Opitz B, Vogelsgesang M, Hippenstiel S, et al. (2006) Legionella pneumophila glucosyltransferase inhibits host elongation factor 1A. Proc Natl Acad Sci U S A 103:16953–16958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Belyi Y, Tabakova I, Stahl M, Aktories K (2008) Lgt: a family of cytotoxic glucosyltransferases produced by Legionella pneumophila. J Bacteriol 190:3026–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Belyi Y, Jank T, Aktories K (2011) Effector glycosyltransferases in Legionella. Front Microbio 2 10.3389/fmicb.2011.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen X, Banga S, Liu Y, Xu L, Gao P, et al. (2009) Targeting eEF1A by a Legionella pneumophila effector leads to inhibition of protein synthesis and induction of host stress response. Cell Microbiol 11:911–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asrat S, Dugan AS, Isberg RR (2014) The frustrated host response to Legionella pneumophila is bypassed by MyD88-dependent translation of pro-inflammatory cytokines. PLoS Pathog 10:e1004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Losick VP, Isberg RR (2006) NF-kappaB translocation prevents host cell death after low-dose challenge by Legionella pneumophila. J Exp Med 203:2177–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abu-Zant A, Jones S, Asare R, Suttles J, Price C, et al. (2007) Anti-apoptotic signalling by the Dot/Icm secretion system of L. pneumophila. Cell Microbiol 9:246–264. [DOI] [PubMed] [Google Scholar]
- 40. Bartfeld S, Engels C, Bauer B, Aurass P, Flieger A, et al. (2009) Temporal resolution of two-tracked NF-kappaB activation by Legionella pneumophila. Cell Microbiol 11:1638–1651. [DOI] [PubMed] [Google Scholar]
- 41. Schmeck B, N'Guessan PD, Ollomang M, Lorenz J, Zahlten J, et al. (2007) Legionella pneumophila-induced NF-kappaB- and MAPK-dependent cytokine release by lung epithelial cells. Eur Respir J 29:25–33. [DOI] [PubMed] [Google Scholar]
- 42. Archer KA, Ader F, Kobayashi KS, Flavell RA, Roy CR (2010) Cooperation between multiple microbial pattern recognition systems is important for host protection against the intracellular pathogen Legionella pneumophila. Infect Immun 78:2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stetson DB, Medzhitov R (2006) Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24:93–103. [DOI] [PubMed] [Google Scholar]
- 44. Stavrou S, Crawford D, Blouch K, Browne EP, Kohli RM, et al. (2014) Different modes of retrovirus restriction by human APOBEC3A and APOBEC3G in vivo. PLoS Pathog 10:e1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu Y, Zhang W (2008) Identification of a new transmembrane adaptor protein that constitutively binds Grb2 in B cells. J Leukoc Biol 84:842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McHugh SL, Yamamoto Y, Klein TW, Friedman H (2000) Murine macrophages differentially produce proinflammatory cytokines after infection with virulent vs. avirulent Legionella pneumophila. J Leukoc Biol 67:863–868. [DOI] [PubMed] [Google Scholar]
- 47. Brieland JK, Remick DG, Freeman PT, Hurley MC, Fantone JC, et al. (1995) In vivo regulation of replicative Legionella pneumophila lung infection by endogenous tumor necrosis factor alpha and nitric oxide. Infect Immun 63:3253–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen Y, Tateda K, Fujita K, Ishii T, Ishii Y, et al. (2010) Sequential changes of Legionella antigens and bacterial load in the lungs and urines of a mouse model of pneumonia. Diagn Microbiol Infect Dis 66:253–260. [DOI] [PubMed] [Google Scholar]
- 49. Fernandez-Serrano S, Dorca J, Coromines M, Carratala J, Gudiol F, et al. (2003) Molecular inflammatory responses measured in blood of patients with severe community-acquired pneumonia. Clin Diagn Lab Immunol 10:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sporri R, Joller N, Albers U, Hilbi H, Oxenius A (2006) MyD88-dependent IFN-gamma production by NK cells is key for control of Legionella pneumophila infection. J Immunol 176:6162–6171. [DOI] [PubMed] [Google Scholar]
- 51. Tateda K, Matsumoto T, Ishii Y, Furuya N, Ohno A, et al. (1998) Serum cytokines in patients with Legionella pneumonia: relative predominance of Th1-type cytokines. Clin Diagn Lab Immunol 5:401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deng JC, Tateda K, Zeng X, Standiford TJ (2001) Transient transgenic expression of gamma interferon promotes Legionella pneumophila clearance in immunocompetent hosts. Infect Immun 69:6382–6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shin S, Case CL, Archer KA, Nogueira CV, Kobayashi KS, et al. (2008) Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog 4:e1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Neild AL, Roy CR (2003) Legionella reveal dendritic cell functions that facilitate selection of antigens for MHC class II presentation. Immunity 18:813–823. [DOI] [PubMed] [Google Scholar]
- 55. Barry KC, Fontana MF, Portman JL, Dugan AS, Vance RE (2013) IL-1alpha signaling initiates the inflammatory response to virulent Legionella pneumophila in vivo. J Immunol 190:6329–6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Park DR, Skerrett SJ (1996) IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-gamma: differential responses of blood monocytes and alveolar macrophages. J Immunol 157:2528–2538. [PubMed] [Google Scholar]
- 57. Yoshizawa S, Tateda K, Matsumoto T, Gondaira F, Miyazaki S, et al. (2005) Legionella pneumophila evades gamma interferon-mediated growth suppression through interleukin-10 induction in bone marrow-derived macrophages. Infect Immun 73:2709–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McCoy-Simandle K, Stewart CR, Dao J, DebRoy S, Rossier O, et al. (2011) Legionella pneumophila type II secretion dampens the cytokine response of infected macrophages and epithelia. Infect Immun 79:1984–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Santic M, Molmeret M, Abu Kwaik Y (2005) Maturation of the Legionella pneumophila-containing phagosome into a phagolysosome within gamma interferon-activated macrophages. Infect Immun 73:3166–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Luo Z-Q (2011) Striking a balance: Modulation of host cell death pathways by Legionella pneumophila. Front Microbio 2 10.3389/fmicb.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Derre I, Isberg RR (2004) Macrophages from mice with the restrictive Lgn1 allele exhibit multifactorial resistance to Legionella pneumophila. Infect Immun 72:6221–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Molmeret M, Zink SD, Han L, Abu-Zant A, Asari R, et al. (2004) Activation of caspase-3 by the Dot/Icm virulence system is essential for arrested biogenesis of the Legionella-containing phagosome. Cell Microbiol 6:33–48. [DOI] [PubMed] [Google Scholar]
- 63. Abu-Zant A, Santic M, Molmeret M, Jones S, Helbig J, et al. (2005) Incomplete activation of macrophage apoptosis during intracellular replication of Legionella pneumophila. Infect Immun 73:5339–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR (2006) A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci U S A 103:18745–18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Banga S, Gao P, Shen X, Fiscus V, Zong WX, et al. (2007) Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc Natl Acad Sci U S A 104:5121–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ge J, Xu H, Li T, Zhou Y, Zhang Z, et al. (2009) A Legionella type IV effector activates the NF-kappaB pathway by phosphorylating the IkappaB family of inhibitors. Proc Natl Acad Sci U S A 106:13725–13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Losick VP, Haenssler E, Moy MY, Isberg RR (2010) LnaB: a Legionella pneumophila activator of NF-kappaB. Cell Microbiol 12:1083–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Diehl N, Schaal H (2013) Make yourself at home: viral hijacking of the PI3K/Akt signaling pathway. Viruses 5:3192–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tachado SD, Samrakandi MM, Cirillo JD (2008) Non-opsonic phagocytosis of Legionella pneumophila by macrophages is mediated by phosphatidylinositol 3-kinase. PLoS One 3:e3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Boon JY, Dusonchet J, Trengrove C, Wolozin B (2014) Interaction of LRRK2 with kinase and GTPase signaling cascades. Front Mol Neurosci 7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kubori T, Hyakutake A, Nagai H (2008) Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions. Mol Microbiol 67:1307–1319. [DOI] [PubMed] [Google Scholar]
- 72. Kubori T, Shinzawa N, Kanuka H, Nagai H (2010) Legionella metaeffector exploits host proteasome to temporally regulate cognate effector. PLoS Pathog 6:e1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ivanov SS, Roy CR (2009) Modulation of ubiquitin dynamics and suppression of DALIS formation by the Legionella pneumophila Dot/Icm system. Cell Microbiol 11:261–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cazalet C, Rusniok C, Bruggemann H, Zidane N, Magnier A, et al. (2004) Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat Genet 36:1165–1173. [DOI] [PubMed] [Google Scholar]
- 75. Price CT, Abu Kwaik Y (2010) Exploitation of host polyubiquitination machinery through molecular mimicry by eukaryotic-like bacterial F-box effectors. Front Microbio 1 10.3389/fmicb.2010.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Corduan A, Lecomte S, Martin C, Michel D, Desmots F (2009) Sequential interplay between BAG6 and HSP70 upon heat shock. Cell Mol Life Sci 66:1998–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Desmots F, Russell HR, Michel D, McKinnon PJ (2008) Scythe regulates apoptosis-inducing factor stability during endoplasmic reticulum stress-induced apoptosis. J Biol Chem 283:3264–3271. [DOI] [PubMed] [Google Scholar]
- 78. Canadien V, Tan T, Zilber R, Szeto J, Perrin AJ, et al. (2005) Cutting edge: microbial products elicit formation of dendritic cell aggresome-like induced structures in macrophages. J Immunol 174:2471–2475. [DOI] [PubMed] [Google Scholar]
- 79. Ivanov SS, Roy CR (2013) Pathogen signatures activate a ubiquitination pathway that modulates the function of the metabolic checkpoint kinase mTOR. Nat Immunol 14:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Franco IS, Shohdy N, Shuman HA (2012) The Legionella pneumophila effector VipA is an actin nucleator that alters host cell organelle trafficking. PLoS Pathog 8:e1002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Guo Z, Stephenson R, Qiu J, Zheng S, Luo ZQ (2014) A Legionella effector modulates host cytoskeletal structure by inhibiting actin polymerization. Microbes Infect 16:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim S, Coulombe PA (2010) Emerging role for the cytoskeleton as an organizer and regulator of translation. Nat Rev Mol Cell Biol 11:75–81. [DOI] [PubMed] [Google Scholar]
- 83. Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150. [DOI] [PubMed] [Google Scholar]
- 84. Di Paolo G, De Camilli P (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443:651–657. [DOI] [PubMed] [Google Scholar]
- 85. Viner R, Chetrit D, Ehrlich M, Segal G (2012) Identification of two Legionella pneumophila effectors that manipulate host phospholipids biosynthesis. PLoS Pathog 8:e1002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Degtyar E, Zusman T, Ehrlich M, Segal G (2009) A Legionella effector acquired from protozoa is involved in sphingolipids metabolism and is targeted to the host cell mitochondria. Cell Microbiol 11:1219–1235. [DOI] [PubMed] [Google Scholar]
- 87. Hilbi H, Weber S, Finsel I (2011) Anchors for effectors: subversion of phosphoinositide lipids by Legionella. Front Microbio 2 10.3389/fmicb.2011.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Weber SS, Ragaz C, Hilbi H (2009) The inositol polyphosphate 5-phosphatase OCRL1 restricts intracellular growth of Legionella, localizes to the replicative vacuole and binds to the bacterial effector LpnE. Cell Microbiol 11:442–460. [DOI] [PubMed] [Google Scholar]
- 89. Frasch SC, Bratton DL (2012) Emerging roles for lysophosphatidylserine in resolution of inflammation. Prog Lipid Res 51:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wieland H, Ullrich S, Lang F, Neumeister B (2005) Intracellular multiplication of Legionella pneumophila depends on host cell amino acid transporter SLC1A5. Mol Microbiol 55:1528–1537. [DOI] [PubMed] [Google Scholar]
- 91. Hoffmann C, Finsel I, Otto A, Pfaffinger G, Rothmeier E, et al. (2014) Functional analysis of novel Rab GTPases identified in the proteome of purified Legionella-containing vacuoles from macrophages. Cell Microbiol 16:1034–1052. [DOI] [PubMed] [Google Scholar]
- 92. Yeramian A, Martin L, Serrat N, Arpa L, Soler C, et al. (2006) Arginine transport via cationic amino acid transporter 2 plays a critical regulatory role in classical or alternative activation of macrophages. J Immunol 176:5918–5924. [DOI] [PubMed] [Google Scholar]
- 93. Barry DP, Asim M, Scull BP, Piazuelo MB, de Sablet T, et al. (2011) Cationic amino acid transporter 2 enhances innate immunity during Helicobacter pylori infection. PLoS One 6:e29046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Das P, Lahiri A, Sen M, Iyer N, Kapoor N, et al. (2010) Cationic amino acid transporters and Salmonella Typhimurium ArgT collectively regulate arginine availability towards intracellular Salmonella growth. PLoS One 5:e15466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sans-Fons MG, Yeramian A, Pereira-Lopes S, Santamaria-Babi LF, Modolell M, et al. (2013) Arginine transport is impaired in C57Bl/6 mouse macrophages as a result of a deletion in the promoter of Slc7a2 (CAT2), and susceptibility to Leishmania infection is reduced. J Infect Dis 207:1684–1693. [DOI] [PubMed] [Google Scholar]
- 96. Wanasen N, MacLeod CL, Ellies LG, Soong L (2007) L-arginine and cationic amino acid transporter 2B regulate growth and survival of Leishmania amazonensis amastigotes in macrophages. Infect Immun 75:2802–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Berger UV, Hediger MA (2006) Distribution of the glutamate transporters GLT-1 (SLC1A2) and GLAST (SLC1A3) in peripheral organs. Anat Embryol (Berl) 211:595–606. [DOI] [PubMed] [Google Scholar]
- 98. Rimaniol AC, Mialocq P, Clayette P, Dormont D, Gras G (2001) Role of glutamate transporters in the regulation of glutathione levels in human macrophages. Am J Physiol Cell Physiol 281:C1964–1970. [DOI] [PubMed] [Google Scholar]
- 99. Rimaniol AC, Haik S, Martin M, Le Grand R, Boussin FD, et al. (2000) Na+-dependent high-affinity glutamate transport in macrophages. J Immunol 164:5430–5438. [DOI] [PubMed] [Google Scholar]
- 100. Tattoli I, Philpott DJ, Girardin SE (2012) The bacterial and cellular determinants controlling the recruitment of mTOR to the Salmonella-containing vacuole. Biol Open 1:1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The total processed microarray data of hMDMs uninfected or infected with wild type or ankB mutant L. pneumophila at 8 h post-infection.
(XLSX)
Metacore enrichment analysis of hMDMs infected with wild type or ankB mutant L. pneumophila at 8 h post-infection showing up-regulated pathways.
(XLS)
Metacore enrichment analysis of hMDMs infected with wild type or ankB mutant L. pneumophila at 8 h post-infection showing down-regulated pathways.
(XLS)
Metacore enrichment analysis of hMDMs infected with wild type or ankB mutant L. pneumophila at 8 h post-infection showing up-regulated metabolic pathways.
(XLS)
Metacore enrichment analysis of hMDMs infected with wild type or ankB mutant L. pneumophila at 8 h post-infection showing down-regulated metabolic pathways.
(XLS)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files. Microarray data was submitted to the Gene Expression Omnibus (GEO) repository and can be accessed through accession number GSE61535.