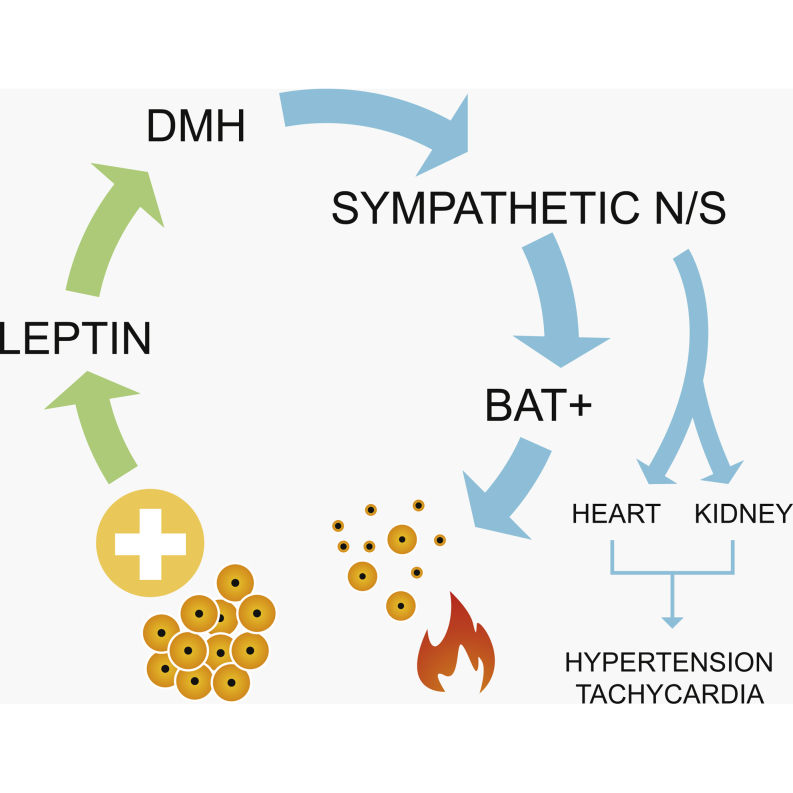
Summary
Obesity is associated with increased blood pressure (BP), which in turn increases the risk of cardiovascular diseases. We found that the increase in leptin levels seen in diet-induced obesity (DIO) drives an increase in BP in rodents, an effect that was not seen in animals deficient in leptin or leptin receptors (LepR). Furthermore, humans with loss-of-function mutations in leptin and the LepR have low BP despite severe obesity. Leptin’s effects on BP are mediated by neuronal circuits in the dorsomedial hypothalamus (DMH), as blocking leptin with a specific antibody, antagonist, or inhibition of the activity of LepR-expressing neurons in the DMH caused a rapid reduction of BP in DIO mice, independent of changes in weight. Re-expression of LepRs in the DMH of DIO LepR-deficient mice caused an increase in BP. These studies demonstrate that leptin couples changes in weight to changes in BP in mammalian species.
Graphical Abstract
Highlights
-
•
Leptin is the link between obesity and increased blood pressure
-
•
Leptin acts through the dorsomedial hypothalamus to increase blood pressure
-
•
Blockade of leptin signaling reduces blood pressure in obese mice
-
•
Humans with defects in leptin signaling are protected from obesity hypertension
Leptin is found to be the link between obesity and increased blood pressure. Blocking leptin action reduces blood pressure in obese mice with clinical studies in humans, suggesting that defects in leptin signaling may protect against hypertension associated with obesity.
Introduction
Obesity increases the risk for hypertension and is a major driver of morbidity and mortality due to cardiovascular diseases (Dustan, 1983, Poirier et al., 2006). Studies in rodents with diet-induced obesity (DIO) suggest that increased sympathetic nerve activity (SNA) is an important mediator of obesity-induced hypertension as α and β adrenergic receptor antagonists and renal denervation significantly blunt the rise in blood pressure (BP) associated with weight gain (Carlyle et al., 2002, Esler et al., 2006, Kassab et al., 1995). However, the precise molecular and neural mechanisms that link changes in weight with changes in BP have not been fully elucidated.
Circulating concentrations of the adipocyte-derived hormone leptin increase in proportion to adipose tissue mass and fall with weight loss (Considine et al., 1996, Maffei et al., 1995). As such, we hypothesized that leptin may be involved in coupling changes in body weight (BW) to changes in BP. Leptin regulates energy homeostasis by acting on hypothalamic neuronal circuits expressing the signaling isoform of the leptin receptor (LepR) to reduce calorie intake and increase energy expenditure (Halaas et al., 1997, Harris et al., 1998, Maffei et al., 1995, Zhang et al., 1994). Leptin can increase SNA, leading to increases in BP and heart rate (HR) (Haynes, 2000, Mark et al., 1999). In the arcuate nucleus of the hypothalamus (ARH), leptin stimulates the expression of pro-opiomelanocortin (POMC) and increases the activity of POMC neurons, which release the POMC peptides (α, β, and γ melanocyte-stimulating hormones [MSHs]) that act on melanocortin 4-receptor (MC4R)-expressing neurons in the paraventricular nucleus of the hypothalamus (PVH) and other brain regions to increase SNA (Cone, 2005, Cowley et al., 1999, Cowley et al., 2001, Haynes et al., 1999). However, POMC neurons become unresponsive to leptin in obesity, and leptin can act independently of MC4R signaling (Enriori et al., 2011, Patterson et al., 2011, Scott et al., 2009). Therefore, leptin’s effects beyond the melanocortin circuits need to be investigated. The dorsomedial hypothalamus (DMH) is critical for leptin’s ability to regulate brown adipose tissue (BAT) temperature and the cardiovascular system (Enriori et al., 2011, Fontes et al., 2001, Horiuchi et al., 2006, Marsh et al., 2003, Rezai-Zadeh et al., 2014).
Here, we investigated the development of obesity-induced hypertension. We demonstrate that, in DIO mice, increasing levels of leptin directly lead to an increase in HR and BP and that blocking the actions of leptin reverses these effects via neural circuits originating in the DMH. Furthermore, humans with loss-of-function mutations in leptin and its receptor have normal BP despite severe obesity, suggesting that these mechanisms are likely to be preserved in humans.
Results
Weight Gain Increases Leptin Levels, Heart Rate, and Blood Pressure
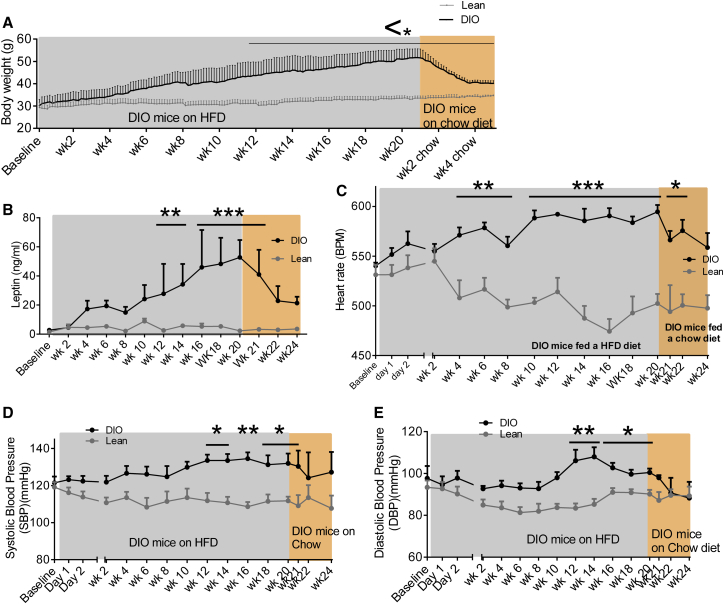
The temporal association between weight gain, changes in circulating leptin levels, HR, and BP were first examined. Four-week-old C57Bl/6J mice on a chow diet were implanted with radiotelemetric BP probes; baseline measurements were recorded at 6 weeks. As BW increased (Figure 1A), plasma leptin levels also progressively increased (Figure 1B). After 4 weeks of HFD, HR became significantly elevated (Figure 1C) and remained elevated throughout the 20 week recording period compared to chow fed mice. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were significantly elevated after 12 weeks of HFD (Figures 1D and 1E), subsequent to the rise in plasma leptin concentration. When HFD-fed mice were returned to a chow diet following 20 weeks of HFD feeding, mice lost 3.9 g in the first week, 3.9 g in the second week, 3.4 g in the third week, and 0.4 g in the fourth week (Figure 1A). DBP reduced after 1 week (Figure 1E), and SBP (Figure 1D) and HR (Figure 1C) reduced after 2 weeks of chow feeding. These findings suggest that leptin appears to increase before HR and BP increases in DIO, effects that are reversed with weight loss.
Figure 1.
Temporal Relationship between Changes in Weight Gain, Plasma Leptin Concentrations, and Blood Pressure
(A) Body weight (g), of mice from 4 weeks of age ad libitum fed either a high-calorie diet (DIO mice) or chow diet (lean mice) for 20 weeks. Following this 20 week period, DIO mice were switched to chow diet for 4 weeks; n = 3–7.
(B–E) (B) Plasma leptin concentration (ng/ml), (C) heart rate BPM (beats per min), (D) SBP, and (E) DBP (mmHg) at 2 week intervals, beginning at baseline (4-week-old mice). Following this baseline period, mice were fed a high-calorie diet (DIO) or chow diet (lean) for 20 weeks ad libitum. After 20 weeks of HFD in DIO mice, these mice were switched to chow diet for 4 weeks. n = 3–18.
Values mean ± SEM; two-way AVOVA, Bonferroni post hoc test. ∗p < 0.05, ∗∗p < 0.01, and ∗∗p < 0.001.
Leptin Signaling Is Required for Obesity-Induced Increases in Blood Pressure
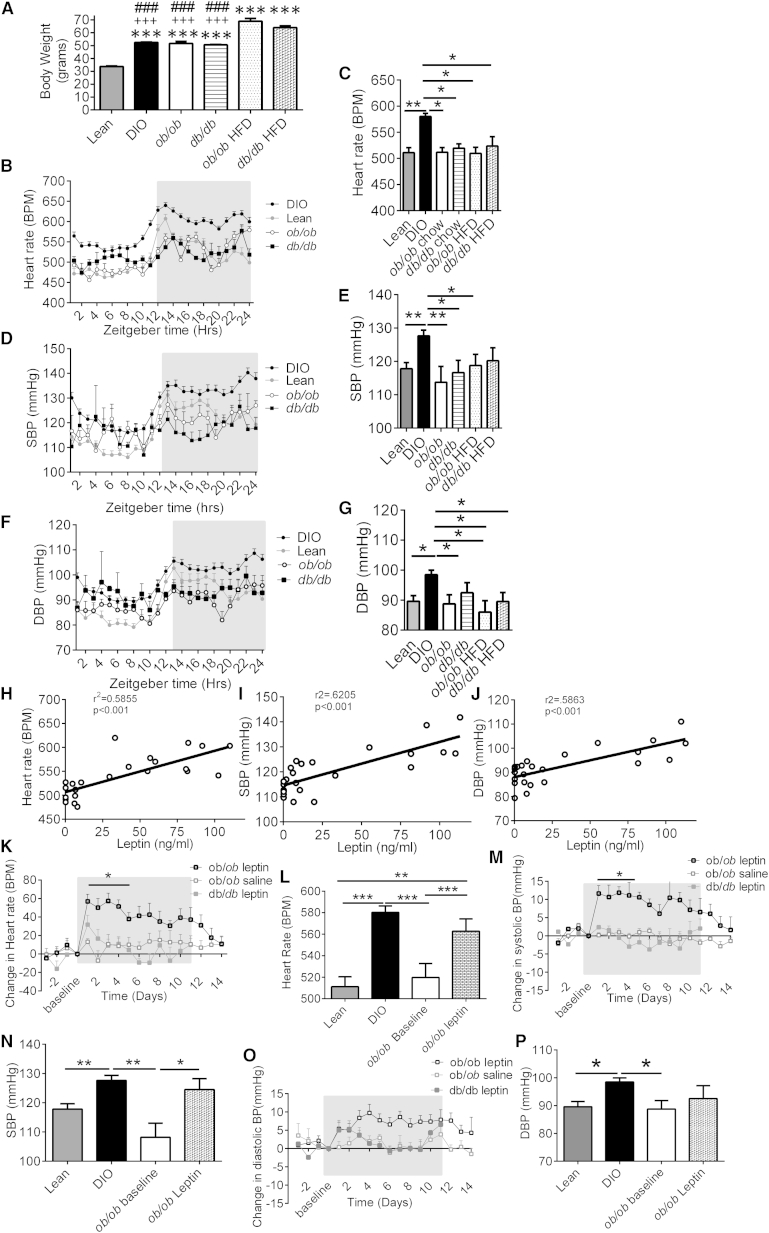
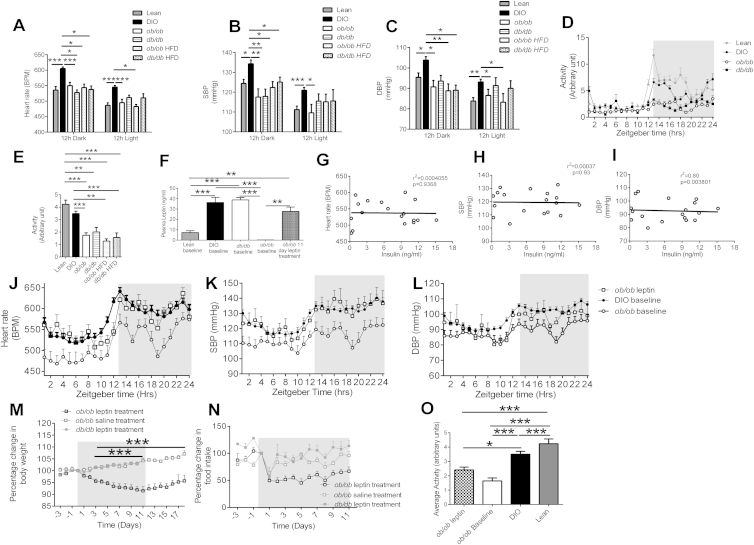
We examined whether the effects seen in DIO mice are dependent upon leptin signaling, using mice lacking leptin (ob/ob) or the LepR (db/db). HFD-fed ob/ob and db/db mice were significantly (p < 0.001) heavier than HFD-fed DIO and chow-fed lean, ob/ob, and db/db mice (Figure 2A). Despite the increased severity of obesity in the ob/ob and db/db mice, only DIO mice exhibited elevated HR, SBP, and DBP (Figures 2B–2G), demonstrating that increased BW alone is not the cause of the increase in BP in obesity. There was a greater difference in HR and BP between DIO mice to other mice during the dark period compared to the light period (Figures S1A–S1C available online). The increase in cardiovascular parameters in DIO mice was not due to increased activity (Figures S1D and S1E).
Figure 2.
Leptin Signaling Is Required for the Obesity-Induced Increases in BP
(A) Body weight (g) of mice fed 20 weeks a high-calorie (DIO, ob/ob, and db/db mice) or chow (lean ob/ob and db/db mice) diet ad libitum, ∗∗∗p < 0.001 versus lean, +++p < 0.001 versus ob/ob HFD fed, ###p < 0.001 versus db/db HFD (lean, n = 14; DIO, n = 37; ob/ob, n = 8; db/db, n = 3; ob/ob, HFD = 3; db/db, HFD = 5).
(B) Average heart rate (BPM) over a 24 hr period of mice fed a high-calorie (DIO mice) or chow (lean mice) diet, ob/ob mice and db/db mice fed a chow diet for over 20 weeks (lean, n = 14; DIO, n = 37; ob/ob, n = 8; db/db, n = 3; ob/ob, HFD = 3; db/db, HFD = 5).
(C) Heart rate (BPM) over a 24 hr period in mice fed a chow diet (lean mice), HFD-fed DIO mice, chow-fed ob/ob and db/db mice, and HFD-fed ob/ob and db/db mice. Measurements at 20 weeks are presented (lean, n = 14; DIO, n = 37; ob/ob, n = 8; db/db, n = 3; ob/ob, HFD = 3; db/db, HFD = 5).
(D) Average SBP (mmHg) over a 24 hr period of mice fed a high-calorie (DIO mice) or chow (lean mice) diet, ob/ob mice and db/db mice fed a chow diet for 20 weeks (lean, n = 14; DIO, n = 37; ob/ob, n = 8; db/db, n = 3; ob/ob, HFD = 3; db/db, HFD = 5).
(E) SBP (mmHg) over a 24 hr period in mice fed a chow diet (lean mice), HFD-fed DIO mice, chow-fed ob/ob and db/db mice, and HFD-fed ob/ob and db/db mice for 20 weeks (lean, n = 14; DIO, n = 37; ob/ob, n = 8; db/db, n = 3; ob/ob, HFD = 3; db/db, HFD = 5).
(F) Average DBP (mmHg) over a 24 hr period of mice fed a high-calorie (DIO mice) or chow (lean mice) diet, ob/ob mice and db/db mice fed a chow diet for 20 weeks (lean, n = 14; DIO, n = 37; ob/ob, n = 8; db/db, n = 3; ob/ob, HFD = 3; db/db, HFD = 5).
(G) DBP (mmHg) over a 24 hr period in mice fed a chow diet (lean mice), HFD-fed DIO mice, chow-fed ob/ob and db/db mice, and HFD-fed ob/ob and db/db mice (lean, n = 14; DIO, n = 37; ob/ob, n = 8; db/db, n = 3; ob/ob, HFD = 3; db/db, HFD = 5).
(H) Plasma leptin (ng/ml) correlates with heart rate (BPM). Linear regression, r2 = 0.5855; p < 0.001; n = 24.
(I) Plasma leptin (ng/ml) correlates with SBP (mmHg). Linear regression, r2 = 0.6205; p < 0.001; n = 24.
(J) Plasma leptin (ng/ml) correlates with DBP. Linear regression, r2 = 0.5883; p < 0.001; n = 24.
(K) Exogenous leptin administration into ob/ob mice increased heart rate. Vehicle (Veh) treatment into ob/ob mice and leptin administration into db/db mice produced no change. Two-way ANOVA, Bonferroni post hoc test. n = 3–8.
(L) Average heart rate (BPM) over 11 day treatment period of leptin-treated ob/ob mice, compared to the baseline HR of ob/ob mice, the baseline of DIO and lean mice. One-way ANOVA, Bonferroni post hoc test, paired t test between leptin-treated ob/ob mice and ob/ob baseline, n = 8–37.
(M) Exogenous leptin administration into ob/ob mice, increased SBP (mmHg). Vehicle treatment into ob/ob mice and leptin administration into db/db mice produced no change. Values represent mean ± SEM. Two-way ANOVA, Bonferroni post hoc test. n = 3–8.
(N) Average SBP (mmHg) over 11 day treatment period of leptin-treated ob/ob mice compared to ob/ob baseline SBP, DIO, and lean baseline SBP measurements. One-way ANOVA, Bonferroni post hoc test, paired t test between leptin-treated ob/ob mice and ob/ob baseline. n = 8–37.
(O) Exogenous leptin administration into ob/ob mice increased diastolic BP (DBP) (mmHg). Vehicle treatment into ob/ob mice and leptin administration into db/db mice produced no change. Two-way ANOVA, Bonferroni post hoc test. n = 3–8.
(P) Average DBP (mmHg) over 11 day treatment period of leptin-treated ob/ob mice compared to ob/ob baseline DBP, DIO baseline DBP, and lean mice DBP. One-way ANOVA, paired t test between leptin-treated ob/ob mice and ob/ob baseline. n = 8–37.
Values represent mean ± SEM, one-way AVOVA, Bonferroni post hoc test, unless specifically stated. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. See also Figure S1.
Figure S1.
Characterization of Leptin Effects on Metabolic and Cardiovascular Parameters, Related to Figure 2
(A) The average 12h dark and 12h light heart rate (HR) of lean, DIO, chow fed ob/ob, db/db and HFD fed ob/ob, db/db mice. Mean ± SEM, Two way ANOVA, ∗p < 0.05, ∗∗∗p < 0.001 Vs DIO, (lean n = 14, DIO n = 37, ob/ob n = 8, db/db n = 3, ob/ob HFD = 3, db/db HFD = 5)
(B) The average 12h dark and 12h light systolic BP (SBP) of lean, DIO, chow fed ob/ob, db/db and HFD fed ob/ob, db/db mice. Mean ± SEM, Two way ANOVA, ∗p < 0.05, ∗∗p < 0.01 ∗∗∗p < 0.001 Vs DIO, (lean n = 14, DIO n = 37, ob/ob n = 8, db/db n = 3, ob/ob HFD = 3, db/db HFD = 5)
(C) The average 12h dark and 12h light diastolic BP (DBP) of lean, DIO, chow fed ob/ob, db/db and HFD fed ob/ob, db/db mice. Mean ± SEM, Two way ANOVA, ∗p < 0.05, ∗∗p < 0.01 ∗∗∗p < 0.001 Vs DIO, (lean n = 14, DIO n = 37, ob/ob n = 8, db/db n = 3, ob/ob HFD = 3, db/db HFD = 5)
(D) Average activity (arbitrary units) throughout 24hr period of mice fed a high calorie (DIO mice) or chow (lean mice) diet, ob/ob mice and db/db mice fed a chow diet for 20 weeks. Values mean ± SEM, (lean n = 14, DIO n = 37, ob/ob n = 8, db/db n = 3, ob/ob HFD = 3, db/db HFD = 5)
(E) Average Activity (arbitrary units) over a 24hr period in mice fed a chow diet (lean mice), HFD fed DIO mice, chow fed ob/ob and db/db mice, HFD fed ob/ob and db/db mice. Values mean ± SEM, One-way AVOVA, Bonferroni post hoc test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, (lean n = 14, DIO n = 37, ob/ob n = 8, db/db n = 3, ob/ob HFD = 3, db/db HFD = 5)
(F) Plasma leptin concentration of lean mice baseline, DIO mice baseline, db/db mice baseline, ob/ob mice baseline and the plasma leptin concentration after 11 days of treatment in ob/ob mice. Mean ± SEM, one way ANOVA, ∗∗p < 0.01, ∗∗∗p < 0.001, n = 3-12
(G) Plasma insulin (ng/ml) does not correlate with heart rate (BPM) in mice on a C57Bl/6J background. Linear regression, r2 = 0.0004055, p = 0.9368, n = 18
(H) Plasma insulin (ng/ml) does not correlate with systolic BP (SBP) in mice on a C57Bl/6J background. Linear regression, r2 = 0.0003732, p = 0.9394, n = 18
(I) Plasma insulin (ng/ml) does not correlate with diastolic BP (DBP) in mice on a C57Bl/6J background. Linear regression, r2 = 0.003801, p = 0.8080, n = 18
(J) 24hr circadian heart rate (BPM) rhythm in leptin treated ob/ob mice, baseline DIO mice measurements and ob/ob baseline mice measurements, day 4 of treatment. Values mean ± SEM n = 8-37
(K) 24 hr circadian systolic BP (SBP) (mmHg) rhythm in leptin treated ob/ob mice, the baselines of DIO and ob/ob mice, day 4 of treatment. Values mean ± SEM, n = 8-37
(L) 24 hr circadian diastolic BP (DBP) (mmHg) rhythm in leptin treated ob/ob mice, the baselines of DIO and ob/ob mice day 4 of treatment. Values mean ± SEM, n = 8-37
(M) Leptin administration to ob/ob mice decreases BW, while vehicle administration into ob/ob mice and leptin administration into db/db mice produced no change in bodyweight. Values mean ± SEM. Two-way ANOVA, ∗∗∗p < 0.001, n = 3-8
(N) Change in food intake in exogenous leptin treated ob/ob mice, vehicle treated ob/ob mice and leptin treated db/db mice. Values mean ± SEM. Two-way ANOVA. (Significance between ob/ob leptin treatment and ob/ob vehicle treated, day 2 p < 0.05, day 4p < 0.05, day 5 p < 0.001, day 11 p < 0.05). (Significance between ob/ob leptin treatment and db/db leptin treated, day 2 p < 0.01, day3 p < 0.05, day 5 p < 0.05, day 6 p < 0.01, day 7p < 0.05, day 8 p < 0.05, day 9 p < 0.01, day 10 p < 0.05, day 11 p < 0.01), n = 3-8
(O) Average activity (arbitrary units) over 11 day treatment period in exogenously leptin administrated ob/ob mice, compared with the baseline activity of ob/ob, DIO and lean mice.
Values mean ± SEM. One way ANOVA, ∗p < 0.05, ∗∗∗p < 0.001 n = 8-37. Additional data for Figure 2.
Strong correlations were found in C57Bl/6J mice between plasma leptin concentration and HR (Figure 2H), SBP (Figure 2I), and DBP (Figure 2J). No correlation was found between plasma insulin concentration and HR, SBP, or DBP (Figures S1G–S1I). To examine the direct effect of leptin on BP, leptin-deficient ob/ob mice were treated for 11 days with either leptin or vehicle. After 11 days of treatment, the average plasma leptin concentration of ob/ob mice treated with leptin was 27.7 ± 2.9 ng/ml compared to 36.3 ± 4.9 ng/ml in DIO mice and 6.6 ± 1.1 ng/ml in lean mice (Figure S1F). Exogenous leptin treatment in ob/ob mice progressively decreased BW (Figure S1M) and food intake (FI) (Figure S1N) compared to leptin-treated db/db mice and vehicle-treated ob/ob mice. Despite this decrease in BW, leptin treatment increased HR (Figures 2K and 2L) and SBP (Figures 2M and 2N) in leptin-treated ob/ob mice compared to leptin-treated db/db mice and vehicle-treated ob/ob mice. DBP also increased in ob/ob leptin-treated mice, however, not to the same extent as SBP (Figures 2O and 2P). The magnitude of the increase in HR and BP in leptin-treated ob/ob mice decreased with time, coincident with their substantial reduction in BW. The 24 hr circadian rhythm of HR (Figure S2J), SBP (Figure S2K), and DBP (Figure S1L) of leptin-treated ob/ob mice was increased toward the baseline recordings of DIO mice. These changes were not explained by increased activity (Figure S1O).
Figure S2.
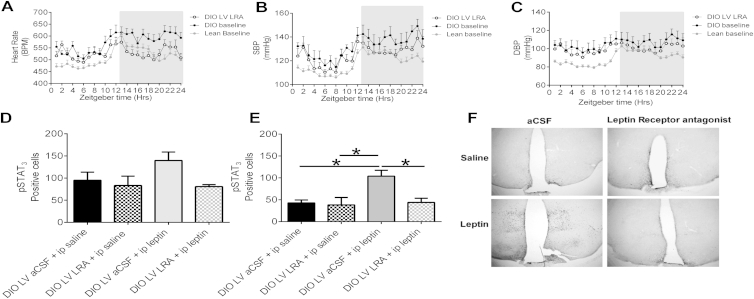
Effects of Leptin Receptor Antagonist on Cardiovascular and Immunohistochemical Parameters, Related to Figure 3
(A) 24 hr heart rate (HR) (BPM) circadian rhythm in DIO mice on day 7 treated in the lateral ventricle (LV) with leptin receptor antagonist (LRA) treatment, compared to the baseline of DIO and lean mice. Mean ± SEM, n = 6-47
(B) 24 hr circadian systolic BP (SBP) (mmHg) in DIO mice on day 7 from treatment in the lateral ventricle (LV) with leptin receptor antagonist (LRA) compared to the baseline of DIO and lean mice. Mean ± SEM, n = 6-37
(C) 24 hr circadian rhythm of diastolic BP (DBP) (mmHg) in DIO mice on day 7 of treatment in the lateral ventricle (LV) with leptin receptor antagonist (LRA) compared to the baselines of DIO and lean mice at baseline. Mean ± SEM, n = 6-37
(D) pSTAT3 expression in the ARH region of DIO mice, following either 0.5 μl of aCSF or leptin antagonist (5μg/μl) ICV injection and then either peripheral administration of Vehicle or leptin (2μg/g). One way ANOVA, Bonferroni post hoc, n = 3-5
(E) pSTAT3 expression in the VMH of DIO mice, following either 0.5 μl of aCSF or leptin antagonist (5μg/μl) ICV injection and then either peripheral administration of Vehicle or leptin (2μg/g). One way ANOVA, Bonferroni post hoc test. ∗p < 0.05, n = 3-5
(F) pSTAT3 Immunochemistry representative figure of hypothalamic sections of DIO mice, following either 0.5 μ of aCSF or leptin antagonist (5uμg/μl) ICV injection and then either peripheral administration of Vehicle or leptin (2μg/g).
The DMH analysis can be found in Lee et al. (2013). Additional data for Figure 3.
Inhibition of Leptin Signaling Reduces Elevated BP
Peripheral administration of a leptin antibody into DIO mice for 5 days caused a significant reduction in HR (Figure 3A), SBP (Figure 3B), and DBP (Figure 3C), although no change in BW was observed (Figure 3D). Central administration of a leptin receptor antagonist (LRA) into the lateral ventricle (LV) of hypertensive DIO mice over a 7 day period significantly reduced the elevated HR (p < 0.001; Figure 3E). By the seventh day of LRA treatment, HR was comparable to the average HR of lean animals (Figures 3F and S2A). Systolic BP also progressively decreased when DIO mice were treated with the LRA, and by day 5 of LRA treatment, SBP was significantly (p ≤ 0.05) decreased compared to vehicle-treated DIO mice (Figure 3G). By the seventh day of LRA treatment, SBP was significantly (p < 0.05) lower compared to the SBP at baseline in DIO mice and was comparable to the baseline SBP of lean mice (Figures 3H and S2B). SBP increased after LRA treatment ended. DBP also decreased throughout the LRA treatment period (Figures 3I, 3J, and S2C). These changes were seen despite the absence of a significant change in FI (Figure 3K), indicating that these changes are mediated by blocking the effects of leptin signaling rather than through changes in FI and BW.
Figure 3.
Blockade of Leptin Actions in DIO Mice Reduces Elevated BP and HR
(A–C) (A) Percentage change in HR, (B) SBP, and (C) DBP over a 5 day treatment period with either leptin antibody or vehicle (IgG control). Student’s t test, n = 4–8.
(D) Percentage daily change in body weight of DIO mice before and during 5 days of treatment and posttreatment of leptin antibody or vehicle (IgG control). Two-way ANOVA, Bonferroni post hoc test. n = 4–8.
(E) Daily change in HR (BPM) in DIO mice treated LV with aCSF or leptin receptor antagonist (LRA). Two way ANOVA, Bonferroni post hoc, n = 4-6
(F) Total 24hr HR(BPM) after 7 days of LRA treatment in DIO mice compared to lean and DIO mice baselines. t test paired (DIO baseline versus LRA), unpaired t test. n = 6–37.
(G) Daily change in SBP (mmHg) from baseline in DIO mice treated in the LV with aCSF or LRA. Two-way ANOVA, Bonferroni post hoc test, n = 4–6.
(H) Total 24 hr SBP (mmHg) after 7 days of LRA treatment in DIO mice compared to the baselines of lean and DIO mice. t test paired (DIO baseline versus LRA), unpaired t test. n = 6–37.
(I) Daily change in DBP (mmHg) in DIO mice treated with LVaCSF or LRA. Two-way ANOVA, Bonferroni post hoc, n = 4–6.
(J) Total 24 hr DBP (mmHg) after 7 days of LRA in DIO mice compared to the baseline measurements of lean and DIO mice. t test paired (DIO baseline versus LRA), unpaired t test. n = 6–37.
(K) Average food intake over the 7 day treatment period of LVaCSF (control) or LRA DIO mice. t test, n = 4–6.
Values represent mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. See also Figure S2.
Leptin Modulates BP via Neural Circuits in the Dorsomedial Hypothalamus
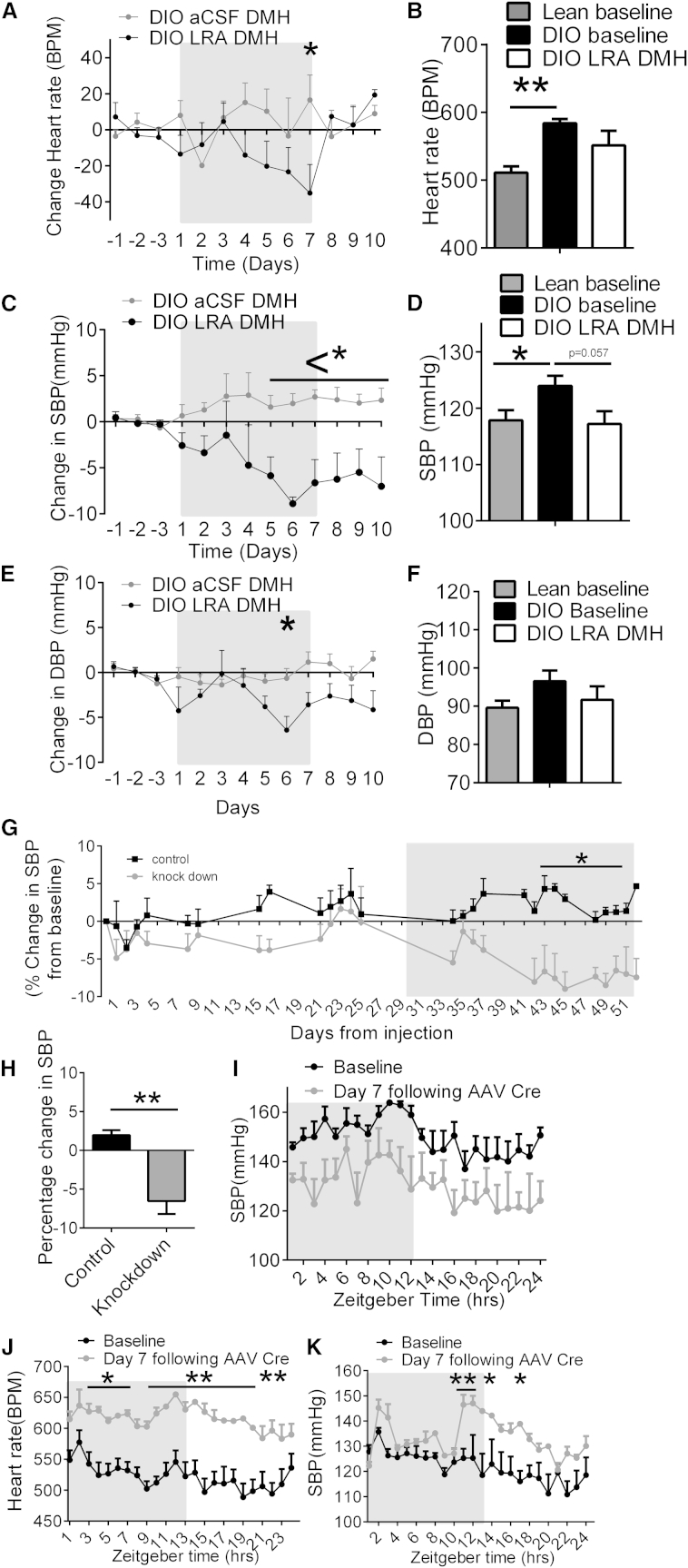
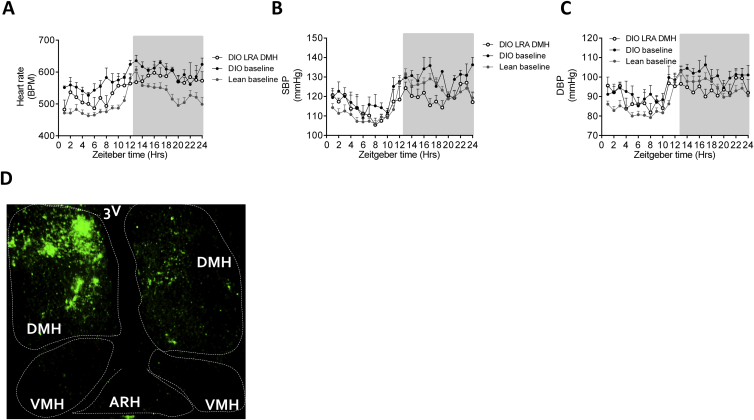
To determine whether leptin’s effects on BP and HR were mediated by neurons in the DMH, the LRA was injected directly into the DMH of hypertensive DIO mice daily over 7 days. LRA treatment in the DMH decreased HR (Figures 4A, 4B, and S3A) and SBP (Figures 4C, 4D, and S3B) by day 7 of treatment. No significant change in DBP (Figures 4E, 4F, and S3C) was observed. Furthermore, injection of an AAV expressing a short hairpin RNA directed against the LepR (Hommel et al., 2006) in the DMH of DIO mice led to a sustained decrease in SBP (Figures 4G, 4H, and S3D) after 4 weeks. Utilizing mice in which the LepR is flanked by loxP sites and can be deleted by an AAV Cre, HFD for 20 weeks induced increased SBP at baseline, whereas AAV Cre administration into the DMH (hence deleting the LepR in only the DMH regions) decreased SBP (Figure 4I). Additionally, we examined the effects of reactivation of the LepR by injection of an AAV-expressing Cre recombinase into the DMH of normotensive morbidly obese LepR null mice (expressing a loxP flanked STOP codon in front of the LepR, called LepR transcriptional blocker or “LepR TB” mice; Berglund et al., 2012). Both HR (Figure 4J) and SBP (Figure 4K) increased rapidly after LepR expression was reactivated in just the DMH of obese LepR-deficient mice.
Figure 4.
The DMH Is Involved in Leptin-Mediated Increases in HR and BP
(A) Daily change in HR (BPM) from baseline in DIO mice treated in the DMH with aCSF or a LRA. Two-way ANOVA, Bonferroni post hoc test, n = 4.
(B) Total 24 hr HR (BPM) after 7 days of LRA treatment in the DMH of DIO mice compared to lean and DIO mice at baseline. t test paired (DIO baseline versus LRA), unpaired t test. n = 4–37.
(C) Daily change in SBP (mmHg) from baseline in DIO mice treated in the DMH with aCSF or a LRA. Two-way ANOVA, Bonferroni post hoc test, n = 4.
(D) Total 24 hr SBP (mmHg) after 7 days of LRA treatment in the DMH of DIO mice compared to lean and DIO mice at baseline. t test paired (DIO baseline versus LRA), unpaired t test. n = 4–37.
(E) Daily change in DBP (mmHg) from baseline in DIO mice treated in the DMH with aCSF or a LRA. Two-way ANOVA, Bonferroni post hoc test, n = 4.
(F) 24 hr DBP (mmHg) after 7 days of LRA treatment in the DMH of DIO mice compared to lean and DIO mice at baseline. t test paired (DIO baseline versus LRA), unpaired t test. n = 4–37.
(G) Percentage change in SBP (mmHg) of DIO mice, following control (scrambled AAV) or leptin receptor knockdown AAV into the DMH. Two-way ANOVA, Bonferroni post hoc test, n = 4–6.
(H) Quantitative analysis from day 31 onward of AAV scrambled control-injected DIO mice and AAV LepR knockdown-treated DIO mice. t test, n = 4–6.
(I) 24 hr circadian rhythm of SBP (mmHg) at baseline and then following the seventh day of AAV Cre injection into the DMH of LepR flox mice. Two-way ANOVA, Bonferroni post hoc test, n = 4.
(J) 24 hr circadian rhythm of heart rate (BPM) at baseline and then following the seventh day of AAV Cre injection into the DMH of LepR flox TB mice. Two-way ANOVA, Bonferroni post hoc test, n = 4.
(K) 24 hr circadian rhythm of SBP (mmHg) at baseline and then following the seventh day of AAV Cre injection into the DMH of LepR flox TB mice. Two-way ANOVA, Bonferroni post hoc test, n = 4.
Values represent mean ± SEM. ∗p < 0.05 and ∗∗p < 0.01. See also Figure S3.
Figure S3.
Cardiovascular Effects of Altering Leptin Signaling in the DMH, Related to Figure 4
(A) 24hr heart rate (BPM) circadian rhythm in DIO mice on day 7 of leptin receptor antagonist (LRA) treatment into the DMH, compared to the baseline of DIO and lean mice. Mean ± SEM, n = 4-37
(B) 24hr systolic BP (SBP) (mmHg) circadian rhythm in DIO mice on day 7 of leptin receptor antagonist (LRA) treatment into the DMH, compared to the baseline of DIO and lean mice. Mean ± SEM, n = 4-37
(C) 24hr diastolic BP (DBP) (mmHg) circadian rhythm in DIO mice on day 7 of leptin receptor antagonist (LRA) treatment into the DMH, compared to the baseline of DIO and lean mice. Mean ± SEM, n = 4-37
(D) Representative image of GFP fluorescence, indicating AAV infection, to knockdown leptin receptor in the DMH of DIO mice. Virus had been injected 55 days prior to sacrifice of mice. Additional data for Figure 4.
Depolarization of DMH LepR-Expressing Neurons
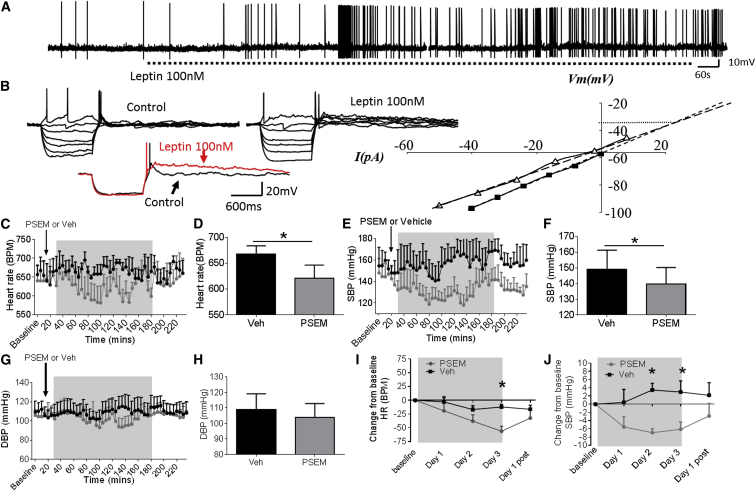
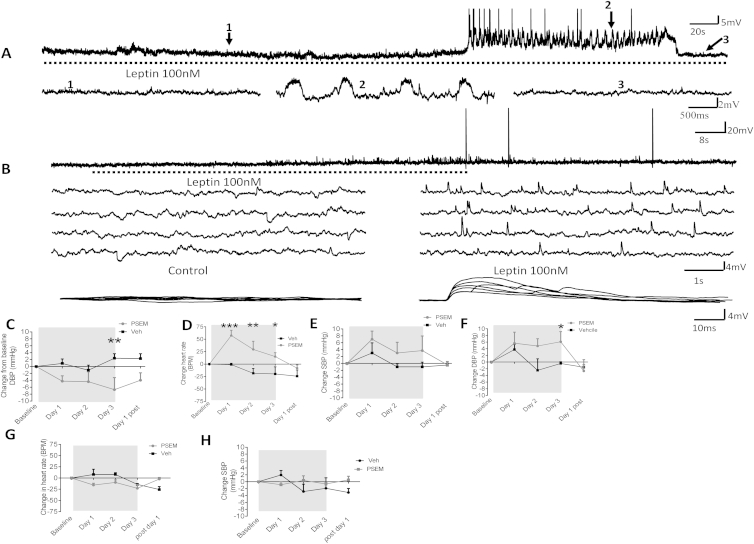
To determine the electrophysiological effect of leptin on DMH neurons, we recorded the electrical activity of neurons expressing LepR in LepR-Cre-YFP mice (Leshan et al., 2012). Whole-cell recordings were obtained from 34 DMH neurons expressing LepR. Application of 100 nM leptin induced membrane depolarization and/or an increase in spontaneous action potential firing rate in 38.2% of recorded neurons (Figure 5A). Leptin induced a mean peak amplitude depolarization of 4.9 ± 1.0 mV from a mean resting potential of −49.9 ± 3.1 mV to a new steady-state membrane potential of −45.0 ± 2.7 mV (p = 0.0004). Leptin-induced excitation was associated with an increase in firing rate from a mean control basal level of 0.48 ± 0.28 Hz to 0.64 ± 0.34 Hz in the presence of leptin (n = 5), effects that were at least in part reversible after washout of leptin (p = 0.59). In two neurons, leptin induced sub- and suprathreshold oscillations in membrane potential (Figure S4A). Voltage-current relations, generated in response to a range of depolarizing and hyperpolarizing rectangular-wave current pulses (−150 to +100 pA, 1,000 ms duration) revealed that leptin-induced excitation was principally associated with a trend for a decrease (p = 0.86) in neuronal input resistance from 841 ± 111 MΩ in the absence to 743 ± 138 MΩ in the presence of leptin. Plots of the voltage-current relations revealed extrapolated reversal potentials for leptin-induced excitation around −35 mV (Figure 5B). Taken together, these data suggest that leptin-induced excitation is mediated via activation of one or more nonselective cation conductances. In addition to these effects on membrane input conductance, leptin also induced modulation of intrinsic subthreshold active conductance in a subpopulation of DMH neurons. In DMH neurons, membrane depolarization from negative holding potentials (< −65 mV) or depolarization at the offset of the membrane response to hyperpolarization from potentials close to resting potential (−45 to −50 mV) evoked a transient depolarizing potential consistent with activation of a low-threshold T-type calcium conductance. In the presence of leptin, this potential was prolonged (Figure 5B), the half-time to decay increasing from 132 ± 59 ms in the absence to 179 ± 61 ms in the presence of leptin. These data are consistent with leptin modulating intrinsic active conductances in a subpopulation of DMH neurons. In addition to these postsynaptic effects, leptin induced an increase in spontaneous excitatory postsynaptic potentials (EPSPs) in a subpopulation (n = 2) of DMH neurons (Figure S4B). The mean frequency of spontaneous EPSPs increased from 0.07 ± 0.02 Hz to 0.31 ± 0.11 Hz in the presence of leptin. These EPSPs could be of sufficient magnitude to reach threshold for firing, suggesting that indirect presynaptic effects of leptin on DMH neurons can contribute to leptin-induced increases in neuronal excitability.
Figure 5.
Leptin Excites Leptin-Receptor-Expressing Neurons in the DMH
(A) Whole-cell patch-clamp electrophysiology in LepR-Cre-YFP mice; bath application of leptin (100 nM) induced an increase in spontaneous firing rate.
(B) Voltage-current relations from the cell shown in Figure S4A before and following exposure to leptin (100 nM). Note the transient rebound depolarization at the offset of the response to hyperpolarizing current injection and its prolongation in the presence of leptin, illustrated below with responses to hyperpolarizing current injection in control (black) and in the presence of leptin (red). Right, plot of data shown in (B); note the decreased slope of the voltage-current relations in the presence of leptin (100 nM) (open triangles) compared to control (closed squares), indicating a decrease in neuronal input resistance and extrapolated reversal potential around −35 mV, consistent activation of a nonselective cation conductance.
(C) Averaged 5 min (data collected every 10 s) heart rate (BPM) of DIO mice injected in the DMH with inhibitor chimeric ion channel virus (pSyn::Flex-rev-PSAM L141F:GlyR-IRES-GFP) and treated i.p. with vehicle or PSEM (5 mg/kg). n = 5.
(D) Average (20–180 min) heart rate (BPM) over treatment period of DIO mice injected in the DMH with inhibitor chimeric ion channel virus with vehicle or PSEM. Paired t test, n = 5.
(E) Averaged 5 min (data collected every 10 s) SBP (mmHg) of DIO mice injected in the DMH with inhibitor chimeric ion channel virus and treated i.p. with vehicle or PSEM (5 mg/kg). Two-way ANOVA, Bonferroni post hoc test, n = 5.
(F) Average (20–180 min) SBP (mmHg) in DIO mice injected in the DMH with inhibitor chimeric ion channel virus in mice treated i.p. with Veh or pharmacologically selective effector molecule (PSEM). Paired t test, n = 5.
(G) Averaged 5 min (data collected every 10 s) DBP (mmHg) of DIO mice injected in the DMH with inhibitor chimeric ion channel virus and treated i.p. with vehicle or PSEM (5 mg/kg). Two-way ANOVA, Bonferroni post hoc test, n = 5.
(H) Average (20–180 min) DBP (mmHg) of DIO mice treated with chimeric ion channel virus and with Veh or PSEM. Paired t test, n = 5.
(I) Average daily HR (BPM) in DIO mice injected in the DMH with inhibitor chimeric ion channel virus treated i.p. with Veh or PSEM for 3 days. Paired t test, n = 5.
(J) Average daily SBP (mmHg) in DIO mice treated with inhibitor chimeric ion channel virus and with Veh or PSEM for 3 days. Paired t test, n = 5.
Values represent mean ± SEM. ∗p < 0.05. See also Figure S4.
Figure S4.
Leptin Activates DMH Neurons to Regulate Blood Pressure, Related to Figure 5
(A) In DMH lepR expressing neurons, leptin (100nM) induced membrane depolarization, increased firing rate and underlying oscillations in membrane potential to close to threshold. Oscillations induced in the presence of leptin shown on an expanded time-scale below (2) compared with control (1). Note oscillations were reset and abolished following membrane hyperpolarization with constant current injection (3).
(B) Leptin (100nM) induced excitation characterized by an increase in firing frequency and spontaneous excitatory postsynaptic potentials (EPSPs), the latter illustrated on an expanded scale below and superimposed.
(C) Average daily diastolic BP (DBP) (mmHg) in DIO mice treated with chimeric ion channel virus (pSyn::Flex-rev-PSAM L141F:GlyR-IRES-GFP) and with Veh or PSEM, for 3 days. Mean ± SEM, Paired t test, ∗∗p < 0.01 n = 5
(D) Average daily heart rate (HR) (BPM) in lean mice treated with activator chimeric ion channel virus (pSyn::Flex-rev-PSAM Y115F:5HT3 HC-IRES-GFP) in the DMH and treated with Veh or PSEM, for 3 days. Mean ± SEM, two way ANOVA, ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05, n = 4
(E) Average daily systolic BP (SBP) (mmHg) in lean mice treated with activator chimeric ion channel virus (pSyn::Flex-rev-PSAM Y115F:5HT3 HC-IRES-GFP) in the DMH and treated with Veh or PSEM, for 3 days. Mean ± SEM, two way ANOVA, n = 4
(F) Average daily diastolic BP (DBP) (mmHg) in lean mice treated with activator chimeric ion channel virus (pSyn::Flex-rev-PSAM Y115F:5HT3 HC-IRES-GFP) in the DMH and treated with Veh or PSEM, for 3 days. Mean ± SEM, two way ANOVA, ∗p < 0.05, n = 4
(G) Average daily HR (BPM) in DIO mice before injection to inhibitor in the DMH and treated i.p. with Veh or PSEM. Mean ± SEM, two way ANOVA, n = 3
(H) Average daily SBP (mmHg) in DIO mice before injection of inhibitor in the DMH and treated with i.p. Veh or PSEM.
Mean ± SEM, two way ANOVA, n = 3. Additional data for Figure 5.
In Vivo Inhibition of LepR DMH-Expressing Neurons Decreases BP
In vivo, the effects of directly altering the neuronal activity of LepR-expressing DMH neurons were assessed using engineered pharmacologically selective chimeric ion channels for activating and silencing neuron activity (Magnus et al., 2011). Briefly, this technology requires the injection of a virus, which only infects and replicates in a Cre-dependent manner, into Cre-expressing mice. The virus drives the Cre-dependent expression of a modified ion channel, containing a PSAM element. After injection (intraperitoneal injection), the otherwise inert molecule PSEM binds to an ion channel that contains a PSAM element, which opens the ion pore and allows ion flux across the cell membrane, which depolarizes or hyperpolarizes virus-infected, Cre-expressing neurons. Using male 20-week-old chow-fed lean LepR-Cre-YFP transgenic mice (Leshan et al., 2012), we investigated whether activation of DMH LepR-expressing neurons could increase HR and BP. Lean LepR-Cre-YFP mice were injected bilaterally with activator virus, an engineered ionotropic serotonin receptor packaged in an AAV2 (pSyn::Flex-rev-PSAM Y115F:5HT3 HC-IRES-GFP) into the DMH. In lean mice 21 days after virus injection, twice daily intraperitoneal (i.p.) administration of the receptor ligand PSEM (i.p. 2X daily [5 mg/kg]) for 3 days significantly increased HR and BP (p ≤ 0.05; Figures S4D–S4F) compared to vehicle-treated mice. In DIO LepR-Cre-YFP mice, an inhibitory virus, an engineered ionotropic glycine receptor packaged into an AAV2 (AAV2: pSyn::Flex-rev-PSAM L141F:GlyR-IRES-GFP) was administered bilaterally into the DMH. Compared to vehicle treated mice, PSEM acutely decreased HR (Figures 5C and 5D) and SBP (Figures 5E and 5F), but no significant change in DBP was observed (Figures 5G and 5H). Sub-chronic daily treatment with PSEM reduced HR (by 72.6 ± 5.6 bpm), SBP (by 6.1 ± 1.7 mmHg) and DBP over 3 days (Figures 5I, 5J, and S4C), effects which were reversed once PSEM treatment ceased. Treatment of mice with PSEM, prior to virus administration had no effect on HR or BP (Figures S4G and S4H). Cumulatively, these findings indicate that leptin signaling is required for the changes in BP seen in DIO and that LepR expressing neurons in the DMH are necessary and sufficient to, cause these effects. These data also support the premise that the hypertension induced by leptin in the DMH is due to leptin-induced depolarization of DMH neurons.
Human Leptin and Leptin Receptor Mutations Are Associated with Low Blood Pressure Despite Severe Obesity
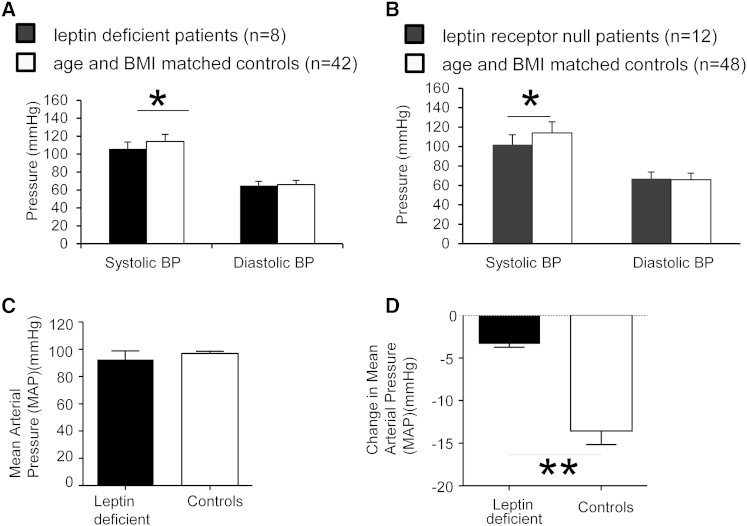
Homozygous complete loss-of-function mutations in the gene encoding leptin (LEP) are associated with undetectable leptin levels and severe early-onset obesity in humans (Montague et al., 1997). We measured BP in the fasted rested state in eight leptin-deficient children and 42 equally obese controls of the same age with normal leptin levels for the degree of obesity studied in the same conditions (Table S1). SBP was significantly lower in leptin-deficient children compared to age- and BMI-matched controls (Figure 6A; p < 0.05); there was no difference in DBP. A statistically significant attenuation of SBP was also seen in severely obese children who were homozygous for complete loss-of-function mutations in the LEPR gene (n = 12) compared to 48 age- and BMI-matched controls (Figure 6B; p < 0.05) (Table S1). There were no significant differences in resting HR between the groups (data not shown). Administration of recombinant methionyl human leptin to individuals with congenital leptin deficiency leads to significant weight loss (Farooqi et al., 1999, Ozata et al., 1999). In a previously reported experimental paradigm (Galgani et al., 2010), three leptin-deficient adults were studied before and after treatment with recombinant leptin for 19 weeks, which was sufficient to cause 15.5 ± 0.5 kg weight loss. In parallel, three age- and BMI-matched controls were studied before and after a diet and exercise intervention, which achieved a comparable degree of weight loss (14.8 ± 1.76 kg). The three adult leptin-deficient individuals were found to have normal BP despite severe obesity (Figure 6C). Whereas the MAP of the obese control group decreased as expected (Figure 6D), the BP of the leptin-deficient adults did not change. No difference in HR was observed (Figure S5). Similarly, there was no statistically significant change in BP after recombinant leptin administration to leptin-deficient children (data not shown). These studies in rare individuals completely lacking leptin or functioning LEPR support the assertion that leptin is necessary for the increased BP associated with obesity in humans.
Figure 6.
Blood Pressure Measurements in Humans with Leptin and Leptin Receptor Mutations
(A) Leptin-deficient children (n = 8) have significantly lower SBP (mmHg) compared to age- and weight-matched control subjects (n = 42).
(B) Leptin-receptor-deficient children (n = 12) have significantly lower SBP (mmHg) compared to age- and weight-matched control subjects (n = 48).
(C) Baseline pretreatment mean arterial pressure (MAP) (mmHg) of leptin-deficient adult and control subjects. n = 3.
(D) Change in MAP (mmHg) after 19 weeks of weight loss treatment. n = 3.
Value represent mean ± SEM. Student’s t test. ∗p < 0.05. See also Figure S5 and Table S1.
Figure S5.
Heart Rate Response to Leptin or Lifestyle-Induced Weight Loss, Related to Figure 6
Change in heart rate (HR) (BPM) after 19 weeks of weight loss in leptin deficient adults treated with recombinant human leptin and BMI matched controls with the same degree of weight loss. Mean ± SEM, n = 3. Additional data for Figure 6.
Discussion
Leptin Is the Peripheral Signal Linking Weight Gain to Changes in Blood Pressure
Cumulatively, these studies demonstrate that leptin signaling is necessary for obesity-induced increased BP. We have used multiple convergent methods, including making lean mice obese, by adding leptin systemically and by restoring LepR to the DMH of LepR-deficient mice. In all these studies, BP increased. Similarly, we have studied reduced leptin signaling in leptin- and LepR-deficient mice, neutralized circulating leptin with a systemic antibody, infused LepR antagonist ICV and intra-DMH; used shRNA to knock down LepR expression; selectively deleted LepR; and inhibited LepR-expressing neurons in the DMH of obese hypertensive mice. In all of these experiments, reducing leptin signaling reduced BP, even in the presence of obesity. Clinical studies in severely obese humans with two different forms of defective leptin signaling show that these observations are relevant to human physiology and pathophysiology.
Leptin in the DMH Regulates Cardiovascular Function in the Absence of Effects on BW
Leptin can acutely increase HR, BP, and BAT, even in anesthetized animals, presumably through activation of the SNS (Enriori et al., 2011, Marsh et al., 2003). Intracerebroventricular (ICV) leptin increases SNA to numerous organs, including the lumbar, kidney, and BAT regions (Haynes et al., 1999). Central antagonism of LepR caused a reduction in BP and HR in DIO hypertensive mice. Although early lesion studies confirm the importance of the DMH for the control of energy homeostasis, little is known about the neurochemical phenotype of the neurons present in the DMH (Elmquist et al., 1999, Lee et al., 2012). The DMH appears to have a critical ability to control thermogenesis and is involved in elevated BAT-mediated thermogenesis found in obese mice (Enriori et al., 2011, Fan et al., 2005, Morrison et al., 2008). In the studies conducted with antagonism or knocking down of the LepR in DIO mice, we did not find significant changes in FI or BW. These results suggest that the DMH LepR-expressing neurons are not essential for leptin’s effects on BW because the mice lost no additional weight when LepRs were inactivated. Although lesion studies have previously demonstrated a critical role of the DMH in the control of BW, we have shown that long-term knockdown of the LepR does not significantly affect BW, despite the significant reduction in BP and previously reported increase in BAT temperature in obesity (Elmquist et al., 1999, Enriori et al., 2011). Additionally, acute activation or inhibition of LepR-expressing neurons did not change FI or BW.
Leptin in Humans
Contrary to our expectations, we did not observe an increase in BP with the administration of leptin to leptin-deficient patients. There are a number of possible explanations. First, the magnitude of leptin’s effect on BP may be too small to be detected given the sensitivity of the tools for measurement of BP in humans and the small number of individuals studied. Notably, despite weight loss with continued leptin administration, we did not see a significant decrease in BP in these patients as would be expected in more common forms of obesity. These effects are comparable to the effects of leptin on total energy expenditure (TEE), another phenotype mediated by sympathetic tone. We have previously shown that, in contrast to weight loss in obese controls where TEE decreases, TEE does not change with leptin administration in leptin deficiency (Galgani et al., 2010). Whether leptin increases BP (to a small degree) or leptin-deficient individuals respond differently to weight loss cannot be deduced from our data. However, our results are in line with the suggestion that the effect of leptin administration may be to prevent the reduction in BP expected in congenitally leptin-deficient individuals as they lose weight. In previous trials of leptin in common obesity, no effects on BP were observed (Heymsfield et al., 1999). Rosenbaum and Leibel have shown that controlled 10% weight loss in an experimental setting is sufficient to reduce leptin levels and is associated with decreased SNA (Rosenbaum et al., 2005).
Could Leptin Be Acting Peripherally?
It is possible that leptin could be producing some of its cardiovascular effects by acting peripherally. LepR are expressed throughout the brain, including the brainstem; additionally, LepR are expressed on cardiac myocytes, the kidney, and arteries, including the coronary arteries (Knudson et al., 2005, Purdham et al., 2004, Serradeil-Le Gal et al., 1997). We treated DIO mice peripherally with a leptin antibody and then in a separate experiment, DIO mice were treated centrally with the LepR antagonist, and in both experiments, similar responses were observed. In mice lacking the LepR, re-expression of the LepR in only the DMH caused a dramatic increase in BP and HR. The opposite effects occurred when the leptin receptors were deleted from only the DMH region in hypertensive mice. Thus, although LepR expression in peripheral regions may play a role, the results presented here clearly demonstrate a key role for the DMH LepR-expressing neurons in mediating the changes in BP in obesity.
Is Leptin Action in the DMH Mediated by the Melanocortin System?
Experiments in rodents and humans support a role for melanocortin signaling in the regulation of BP. Central administration of α-MSH increases BP and HR in wild-type mice, but not in Mc4r−/− mice, which maintain a normal BP despite severe obesity (Kuo et al., 2003, Morgan et al., 2008, Tallam et al., 2005). In humans, heterozygous loss-of-function mutations in MC4R are associated with a reduced prevalence of hypertension, low SBP, lower urinary noradrenaline excretion, and reduced peripheral nerve SNA (Greenfield et al., 2009, Sayk et al., 2010). Moreover, systemic administration of a centrally active melanocortin receptor agonist acutely increased BP in obese volunteers (Greenfield et al., 2009). As such, some of leptin’s effects on BP may be mediated by the melanocortin system. However, there are some indications that, in obesity, the POMC neurons in the ARH become nonresponsive to leptin (this has been termed leptin resistance), and this may limit how much the actions of leptin can be transduced by the POMC neurons in the ARH (Enriori et al., 2007, Knight et al., 2010, Münzberg et al., 2004). Previous research has implicated the POMC neurons of the ARH in the regulation of obesity-induced hypertension via activation of the IKKβ/NF-κB pathway (Purkayastha et al., 2011, Zhang et al., 2008). How POMC neurons interact with the DMH LepR-expressing neurons has not been addressed in these studies, but the DMH LepR-expressing neurons do send direct efferent projections to ARH neurons (Gautron et al., 2010). It is possible that the LepR-expressing neurons in the DMH express Mc4r or that the DMH LepR-expressing neurons act through other neurons that express Mc4r (Liu et al., 2003). The degree to which leptin’s effects on BP in obesity are dependent upon intact melanocortin signaling remains to be determined (do Carmo et al., 2011, Harlan et al., 2011).
DMH Neurons Regulate Autonomic Outflow to the Heart and Peripheral Vasculature
DMH LepR-expressing neurons are known to project to numerous brain regions, including the PVH (Elmquist et al., 1998, Gautron et al., 2010). DMH neurons also project to other nuclei, including the Raphe pallidus nucleus (RPa) and rostral ventrolateral medualla (RVLM) (Cao and Morrison, 2003, Horiuchi et al., 2004, Simonds and Cowley, 2013). Microinjection of leptin into the DMH of anesthetized rats can induce an acute increase in HR and BP (Marsh et al., 2003). The DMH-RPa connection has already been recognized to be important in the regulation of BAT thermogenesis in response to leptin (Zhang et al., 2011). The RVLM, however, appears to have a greater control over the regulation of BP, compared to regulation of thermogenesis and HR (Horiuchi et al., 2006, Morrison et al., 2008). The neurochemical phenotype of the LepR-expressing neuron populations in the DMH is still debated (Lee et al., 2012). Further studies will be necessary to characterize the DMH circuits that contribute to leptin’s effects on blood pressure and to characterize the mechanisms by which these neurons modulate sympathetic outputs to the heart and peripheral blood vessels.
Why Leptin—What about Insulin?
Although results here strongly demonstrate the role of leptin in contributing to elevated BP in obesity, a number of other hormones that change in response to weight gain could contribute. As expected, we found that, compared to lean mice, plasma insulin was elevated in all obese models. Despite this increase in plasma insulin, it was only DIO mice that exhibited significantly elevated BP, and insulin levels were not correlated with HR or BP, suggesting that insulin contributes little to the chronic elevation of BP seen in obesity. Also, insulin sensitivity as measured by hyperinsulinemic euglycemic clamps is comparable in human MC4R deficiency versus obese controls despite a lower BP and reduced urinary catecholamine excretion in MC4R-deficient subjects (Greenfield et al., 2009). Therefore, our data do not support a role for insulin in mediating obesity-induced increases in BP.
In conclusion, these observations suggest that pharmacological approaches based on the modulation of leptin’s effects on specific subpopulations of neurons could represent a potentially useful therapeutic strategy for the treatment of obesity-associated hypertension and for the prevention of some obesity-associated cardiovascular disease.
Experimental Procedures
All animal procedures were approved by Monash University animal ethics committee. All mice were housed in a controlled environment in which lights were on a 12 hr light/12 hr dark cycle; temperature and humidity remained constant. In experiments to examine the development of hypertension, 4-week-old male C57bl/6J mice were implanted with radiotelemetry probes (DSI, USA, model TA11PA-C10). These mice were allowed 2 weeks recovery postsurgery and, following baseline recordings, mice were spilt onto either chow (4.8% of fat, mouse and rat rodent chow diet, Specialty Feeds, Glen Forrest, Australia) or HFD (43% of fat, SF04-001, Specialty Feeds, Western Australia, Australia) for 20 weeks (140 days) in which recordings were taken every 13 to 15 days. After 20 weeks (140 days), the HFD fed mice were swapped onto a chow diet (4.8% of fat, SF04-001, Specialty Feeds, Western Australia, Australia) for 4 weeks. In all other experiments, male C57Bl/6J, leptin-deficient (ob/ob), LepR-deficient (db/db), LepR-deficient (LepRTB (Berglund et al., 2012), LepR flox, and LepR-Cre-YFP mice (Leshan et al., 2012) were placed on either a chow diet or HFD diet at 4 weeks of age and continuing for 20 consecutive weeks, after which mice were used for experiments. All mice were on a C57Bl/6J background. In all experiments, the animals’ BW and FI were monitored daily. Specific experimental procedures for radiotelemetry, pharmacological studies, genetic manipulations, and electrophysiological studies are detailed in the Supplemental Information.
Human Studies
All human studies were conducted according to the principles outlined in the Declaration of Helsinki and after approval by local ethical committees. All individuals or their parents (for children) gave written informed consent. Systolic and diastolic BP were measured in the rested, fasted state using wrist BP monitors (OMRON Healthcare, Hamburg, Germany) in leptin-deficient (n = 8) and leptin-receptor-deficient children (n = 12) (Farooqi et al., 2002, Farooqi et al., 2007). Control subjects were recruited from the Genetics of Obesity Study (GOOS) cohort. These control subjects had been tested for mutations in leptin, leptin receptor, and MC4R. Control subjects were age and BMI matched to leptin-deficient and leptin-receptor-deficient subjects (n = 42 and 48, respectively) (Wheeler et al., 2013).
Adult Studies
Leptin-deficient adult patients received pretreatment testing at the Pennington center, along with weight- and BP-matched control subjects. The leptin-deficient patients then received a 3 month treatment of recombinant leptin (Leptin [Amgen Inc., Thousand Oaks; Galgani et al., 2010]). Subjects were all provided a nutritionally balanced mixed diet during this period. Subjects then returned to the Pennington center for posttesting. Control subjects with “normal” leptin levels were administered a low-calorie diet (Galgani et al., 2010) for 9–20 weeks to cause the same weight loss and also returned to the Pennington center for posttesting. Mean ± SEM, t test, ∗∗p < 0.01. All subjects provided written informed consent. The study design was approved by the ethics committee of the Pennington Biomedical Research Center. Approval for leptin replacement included UCLA IRB approval (April 9, 2001) and FDA approval (IND application number 61690).
Statistics
Data are represented as mean ± SEM, and error bars also indicate SEM. p values were calculated by either unpaired or paired two-tailed Student’s t test, One-way ANOVA with Bonferroni post hoc test or two-way ANOVA with Bonferroni post hoc test. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
Extended Experimental Procedures.
Radiotelemetry
Radiotelemetry probes were implanted into the left carotid artery (TA11PA-C10 Data Science International) under isoflurane anesthesia. All mice received 2 weeks recovery before any baseline recordings were measured.
Chronic Leptin Infusion
Chow fed, ob/ob or db/db mice were implanted with radiotelemetry probes, and baseline recordings were made. Alzet 14 day osmotic minipumps (model 1002, Cupertino, CA) to administer either vehicle or leptin (Peprotech, Rocky USA) (30 μg/per day in 6 μl of solution [1.25 μg/0.25 μl per hour]) were implanted into a subcutaneous pocket 3hrs prior to the dark period. Pumps had been prepared and incubated at 37°C, 8-9hrs prior to implantation. Pumps where removed after 11 days (prior to the onset of the dark period) and the mice recovered from treatment while radiotelemetry measurements continued to be recorded (db/db mice were killed at day 11).
Immunohistochemistry and Radioimmunoassay
Immunohistochemistry (IHC) for pSTAT3 and radioimmunoassay (RIA) measurement to determine plasma leptin concentration were performed as previously described (Enriori et al., 2007).
Peripheral Neutralization of Endogenous Leptin
To neutralize leptin’s actions a goat IgG against recombinant mouse leptin (AF498 from R and D systems, Minneapolis) was peripherally (i.p.) administered into DIO mice for 5 consecutive days, following a sham injection period. Mice received i.p. injections of 24 μg in 90 μl of antibody solution or 90 μl of IgG control (Antibodies Australia, Victoria, Australia). The dose of the antibody was determined from previous research (Konstantinides et al., 2004). All mice where randomly treated.
Central Blockade of Endogenous Leptin
To reduce the activation of lepR expressing neurons in the brain, a leptin receptor antagonist (LRA) (mouse leptin antagonist mutant L39A/D40A/F41A, 0.5 μl of 5μg/μl, Protein Laboratories, Rehovot, Israel) was used as previously reported (Enriori et al., 2011). In DIO mice that where implanted with radiotelemetry probes, they were additionally equipped with either a lateral ventricle (LV) (Plastics one, 2 mm long 22 gauge, implanted 0.5 mm posterior and 1 mm lateral to bregma, 2 mm below the surface of the skull) or DMH cannula (Plastics one, 4 mm long, 26 gauge, implanted 1.9 mm posterior, 0.4 mm lateral to bregma, 4 mm below the surface of the skull) as described previously (Enriori et al., 2011). Following a 7 day recovery period, mice where all injected for 5 days twice daily with sham aCSF injection (0.5 μl) into cannulas. Mice then received aCSF or the LRA, injected directly into cannulas, twice daily for 7 days, immediately following the dark period and immediately prior to the dark period. Following the 7 day injection period mice where monitored daily for a 7 day recovery period.
Chronic Knockdown of Leptin Receptors in the Dorsomedial Hypothalamus
DIO mice were implanted with radiotelemetry probes and after 14 days of recovery baseline recordings were measured. In a stereotaxic apparatus (Stoelting, USA) mice were injected bilaterally into the DMH region (coordinated 1.8 mm posterior, 0.3/-0.3 mm lateral from bregma and 5.2 mm below the surface of the skull) 0.5 μl of either an AAV scrambled shRNA or AAV lepRshRNA over a 20 min period each side (Hommel et al., 2006). BW and FI were measurements daily and radiotelemetry recordings were measured periodically throughout the treatment period.
Reactivation and Deletion of Leptin Receptor in the DMH
LepR loxTB mice and LepR flox mice (Berglund et al., 2012) where placed on a HFD for 20 weeks. After which mice were implanted with radiotelemetry probes. Following the surgery (2 weeks), baseline recordings where measured. Mice where then injected into the DMH (coordinates 1.8 mm posterior, 0.3/-0.3 mm lateral from bregma and 5.2 mm below the surface of the skull) with an AAV cre virus. Mice where then monitored and cardiovascular parameters measured throughout.
Neuron Activation and Silencing with PSAM/PSEM Systems
To examine the effect of activation or inhibition of DMH lepR expressing neurons, we injected a virus that only infects and replicates in a Cre dependent manner, into LepR-Cre-YFP mice (Leshan et al., 2012). The virus drives the expression of a modified ion channel. After injection, the otherwise inert molecule PSEM binds to an ion channel that contains a PSAM element which causes opening of ion pore in the channel and allows ion flux across the cell membrane, which causes depolarization or hyperpolarization of Cre-expressing neurons. The PSAM element only binds PSEM, this system is similar to DREADDS, but uses direct ligand gated ion channels rather than GPCR’s to drive neuron specific effects (Magnus et al., 2011). These mice where placed on either the chow or HFD for 20 weeks then implanted with radiotelemtry probes and 2 weeks later BP radiotelemetry baseline recordings were made. Following this the lepR-Cre-YFP DIO mice were injected in the DMH (coordinates 1.8 mm posterior, 0.3/-0.3 mm lateral from bregma and 5.2 mm below the surface of the skull) with the inhibitor virus, pSyn::Flex-rev-PSAM L141F:GlyR-IRES-GFP (AAV2, UNC gene therapy center vector core, NC, USA), lean chow fed LepR-Cre-YFP mice were injected in the DMH (coordinated 1.8 mm posterior, 0.3/-0.3 mm lateral from bregma and 5.2 mm below the surface of the skull) with the activator virus, pSyn::Flex-rev-PSAM Y115F:5HT3 HC-IRES-GFP (AAV2, UNC gene therapy center vector core, NC, USA). Injection of either of these virus’s did not change baseline cardiovascular function, until the peripheral injection of the inert PSEM ligand, that caused opening of ion pores and activation or inhibition of neurons. After a 21 day recovery period cardiovascular parameters were re-examined and then mice where sham injected followed by acute or 3 consecutive days of treatment with PSEM (5 mg/kg, i.p., twice daily; Apex Scientific Inc. Stony Brook, NY) or Vehicle, randomized, crossover design (Magnus et al., 2011).
Electrophysiology
Experiments were undertaken on LepR-YFP-Cre mice (Leshan et al., 2012) following 20 weeks of chow diet feeding, ad lib. On the day of electrophysiological experiment, animals were terminally anaesthetized with isoflurane and decapitated. The brain was rapidly removed, submerged in ice cold artificial cerebrospinal fluid (aCSF) and coronal slices (200-250 μm thick) cut using a Vibratome (Leica, VT1000S, UK). Slices were maintained at room temperature in oxygenated aCSF (gassed with 95% 02, 5% CO2) for at least an hour prior to recording. For recording, slices were continuously perfused at room temperature with aCSF of the following composition (in mM): 127.0 NaCl, 1.9 KCl,1.2 KH2PO4, 26.0 NaHCO3, 2.0 D-glucose, 8.0 D-mannitol, 1.3 MgCl2, 2.4 CaCl2, equilibrated with 95% O2, 5% CO2, pH 7.3 – 7.4. LepR-YFP expressing neurons in the DMH were visualized using fluorescence, fitted with appropriate filters and infrared video microscopy with DIC optics and recordings obtained from these neurons with an Axopatch 700A amplifier. Patch pipettes were pulled using a horizontal puller (Sutter Instrument Co., Novato, CA, USA) from thin-walled, filamented borosilicate glass (GC150TF-10; Harvard Apparatus LTD) with resistances between 4 and 8 MΩ when filled with intracellular solution. The pipette solution comprised (mM): 140 K-gluconate, 10 HEPES, 10 KCl, 1.0 EGTA, 2 Na2ATP. The pH was adjusted to 7.3 with KOH and the osmolarity to around 310 mOsm with sucrose. Data were filtered at 2 – 5 kHz, digitized at 2 – 10 kHz (Digidata 1322, MDS Analytical Technologies) and stored on PC running PClamp 9 data acquisition software. Analysis of electrophysiological data was carried out using Clampfit 9.2 software (MDS Analytical Technologies). Results are expressed as mean ± S.E.M., and statistical significance (p) was determined using unpaired, non-parametric Student’s t test with p < 0.05 considered as significant. Leptin was made up as a concentrated stock solution and diluted to the concentration required (100nM) in aCSF immediately before commencement of electrophysiological recording.
Author Contributions
The design and performance of animal experiments was conducted by S.E.S., J.T.P., J.B., R.D.B., P.J.E., D.C.S., and M.A.C. Human clinical experiments were designed and performed by E.R., F.L.G., J.L., E.H., J.M.K., S.O., and I.S.F. Experiments were assisted by contributions from R.D., A.M.A., M.G.M., K.L.G., S.M.S., and J.K.E. Data analysis was conducted by S.E.S., J.T.P., E.R., F.L.G., J.L., D.C.S., I.S.F., and M.A.C. The manuscript was prepared by S.E.S., J.T.P., D.C.S., I.S.F., and M.A.C.
Acknowledgments
This work was supported by the Heart Foundation of Australia (M.A.C. and S.E.S.), the National Health and Medical Research Council of Australia (1029188 to M.A.C. and 1063955 to D.C.S.), Monash University (S.E.S.), Pfizer Australia (M.A.C.), a NORC Center grant (P30DK072476) at the Pennington Biomedical Research Center (E.R., F.L.G., and J.L.), the Leverhulme Trust (J.T.P.), the Wellcome Trust (082390/Z/07/Z) (I.S.F.), Medical Research Council (I.S.F.), NIHR Cambridge Biomedical Research Centre (I.S.F. and S.O.R.), and the Bernard Wolfe Health Neuroscience Fund (I.S.F.). We thank Professor Streamson Chua for the LepR flox mice. J.L. and I.S.F. treat patients with recombinant human leptin which is provided by BMS/AstraZeneca.
Published: December 4, 2014
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information includes Extended Experimental Procedures, five figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2014.10.058.
Contributor Information
I. Sadaf Farooqi, Email: isf20@cam.ac.uk.
Michael A. Cowley, Email: michael.cowley@monash.edu.
Supplemental Information
References
- Berglund E.D., Vianna C.R., Donato J., Jr., Kim M.H., Chuang J.C., Lee C.E., Lauzon D.A., Lin P., Brule L.J., Scott M.M. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J. Clin. Invest. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W.H., Morrison S.F. Disinhibition of rostral raphe pallidus neurons increases cardiac sympathetic nerve activity and heart rate. Brain Res. 2003;980:1–10. doi: 10.1016/s0006-8993(03)02981-0. [DOI] [PubMed] [Google Scholar]
- Carlyle M., Jones O.B., Kuo J.J., Hall J.E. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension. 2002;39:496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- Cone R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Considine R.V., Sinha M.K., Heiman M.L., Kriauciunas A., Stephens T.W., Nyce M.R., Ohannesian J.P., Marco C.C., McKee L.J., Bauer T.L. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Cowley M.A., Pronchuk N., Fan W., Dinulescu D.M., Colmers W.F., Cone R.D. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Cowley M.A., Smart J.L., Rubinstein M., Cerdán M.G., Diano S., Horvath T.L., Cone R.D., Low M.J. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- do Carmo J.M., da Silva A.A., Cai Z., Lin S., Dubinion J.H., Hall J.E. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustan H.P. Mechanisms of hypertension associated with obesity. Ann. Intern. Med. 1983;98:860–864. doi: 10.7326/0003-4819-98-5-860. [DOI] [PubMed] [Google Scholar]
- Elmquist J.K., Ahima R.S., Elias C.F., Flier J.S., Saper C.B. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc. Natl. Acad. Sci. USA. 1998;95:741–746. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist J.K., Elias C.F., Saper C.B. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Enriori P.J., Evans A.E., Sinnayah P., Jobst E.E., Tonelli-Lemos L., Billes S.K., Glavas M.M., Grayson B.E., Perello M., Nillni E.A. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Enriori P.J., Sinnayah P., Simonds S.E., Garcia Rudaz C., Cowley M.A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M., Straznicky N., Eikelis N., Masuo K., Lambert G., Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- Fan W., Voss-Andreae A., Cao W.H., Morrison S.F. Regulation of thermogenesis by the central melanocortin system. Peptides. 2005;26:1800–1813. doi: 10.1016/j.peptides.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Farooqi I.S., Jebb S.A., Langmack G., Lawrence E., Cheetham C.H., Prentice A.M., Hughes I.A., McCamish M.A., O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- Farooqi I.S., Matarese G., Lord G.M., Keogh J.M., Lawrence E., Agwu C., Sanna V., Jebb S.A., Perna F., Fontana S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi I.S., Wangensteen T., Collins S., Kimber W., Matarese G., Keogh J.M., Lank E., Bottomley B., Lopez-Fernandez J., Ferraz-Amaro I. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N. Engl. J. Med. 2007;356:237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes M.A., Tagawa T., Polson J.W., Cavanagh S.J., Dampney R.A. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2891–H2901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- Galgani J.E., Greenway F.L., Caglayan S., Wong M.L., Licinio J., Ravussin E. Leptin replacement prevents weight loss-induced metabolic adaptation in congenital leptin-deficient patients. J. Clin. Endocrinol. Metab. 2010;95:851–855. doi: 10.1210/jc.2009-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron L., Lazarus M., Scott M.M., Saper C.B., Elmquist J.K. Identifying the efferent projections of leptin-responsive neurons in the dorsomedial hypothalamus using a novel conditional tracing approach. J. Comp. Neurol. 2010;518:2090–2108. doi: 10.1002/cne.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield J.R., Miller J.W., Keogh J.M., Henning E., Satterwhite J.H., Cameron G.S., Astruc B., Mayer J.P., Brage S., See T.C. Modulation of blood pressure by central melanocortinergic pathways. N. Engl. J. Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- Halaas J.L., Boozer C., Blair-West J., Fidahusein N., Denton D.A., Friedman J.M. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan S.M., Morgan D.A., Agassandian K., Guo D.F., Cassell M.D., Sigmund C.D., Mark A.L., Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ. Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.B., Zhou J., Redmann S.M., Jr., Smagin G.N., Smith S.R., Rodgers E., Zachwieja J.J. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139:8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- Haynes W.G. Interaction between leptin and sympathetic nervous system in hypertension. Curr. Hypertens. Rep. 2000;2:311–318. doi: 10.1007/s11906-000-0015-1. [DOI] [PubMed] [Google Scholar]
- Haynes W.G., Morgan D.A., Djalali A., Sivitz W.I., Mark A.L. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- Heymsfield S.B., Greenberg A.S., Fujioka K., Dixon R.M., Kushner R., Hunt T., Lubina J.A., Patane J., Self B., Hunt P. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- Hommel J.D., Trinko R., Sears R.M., Georgescu D., Liu Z.W., Gao X.B., Thurmon J.J., Marinelli M., DiLeone R.J. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Horiuchi J., McAllen R.M., Allen A.M., Killinger S., Fontes M.A., Dampney R.A. Descending vasomotor pathways from the dorsomedial hypothalamic nucleus: role of medullary raphe and RVLM. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R824–R832. doi: 10.1152/ajpregu.00221.2004. [DOI] [PubMed] [Google Scholar]
- Horiuchi J., McDowall L.M., Dampney R.A. Differential control of cardiac and sympathetic vasomotor activity from the dorsomedial hypothalamus. Clin. Exp. Pharmacol. Physiol. 2006;33:1265–1268. doi: 10.1111/j.1440-1681.2006.04522.x. [DOI] [PubMed] [Google Scholar]
- Kassab S., Kato T., Wilkins F.C., Chen R., Hall J.E., Granger J.P. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–897. doi: 10.1161/01.hyp.25.4.893. [DOI] [PubMed] [Google Scholar]
- Knight Z.A., Hannan K.S., Greenberg M.L., Friedman J.M. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE. 2010;5:e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson J.D., Dincer U.D., Zhang C., Swafford A.N., Jr., Koshida R., Picchi A., Focardi M., Dick G.M., Tune J.D. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H48–H56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- Konstantinides S., Schäfer K., Neels J.G., Dellas C., Loskutoff D.J. Inhibition of endogenous leptin protects mice from arterial and venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 2004;24:2196–2201. doi: 10.1161/01.ATV.0000146531.79402.9a. [DOI] [PubMed] [Google Scholar]
- Kuo J.J., Silva A.A., Hall J.E. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension. 2003;41:768–774. doi: 10.1161/01.HYP.0000048194.97428.1A. [DOI] [PubMed] [Google Scholar]
- Lee S., Bookout A.L., Lee C.E., Gautron L., Harper M.J., Elias C.F., Lowell B.B., Elmquist J.K. Laser-capture microdissection and transcriptional profiling of the dorsomedial nucleus of the hypothalamus. J. Comp. Neurol. 2012;520:3617–3632. doi: 10.1002/cne.23116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshan R.L., Greenwald-Yarnell M., Patterson C.M., Gonzalez I.E., Myers M.G., Jr. Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat. Med. 2012;18:820–823. doi: 10.1038/nm.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Kishi T., Roseberry A.G., Cai X., Lee C.E., Montez J.M., Friedman J.M., Elmquist J.K. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J. Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M., Halaas J., Ravussin E., Pratley R.E., Lee G.H., Zhang Y., Fei H., Kim S., Lallone R., Ranganathan S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Magnus C.J., Lee P.H., Atasoy D., Su H.H., Looger L.L., Sternson S.M. Chemical and genetic engineering of selective ion channel-ligand interactions. Science. 2011;333:1292–1296. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark A.L., Shaffer R.A., Correia M.L., Morgan D.A., Sigmund C.D., Haynes W.G. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J. Hypertens. 1999;17:1949–1953. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- Marsh A.J., Fontes M.A., Killinger S., Pawlak D.B., Polson J.W., Dampney R.A. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42:488–493. doi: 10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]
- Montague C.T., Farooqi I.S., Whitehead J.P., Soos M.A., Rau H., Wareham N.J., Sewter C.P., Digby J.E., Mohammed S.N., Hurst J.A. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Morgan D.A., Thedens D.R., Weiss R., Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1730–R1736. doi: 10.1152/ajpregu.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.F., Nakamura K., Madden C.J. Central control of thermogenesis in mammals. Exp. Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzberg H., Flier J.S., Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- Ozata M., Ozdemir I.C., Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J. Clin. Endocrinol. Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- Patterson C.M., Leshan R.L., Jones J.C., Myers M.G., Jr. Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res. 2011;1378:18–28. doi: 10.1016/j.brainres.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier P., Giles T.D., Bray G.A., Hong Y., Stern J.S., Pi-Sunyer F.X., Eckel R.H., American Heart Association. Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- Purdham D.M., Zou M.X., Rajapurohitam V., Karmazyn M. Rat heart is a site of leptin production and action. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2877–H2884. doi: 10.1152/ajpheart.00499.2004. [DOI] [PubMed] [Google Scholar]
- Purkayastha S., Zhang G., Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat. Med. 2011;17:883–887. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K., Yu S., Jiang Y., Laque A., Schwartzenburg C., Morrison C.D., Derbenev A.V., Zsombok A., Münzberg H. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol. Metab. 2014;3:681–693. doi: 10.1016/j.molmet.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M., Goldsmith R., Bloomfield D., Magnano A., Weimer L., Heymsfield S., Gallagher D., Mayer L., Murphy E., Leibel R.L. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayk F., Heutling D., Dodt C., Iwen K.A., Wellhoner J.P., Scherag S., Hinney A., Hebebrand J., Lehnert H. Sympathetic function in human carriers of melanocortin-4 receptor gene mutations. J. Clin. Endocrinol. Metab. 2010;95:1998–2002. doi: 10.1210/jc.2009-2297. [DOI] [PubMed] [Google Scholar]
- Scott M.M., Lachey J.L., Sternson S.M., Lee C.E., Elias C.F., Friedman J.M., Elmquist J.K. Leptin targets in the mouse brain. J. Comp. Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serradeil-Le Gal C., Raufaste D., Brossard G., Pouzet B., Marty E., Maffrand J.P., Le Fur G. Characterization and localization of leptin receptors in the rat kidney. FEBS Lett. 1997;404:185–191. doi: 10.1016/s0014-5793(97)00125-7. [DOI] [PubMed] [Google Scholar]
- Simonds S.E., Cowley M.A. Hypertension in obesity: is leptin the culprit? Trends Neurosci. 2013;36:121–132. doi: 10.1016/j.tins.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Tallam L.S., Stec D.E., Willis M.A., da Silva A.A., Hall J.E. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46:326–332. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- Wheeler E., Huang N., Bochukova E.G., Keogh J.M., Lindsay S., Garg S., Henning E., Blackburn H., Loos R.J., Wareham N.J. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nat. Genet. 2013;45:513–517. doi: 10.1038/ng.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Kerman I.A., Laque A., Nguyen P., Faouzi M., Louis G.W., Jones J.C., Rhodes C., Münzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J. Neurosci. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplemental Reference
- Lee S.J., Verma S., Simonds S.E., Kirigiti M.A., Kievit P., Lindsley S.R., Loche A., Smith M.S., Cowley M.A., Grove K.L. Leptin stimulates neuropeptide Y and cocaine amphetamine-regulated transcript coexpressing neuronal activity in the dorsomedial hypothalamus in diet-induced obese mice. J. Neurosci. 2013;33:15306–15317. doi: 10.1523/JNEUROSCI.0837-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.