Abstract
Venous thromboembolism (VTE) is a common complication in patients with malignant disease. Emerging data have enhanced our understanding of cancer-associated thrombosis, a major cause of morbidity and mortality in patients with cancer. In addition to VTE, arterial occlusion with stroke and anginal symptoms is relatively common among cancer patients, and is possibly related to genetic predisposition. Several risk factors for developing venous thrombosis usually coexist in cancer patients including surgery, hospital admissions and immobilization, the presence of an indwelling central catheter, chemotherapy, use of erythropoiesis-stimulating agents (ESAs) and new molecular-targeted therapies such as antiangiogenic agents. Effective prophylaxis and treatment of VTE reduced morbidity and mortality, and improved quality of life. Low-molecular-weight heparin (LMWH) is preferred as an effective and safe means for prophylaxis and treatment of VTE. It has largely replaced unfractionated heparin (UFH) and vitamin K antagonists (VKAs). Recently, the development of novel oral anticoagulants (NOACs) that directly inhibit factor Xa or thrombin is a milestone achievement in the prevention and treatment of VTE. This review will focus on the epidemiology and pathophysiology of cancer-associated thrombosis, risk factors, and new predictive biomarkers for VTE as well as discuss novel prevention and management regimens of VTE in cancer according to published guidelines.
Keywords: cancer, thrombosis, management, low-molecular-weight heparin
Introduction
Historically, in 1823, the French physician Jean-Baptiste Bouillaud published what appears to be the first report of an association between cancer and thrombosis.1 In 1865, another French physician Armand Trousseau reported an association between gastric cancer and venous thrombosis.2 These reports considered the beginning of attention that malignant disease and hemostasis interact together.
Currently, cancer and its treatments are well-recognized risk factors for venous thromboembolism (VTE). Evidence suggests that the absolute risk depends on the tumor type, the stage of the cancer, and treatment with antineoplastic agents.3
Venous manifestations of cancer-associated thrombosis include deep vein thrombosis (DVT) and pulmonary embolism (PE), as well as visceral or splanchnic vein thrombosis, together described as VTE. In addition to VTE, arterial occlusion with stroke and anginal symptoms is relatively common among cancer patients, and is possibly related to genetic predisposition.4
Several risk factors for developing venous thrombosis usually coexist in cancer patients including surgery, hospital admissions, and immobilization; the presence of an indwelling central catheter; chemotherapy; and new molecular targeted therapies.5,6 Furthermore, other comorbid features will also influence the overall of thrombotic complications, as they do in patients without cancer.3
In addition to the above-mentioned clinical factors, the presence of tumor cells induces a hypercoagulable state.7 More recently, novel risk factors, including platelet and leukocyte counts and tissue factor (TF), are associated with high risk of VTE in cancer patients.8 Furthermore, cancer-associated thrombosis is linked with poor prognosis, and it is the second leading cause of death in cancer patients.9,10
Effective prophylaxis and treatment of VTE reduced mortality and morbidity, and improved quality of life. Low-molecular-weight heparin (LMWH) is preferred as an effective and safe means for prophylaxis and treatment of VTE. It has largely replaced unfractionated heparin (UFH) and vitamin K antagonists (VKAs).11
This brief review will focus on the epidemiology and pathophysiology of cancer-associated thrombosis, risk factors, and new predictive biomarkers for VTE as well as discuss novel prevention and management strategies of VTE in cancer.
Epidemiology of Cancer-Associated Thrombosis
Cancer patients are characterized by an acquired thrombophilic condition predisposing to increased risk of VTE.12 VTE in patients with cancer may present as a vast range of clinically significant thrombotic complications including DVT, PE, arterial thrombosis, nonbacterial thrombotic endocarditis, superficial thrombophlebitis, catheter-related thrombosis, and hepatic veno-occlusive disease.13–15
It is well established that cancer patients are at an increased risk of VTE; the risk of VTE is four-fold to sevenfold higher in patients than in those without cancer16 the reported incidence varies widely between studies depending on patient population, start and duration of follow-up, and the method of detecting and reporting thrombotic events.17 The recent meta-analysis by Horsted et al described incidence rates of venous thrombosis in cancer patients, stratified by background risk of venous thrombosis; the incidence among cohorts with average-risk patients was estimated to be 13 per 1000 person-years (95% CI: 7–23). Among cohorts with high-risk patients, the overall incidence rate was 68 per 1000 person-years (95% CI: 48–96).18
It is estimated that about 4–20% of patients with cancer experience venous thrombosis9 with the annual incidence of 0.5% in them compared to 0.1% in the general population.19 Overall, cancer patients constitute 15–20% of the patients diagnosed with VTE.20
VTE and thrombotic complications are the second most frequent cause of mortality in patients with cancer.14 Several studies have showed that the incidence of VTE is associated with the duration of the underlying illness. The highest rate of VTE is seen in the initial period after diagnosis,21,22 and mortality from VTE is highest in one year after diagnosis.23
The heterogeneity of the studies makes it difficult to compare rates of venous thrombosis between these studies; but over the years, the incidence of VTE in cancer is on the rise.24 Novel anti-cancer drugs, particularly antiangiogenic agents, may be contributing to this increase.25,26 VTE in cancer is associated with a 21% annual risk of recurrent VTE, a 12% annual risk of bleeding complications, requirement of long-term anticoagulation, and interruption of chemotherapy.27,28
A high incidence of VTE following chemotherapy was reported in cancers.29 Chemotherapy increased the risk of VTE and recurrent VTE six-fold and two-fold, respectively, in patients with cancer, and it is estimated that the annual incidence of VTE in cancer patients undergoing chemotherapy is about 10.9%.14
Pathophysiology and Risk Factors for Cancer-Associated Thrombosis
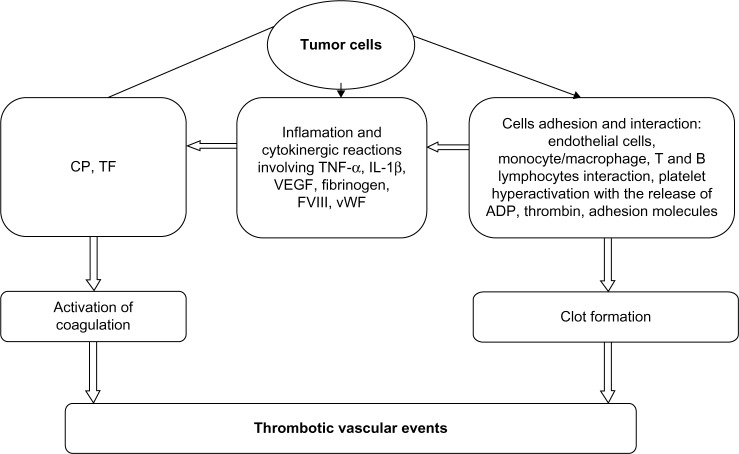
The pathophysiology of cancer-associated thrombosis is not entirely understood. The hypercoagulable state in cancer involves several complex interdependent mechanisms (Fig. 1), including interaction among cancer cells, host cells, and the coagulation system.30 Key roles in pathophysiology are played by TF, inflammatory cytokines, and platelets. Tumor cells can activate blood coagulation through multiple mechanisms, including production of procoagulant, fibrinolytic, and proaggregating activities; release of proinflammatory and proangiogenic cytokines; and direct interaction with host vascular and blood cells through adhesion molecules.7 Novel risk factors include platelet and leukocyte counts and TF.8
Figure 1.
Factors involved in cancer-associated thrombosis.
Abbreviations: CP, Cancer procoagulant; TF, Tissue factor; TNF-α, Tumor necrosis factor-α; IL-1β, Interleukin-1β; VEGF, Vascular endothelial growth factor; FVIII, Factor VIII; vWF, Von Willebrand factor; ADP, Adenosine diphosphat.
Source: Karimi M, Cohan N. Cancer-Associated Thrombosis. The Open Cardiovascular Medicine Journal 2010;4:78–82. doi:10.2174/1874192401004020078.
Many tumors have been shown to activate blood coagulation through an abnormal expression of high levels of the procoagulant molecule TF.31 In normal vascular cells, expression of TF is normally not expressed, except when induced by inflammatory cytokines or by bacterial lipopolysaccharides. In tumor cells, TF is expressed constitutively. Constitutive activation of the extrinsic pathway has been shown in patients with cancer. In a study conducted by Kakkar et al.32 plasma levels of TF, factor VIIa, factor XIIa, the thrombin–antithrombin complex, and prothrombin fragments were elevated in patients with cancer compared with those in healthy controls. TF and factor VIIa levels were both significantly higher, suggesting that the extrinsic pathway was strongly activated. Levels of factor XIIa were only slightly elevated, suggesting that the intrinsic pathway is not involved to a significant extent in the hypercoagulable state seen in patients with cancer.33 Also, Hoffman et al.34 revealed that the majority of patients with cancer have increased levels of coagulation factors V, VIII, IX, and XI as well as increased levels of markers of coagulation activation.
The risk factors associated with the development of thromboembolic complications can be divided into patient characteristics, tumor factors, and treatment-related factors (Table 1).35 Patient characteristics include old age; female sex; black ethnicity; elevated D-dimer levels; C-reactive protein and soluble P-selectin (sP-selectin); platelet count over 350 × 106/L or leukocyte count over 11 × 106/L; pro-thrombotic mutations, factor V Leiden, and prothrombin 20210A;23,35–37 and commonly recognized risk factors for the development of thromboembolism, such as obesity and a history of VTE. Tumor-related factors include anatomical site of tumor, and tumor histology, stage, and duration of cancer.38,39 Treatment-related factors include both pharmacologic agents, such as chemotherapeutic agents, hormonal agents, antiangiogenic agents, and erythropoiesis-stimulating agents (ESAs), and mechanical causes like surgery and central venous catheters.35,39
Table 1.
Summarize the risk factors for cancer associated thrombosis.
| PATIENT CHARACTERISTICS | TUMOR-RELATED FACTORS | TREATMENT-RELATED FACTORS | BIOMARKERS |
|---|---|---|---|
| Female gender | Anatomical site of tumor | Major surgery | High TF expression by tumor cells31 |
| Older age | Tumour histology | Hospitalization | Pre-chemotherapy platelet count >350,000/mm38,35 |
| Race (black ethnicity) | Advanced stage of cancer | Cancer therapy | Pre-chemotherapy leukocyte count >11,000/mm38 |
| Common comorbidities: DM, Obesity, Previous VTE, atherosclerosis, inflammation, others | Initial period after diagnosis of cancer | Erythropoiesis-stimulating agents | Elevated D-dimer34,35 |
| Inherited prothrombotic mutations | – | Central venous catheters | High level of35 - TF plasma levels - soluble P-selectin - C-reactive protein |
Abbreviations: TF, tissue factor; DM, diabetic mellitus; VTE, Venous thromboembolism.
According to a systematic review, up to 10% of patients presenting with idiopathic VTE are subsequently diagnosed with cancer during the first year of follow-up.38,39
Management of Cancer-Associated Thrombosis
Initial treatment of cancer-associated VTE
The treatment of VTE in cancer patients aims at reducing mortality and morbidity, and improving quality of life. Until the mid-2000s, the standard treatment for acute VTE consisted of initial therapy with LMWH or UFH followed by long-term therapy with an oral anticoagulant, namely VKAs.11 Oncology patients have a higher rate of VTE recurrences during oral-anticoagulant therapy with VKAs and a higher anticoagulation-associated hemorrhagic risk as compared with non-cancer patients.40
The most important guidelines, namely, from the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO), the American College of Chest Physicians (ACCP), and the National Cancer Comprehensive Network (NCCN) recommend LMWH-based therapy over warfarin-based therapy as the preferred VTE treatment in cancer patients for the initial therapy (Table 2).40,41
Table 2.
Summary of guidelines on treatment of VTE in patients with cancer.
| NCCN 2011 | ACCP 2012 | ASCO 2013 | |
|---|---|---|---|
| Initial treatment | LMWH Dalteparin 200 U/kg OD Enoxaparin 1 mg/kg BID Tinzaparin 175 U/kg OD Fondaparinux 5 mg (<50 kg), 7.5 mg (50–100 kg), or 10 mg (>100 kg) OD APTT-adjusted UFH infusion |
Not addressed in cancer patients | LMWH is recommended for the initial 5 to 10 days of treatment for DVT and PE in patients with a CrCI>30 mL/min. |
| Long-term treatment | - LMWH is recommended for first 6 months as monotherapy without warfarin in patients with proximal DVT or PE and metastatic or advanced cancer. - Warfarin 2.5–5 mg every day initially with dynamic dosing strategy based on INR value targeted at 2–3. |
-LMWH preferred to VKA - In patients not treated with LMWH, VKA therapy is preferred to dabigatran or rivaroxaban - Patients receiving extended therapy should continue with the same agent used for the first 3 months of treatment |
- LMWH is recommended for long-term therapy for DVT and PE - VKAs (target INR, 2–3) are acceptable for long-term therapy if LMWH is not available. - Use of novel oral anticoagulants is not recommended - Patients with cancer should be periodically assessed for VTE risk |
| Duration of therapy | Minimum 3 months. Indefinite anticoagulant if active cancer or persistent risk factors. |
Extended therapy is preferred to 3 months of treatment | At least 6 months duration. Extended anticoagulation with LMWH or VKA beyond 6 months for patients with: - metastatic disease -receiving chemotherapy -recurrent thrombosis |
Abbreviations: ACCP, American College of Chest Physicians; BID, twice-daily dosing; OD, once-daily dosing; NCCN, National Comprehensive Cancer Network; OD, once-daily dosing; ASCO, American Society of Clinical Oncology; EMSO, European Society of Medical Oncology; ACCP, American College of Chest Physicians; NCCP, National Cancer Comprehensive Network; DVT, Deep vein thrombosis; PE, Pulmonary embolism.
The initial treatment of cancer-associated thrombosis includes LMWH, UFH, or fondaparinux.41 Data analysis of trials showed no difference in efficacy between LMWH and UFH in patients with cancer.42 However, LMWH is preferred as an effective and safe for treatment of VTE. It has largely replaced UFH and VKAs because LMWH does not need hospitalization and laboratory monitoring like UFH. Also, LMWH is associated with a lower risk of heparin-induced thrombocytopenia (HIT) and simple dosing (once-daily, weight-based subcutaneous injection).43 Moreover, a statistically significant reduction in mortality risk with LMWH at three months of follow-up has been noted. The reason for this survival benefit is unknown. Some studies of the efficacy of LMWH in the treatment of malignancy-associated VTE have reported a survival benefit not only because of resolution of the thrombus but also because of an antineoplastic effect.44,45 However, the reduction in mortality observed in favor of LMWH has been found in a subgroup analysis of a systematic review and has never been confirmed in subsequent randomized clinical trials.
Fondaparinux is administered as a once-daily, weight-based subcutaneous injection as LMWH, and is also rarely associated with drug-induced thrombocytopenia.46 However, its use in cancer patients is limited because of long half-life of 17–21 hours, the lack of a reversal agent, and 100% dependence on renal clearance.47 However, UFH can be used in those with severe renal impairment as it depends on hepatic clearance and fondaparinux is a reasonable choice in patients with a history of HIT.41
Long-term management of cancer-associated VTE
According to current international recommendations,48–50 LMWH is the standard anticoagulant therapy during the first three months after the VTE. LMWH is also routinely recommended for 6–12 months or indefinitely for patients with active neoplastic disease, for patients receiving chemotherapy, if thrombosis is recurrent in patients, or if the patient had inherited thrombophilia.44
VKAs have been the mainstay agents for long-term management and secondary prophylaxis of acute VTE in patients without cancer.50 However, its use is problematic in oncology patients because of lower efficacy and high rates of recurrence (three times than in patients without cancer) despite maintenance of the international normalized ratio (INR) within the therapeutic range.27
In CLOT trial,11 patients with cancer who had acute VTE were randomly assigned to receive LMWH (dalteparin) at a dose of 200 IU/kg of body weight subcutaneously once daily for five to seven days and a coumarin derivative for six months (target INR, 2.5) or dalteparin alone for six months (200 IU/kg once daily for one month, followed by a daily dose of approximately 150 IU/kg for five months). During the six-month follow-up, 27 of 336 patients in the dalteparin group had recurrent VTE, compared with 53 of 336 patients in the oral-anticoagulant group (hazard ratio, 0.48; P = 0.002). The probability of recurrent VTE at six months was 17% in the oral-anticoagulant group and 9% in the dalteparin group. No significant difference between the dalteparin group and the oral-anticoagulant group was detected in the rate of major bleeding (6% and 4%, respectively) or any bleeding (14% and 19%, respectively), and the authors conclude that dalteparin was more effective than an oral anticoagulant in reducing the risk of recurrent VTE without increasing the risk of bleeding. In addition to lower efficacy, VKAs also need laboratory monitoring of their anticoagulant activity; and their absorption affected by food interactions has a longer half-life that makes interruption for procedures, or thrombocytopenia is difficult.
Whenever possible, outpatient management of the VTE is preferred. Criteria for hospital admission were adapted in Siragusa et al.51 study: poor clinical conditions because of the VTE event and/or concomitant medical comorbidities, poor compliance, high risk of bleeding or active bleeding, renal insufficiency, and platelet count less than 50 × 109/L.
In brief, LMWH is recommended for both initial and long-term anticoagulation in cancer-associated thrombosis by major consensus guidelines.40,50,52,53 If LMWH is unavailable, the ASCO 2013 VTE Prevention and Treatment Guideline recommends the use of VKAs with a target INR of 2–3 as an acceptable alternative.54
Duration of anticoagulant therapy
Studies regarding the optimal duration of anticoagulant therapy are lacking in oncology patients. The decision regarding the continuation of anticoagulation beyond the first three to six months is largely based on weighing the risk for recurrent thrombosis against the risk of major bleeding. The patient assessment should be done to determine whether biomarkers, radiologic imaging, and clinical prediction models can identify patients with a sufficiently high risk for recurrent thrombosis to benefit from extended anticoagulation.55
Management of Selected Cases of Cancer-Associated VTE
Treatment of patients with renal impairment
Abnormal renal function is a common condition in patients with malignancy. Because LMWH is partially cleared by renal excretion and metabolism, drug accumulation is expected with long-term management in those with significant renal insufficiency. Limited data are available on the use of LMWH in patients with significant renal dysfunction, but they do indicate that the risk of bleeding is higher in patients with renal impairment.56 Manufacturer-recommended dose reduction in renal impairment exists for enoxaparin but not for other LMWH preparations.57 Compared with other LMWHs, tinzaparin does not exhibit significant accumulation in patients with renal impairment, allowing for utilization without dose adjustment.58 The difference favoring tinzaparin clearance in patients with severe renal insufficiency compared to other LMHWs is possibly related to the drug’s metabolism by hepatic mechanisms because of the higher molecular weight of tinzaparin.59,60
However, the results regarding tinzaparin have not been confirmed in a clinical trial.
Most experts and guidelines recommend dose adjustment based on anti-factor Xa activity in patients with a CrCl <30 mL/minute.50,52 If anti-factor Xa monitoring is not readily available, VKA therapy is likely a safer option for long-term anticoagulation in these patients.
Inferior vena cava (IVC) filters insertion
Data on the efficacy and safety to insert IVC filters in oncology patients are limited, and its use remains controversial. Complications associated with IVC filters include recurrent VTE up to 32%, and fatal PE has been well documented.28 Also, insertion problems occur in 4–11% of patients, and long-term adverse effects, such as thrombosis, which can occur proximally or distally to the filter, occur in 4–32% of patients.61 So, because of the absence of data to support their efficacy and high rates of complications, IVC filters should be restricted to patients with acute VTE when anticoagulant therapy is not tolerated or contraindicated. Abdel-Razeq et al revealed that the IVC filter should be considered for patients who are currently bleeding or are at high risk for bleeding, patients who have recurrent VTE despite anticoagulation or develop VTE immediately postoperatively, and patients who present with a large primary or metastatic CNS tumor or present with cor pulmonale.62
Treatment of incidental VTE
Incidental or unsuspected VTE is defined as evidence of thrombosis detected on imaging studies performed for other indications such as cancer staging.63 Retrospective studies in unselected oncology patients demonstrated incidental VTE rates of up to 6%.64 Currently, based on published literature, it is recommended that patients with incidental DVT and PE receive therapeutic anticoagulation if there are no contraindications.50,54 However, confirming the diagnosis with the appropriate testing is strongly required in such cases.
Treatment of catheter-related thrombosis
To date, published data and clinical experience suggest that catheter-related thrombosis is associated with a low risk for thrombosis recurrence and postthrombotic syndrome.65 Therefore, conservative treatment is recommended. Also, removal of the catheter is indicated if there is evidence of concomitant DVT, line-related sepsis is suspected or documented, or the access is no longer required or nonfunctioning. Anticoagulant therapy should be given using either LMWH alone or LMWH followed by warfarin therapy. A short period of anticoagulation (three to five days of LMWH) may even salvage some thrombosed catheters and obviate the need to remove and replace the line. Anticoagulation is recommended for a minimum of three months while the catheter remains in place.41
Management of Challenging Cases of Patients with Cancer-Associated Thrombosis
Management of VTE in patients with thrombocytopenia
Thrombosis is commonly diagnosed in patients with malignancy and thrombocytopenia.66 The possible etiologies of the thrombocytopenia in cancer patients are HIT, thrombotic thrombocytopenic purpura, immune thrombocytopenia, or chemotherapy effect. Clinicians need to assess the severity; whether there are potentially reversible causes that can be corrected; and whether there are other risk factors for bleeding, such as advanced age or renal insufficiency. Anticoagulation in patients with thrombocytopenia should be applied on an individual patient basis after assessment of the risks and benefits. In the initial month following the diagnosis of VTE, the risk of recurrent thrombosis is highest.11,67 Consequently, giving maximal or therapeutic anticoagulant therapy is important. In patients with acute cancer-associated thrombosis and platelet count ≥50 × 109/L, full therapeutic anticoagulation without platelet transfusion is appropriate. But, in patients with a platelet count <50 × 109/L, platelet transfusion support to maintain a platelet count ≥50 × 109 L to allow full, therapeutic anticoagulation should be considered.68 The cut-off of 50 × 109/L is empirical, but there is general consensus that the risk of spontaneous bleeding is very low above this level.
However, platelet transfusion support to maintain a platelet count of ≥50 × 109 L just to allow full, therapeutic anticoagulation may not be practical. In retrospective study of 53 patients with cancer-associated thrombosis and thrombocytopenia <50 × 109/L, the impact of anticoagulation dose reduction on the risk of recurrent cancer-associated thrombosis appeared to be minor. In all, 23 patients received anti-coagulation for less than three months, including 11 patients who received it for >14 days. Fifteen patients had ≥25% dose reductions of anticoagulants. At six-month follow-up, the recurrent thrombosis rate was 1.8%.69
In such cases, most experts agree that dynamic dosing strategy for anticoagulants, irrespective of the initial one-month period, appeared the best option70: for a platelet count of ≥50 × 109/L, full therapeutic anticoagulation; for a platelet count of 25–50 × 109/L, reducing the dose of LMWH to 50% of the therapeutic dose or using a prophylactic dose of LMWH in patients with a platelet count of 25–50 × 109/L; and for a platelet count of <25 × 109/L, no anticoagulation.68,70
Treatment of recurrent VTE during anticoagulant therapy
Recurrent VTE despite appropriate anticoagulation is common among cancer patients. Approximately 10–17% of patients with cancer-associated thrombosis treated with a VKA and 6–9% of patients treated with LMWH will have recurrent VTE during follow-up.11,67,71 The causes for VKA failure are multifactorial, and cancer patients can develop recurrent VTE despite maintaining therapeutic INR values.11 LMWHs for at least the first three months are known to be more effective than VKAs in the treatment of cancer-associated thrombosis.11,67 Raising the anticoagulation target of the VKA (eg, INR 2.5–3.5) is not recommended, given the lack of cancer-specific data and the increase in the risk of bleeding with a higher target INR.27
Once recurrent VTE is confirmed, it is essential that HIT be excluded in patients who were first exposed to LMWH or UFH within the past 10–14 days and also to confirm drug compliance. An approach to managing cancer patients with symptomatic recurrent VTE despite anticoagulation has been proposed.72,73 Patients with recurrent event who are being treated with VKAs should be switched to LMWH mono-therapy or continuation of VKA after a bridging period with LMWH (or UFH) and those managed with LMWH should have their dose increased by 25% (or increased to therapeutic, weight-adjusted doses if they are receiving lower doses). A retrospective study of 70 patients with cancer with recurrent VTE demonstrated that transition to LMWH (in patients receiving VKA therapy at the time of recurrence) or LMWH dose escalation by 20–25% (in patients receiving LMWH at recurrence) prevented additional VTE in 91% of patients during a minimum of three months of follow-up.74
All patients should be reassessed in five to seven days to ensure symptomatic improvement. Patients without symptomatic improvement should be considered for another dose escalation, and the anti-FXa level can be used to estimate the next dose escalation, although there is no published evidence to support this strategy.41
Other therapeutic options, including the insertion of an IVC filter or switching to a different anticoagulant (eg, fonda-parinux or VKA), have been proposed.
Thromboprophylaxis in Patients with Cancer
Prediction of VTE in cancer patients
Multiple clinical risk factors including primary site of cancer and systemic therapy, and biomarkers including leukocyte and platelet counts and TF are associated with increased risk of VTE. However, risk cannot be reliably predicted based on single risk factors or biomarkers, and the patients with cancer should be assessed for VTE risk at the time of chemotherapy initiation and periodically thereafter.75 Based on known risk factors, a simple model for predicting chemotherapy-associated VTE in ambulatory cancer patients was developed by Khorana et al.76 The study included patients with breast, colorectal, lung, gynecologic, gastric, and pancreatic cancers, and lymphoma who were to receive systemic chemotherapy. Other cancer sites made up the 10% of remaining patients. The five predictive variables identified included cancer site, elevated prechemotherapy platelet count, anemia or use of red blood cell growth factors, elevated prechemotherapy leukocyte count, and elevated body mass index (BMI).8,76
This risk model was subsequently validated in another cohort of cancer patients and expanded with two additional laboratory markers, sP-selectin and D-dimer, to increase the predictive value of estimating a patient’s risk of chemotherapy-associated thrombosis (Table 3).77,78 The patient population used to generate the expanded risk model consisted of 819 patients from Vienna Cancer and Thrombosis Study (CATS) enrolled at the time of newly diagnosed cancer or progression of the disease. The median follow-up was much longer in this study than in that of Khorana et al (21.4 months vs 73 days). Primary brain tumors were added to the very-high-risk category. Kidney cancer and multiple myeloma were added to the high-risk category. This model was better able to stratify high-risk patients from low-risk patients. Yet the application of this extended risk-assessment tool is limited by the fact that the sP-selectin assay is not routinely performed in clinical centers and that there is significant variability of D-dimer assays employed.79
Table 3.
| PATIENT CHARACTERISTICS | VTE RISK SCORE |
|---|---|
| Site of cancer | |
| Very high risk (primary brain, stomach or pancreas) | 2 |
| High risk (lung, lymphoma, gynecologic, genitourinary excluding prostate or multiple myeloma) | 1 |
| Low risk (breast, colorectal or head and neck) | 0 |
| Other characteristics | |
| Platelet count ≥350 × 109/l | 1 |
| Hemoglobin <100 g/l or use of red blood cell growth factors | 1 |
| Leukocyte count >11 × 109/l | 1 |
| BMI ≥35 kg/m2 | 1 |
| sP-selectin ≥53.1 ng/ml | 1 |
| D-dimer ≥1.44 μg/ml | 1 |
Notes: 0 score, Low risk. 1 or 2 score, Intermediate risk. 3 or higher, High risk.
In a multicenter trial (SAVE-ONCO),80 once-daily, subcutaneous semuloparin (a hemisynthetic, ultra-LMWH with high anti-Xa activity) at 20 mg administered to 1608 patients receiving chemotherapy for locally advanced or metastatic solid tumors significantly prevented VTE without increasing major bleeding. In this trial, the median treatment duration was 3.5 months. VTE occurred in 20 of 1608 patients (1.2%) receiving semuloparin, as compared with 55 of 1604 (3.4%) receiving placebo (hazard ratio, 0.36; 95% confidence interval [CI], 0.21–0.60; P < 0.001). The incidence of clinically relevant bleeding was 2.8% and 2.0% in the semuloparin and placebo groups, respectively (hazard ratio, 1.40; 95% CI, 0.89–2.21). Major bleeding occurred in 19 of 1589 patients (1.2%) receiving semuloparin and 18 of 1583 (1.1%) receiving placebo (hazard ratio, 1.05; 95% CI, 0.55–1.99). The authors show that semuloparin is safe and effective for prophylaxis against VTE in patients receiving chemotherapy for solid tumors.
Thromboprophylaxis in cancer patients
The risk for recurrent thrombosis in patients with active cancer is high even while they are receiving anticoagulation; it is generally recommended that extended anticoagulation prophylaxis be considered in this population.50,54 Patients given extended anticoagulation require frequent reassessment to review the risk–benefit balance of continuing anticoagulant therapy.
Despite the existence of several evidence-based guidelines that delineate appropriate anticoagulation regimens for primary and secondary prophylaxis of VTE and long-term anticoagulation in cancer patients,49,81–84 up to 75% of cancer patients do not receive appropriate prophylaxis.85
Indeed, the use of thromboprophylaxis in cancer patients is complicated by the fact that although they are at an increased risk of VTE, they are also at an increased risk of bleeding.86 So, the use of antithrombotic agents that provide stable anticoagulation while minimizing bleeding complications is especially important in this high-risk group.
Controversy exists regarding the benefits of extended prophylaxis on an outpatient basis for ambulant patients receiving chemotherapy, as guidelines currently do not recommend routine prophylaxis for this group or are not always consistent in their recommendations.81–84
Both UFH and the LMWHs are recommended for primary prophylaxis following cancer surgery. Studies show that the LMWHs are at least as effective as UFH in this setting, but associated bleeding tendency is lower than UFH.44
The LMWHs are recommended for use in secondary/long-term prophylaxis where, compared with warfarin, they display increased efficacy with a good safety profile and reliability, and are associated with increased quality of life. In addition, the LMWHs have been associated with potential antineoplastic effects that may contribute to improved survival times in cancer patients.44,45 However, more studies are needed to understand this effect and the potential role of the LMWHs as antineoplastic therapy.
Novel Oral Anticoagulants
Recently, the development of novel oral anticoagulants (NOACs) that directly inhibit factor Xa (eg, rivaroxaban, apixaban, or betrixaban) or thrombin (for example, dabigatran etexilate) is a milestone achievement in the prevention and treatment of VTE.87 Unlike LMWHs and warfarin, which inhibit multiple coagulation factors, NOACs target specific clotting cascade factors; NOACs are more attractive to patients and clinicians because they do not require laboratory monitoring to achieve therapeutic anticoagulation, they can be taken orally in fixed doses once or twice daily, and they have minimal food and drug–drug interactions.41,54,88 The major limitation is the lack of specific antidotes to reverse the anticoagulant effect and the absence of readily available assays to measure the anticoagulant effect, which can be an issue when facing bleeding events or treatment failure.55,87 To date, NOACs have not been rigorously evaluated in cancer patients. A recent randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer89 showed that apixaban is safe and feasible to use as VTE prophylaxis for high-risk cancer patients receiving chemotherapy. However, no published clinical trials have specifically addressed the treatment of cancer-associated VTE using these direct inhibitors. Also, ASCO guideline does not recommend the use of these new agents.41,54
Summary
VTE is a serious complication, and the second most frequent cause of death in patients with cancer and adversely affects quality of life. Studies showed that anticoagulant therapy and thromboprophylaxis are efficacious and can protect patients from VTE.
Based on clinical trial findings, subcutaneous LMWH is the first-line therapy for VTE in patients with cancer and has largely replaced UFH and VKAs. The treatment should be delivered for an extended period between three and six months, or even indefinitely, in the presence of active neoplastic disease or a very high risk of recurrence. The results of the studies indicate that patients demonstrate a survival benefit with LMWH, but these possible benefits must be weighed against the risks, costs, and inconvenience of chemopreventive anticoagulation. Recently, the results of clinical trials of use of NOACs that directly inhibit factor Xa or thrombin are promising in the prophylaxis of high-risk cancer patients receiving chemotherapy.
Footnotes
ACADEMIC EDITOR: William CS Cho, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: GE. Analyzed the data: GE, AMA, EB. Wrote the first draft of the manuscript: GE, AMA. Contributed to the writing of the manuscript: GE, AMA, EB. Agree with manuscript results and conclusions: GE, AMA, EB. Jointly developed the structure and arguments for the paper: GE, AMA, EB. Made critical revisions and approved final version: GE, AMA, EB. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Bouillard JB, Bouillaud S. De l’Obliteration des veines et de son influence sur la formation des hydropisies partielles: consideration sur la hydropisies passive et general. Arch Gen Med. 1823;1:188–204. [Google Scholar]
- 2.Trousseau A. Phlegmasia alba dolens. Clin Med Hotel-dieu Paris. 1865;3:654–712. [Google Scholar]
- 3.Brose KM, Lee AY. Cancer-associated thrombosis: prevention and treatment. Curr Oncol. 2008;15(suppl 1):S58–67. doi: 10.3747/co.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khorana AA. Cancer-associated thrombosis: updates and controversies. Hematology Am Soc Hematol Educ Program. 2012;2012:626–30. doi: 10.1182/asheducation-2012.1.626. [DOI] [PubMed] [Google Scholar]
- 5.Lee AY. Epidemiology and management of venous thromboembolism in patients with cancer. Thromb Res. 2003;110(4):167–72. doi: 10.1016/s0049-3848(03)00347-5. [DOI] [PubMed] [Google Scholar]
- 6.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6(6):401–10. doi: 10.1016/S1470-2045(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 7.Rickles FR. Mechanisms of cancer-induced thrombosis in cancer. Pathophysiol Haemost Thromb. 2006;35(1–2):103–10. doi: 10.1159/000093551. [DOI] [PubMed] [Google Scholar]
- 8.Khorana AA. Cancer and thrombosis: implications of published guidelines for clinical practice. Ann Oncol. 2009;20(10):1619–30. doi: 10.1093/annonc/mdp068. [DOI] [PubMed] [Google Scholar]
- 9.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–4. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 10.Donati MB. Cancer and thrombosis. Haemostasis. 1994;24:128–31. doi: 10.1159/000217092. [DOI] [PubMed] [Google Scholar]
- 11.Lee AY, Levine MN, Baker RI, et al. Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–53. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 12.Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49:1404–13. doi: 10.1016/j.ejca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Deitcher SR. Cancer and thrombosis: mechanisms and treatment. J Thromb Thrombolysis. 2003;16:21–31. doi: 10.1023/B:THRO.0000014589.17314.24. [DOI] [PubMed] [Google Scholar]
- 14.Haddad TC, Greeno EW. Chemotherapy-induced thrombosis. Thromb Res. 2006;118:555–68. doi: 10.1016/j.thromres.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Gomes MP, Deitcher SR. Diagnosis of venous thromboembolic disease in cancer patients. Oncology (Williston Park) 2003;17:126–35. [PubMed] [Google Scholar]
- 16.Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119:60–8. doi: 10.1016/j.amjmed.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 17.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–23. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 18.Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275. doi: 10.1371/journal.pmed.1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sud R, Khorana AA. Cancer-associated thrombosis: risk factors, candidate biomarkers and a risk model. Thromb Res. 2009;123(suppl 4):S18–21. doi: 10.1016/S0049-3848(09)70137-9. [DOI] [PubMed] [Google Scholar]
- 20.Lee AY. Management of thrombosis in cancer: primary prevention and secondary prophylaxis. Br J Haematol. 2005;128(3):291–302. doi: 10.1111/j.1365-2141.2004.05292.x. [DOI] [PubMed] [Google Scholar]
- 21.Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25:70–6. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 22.Prandoni P, Falanga A, Piccioli A. Cancer, thrombosis and heparin induced thrombocytopenia. Thromb Res. 2007;120(suppl 2):S137–40. doi: 10.1016/S0049-3848(07)70143-3. [DOI] [PubMed] [Google Scholar]
- 23.Wun T, White RH. Venous thromboembolism (VTE) in patients with cancer: epidemiology and risk factors. Cancer Invest. 2009;27(suppl 1):63–74. doi: 10.1080/07357900802656681. [DOI] [PubMed] [Google Scholar]
- 24.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–46. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 25.Zangari M, Barlogie B, Anaissie E, et al. Deep vein thrombosis in patients with multiple myeloma treated with thalidomide and chemotherapy: effects of prophylactic and therapeutic anticoagulation. Br J Haematol. 2004;126:715–21. doi: 10.1111/j.1365-2141.2004.05078.x. [DOI] [PubMed] [Google Scholar]
- 26.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277–85. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 27.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–8. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 28.Elting LS, Escalante CP, Cooksley C, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164(15):1653–61. doi: 10.1001/archinte.164.15.1653. [DOI] [PubMed] [Google Scholar]
- 29.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 30.Karimi M, Cohan N. Cancer-associated thrombosis. Open Cardiovasc Med J. 2010;4:78–82. doi: 10.2174/1874192401004020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–22. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 32.Kakkar AK, DeRuvo N, Chinswangwatanakul V, Tebbutt S, Williamson RC. Extrinsic-pathway activation in cancer with high factor VIIa and tissue factor. Lancet. 1995;346(8981):1004–5. doi: 10.1016/s0140-6736(95)91690-3. [DOI] [PubMed] [Google Scholar]
- 33.Rickles FR, Brenner B. Tissue factor and cancer. Semin Thromb Hemost. 2008;34(2):143–5. doi: 10.1055/s-2008-1079253. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman R, Haim N, Brenner B. Cancer and thrombosis revisited. Blood Rev. 2001;15:61–7. doi: 10.1054/blre.2001.0149. [DOI] [PubMed] [Google Scholar]
- 35.Khorana AA, Liebman HA, White RH, et al. The Risk of Venous Thromboembolism in Patients with Cancer. Alexandria, VA: American Society of Clinical Oncology; ASCO Educational Book; 2008. pp. 240–8. [Google Scholar]
- 36.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., III Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–15. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 37.Horowitz N, Brenner B. Thrombophilia and cancer. Pathophysiol Haemost Thromb. 2008;36:131–6. doi: 10.1159/000175151. [DOI] [PubMed] [Google Scholar]
- 38.Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149(5):323–33. doi: 10.7326/0003-4819-149-5-200809020-00007. [DOI] [PubMed] [Google Scholar]
- 39.Khorana AA. Targeted prophylaxis in cancer: the evidence accumulates. Intern Emerg Med. 2013;8(3):187–9. doi: 10.1007/s11739-012-0883-9. [DOI] [PubMed] [Google Scholar]
- 40.Mandalà M, Falanga A, Roila F, ESMO Guidelines Working Group Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(suppl 6):vi85–92. doi: 10.1093/annonc/mdr392. [DOI] [PubMed] [Google Scholar]
- 41.Lee AY, Peterson EA. Treatment of cancer-associated thrombosis. Blood. 2013;122(14):2310–7. doi: 10.1182/blood-2013-04-460162. [DOI] [PubMed] [Google Scholar]
- 42.Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011;6:CD006649. doi: 10.1002/14651858.CD006649.pub4. [DOI] [PubMed] [Google Scholar]
- 43.Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106(8):2710–5. doi: 10.1182/blood-2005-04-1546. [DOI] [PubMed] [Google Scholar]
- 44.Robert F. The potential benefits of low-molecular-weight heparins in cancer patients. J Hematol Oncol. 2010;3:3. doi: 10.1186/1756-8722-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walenga JM, Lyman GH. Evolution of heparin anticoagulants to ultra-low-molecular-weight heparins: a review of pharmacologic and clinical differences and applications in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):1–18. doi: 10.1016/j.critrevonc.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Warkentin TE, Pai M, Sheppard JI, Schulman S, Spyropoulos AC, Eikel-boom JW. Fondaparinux treatment of acute heparin-induced thrombocytopenia confirmed by the serotonin-release assay: a 30-month, 16-patient case series. J Thromb Haemost. 2011;9(12):2389–96. doi: 10.1111/j.1538-7836.2011.04487.x. [DOI] [PubMed] [Google Scholar]
- 47.Nagler M, Haslauer M, Wuillemin WA. Fondaparinux - data on efficacy and safety in special situations. Thromb Res. 2012;129(4):407–17. doi: 10.1016/j.thromres.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 48.Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):401S–28. doi: 10.1378/chest.126.3_suppl.401S. [DOI] [PubMed] [Google Scholar]
- 49.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). 47. Chest. 2008;133(6 suppl):454S–545. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 50.Kearon C, Akl EA, Comerota AJ, et al. American College of Chest Physicians Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis,9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):419S–94. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siragusa S, Arcara C, Malato A, et al. Home therapy for deep vein thrombosis and pulmonary embolism in cancer patients. Ann Oncol. 2005;16(suppl 4):iv136–9. doi: 10.1093/annonc/mdi923. [DOI] [PubMed] [Google Scholar]
- 52.Streiff MB, Bockenstedt PL, Cataland SR, et al. National comprehensive cancer network. Venous thromboembolic disease. J Natl Compr Canc Netw. 2013;11(11):1402–29. doi: 10.6004/jnccn.2013.0163. [DOI] [PubMed] [Google Scholar]
- 53.Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490–3. doi: 10.1016/j.thromres.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyman GH, Khorana AA, Kuderer NM, et al. American Society of Clinical Oncology Clinical Practice. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(17):2189–204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 55.Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH) J Thromb Haemost. 2012;10(6):1019–25. doi: 10.1111/j.1538-7836.2012.04735.x. [DOI] [PubMed] [Google Scholar]
- 56.Lim W. Low-molecular-weight heparin in patients with chronic renal insufficiency. Intern Emerg Med. 2008;3(4):319–23. doi: 10.1007/s11739-008-0164-9. [DOI] [PubMed] [Google Scholar]
- 57.Nutescu EA, Spinler SA, Wittkowsky A, Dager WE. Low-molecular-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother. 2009;43(6):1064–83. doi: 10.1345/aph.1L194. [DOI] [PubMed] [Google Scholar]
- 58.Mousa SA, Petersen LJ. Anti-cancer properties of low-molecular-weight heparin: preclinical evidence. Thromb Haemost. 2009;102(2):258–67. doi: 10.1160/TH08-12-0832. [DOI] [PubMed] [Google Scholar]
- 59.Garcia DA, Baglin TP, Weitz JI, Samama MM. American College of Chest Physicians. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):24S–43. doi: 10.1378/chest.11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johansen KB, Balchen T. Tinzaparin and other low-molecular-weight heparins: what is the evidence for differential dependence on renal clearance? Exp Hematol Oncol. 2013;2(1):21. doi: 10.1186/2162-3619-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000;95(12):3669–77. [PubMed] [Google Scholar]
- 62.Abdel-Razeq H, Mansour A, Ismael Y, Abdulelah H. Inferior vena cava filters in cancer patients: to filter or not to filter. Ther Clin Risk Manag. 2011;7:99–102. doi: 10.2147/TCRM.S17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khorana AA, O’Connell C, Agnelli G, Liebman HA, Lee AY. Subcommittee on Hemostasis and Malignancy of the SSC of the ISTH. Incidental venous thromboembolism in oncology patients. J Thromb Haemost. 2012;10(12):2602–4. doi: 10.1111/jth.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Douma RA, Kok MG, Verberne LM, Kamphuisen PW, Büller HR. Incidental venous thromboembolism in cancer patients: prevalence and consequence. Thromb Res. 2010;125(6):e306–9. doi: 10.1016/j.thromres.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Frank DA, Meuse J, Hirsch D, Ibrahim JG, van den Abbeele AD. The treatment and outcome of cancer patients with thromboses on central venous catheters. J Thromb Thrombolysis. 2000;10(3):271–5. doi: 10.1023/a:1026503526188. [DOI] [PubMed] [Google Scholar]
- 66.Gerber DE, Segal JB, Levy MY, Kane J, Jones RJ, Streiff MB. The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention. Blood. 2008;112:504–10. doi: 10.1182/blood-2007-10-117051. [DOI] [PubMed] [Google Scholar]
- 67.Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062–72. doi: 10.1016/j.amjmed.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 68.Carrier M, Khorana A, Zwicker J, Noble S, Lee A, The Subcommittee on Haemostasis Malignancy for the SSC of the ISTH Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: guidance from the SSC of the ISTH. J Thromb Haemost. 2013;1:1–9. doi: 10.1111/jth.12338. [DOI] [PubMed] [Google Scholar]
- 69.Pemmaraju N, Kroll MB, Afshar-Kharghan V, Oo TH. Bleeding risk in thrombocytopenic cancer patients with venous thromboembolism (VTE) receiving anticoagulation. Blood. (ASH Annual Meeting Meeting Abstracts) 120, 11/2012. e-Pub 11/2011. [Google Scholar]
- 70.Soff GA. Pathophysiology and management of thrombosis in cancer: 150 years of progress. J Thromb Thrombolysis. 2013;35:346–51. doi: 10.1007/s11239-013-0897-9. [DOI] [PubMed] [Google Scholar]
- 71.Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729–35. doi: 10.1001/archinte.162.15.1729. [DOI] [PubMed] [Google Scholar]
- 72.Lee AY. Thrombosis in cancer: an update on prevention, treatment, and survival benefits of anticoagulants. Hematology Am Soc Hematol Educ Program. 2010;2010:144–9. doi: 10.1182/asheducation-2010.1.144. [DOI] [PubMed] [Google Scholar]
- 73.Lee AY. Treatment of established thrombotic events in patients with cancer. Thromb Res. 2012;129:S146–53. doi: 10.1016/S0049-3848(12)70035-X. [DOI] [PubMed] [Google Scholar]
- 74.Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7(5):760–5. doi: 10.1111/j.1538-7836.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 75.Khorana AA, McCrae KR. Risk stratification strategies for cancer-associated thrombosis: an update. Thromb Res. 2014;133(suppl 2):S35–8. doi: 10.1016/S0049-3848(14)50006-0. [DOI] [PubMed] [Google Scholar]
- 76.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–6. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377–82. doi: 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 78.Chen M, Geng JG. P-Selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch Immunol Ther Exp (Warsz) 2006;54:75–84. doi: 10.1007/s00005-006-0010-6. [DOI] [PubMed] [Google Scholar]
- 79.Piatek C, O’Connell CL, Liebman HA. Treating venous thromboembolism in patients with cancer. Expert Rev Hematol. 2012;5(2):201–9. doi: 10.1586/ehm.11.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agnelli G, George DJ, Kakkar AK, et al. SAVE-ONCO Investigators Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366(7):601–9. doi: 10.1056/NEJMoa1108898. [DOI] [PubMed] [Google Scholar]
- 81.Geerts WH, Bergqvist D, Pineo GF, et al. American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2008;133(6 suppl):381S–453. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 82.Mandalà M, Falanga A, Piccioli A, et al. Working Group AIOM Venous thromboembolism and cancer: guidelines of the Italian Association of Medical Oncology (AIOM) Crit Rev Oncol Hematol. 2006;59:194–204. doi: 10.1016/j.critrevonc.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 84.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology Venous Thromboembolic Disease version 2.2008. May 29, 2008. Available at: http://www.nccn.org/professionals/physician_gls/pdf/vte.pdf.
- 85.Masci G, Magagnoli M, Zucali PA, et al. Minidose warfarin prophylaxis for catheter-associated thrombosis in cancer patients: can it be safely associated with fluorouracil-based chemotherapy? J Clin Oncol. 2003;21(4):736–9. doi: 10.1200/JCO.2003.02.042. [DOI] [PubMed] [Google Scholar]
- 86.Deitcher SR, Kessler CM, Merli G, et al. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin Appl Thromb Hemost. 2006;12(4):389–96. doi: 10.1177/1076029606293692. [DOI] [PubMed] [Google Scholar]
- 87.Tun NM, Oo TH. Prevention and treatment of venous thromboembolism with new oral anticoagulants: a practical update for clinicians. Thrombosis. 2013;2013:183616. doi: 10.1155/2013/183616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barbosa M. What is the best treatment for a cancer patient with thrombosis? Clin Med Insights Oncol. 2014;8:49–55. doi: 10.4137/CMO.S13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levine MN, Gu C, Liebman HA, et al. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost. 2012;10(5):807–14. doi: 10.1111/j.1538-7836.2012.04693.x. [DOI] [PubMed] [Google Scholar]