Abstract
The South Texas region has a historical record of occasional dengue outbreaks. The recent introduction of chikungunya virus to the Caribbean suggests that this disease may be a concern as well. Six different cities and three field habitat types (residential, tire shops, and cemeteries) were examined for evidence of habitat and longitudinal preference of two vector species, Aedes aegypti and Aedes albopictus. A. aegypti was more prevalent in tire shop sites, while A. albopictus was more prevalent in cemetery sites. In residential sites, the relative abundance of the two species varied with longitude, with A. albopictus being more abundant near the coast, and A. aegypti being more abundant inland. There was also a temporal variation, with A. aegypti declining in frequency over time in residential sites. These results have implications for control strategies and disease risk and suggest a greater need for increased surveillance and research in the region.
Keywords: Aedes aegypti, Aedes albopictus, habitat preference, dengue risk
Introduction
Vector-borne diseases are a critical worldwide issue.1,2 Increased travel by people and range expansion of mosquitoes due to global warming has resulted in spreading mosquito-borne pathogens to places where they have previously been eradicated or were nonexistent.3,4 Dengue virus is one such pathogen. This virus has been reintroduced to previously eradicated locations, and is spreading to areas where it has never been observed.5 Dengue virus is a vector-borne RNA virus from the family Flaviviridae that has become one of the most common arboviral diseases in the world, with an estimated 390 million people affected worldwide.6–8 Dengue virus historically has been transmitted in Northern Mexico; however, there is an extreme disparity between the infections rates of dengue virus in South Texas and Mexico. From 1980 to 1999, over 60,000 dengue cases were reported in Mexican border states, while Texas only reported 64 cases along the border region.9 Previously reported cases of endemic transmission occurred during corresponding outbreaks in Northern Mexico in 1995, 1999, and 2005. In late 2013, 10 positive cases were identified in Hidalgo County, and another 11 positive cases were observed in Cameron County, both of which are border counties. These data suggest that dengue is an ongoing concern in South Texas and the border region.
Chikungunya virus has shown a similar pattern as an emerging pathogen. Prior to 2007, chikungunya virus was localized in the Indian Ocean region, but since then it has experienced a rapid spread throughout the world.10,11 Chikungunya virus is an RNA, vector-borne virus in the togaviridae family.12–15 In late 2013, there were reported cases of endemic transmission in the Caribbean island of Saint Martin.16 This was the first reported case of endemic transmission in the Western hemisphere. As of May 2014, this region has reported over 100,000 suspect cases and over 4,000 laboratory confirmed cases.16
Knowledge of distribution patterns, temporal abundance, and habitat preferences of the disease vectors will allow public health officials to more accurately predict the location and timing of potential outbreak events. Aedes aegypti is the primary vector of dengue virus, although A. albopictus has been shown to be capable of transmitting the disease as well.17–20 Both species are also capable of transmitting chikungunya virus.3,13–15 Both species are commonly found in habitats that are associated with human presence and activity.11 A. aegypti and A. albopictus share similar biological and behavioral characteristics; they are both container-breeding mosquitos and often compete with each other for resources.21–25 Many studies have focused on these species in the Florida environment.22,26–31 While these studies have suggested that A. albopictus may outcompete A. aegypti in more humid areas, they have also suggested that in drier, hotter areas, A. aegypti may be able to persist.30 Studies have also shown a longitudinal variation in the relative abundance of both species.31 This result was hypothesized to be due to the relative lack of cover near the coastline, resulting in a more favorable habitat for A. aegypti. In a study comparing the land categories of residential, industrial, and commercial areas, A. aegypti were more abundant in residential areas than in industrial and commercial areas, whereas A. albopictus had the lowest abundance in residential areas, followed by abundance in industrial and commercial areas.29
Although there have been numerous studies that have examined both these vectors, there has been limited research of these vectors in the Lower Rio Grande Valley, Texas (consisting of Starr, Hidalgo, Willacy, and Cameron counties in Texas), a region that may represent a potential location for introduction of diseases. South Texas, and specifically the Lower Rio Grande Valley, is a subtropical region of Texas similar to Florida,32 although historically it has a hotter and drier climate. Factors that influence the abundance and distribution of these two vectors in Florida may differ in this region of South Texas. In addition, the Lower Rio Grande Valley region is adjacent to Northern Mexico where endemic dengue is present, while Florida is a peninsula that does not directly border a country with endemic dengue. Immigration of individuals from Mexico may present a direct source of introduction of vector-borne disease. Previous research has identified both vectors in this region.33 This research was conducted in only one location and did not address how environmental factors such as distance from the coast, temperature, or humidity may influence vector distribution.
We hypothesize that the relative abundance of the two vector species will vary directly with distance from the coast. The coastal region of Florida is more developed, making it a prime habitat for A. aegypti, while moving inland results in increased cover and vegetation, a more suitable habitat for A. albopictus. We hypothesized that the Lower Rio Grande Valley would show a different pattern due to differential development and land use, as well as local environmental factors. We conducted a field survey for A. aegypti and A. albopictus to examine habitat preference and abundance based on habitat type, as well as changes in abundance and distribution relative to distance to the coast. We examined three habitat types, tire shops, cemeteries, and residential areas, within six cities in the lower Rio Grande Valley along a longitudinal gradient. We hypothesize that a greater abundance of A. aegypti would occur further away from the shore-line, where there is less vegetation to provide shade, lower humidity, and warmer temperatures. We also hypothesize that we would find more A. aegypti in tire shops, similar to patterns expected from studies in Florida.22,29,34,35 The current study continues and expands on a previous study with additional results and analysis.36
Materials and Methods
Field sites
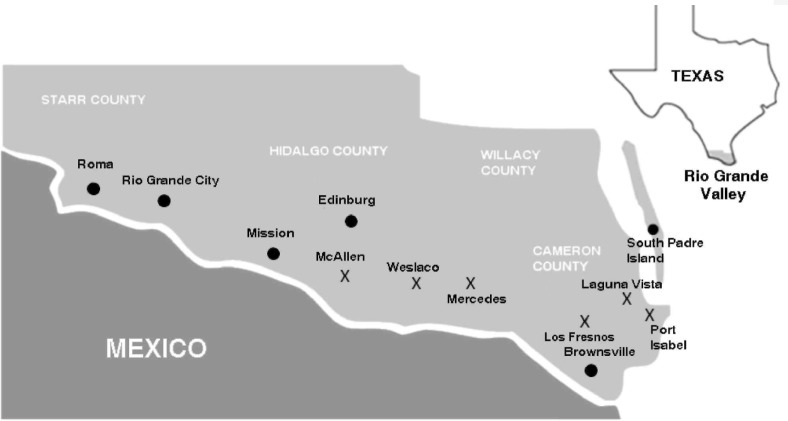
Eleven field sites were selected in South Texas, within the cities of McAllen (3 sites), Weslaco (1 site), Mercedes (2 sites), Los Fresnos (2 sites), Laguna Vista (1 site), and Port Isabel (2 sites) (Fig. 1). The cities were chosen based on their relative proximity to the Gulf of Mexico and the presence of different habitat types. Table 1 shows each site, along with the type of habitat, coordinates, and mean temperature and humidity during the collection periods, the number of oviposition cups at each site, and the number of eggs collected at each site. Trapping sites were classified as cemeteries, tire shops, and residential areas. Cemeteries were well shaded with well-tended lawns, many trees, but little understory vegetation. Tire shops had little to no shade but contained a number of potential oviposition sites in used tires that were scattered around the locations. Residential areas were well shaded, often with many shrubs, or bushes, and many had well-tended lawns. Ten to 20 oviposition traps were placed in shaded or protected regions in the sites depending on the size of the site.
Figure 1.
The six city locations where field sites were established. Each city contained between 1 and 3 field sites. The approximate locations of the field sites are labeled with an “X” on the map.
Table 1.
Site data from the field sites.
| CITY | HABITAT TYPE | LONGITUDE | MEAN TEMPERATURE (°C) | MEAN HUMIDITY | NUMBER OF OVIPOSITION TRAPS | TOTAL NUMBER OF EGGS COLLECTED |
|---|---|---|---|---|---|---|
| McAllen | Cemetery | 98.2350 | 30.1 | 63% | 20 | 34.03 |
| Tire Shop | 98.2606 | 31.3 | 58% | 10 | 215 | |
| Residential | 98.2401 | 33.1 | 52% | 20 | 297 | |
| Weslaco | Cemetery | 97.9932 | 29.9 | 67% | 20 | 1262 |
| Mercedes | Tire Shop | 97.9151 | 29.8 | 69% | 10 | 1752 |
| Residential | 97.9014 | 29.7 | 67% | 10 | 2024 | |
| Los Fresnos | Cemetery | 97.4971 | 28.1 | 77% | 15 | 905 |
| Residential | 97.4782 | 30 | 68% | 10 | 379 | |
| Laguna Vista | Residential | 97.2568 | 29.7 | 67% | 10 | 1369 |
| Port Isabel | Cemetery | 97.2114 | 30.5 | 66% | 10 | 909 |
| Tire Shop | 97.2098 | 29.8 | 69% | 10 | 201 |
Note: Temperature and humidity were recorded weekly at each of the field sites during oviposition collections.
Field collecting
From May to August 2013, field collections for A. aegypti and A. albopictus were conducted weekly at the field sites. The collection period was based on previous research indicating when the two mosquito species of interest were most likely to be collected.33 Oviposition traps consisted of a black cups filled with 300 mL of deionized water and a wooden tongue depressor (stake) secured to the cup with a binder clip. Wooden stakes were scored with a razor blade prior to use to create a more realistic natural surface for mosquito oviposition. All stakes were collected and replaced weekly. Water in the cups was refilled as needed. Missing or damaged cups were also replaced as needed. Temperature and humidity for each site was recorded weekly during each field collection, primarily between 8:00am and 10:00am, using a handheld Kestrel probe. All stakes were returned to the laboratory for inspection.
Eggs were counted on each wooden stake following removal from the field. Eggs were visually identified as hatched or unhatched when possible. Stakes containing unhatched eggs were hatched in an aerated 1.0 g/L nutrient broth solution. Stakes were submerged for 24 hours as described by Vitek and Livdahl.37 After the eggs were hatched, larvae were counted and placed in rearing pans at low densities (50 larvae per small pan (9.75 × 7.75 in) or 150 larvae per large pan (13.5 × 10 in)). Larvae were fed 0.1 g of liver powder ad libitum. Pupated larvae were transferred into smaller containers, 10 pupae per container, and left to emerge to adults. Adult mosquitos were frozen and identified to species and recorded as A. aegypti or A. albopictus. After hatching, wooden stakes were inspected again and total number of hatched, unhatched viable, and unhatched nonviable eggs were recorded.
A. aegypti has been shown to be at a competitive disadvantage when reared in the presence of A. albopictus.3,13,38 Species identification was not conducted until adult emergence. To negate the possibility that larval competition was influencing the survivorship of one of the species, we also examined total larval mortality and the number of A. aegypti adults from each rearing pan compared to the number of A. albopictus.
Statistical analysis
Hatch rates were calculated based on the percentage of viable eggs that hatched at each hatching attempt. Larval survival was calculated by dividing the total emerged adults from the total number of hatched larvae (counted immediately after hatching). Species percentage consisted of the relative abundance of A. albopictus to total hatched larvae. Egg counts per week per trap were used as a relative estimate of mosquito abundance.
Multifactor ANOVA was used to analyze the effect of temperature, humidity, habitat type (tire, cemetery, residential), and proximity to the coast on mosquito abundance. Egg collection data were log transformed to meet assumptions of normality. When needed, pairwise comparisons were conducted using Tukey–Kramer highest significant difference tests. Species composition was analyzed using a multinomial logistic regression, with longitude, habitat type, and epiweek as potential factors, affecting the probability of eggs being either A. aegypti or A. albopictus. Nonsignificant interactions were removed from all statistical models to avoid inflation of the degrees of freedom. All statistical analyses were conducted using JMP version 11.0.39
Results
Field sites
The mean temperature was 30.2 °C, with a standard deviation of 2.94 °C. The mean humidity was 65.47%, with a standard deviation of 11.7%. Both temperature and humidity were correlated with the site longitude. Regression analysis suggests that temperature decreased significantly F = 5.068) relative to proximity to the coast, while humidity showed a significant increase (F = 14.034, P = 0.0003) in sites closer to the coast. Neither temperature (F = 1.088, P = 0.299) nor humidity (F = 3.279, P = 0.731) were significantly correlated with the week of collection. In addition, using an ANOVA analysis, neither temperature (F = 1.587, P = 0.209) nor humidity (F = 1.833, P = 0.165) differed between residential, cemetery, and tire shop field sites. Temperature and humidity were included as variables in all subsequent analyses, when possible.
Field collections
A total of 12,716 eggs were collected from all field sites over the trapping period. The collection numbers were converted into an egg/cup measurement for each day, at each site, to account for any differences between the total numbers of cups at each site. This number was used for comparisons and analysis. The total number of cups at each site did not significantly influence the number of eggs per cups (F = 2.685, P = 0.104). A multiple ANCOVA was used to identify the influence of epiweek, habitat type (cemetery, residential, or tire shop), and longitude on the numbers of eggs (transformed to meet assumptions of normality) per cup over the entire collection period, with temperature and humidity and covariates. Epiweek refers to epidemiological week, beginning on Sunday and ending on Saturday, and is a standardized method of counting time intervals across years. The overall ANCOVA was significant (F = 6.40, P < 0.0001). Two significant interactions were identified, habitat type*longitude (F = 10.068, P = 0.0001) and longitude*humidity (F = 5.427, P = 0.0218). In addition, longitude (F = 8.567, P = 0.0042) and habitat type (F = 4.353, P = 0.0153) were both significant. Pairwise comparisons of the habitat types indicated that tire shops were significantly different from cemeteries (with cemeteries having more eggs collected), while residential areas were not significantly different from either. When habitat type was considered, tire shops showed no significant difference with the different longitudes, while both cemeteries (F = 32.310, P < 0.0001) and residential areas (F = 14.110, P = 0.0005) showed negative relationship between the increasing longitude and the number of eggs (transformed) per cup (Fig. 2). Week (F = 1.032, P = 0.312), temperature (F = 0.016, P = 0.901), and humidity (F = 0.080, P = 0.779) were all nonsignificant factors.
Figure 2.
The number of eggs collected per cup (transformed via a log transformation to meet assumptions of normality) were significantly different based on longitude in both cemetery and residential habitats, while tire shop habitats did not vary with longitude.
Species abundance and habitat preferences
Mortality rates of larvae were calculated by determining the total number of hatched larvae in each pan and the final number of emerging adults in each pan. Mortality rates of larvae per pan ranged from 0% to 50%. Mortality was significantly higher in pans with fewer larval (F = 8.970, P = 0.003). This was primarily due to presence of fewer larvae in pans; a single dead larva represented a higher percentage of the entire larval population. Mortality and relative percentage of A. aegypti were not correlated with each other (P = 0.393) and total number of larvae and relative percentage of A. aegypti were not correlated with each other (P = 0.119). These data suggest that competitive interactions did not significantly influence the relative numbers of A. aegypti and A. albopictus used in the analysis.
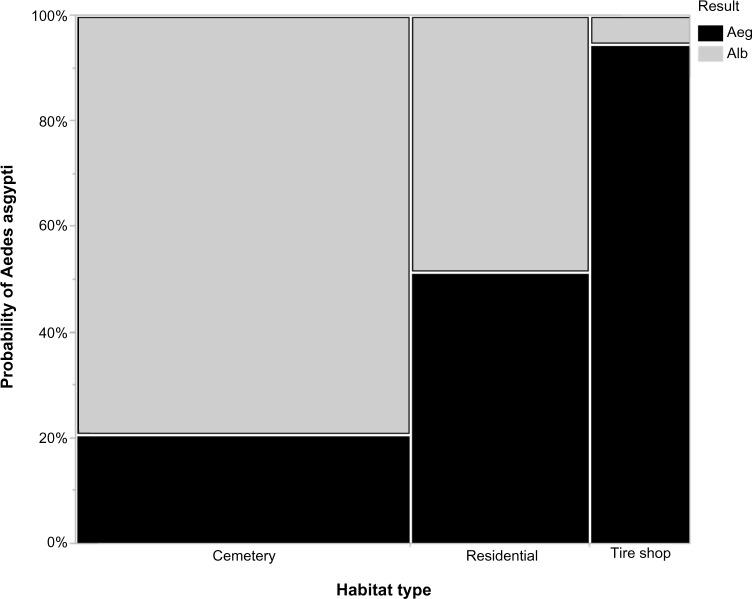
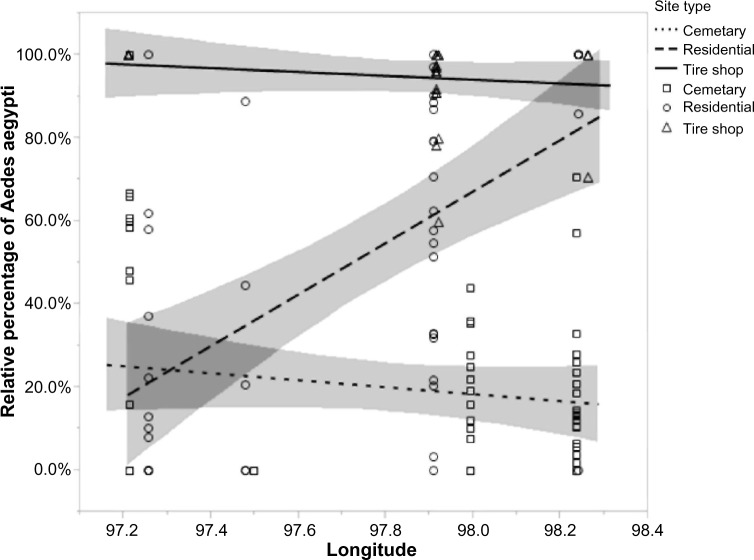
The effect of habitat type, epiweek, and longitude on the numbers of A. aegypti and A. albopictus was analyzed with a multinomial logistic regression, with nonsignificant interaction terms removed from the analysis. The overall regression was significant (χ2 = 2778.465, P < 0.0001, df = 9), with an R2 value of 0.293. Two individual terms (habitat type and longitude) were significant while epiweek was not, and all two-way interactions were significant (Table 2). The reported odds ratios indicate that increasing longitude approximately doubles the probability of A. aegypti mosquitoes (Table 3). In addition, residential sites and tire shops had a higher probability of A. aegypti mosquitoes than cemetery sites, and tire shops had a higher probability of A. aegypti mosquitoes than residential sites (Table 3, Fig. 3). The significant interaction between longitude and habitat type on the probability of finding A. aegypti is shown in Figure 4, indicating that residential sites had a significant change in species composition relative to longitude. Residential sites, and to a lesser degree tire shop sites, also showed a decrease in A. aegypti probability over time (Fig. 5).
Table 2.
Statistical results from examining the multinomial logistic regression larvae (A. aegypti or A. albopictus).
| SOURCE | DEGREES OF FREEDOM | Chi SQUARE | P VALUE |
|---|---|---|---|
| Epiweek | 1 | 0.003 | 0.9534 |
| Longitude | 1 | 9.235 | 0.0024 |
| Habitat Type | 2 | 1719.035 | <0.0001 |
| Epiweek*Longitude | 1 | 75.535 | <0.0001 |
| Longitude*Habitat Type | 2 | 260.967 | <0.0001 |
| Epiweek*Habitat Type | 2 | 29.981 | <0.0001 |
Note: The χ2 and significance values are reported from the Effect Likelihood ratio test. Significant results are bolded.
Table 3.
The reports odds ratios for each of the independent variables from the multinomial logistic regression analysis.
| SOURCE | ODDS RATIO |
|---|---|
| Epiweek (per unit change) | 1.001 |
| Longitude (per unit chage) | 2.176 |
| Habitat Type | |
| Residential:Cemetery | 5.915 |
| Residential:Tire shop | 0.093 |
| Tire shop:Residential | 5.834 |
| Tire shop:Cemetery | 40.228 |
| Cemetery:Residential | 0.127 |
| Cemetery:Tire shop | 0.137 |
Notes: All values were reported as significant. Each of the habitat types represents a comparison of the odds ratios relative to one of the other habitat types. The χ2 and significance values are reported from the Effect Likelihood ratio test. Significant results are bolded.
Figure 3.
A contingency plot with the differences in collecting A. aegypti from the three different habitat types. A. aegypti were significantly more likely in tire shop sites, while A. albopictus were more likely in cemetery sites. Residential sites were intermediate between the other two field sites. The contingency values are based on the multinomial logistic regression results.
Figure 4.
The relative percentage of A. aegypti in the different field sites, by longitude. Both tire shop and cemetery sites did not change the relative percentage with longitude; however, there existed a significant positive relationship between the percentage of A. aegypti and the longitude in residential sites. The 95% confidence interval of the fit is included in the graph. Modified from Champion and Vitek.36
Figure 5.
The relative percentage of A. aegypti in different field sites, by epiweek. A decline in the percentage of A. aegypti is seen in residential sites. Previous research33 has observed this pattern in residential sites and park areas located in McAllen, TX, USA. This interaction is supported with the multinomial logistic regression, where epiweek by itself was not significant (P = 0.9534), but did significantly interact with habitat type (P < 0.0001). The 95% confidence interval of the fit is included in the graph.
Discussion
Overall, cemeteries had significantly more mosquitos than tire shops, although residential sites were not significantly different from either of the other locations. We observed the highest proportion of A. aegypti in tire shop sites, while the probability of finding A. albopictus was greatest in the cemeteries. These results are similar to studies in Florida, where habitat variables in urban and rural areas were measured.22,29,34,35 These studies showed that A. aegypti had a positive association with urbanization variables (eg, building coverage) and a negative association with rural variables (eg, canopy and mixed vegetation coverage). The tire sites in our study consisted primarily of concrete lots and covered garages, with little to no vegetation or bushes and trees for shade. Some of the tire shops had water collected in the piles of tires, but many of them were completely dry for the duration of the field collections. The cemetery and residential sites all contained substantial vegetation and trees that may provide shade. Many cemetery and residential sites also had watering systems that would allow these sites to remain moist even during long dry spells.
We hypothesized that we would see changes in both abundance and relative species composition correlated with increased longitude. This pattern was observed in Florida31 on a smaller scale and was hypothesized to be due to local temperature and humidity conditions and other abiotic factors. Our data support our hypothesis and suggest that longitude may be correlated with mosquito abundance and species composition, perhaps due to changes in temperature and humidity, which were correlated with longitude. The habitat types showed significant differences at varying longitudes (Fig. 2), with both cemeteries and residential areas showing a decrease in overall mosquito abundance as longitude increased. This also corresponded with an increasing probability of finding A. aegypti in residential sites as longitude increased (Fig. 4). While residential sites were similar to cemetery sites with shading, vegetation cover, and watering systems, there is a clear statistical difference between the two in species composition, suggesting that an unknown (abiotic or biotic) factor is influencing species composition. Casual observation during the field collection suggests one possible factor. There was little human activity in the cemeteries and the tire shops during collection times, while the residential areas had a large number of individuals outside that may serve as potential blood meal sources. Different plant species composition may also be involved, as differential plant litter has been shown to affect species composition.40 Residential areas may have different plants communities than commonly found in cemeteries.
Due to the overall significant decline in the probability of finding A. albopictus as longitude increased, our data suggest that habitats for A. albopictus may be more difficult to find with increased longitude in South Texas, USA. Previous research suggests that A. albopictus tolerance for high heat and low humidity may be a limiting factor in their distribution.30,41,42 Temperature increased significantly with longitude while humidity decreased significantly in our field sites. This may be a controlling factor in determining A. albopictus distribution in the Lower Rio Grande Valley and would explain the decrease in A. albopictus as longitude increased. The slight decrease in the probability of collecting A. aegypti in tire shops as longitude increased habitats suggests that despite an overall higher probability of finding A. aegypti in these habitat, tire shops may also be less suitable for A. aegypti as temperature increases and humidity decreases.
In addition to spatial variation, we found a significant temporal component in the data. In the 2010 study,33 one unexpected result was a change in relative species composition over time. Despite uniform weather conditions, a significant trend of decreased proportion of A. aegypti was observed in 2010. This could not be explained due to a change such as an oncoming “rainy season” due to a lack of rainfall through the entire year.33 Our results in this study also identified a change in species composition over time, although overall mosquito abundance did not vary. Epiweek significantly interacted with the habitat type to influence the probability of finding A. aegypti. We were more likely to collect A. aegypti in residential areas over the course of the collection period (Fig. 5), a trend which was not as strong in tire shops (where A. aegypti were more likely to be found at all times). The field collection sites in the 2010 study consisted primarily of residential areas and public parks, matching the pattern observed in this study. The difference between cemetery and residential sites again suggests an unidentified difference in these two habitats, despite similar appearances.
Vector-borne diseases continue to pose a risk to human health, and the increased prevalence of emerging diseases such as dengue and chikungunya virus highlights the need for continued research in the biology and behavior of vector species. Dengue virus is more efficiently transmitted by A. aegypti,5 suggesting that residential areas far from the coast may be at greater risk due to the increased proportion of A. aegypti mosquitoes. In addition, the chikungunya virus strain that originated in the Indian Ocean and resulted in the epidemics in India is more effectively transmitted by A. albopictus.14 If the Western hemisphere strain of chikungunya is similar to the Indian Ocean strain, the relative risk of this disease may also vary based on longitude. Knowledge of differential spatial and temporal mosquito species patterns can assist in identifying disease outbreaks and aiding in accurate disease diagnosis, especially when diseases share similar manifestations and clinical symptoms. As chikungunya spreads in the western hemisphere and dengue continues to become more prevalent, vector control efficiency and outbreak prediction may be enhanced through understanding the mosquito population dynamics and species distribution. Our data contribute to this understanding by demonstrating the existence of differential habitat preferences, as well as spatial and temporal trends in the distribution and abundance of A. aegypti and A. albopictus in the region. Continued research on these and other disease vectors in the region will become more critical as existing and new pathogens become more likely to be found in South Texas.
Acknowledgments
The authors would like to acknowledge the assistance of Neyssy Elizondo, Alejandro Herrera, and Amanda Champion for their assistance in the field collection and sorting. They would also like to acknowledge Dr. Frank Dirrigl for his assistance in preparing this manuscript. This research was conducted as part of the Center for Subtropical Studies at the University of Texas – Pan American.
Footnotes
ACADEMIC EDITOR: Timothy Kelley, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: CJV, SRC. Analyzed the data: CJV. Wrote the first draft of the manuscript: SRC. Contributed to the writing of the manuscript: CJV. Agree with manuscript results and conclusions: CJV, SRC. Jointly developed the structure and arguments for the paper: CJV, SRC. Made critical revisions and approved final version: CJV, SRC. Both authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Gratz NG. Emerging and resurging vector-borne diseases. Annu Rev Entomol. 1999;44:51–75. doi: 10.1146/annurev.ento.44.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Gubler DJ. Resurgent vector-borne disease as a global health problem. Emerging Infect Dis. 1998;4:442–50. doi: 10.3201/eid0403.980326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yee DA, Himel E, Reiskind MH, Vamosi SM. Implications of saline concentrations for the performance and competitive interactions of the mosquitoes Aedes aegypti (Stegomyia aegypti) and Aedes albopictus (Stegomyia albopictus) Med Vet Entomol. 2014;28:60–9. doi: 10.1111/mve.12007. [DOI] [PubMed] [Google Scholar]
- 4.Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol. 2002;47:233–66. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- 5.Guzman MG, Kouri G. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J Clin Virol. 2003;27:1–13. doi: 10.1016/s1386-6532(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiter P, Lathrop S, Bunning M, et al. Texas lifestyle limits transmission of dengue virus. Emerg Infect Dis. 2003;9:86–9. doi: 10.3201/eid0901.020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charrel RN, de Lamballerie X, Raoult D. Chikungunya outbreaks – the globalization of vectorborne diseases. N Engl J Med. 2007;356:769–71. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- 9.Enserink M. Chikungunya: no longer a third world country disease. Science. 2007;318:1860–1. doi: 10.1126/science.318.5858.1860. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons RV, Vaughn DW. Dengue: an escalating problem. Br Med J. 2002;324:1563–6. doi: 10.1136/bmj.324.7353.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rigau-Perez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vordam AV. Dengue and dengue hemorrhagic fever. Lancet. 1998;352:971–7. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 12.Staikowsky F, Le Roux K, Schuffenecker I, et al. Retrospective survey of chickengunya disease in Reunion Island hospital staff. Epidemiol Infect. 2008;136:196–206. doi: 10.1017/S0950268807008424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–27. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 14.Cebula-Byrska I, Kucharz EJ. Chikungunya fever. Eur J Intern Med. 2012;23:325–9. doi: 10.1016/j.ejim.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet. 2012;379:662–71. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- 16.Fischer M, Staples JE. Chikungunya virus spreads in the Americas-Caribbean and South America, 2013–2014. Morbidity Mortality Wkly Rep. 2014;63(22):500. [PMC free article] [PubMed] [Google Scholar]
- 17.Ibanez-Bernal S, Brieseno B, Mutebi JP, et al. First record in America of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak at Reynosa, Mexico. Med Veterinary Entomol. 1997;11:305–9. doi: 10.1111/j.1365-2915.1997.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 18.Gratz NG. Critical review of the status of Aedes albopictus. Med Vet Entomol. 2004;18:215–27. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 19.Erickson RA, Presley SM, Allen LJS, Long KR, Cox SB. A dengue model with a dynamic Aedes albopictus vector population. Ecol Model. 2010;221:2899–908. [Google Scholar]
- 20.Kurane I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp Immunol Microbiol Infect Dis. 2007;30:329–40. doi: 10.1016/j.cimid.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Sota T, Mogi M. Interspecific variation in desiccation survival time and Aedes (Stegomyia) mosquito egg is correlated with habitat and egg size. Oecologia. 1992;90:353–8. doi: 10.1007/BF00317691. [DOI] [PubMed] [Google Scholar]
- 22.O’Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995;32:554–62. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- 23.Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc Biol Sci. 2008;275:463–71. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frances SP, Sithiprasasna R, Linthicum KJ. Laboratory evaluation of the response of Aedes aegypti and Aedes albopictus uninfected and infected with dengue virus to DEET. J Med Entomol. 2011;48:334–6. doi: 10.1603/me10120. [DOI] [PubMed] [Google Scholar]
- 25.Richards SL, Anderson SL, Alto BW. Vector competence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) for dengue virus in Florida Keys. J Med Entomol. 2012;48:942–6. doi: 10.1603/me11293. [DOI] [PubMed] [Google Scholar]
- 26.Harper JP, Paulson SL. Reproductive isolation between Florida strains of Aedes aegypti and Aedes albopictus. J Am Mosq Control Assoc. 1994;10:88–92. [PubMed] [Google Scholar]
- 27.Daugherty MP, Alto BW, Juliano SA. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2000;37:364–72. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–74. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leisnham PT, Juliano SA. Spatial and temporal patterns of coexistence between competing Aedes mosquitoes in urban Florida. Oecologia. 2009;160:343–52. doi: 10.1007/s00442-009-1305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130:458–69. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiskind MH, Lounibos LP. Spatial and temporal patterns of abundance of Aedes aegypti and Aedes albopictus in Southern Florida. Med Vet Entomol. 2013;27:421–9. doi: 10.1111/mve.12000. [DOI] [PubMed] [Google Scholar]
- 32.Hotez PJ, Bottazzi ME, Dumonteil E, et al. Texas and Mexico: sharing a legacy of poverty and neglected tropical diseases. PLoS Negl Trop Dis. 2012;6:e1497. doi: 10.1371/journal.pntd.0001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vitek CJ, Gutierrez JA, Dirrigl FJ. Dengue vectors, human activity, and dengue virus transmission potential in the Lower Rio Grande Valley, Texas, United States. J Med Entomol. 2014;51:1019–28. doi: 10.1603/me13005. [DOI] [PubMed] [Google Scholar]
- 34.O’Meara GF, Gettman AD, Evand LF, Scheel FD. Invasion of cemeteries in Florida by Aedes albopictus. J Am Mosq Control Assoc. 1992;8:1–10. [PubMed] [Google Scholar]
- 35.Rey JR, Nishimura N, Wagner B, Braks MAH, O’Connell SM, Lounibos LP. Habitat segregation of mosquito arbovirus vectors in South Florida. J Med Entomol. 2006;43(6):1134–41. doi: 10.1603/0022-2585(2006)43[1134:hsomav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Champion S, Vitek CJ. Dengue vector distribution and abundance in Lower Rio Grande Valley, Texas USA; Proceedings of the 8th International Conference on Urban Pests; July 20–23 2014; Zurich, Switzerland. Hungary: OOK-Press Kft; 2014. [Google Scholar]
- 37.Vitek CJ, Livdahl T. Field and laboratory comparison of hatch rates in Aedes albopictus (Skuse) J Am Mosq Control Assoc. 2006;22:609–14. doi: 10.2987/8756-971X(2006)22[609:FALCOH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Juliano SA. Coexistence exclusion or neutrality? A meta analysis of competition between Aedes albopictus and resident mosquitos. Isr J Ecol Evol. 2010;56:251–325. doi: 10.1560/IJEE.55.3-4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SAS Institute. JMP Statistical Discovery Software Version 11. Cary, NC: SAS Institute; 2013. [Google Scholar]
- 40.Reiskind MH, Greene KL, Lounibos LP. Leaf species identity and combination affect performance and oviposition choice of two container mosquito species. Ecol Entomol. 2009;34:447–56. doi: 10.1111/j.1365-2311.2008.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutchinson G. Concluding remarks. Cold Spring Harbor Symp Quant Biol. 1957;22:4150427. [Google Scholar]
- 42.Mogi M, Miyagi I, Abadi K, Syafruddin AK. Inter- and intraspecific variation in resistance to desiccation by adult Aedes (Stegomyia) spp. (Diptera: Culicidae) from Indonesia. J Med Entomol. 1996;33:53–7. doi: 10.1093/jmedent/33.1.53. [DOI] [PubMed] [Google Scholar]