Abstract
Radiotherapy is an important treatment option for many human cancers. Current research is investigating the use of molecular targeted drugs in order to improve responses to radiotherapy in various cancers. The cellular response to irradiation is driven by both direct DNA damage in the targeted cell and intercellular signalling leading to a broad range of bystander effects.
This study aims to elucidate radiation-induced DNA damage response signalling in bystander cells and to identify potential molecular targets to modulate the radiation induced bystander response in a therapeutic setting.
Stalled replication forks in T98G bystander cells were visualised via bromodeoxyuridine (BrdU) nuclear foci detection at sites of single stranded DNA. γH2AX co-localised with these BrdU foci. BRCA1 and FANCD2 foci formed in T98G bystander cells. Using ATR mutant F02-98 hTERT and ATM deficient GM05849 fibroblasts it could be shown that ATR but not ATM was required for the recruitment of FANCD2 to sites of replication associated DNA damage in bystander cells whereas BRCA1 bystander foci were ATM-dependent. Phospho-Chk1 foci formation was observed in T98G bystander cells. Clonogenic survival assays showed moderate radiosensitisation of directly irradiated cells by the Chk1 inhibitor UCN-01 but increased radioresistance of bystander cells.
This study identifies BRCA1, FANCD2 and Chk1 as potential targets for the modulation of radiation response in bystander cells. It adds to our understanding of the key molecular events propagating out-of-field effects of radiation and provides a rationale for the development of novel molecular targeted drugs for radiotherapy optimisation.
Keywords: Radiation-induced bystander effect, ionising radiation, DNA damage response, BRCA, Fanconi anaemia
1. Introduction
Radiotherapy is a main treatment option for cancer patients, often combined with surgery and chemotherapy. Direct effects of radiation and their modulation for the benefit of treatment outcome (e.g. fractionation) have been extensively studied and this has led to much improved survival rates. In the last decade, radiation-induced non-targeted bystander responses have increasingly been a focus of research, and may have significant potential for radiotherapy treatment optimisation [1-3]. Radiation induced non-targeted effects have been reported for a range of biological endpoints [4-9] including the induction of the DNA damage marker γH2AX [10-15].
Most recently, ataxia-telangiectasia and Rad3-related (ATR) has been identified as a central player within the bystander signalling cascade that is responsible for H2AX phosphorylation. The ataxia-telangiectasia mutated (ATM) protein was found to be activated downstream of ATR [16] and ATR-mediated, S-phase dependent γH2AX and 53BP1 foci induction was observed [11]. These observations support the hypothesis of an accumulation of replication-associated DNA damage in bystander cells. DNA replication fork stalling can be caused by DNA damage through reactive oxygen or nitrogen species which are thought to play a central role in DNA damage induction in bystander cells. ATR is involved in the recognition of stalled replication forks, failure to stabilise them results in their collapse and ultimately in genetic instability (reviewed in [17]). The report of S-phase specific DNA damage recognised through an ATR and H2AX dependent mechanism in bystander cells strongly suggests the subsequent activation of the Fanconi Anaemia (FA)/BRCA network which is a key pathway in the homologous recombination-dependent resolution of stalled replication and regulation of the intra-S-phase cell cycle checkpoint [18-20]. Phosphorylation of FANCD2 by either ATR or ATM is required for the induction of an intra-S-phase arrest. FA core proteins, ATR and RPA1 [21] are required for the ubiquitination of the FANCD2 protein in S-phase, a modification that is prerequisite for the accumulation at sites of DNA damage to form microscopically visible nuclear foci which associate with BRCA1, BRCA2 and RAD51. γH2AX in connection with BRCA1 recruits FANCD2 to chromatin at stalled replication forks [22] suggesting that H2AX is functionally linked to the FA/BRCA pathway to resolve stalled replication forks and prevent chromosome instability.
The cell cycle checkpoint kinase Chk1 is regulated by ATR and is involved in the activation of the FA/BRCA pathway through phosphorylation of FANCE [23]. The G(2)/M [24] and S-phase DNA damage checkpoints require Chk1 activation [25]. The FA/BRCA DNA repair pathway is frequently affected in breast cancer where BRCA1 or BRCA2 mutations can be found in approximately 10% of cases. Epigenetic silencing of BRCA1 occurs in 13% of breast cancers, 6% of cervical cancers and 4% of non-small-cell lung cancers. FANCF methylation is found in 30% of cervical cancer, 14% of squamous cell head and neck cancers, 6.7% of germ cell tumours of testis, and 15% of non-small-cell lung cancers [26].
This study investigates the hypothesis of an activation of the FA/BRCA network in the radiation-induced bystander response at sites of stalled replication promoting both DNA repair by homologous recombination and intra-S-phase checkpoint activation in bystander cells which is supported by our previous report of an S-phase restricted, ATR dependent formation of γH2AX and 53BP1 foci in bystander cells [11,16].
2. Materials and Methods
2.1 Cell Culture and irradiations
T98G glioma cells and GM05849 ATM deficient fibroblasts were cultured in RPMI 1640 medium (Cambrex, Verviers, Belgium) supplemented with 10 % FBS (PAA, Pasching, Austria), 2 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin (all Cambrex, Verviers, Belgium). ATR mutated Seckel cells (F02/98 hTERT) and 48BR hTERT fibroblasts were cultured in MEM medium supplemented with 15 % FBS (PAA, Pasching, Austria), 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin (all Cambrex, Verviers, Belgium) and 0.4 μg/ml puromycin (Sigma, Poole, UK). All cells were incubated at 37°C, 5 % CO2. For all experiments sub-confluent cell cultures were used.
For medium transfer experiments cells were seeded on 22 × 22 mm2 coverslips placed in 6-well tissue culture dishes and treated with filtered medium obtained from T98G cells, which had been irradiated with 2 Gy of X-rays (Pantak, X-ray set, 240KV) at 37°C. Following 30 min incubation the medium was taken off and filtered using a 0.45 μm syringe filter. The recipient cells were incubated with the conditioned medium at 37°C, 5 % CO2 for 24 h followed by fixation and immunocytochemistry. Sham-treated cells were used as controls. For direct irradiations, cells were seeded on 22 × 22 mm2 coverslips placed in 6-well tissue culture dishes and irradiated in cell culture medium at 37°C with 1 and/or 5 Gy of X-rays (Pantak, X-ray set, 240KV) to induce similar BRCA1 and FANCD2 foci yields as those observed in bystander cells. Cells were incubated at 37°C, 5 % CO2 for 4 h after direct radiation before fixation and immunocytochemistry. Caffeine (Sigma, Poole, UK) was used at a concentration of 1 mM which has been demonstrated and is being widely used to inhibit ATR and ATM [27-29]. The specific ATM inhibitor (ATMi) Ku55933 was used at a concentration of 10 μM.
2.2 Bromodeoxyuridine labelling of DNA
In order to visualise stalled replication forks in bystander cells, sub-confluent T98G cells were labelled with bromodeoxyuridine (BrdU) for 21 h, treated with conditioned medium from irradiated cells for 3 h, then stained for BrdU nuclear foci (rat anti-BrdU, Cancer Research UK) applying a protocol without prior DNA denaturation [30] which relies on the recognition of single stranded, BrdU-labelled DNA at sites of replication fork stalling. For γH2AX co-localisation with BrdU foci, the BrdU staining protocol was combined with the γH2AX staining protocol described below.
A routine BrdU pulse labelling method was used to determine the fraction of actively replicating cells in control and bystander cell cultures treated with conditioned medium from 2 Gy-irradiated cells. 20 μM BrdU was added for 15 min prior to ethanol fixation at −20°C overnight. Following treatment with 2 M hydrochloric acid for 15 min at room temperature and neutralisation with 0.1M sodium borate buffer, samples were washed in 0.5% Triton X100 in PBS, blocked in 3% foetal calf serum in PBS for 30 min, incubated with anti-BrdU antibody, washed in 0.1% Triton X 100 in PBS, incubated in Alexa Fluor 488-conjugated secondary antibody (Molecular Probes, Leiden, The Netherlands) for 30 min, washed in PBS and resuspended in propidium iodide/RNAse in PBS. Flow cytometry was performed using a Becton Dickinson FACScan and the fraction of BrdU-positive and -negative cells was determined using CellQuest software (Becton Dickinson).
2.3 Immunocytochemistry
For immunocytochemistry, cells grown on coverslips were fixed for 15 min with 4 % paraformaldehyde, permeabilized with 0.5 % Triton-X 100 (both Sigma, Poole, UK) and blocked with 3 % FBS (PAA, Pasching, Austria) in PBS for 30 min at room temperature. Incubation with a primary antibody specific for H2AX p139S (Upstate, Chandlers Ford, UK), 53BP1 (Novus Biologicals, Littleton, CO, USA), BRCA1 (Oncogene, Cambridge, MA, USA), phospho-Chk1 (p348S; (Santa Cruz Biotechnology), BRCA1 (Oncogene Research Products; Ab-1) or FANCD2 (Santa Cruz Biotechnology; sc28194) for 1 h at room temperature was followed by three washes of 10 min each in PBS/3% FBS and incubation with a matching Alexa Fluor 488 or 568 conjugated secondary antibody (Molecular Probes, Leiden, The Netherlands). DAPI-stained coverslips were mounted onto glass slides using Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and the edges were sealed with clear nail varnish. A fluorescence microscope was used for imaging and analysis (Zeiss, Welwyn Garden City, UK). Foci were scored by eye in all cells visible in each field of view. Average foci per cell and distribution of foci levels in the analysed cell population were calculated using Microsoft Excel. For the calculation of induced foci per cell, background foci numbers in untreated control cells were subtracted.
2.4 Western Blot
Cells were lysed in buffer (1% Igepal, 0.1% SDS, 0.05% sodium deoxycholate, 1 protease inhibitor tablet and 1 PhosSTOP tablet (Roche, UK)) and protein concentration determined using the BCA assay (Pierce, UK) according to manufacturer’s instructions. Samples were then subject to SDS-PAGE electrophoresis using the XCell SureLock Mini-Cell system (Invitrogen, UK), transferred onto nitrocellulous membranes (Millipore, UK), blocked for 1h in 3% skim milk blocking solution and probed accordingly. Antibody binding was detected using Supersignal West Pico or Femto Chemiluminescent substrate (Pierce, UK) according to manufacturer’s instructions. Antibodies: anti-phospho Chk1 S345 diluted 1:500 (Cell Signalling, UK), anti-BRCA1 diluted 1:500 (Santa Cruz) and anti-β Actin diluted 1:2500 (Sigma, UK).
2.5 Clonogenic Assay
500 cells were seeded in each T25 tissue culture flask containing filtered medium derived from irradiated (2 Gy, X-rays) or sham-irradiated cells, and incubated for 10-14 days at 37°C, 5 % CO2. For experiments involving direct irradiation, individual radiation doses were 0, 1, 2 and 5 Gy, with 500, 1000, 2000 and 5000 cells seeded per flask, respectively. Flasks were stained with crystal violet staining solution, and individual colonies were counted. Average surviving fractions and standard errors (SEM) of 2-4 independent experiments performed in triplicates were calculated. The Chk1 inhibitor UCN-01 (7-hydroxystauosporine) [31,32] was added to the culture medium at a final concentration of 10 nM 15 min prior to direct irradiation, or was added into the conditioned medium prior to transfer to recipient cells for bystander experiments.
2.6 Data analysis
Average foci numbers per cell, average survival fractions and standard errors were calculated using Microsoft Excel for Windows 2003 or 2010. The significance of reported findings was tested with a t-test comparing treated samples with their sham treated controls. Significance levels are provided in the text.
Colocalisation analysis of BrdU and γH2AX foci was analysed with ImageJ software using Pearson correlation coefficients [33] and Costes’ spatial statistics method [34].
3. Results
3. 1 Stalled replication forks in bystander cells
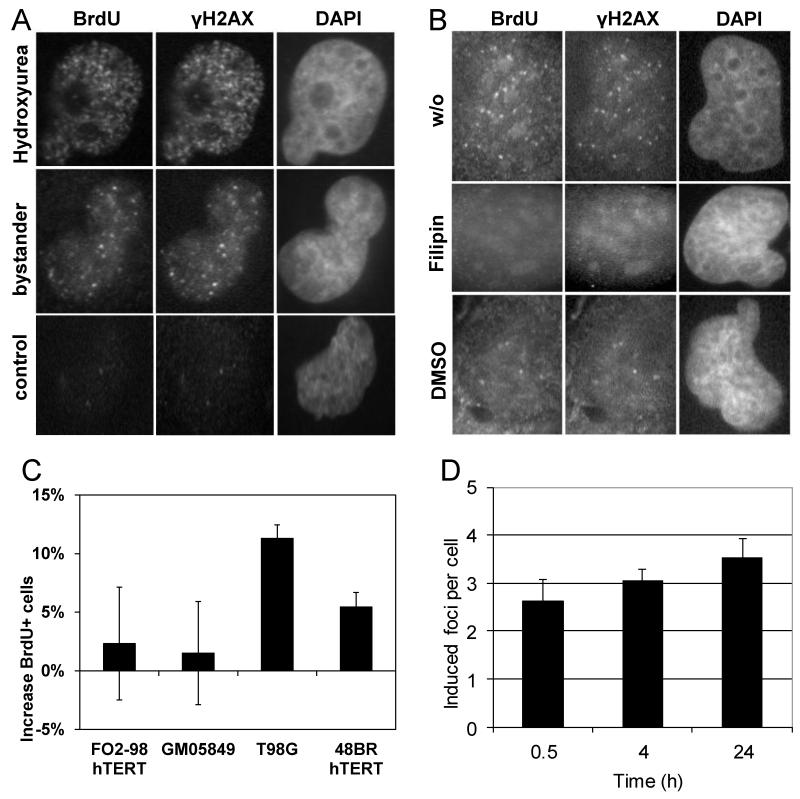
On the basis of published cell cycle distribution data of bystander cells and the S-phase specificity of bystander foci formation [11,16] we postulated that γH2AX and 53BP1 foci form at sites of stalled replication forks during S phase in bystander cells. To test this hypothesis, T98G glioma cells were cultured in BrdU-containing medium for 21 h resulting in overall labelling of DNA during one cycle of DNA replication followed by incubation with filtered conditioned medium derived from irradiated T98G cells for 3 h. Stalled replication forks that expose short patches of single stranded, BrdU-labelled DNA were detected by anti-BrdU immunofluorescence microscopy and appeared as nuclear foci. Treatment with 20 μM hydroxyurea for 3 h was included as a positive control. Immunofluorescence co-staining with a γH2AX specific antibody confirmed co-localisation of BrdU foci at sites of stalled replication with γH2AX foci in bystander cells (Figure 1A), with a mean Pearson correlation coefficient of 0.85 and significant correlation in all 20 analysed cells, according to Costes’ spatial statistics method. Additional representative images are included in Supplementary Figure S1. Formation of both BrdU foci and co-localising γH2AX foci were inhibited by the ROS scavenger DMSO and by Filipin, a disruptor of lipid rafts in the cell membrane and inhibitor of membrane signalling (Figure 1B). Additional representative images are included in Supplementary Figure S1. These findings confirm a role for ROS and membrane signalling in the induction of oxidative DNA damage in non-targeted cells.
Figure 1.
Immunofluorescence microscopic visualisation of stalled replication forks in bystander T98G cells. (A) Co-localisation of BrdU foci with γH2AX nuclear foci at sites of stalled replication in bystander cells and cells treated with 20 μM hydroxyurea; (B) Inhibition of BrdU and γH2AX nuclear foci in bystander cells by Filipin and DMSO. (C) Increase in the fraction of BrdU-positive cells detected by flow cytometry following pulse-labelling of bystander and corresponding control cultures. Bars show average values from 3 independent experiments for each cell line. Error bars show the associated standard errors. (D) Persistent increase of bystander gamma-H2AX foci numbers in T98G cell cultures treated for 0.5, 4 or 24 h with conditioned medium. Bars show average values from 2-6 independent experiments for each time point. Error bars show the associated standard errors.
Replication stalling at sites of oxidative DNA damage in non-targeted cells can be expected to activate the intra-S phase checkpoint and slow down replication progression, resulting in a larger S phase fraction in bystander cells compared to untreated cells. To test this hypothesis, control and bystander cell cultures were pulse-labelled with BrdU and the fraction of BrdU-positive cells determined using flow cytometry. Figure 1C shows that the percentage of BrdU-positive cells increases significantly in T98G (p=0.01) and 48BR hTERT bystander cells (p=0.05), compared to controls. In contrast, no significant increase was detected in ATR-deficient FO2/98 hTERT and ATM-deficient GM05849 human fibroblasts. Figure 1D shows the persistent increase of γH2AX foci in cultures of bystander T98G cells over a period of 24 h.
3.2 FANCD2 and BRCA1 foci induction in bystander cells
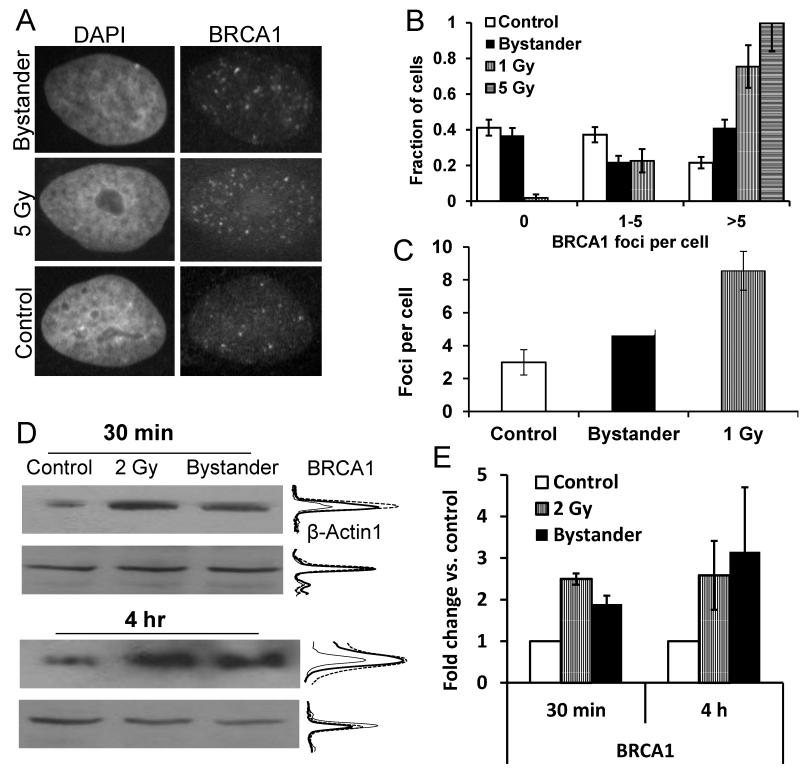
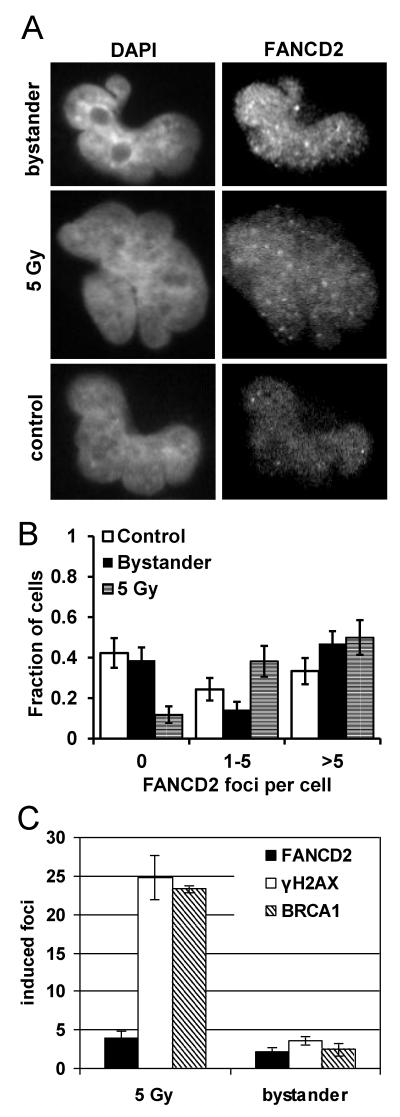
Immunostaining for BRCA1 and FANCD2 was performed to determine the involvement of the BRCA/FA network in bystander signalling. Directly irradiated T98G cells received an X-ray dose of 5 Gy and were subsequently stained for BRCA1 nuclear foci after 4 h incubation time. For the detection of BRCA1 bystander foci, cells were treated for 24 h with filtered medium derived from T98G cells irradiated with 2 Gy of X-rays (Figure 2A; see Supplementary Figure S2 for additional representative immunofluorescence microscopy images). Bystander cells showed a significant induction of BRCA1 foci above background level (p=0.02, Figure 2C) although the fraction of cells without any foci remained at control level (Figure 2B). This finding is consistent with the S-phase dependence of bystander foci formation, as cells without foci are known to be mostly in the G1 phase of the cell cycle [11,16]. In contrast, high numbers of nuclear BRCA1 foci were induced in all directly irradiated cells, without any sign of a ‘resistent’ subpopulation (Figure 2B). In this context it should be noted that the T98G control cultures analysed here contained 42±4 % S phase cells (BrdU-positive following pulse-labelling for 15 min), rising to 53±4 % in bystander cells, measured using flow cytometry (raw data not shown). Western blot analysis of lysates from T98G cells treated with conditioned medium for 30 min or 4 h demonstrated a 2-3-fold increase of BRCA1 protein compared with control cells (p=0.04, Figure 2D,E). Cells irradiated directly with 2 Gy were included as a positive control in the experiment. Induction of FANCD2 foci (Figure 3A & B, see also Supplementary Figure S2 for additional representative immunofluorescence microscopy images) was detected both in directly irradiated and bystander cells, but only in a fraction of the cells (Figure 3B), which are most likely S-phase cells as FANCD2 nuclear foci are known to accumulate at stalled replication forks [22].
Figure 2.
BRCA1 induction in directly irradiated and bystander T98G cells. (A) Anti-BRCA1 immunofluorescence microscopic images of control, bystander and directly irradiated cells. Nuclei were counterstained with DAPI. (B) Distribution and (C) average values of BRCA1 foci in control, bystander and directly irradiated cells. Foci data were collected from a total of 204 control and 204 bystander cells in four independent experiments and 53 1 Gy and 39 5 Gy-irradiated cells in one experiment. (D) Western blot analysis for BRCA1 and β-actin expression. Densitometric intensity profiles are shown for control (thin line), 2 Gy-irradiated (dashed line) and bystander samples (thick line). Densitometry readings are quantified on a linear scale. (E) Fold change relative to unirradiated controls in BRCA1 expression levels of irradiated and bystander samples following normalisation to β-actin levels. Error bars show the standard errors.
Figure 3.
(A&B) Induction of FANCD2 foci in directly 5 Gy-irradiated and bystander T98G cells. The diagram shows the distribution of foci. Foci data were collected from a total of 78 control and 98 bystander cells in two independent experiments and 68 5 Gy-irradiated cells in one experiment. Error bars show the standard errors (C) Bystander and 5 Gy-induced γH2AX, FANCD2 and BRCA1 foci in T98G cells. Foci counts in control samples were subtracted. Bars represent average induced foci per cell; error bars show SEM from 2-5 experiments.
Comparison of induced BRCA1 and FANCD2 foci in directly irradiated and bystander cells with γH2AX foci induction (Figure 3C) revealed a similar pattern of BRCA1 and γH2AX foci induction in cells directly irradiated with 5 Gy with an average of 23 and 25 induced foci per cell at 4 h after irradiation, whereas FANCD2 foci induction was on average 3.9 foci per cell. In bystander cells, BRCA1, FANCD2 and γH2AX foci induction was at a similar level with 2.4, 2.1 and 3.6 induced foci per cell after 24 h of incubation with conditioned medium. Supplementary figure 4 (S4) shows the average number of foci per cell in foci-containing cells only versus the whole cell population for p-Chk1, FANCD2, BRCA1 and γH2AX. These observations, taken together with the evident S-phase dependency of bystander foci induction which has previously been addressed in greater detail [11,16], support the hypothesis of an S-phase dependent bystander DNA damage response involving γH2AX, BRCA1 and FANCD2.
3.3 BRCA1 and FANCD2 foci in ATR/ATM mutant cell lines, ATM-inhibited and caffeine treated cells
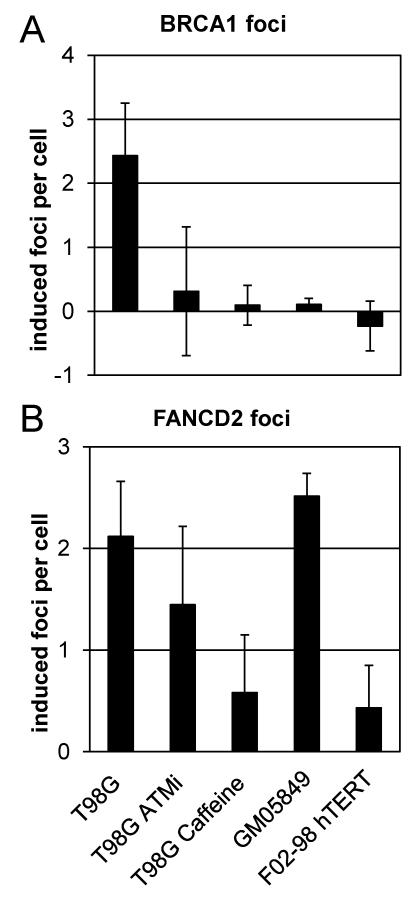
The reduction in clonogenic survival of bystander cells depends on ATR function and subsequent ATM activation [16] whereas bystander γH2AX foci induction requires ATR but not ATM [11]. To determine whether ATR and/or ATM are involved in the activation of the BRCA/FA network in bystander cells, ATR mutant F02-98 hTERT cells, ATM deficient GM05849 cells and ATMi or caffeine treated T98G cells were treated with conditioned medium derived from irradiated T98G cells and analysed for the induction of BRCA1 and FANCD2 foci (Figure 4A and 4B) in comparison to T98G cells.
Figure 4.
Bystander BRCA1 (A) and FANCD2 foci (B) in T98G cells, T98G treated with the specific ATM inhibitor (ATMi) KU55933 at 10 μM or the ATR/ATM inhibitor caffeine at 1 mM, as well as in GM05849 AT cells and in ATR mutated FO2-98 hTERT cells. Foci counts in control samples were subtracted. Bars represent average induced foci per cell; error bars show SEM from 3-5 experiments.
FANCD2 bystander foci were detected in T98G (p<0.01), ATMi-treated and ATM-deficient GM05849 cells (p<0.01) but not in ATR mutated F02-98 hTERT cells and T98G cells treated with the ATR/ATM inhibitor caffeine. This indicates that ATR, but not ATM, is required for the recruitment of FANCD2 to sites of replication associated DNA damage in bystander cells.
BRCA1 bystander foci were induced in T98G cells (p=0.02) but not in ATR mutated F02-98 hTERT, ATM deficient GM05849 or ATMi or caffeine treated T98G cells. These results suggest an essential role for ATM in the formation of nuclear BRCA1 foci in bystander cells. As shown previously [16], the activation of ATM in bystander cells depends on functional ATR. Therefore, the inactivation of either ATR or ATM prevents the formation of BRCA1 nuclear foci in bystander cells.
3.4 Phosphorylation of Chk1 in bystander cells and inhibition of a clonogenic bystander effect by the Chk1 inhibitor UCN-01
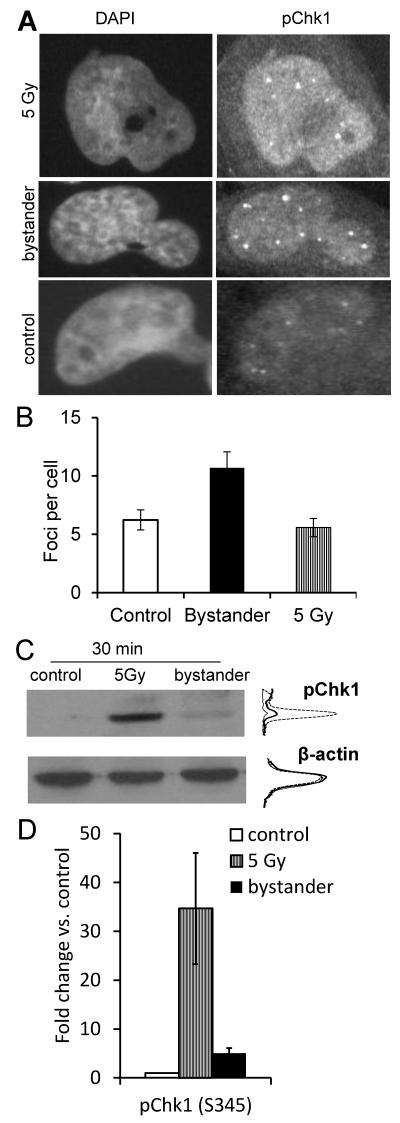
Chk1 is a main downstream target of ATR and is involved in the activation of the BRCA/FA pathway [23]. Therefore, we tested the hypothesis of bystander Chk1 activation which could provide a functional link between activation of ATR and BRCA/FA in bystander cells. Cells were treated for 24 h with filtered medium derived from 2 Gy X-irradiated T98G cells and immunostained for phospho-Chk1 (p348S). Significantly increased phospho-Chk-1 foci formation was observed in T98G bystander cells (p<0.01, Figure 5A,B; see Supplementary Figure S3 for additional representative immunofluorescence microscopy images). This was confirmed by Western blot analysis of protein lysate received from T98G cells treated for 30 min with filtered medium from irradiated cells (p=0.07). Lysate from cells irradiated directly with 5 Gy was included in this experiment as a positive control (Figure 5C,D).
Figure 5.
Phospho-Chk1 foci induction in bystander T98G cells. (B) Bars represent average p-Chk1 foci per cell; error bars show SEM from 2 experiments. (C) Western blot analysis of Chk1 phosphorylation and β-actin expression. Densitometric intensity profiles are shown for control (thin line), 5 Gy-irradiated (dashed line) and bystander samples (thick line). Densitometry readings are quantified on a linear scale. (D) Fold change relative to unirradiated controls in pChk1 expression levels of irradiated and bystander samples following normalisation to β-actin levels. Error bars show the standard errors.
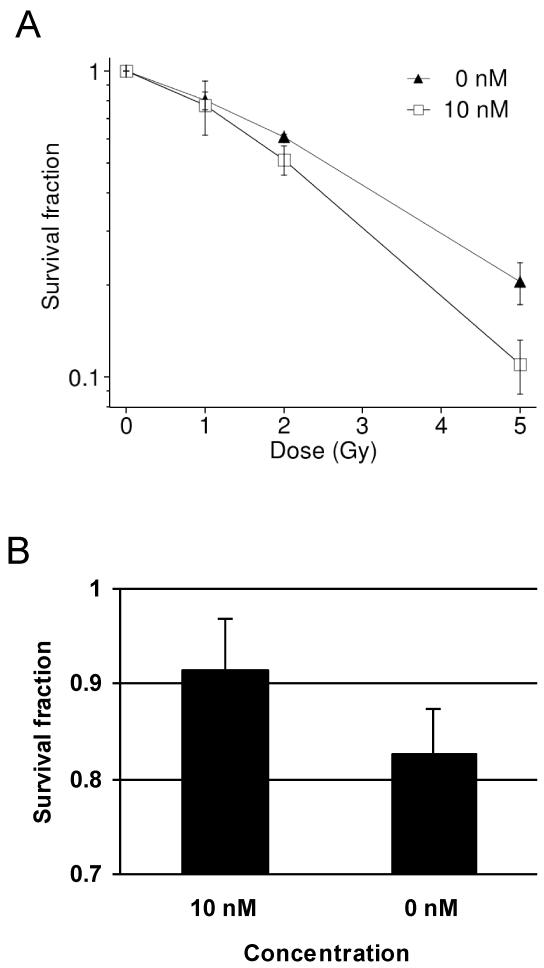
In order to test the functional importance of Chk1 in bystander responses, T98G cells were treated with the Chk1 inhibitor UCN-01 one hour prior to radiation and during subsequent incubation for colony formation in a clonogenic assay. A concentration of 10 nM resulted in a moderate radiosensitisation of T98G cells (p<0.05, Figure 6A). Bystander cells were treated with medium derived from T98G cells treated with 10 nM UCN-01 and irradiated with 2 Gy. Whereas sham-treated controls showed a significant reduction of the clonogenic survival of bystander cells (p<0.05), UCN-01-treated cultures were protected and showed only a minor non-significant bystander effect (Figure 6B). In T98G cells, higher doses of UCN-01 were toxic and could therefore not be tested for a further increase of the effect.
Figure 6.
Clonogenic survival of directly irradiated (A) and bystander T98G cells (B) treated with 0 nM or 10 nM of Chk1 inhibitor UCN-01; error bars show SEM from 2-4 experiments.
4. Discussion
This study aims to further clarify the DNA damage response network in bystander cells and to identify potential targets for novel molecular inhibitors. We have previously shown the S-phase restricted induction of γH2AX nuclear foci in an ATR dependent manner and the activation of ATM downstream of ATR in bystander cells [11,16]. Over the past few years, a novel FA/BRCA DNA damage response pathway has been uncovered which is a key pathway in the resolution of stalled replication and regulation of the intra-S-phase cell cycle checkpoint [20]. Functional loss of members of this pathway is linked to genomic instability [35].
The DNA damage response during DNA replication in S-phase includes an ATM/ATR mediated intra-S-phase checkpoint which suppresses DNA replication in response to DNA damage and also involves Chk1. Stabilisation and restarting of stalled replication forks involves proteins related to homologous recombination (HR) like Rad51 and various other proteins including RPA, ATR, 53BP1, BLM, γH2AX and BRCA1 (reviewed in [17]).
This study demonstrates the accumulation of stalled replication forks in bystander cells and their co-localisation with γH2AX foci. As bystander cells show an ATR- and ATM-dependent intra-S-phase arrest, it is important to note that bystander foci induction in S-phase cells may cause a further increase in overall foci numbers through subsequent accumulation of cells in S phase. However, full characterisation of such a response would require complex, long-term time-lapse microscopy studies of foci formation in a bystander cell population.
Bystander cells show the induction of FANCD2 and BRCA1 nuclear foci which are central proteins of the FA/BRCA DNA damage response network. Whilst the induction of bystander FANCD2 foci is ATR dependent but independent of ATM, the induction of BRCA1 bystander foci requires both ATR and ATM function. In this context, the question arises whether further molecules are involved as intermediaries in this pathway activation, given the complexity of the FA DNA repair network that has been uncovered in recent years [18-23,56]. Future studies are required as this is beyond the scope of this current study.
While this study does not directly demonstrate that FANCD2 bystander foci form in S-phase cells, there is a strong line of argumentation from our previously published bystander studies and this current study to support this interpretation: (i) It has been thoroughly demonstrated that γH2AX foci are formed in S-phase cells via co-staining with CENP-F [11]; (ii) γH2AX foci co-localise with BrdU foci, highlighting replication fork stalling; (iii) γH2AX foci co-localise with BRCA1, ATR and ATM foci in bystander cells [16]; (iv) FANCD2 and γH2AX foci co-localise in bystander cells (Supplementary Figure 5).
Interestingly, a recent study by Redon et al. suggests that malignant tumours induce a DNA damage response in proliferating distant tissue similar to the DNA damage response in the proposed bystander model [36,37].
A previous study has demonstrated that the bystander effect observed in a co-culture of targeted and non-targeted cells persists for at least 24 h following irradiation, using γH2AX foci induction in T98G cells as experimental system (Figure 3 in [11]). In this study, a persistent increase of γH2AX bystander foci for at least 24 h has also been confirmed for medium transfer experiments. This observation is supported by two independent reports by Facoetti et al. [38] and Zhang et al. [39] who also report a persistent bystander response in medium transfer experiments involving different endpoints than gamma-H2AX foci formation. However, no data have been obtained yet on the dose dependence of bystander foci induction. Yet, based on the general observation of dose-independent bystander effects (e.g. Figure 4 from [4]) one would not expect to see a difference for conditioned medium obtained from cells exposed to different doses.
The nature of the signal triggering a DNA damage response in non-targeted cells is still not entirely clear. Subnuclear foci of γH2AX and 53BP1 are well known to be associated with DNA double-strand breaks but their formation in bystander cells likely reflects only a secondary event triggered by stalled replication forks rather than prompt “two-ended” DSBs. These stalled replication forks have been postulated to be a consequence of an accumulation of ROS leading to DNA damage, including base damage which accumulates in S-phase leading to replication fork stalling and secondary production of DSBs and chromosomal aberrations [40]. Increasing ROS levels following treatment with conditioned medium from irradiated cells have previously been reported [41]. The results presented suggest that bystander signals act in a similar manner as inter-strand crosslinks [42], ultraviolet light-induced damage [43], and human papillomavirus type 16 E7 oncoprotein-mediated replication stress [44]. All these factors activate the FA/BRCA pathway and may not directly induce DSBs but rather lead to secondary DNA breaks that form during replication, for example when the replication fork encounters an unrepaired single-strand break. Such “one-ended” breaks are normally repaired via homologous recombination-dependent processes. The previous observation that sister chromatid exchanges are induced in bystander cells [45] in a homologous recombination-dependent manner [46] is consistent with this concept.
A central role for Chk1 in the activation of the BRCA/FA network has been reported previously [23]. Our results show an activation of Chk1 in the radiation-induced bystander DNA damage response and suggest the suitability of Chk1 as a further potential target to differentially modulate radiation effects on directly irradiated and non-targeted bystander cells. In a similar manner to the Chk1 response reported here, where the Chk1 inhibitor UCN-1 acted as a radiosensitizer in directly radiated cells but increased survival in bystander cells (Figure 6), ATR and ATM inhibitors have previously been demonstrated to differentially modulate targeted and bystander responses for the endpoint of clonogenic cell survival [16]. It is thought that inhibition of the repair proteins involved in the bystander response may increase the survival of damaged cells which may otherwise have undergone cell cycle arrest or apoptosis. However, this hypothesis has not been tested yet and will be the subject of future studies. The FA/BRCA pathway is affected in many human tumours including bladder [47], breast [48] and cervical [49] cancer either through gene mutations or epigenetic changes, and inherited mutation in either BRCA1 or BRCA2 causes a hereditary breast and ovarian cancer syndrome. Many of these cancers are treated with radiotherapy. Investigating the role of this pathway in the response to radiation-induced DNA damage is therefore important for treatment optimisation. Especially pathways targeted by novel molecular inhibitors (e.g. PARP inhibitors in BRCA mutated tumours [50], ATM inhibitors or Chk1 inhibitors) with the potential for combined radiotherapy [51] need to be investigated regarding their effects on directly irradiated and bystander cells. Furthermore, increased clinical as well as cellular radiosensitivity has been reported for some but not all patients with inherited defects in the FA/BRCA pathway [52-59], adding to the complexity of radiotherapy treatment optimisation.
Limiting the radiation induced genomic damage of normal cells, which may further lead to cancer formation is key in the field of radiation protection. Furthermore, radiation induced bystander cells are at risk for late genomic instability, which is associated with many cancers. Further studies on genomic instability in bystander cells will be needed to understand the possible mechanisms of secondary cancers after radiation exposure.
Supplementary Material
Acknowledgments
Grant support was received from Cancer Research UK (C1513/A7047) and from Breast Cancer Campaign (2009MayPR03).
References
- [1].Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mothersill C, Seymour CB. Radiation-induced bystander effects--implications for cancer. Nat Rev Cancer. 2004;4:158–164. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- [3].Goldberg Z, Lehnert BE. Radiation-induced effects in unirradiated cells: a review and implications in cancer. Int J Oncol. 2002;21:337–349. [PubMed] [Google Scholar]
- [4].Shao C, Stewart V, Folkard M, Michael BD, Prise KM. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res. 2003;63:8437–8442. [PubMed] [Google Scholar]
- [5].Shao C, Lyng FM, Folkard M, Prise KM. Calcium fluxes modulate the radiatin-induced bystander responses in targeted glioma and fibroblast cells. Radiat Res. 2006;166:479–487. doi: 10.1667/RR3600.1. [DOI] [PubMed] [Google Scholar]
- [6].Mothersill C, Seymour RJ, Seymour CB. Bystander effects in repair-deficient cell lines. Radiat Res. 2004;161:256–263. doi: 10.1667/rr3136. [DOI] [PubMed] [Google Scholar]
- [7].Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD. Bystander-induced apoptosis and premature differentiation in primary urothelial explants after charged particle microbeam irradiation. Radiat Prot Dosimetry. 2002;99:249–251. doi: 10.1093/oxfordjournals.rpd.a006775. [DOI] [PubMed] [Google Scholar]
- [8].Lyng FM, Maguire P, Kilmurray N, Mothersill C, Shao C, Folkard M, Prise KM. Apoptosis is initiated in human keratinocytes exposed to signalling factors from microbeam irradiated cells. Int J Radiat Biol. 2006;82:393–399. doi: 10.1080/09553000600803904. [DOI] [PubMed] [Google Scholar]
- [9].Lyng FM, Seymour CB, Mothersill C. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: a possible mechanism for bystander-induced genomic instability? Radiat Res. 2002;157:365–370. doi: 10.1667/0033-7587(2002)157[0365:ioaice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [10].Sokolov MV, Smilenov LB, Hall EJ, Panyutin IG, Bonner WM, Sedelnikova OA. Ionizing radiation induces DNA double-strand breaks in bystander primary human fibroblasts. Oncogene. 2005;24:7257–7265. doi: 10.1038/sj.onc.1208886. [DOI] [PubMed] [Google Scholar]
- [11].Burdak-Rothkamm S, Short SC, Folkard M, Rothkamm K, Prise KM. ATR-dependent radiation-induced gammaH2AX foci in bystander primary human astrocytes and glioma cells. Oncogene. 2007;26:993–1002. doi: 10.1038/sj.onc.1209863. [DOI] [PubMed] [Google Scholar]
- [12].Han W, Wu L, Chen S, Bao L, Zhang L, Jiang E, Zhao Y, Xu A, Hei TK, Yu Z. Constitutive nitric oxide acting as a possible intercellular signaling molecule in the initiation of radiation-induced DNA double strand breaks in non-irradiated bystander cells. Oncogene. 2006 doi: 10.1038/sj.onc.1210024. [DOI] [PubMed] [Google Scholar]
- [13].Yang H, Asaad N, Held KD. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene. 2005;24:2096–2103. doi: 10.1038/sj.onc.1208439. [DOI] [PubMed] [Google Scholar]
- [14].Hu B, Wu L, Han W, Zhang L, Chen S, Xu A, Hei TK, Yu Z. The time and spatial effects of bystander response in mammalian cells induced by low dose radiation. Carcinogenesis. 2006;27:245–251. doi: 10.1093/carcin/bgi224. [DOI] [PubMed] [Google Scholar]
- [15].Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- [16].Burdak-Rothkamm S, Rothkamm K, Prise KM. ATM acts downstream of ATR in the DNA damage response signaling of bystander cells. Cancer Res. 2008;68:7059–7065. doi: 10.1158/0008-5472.CAN-08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Andreassen PR, Ho GP, D’Andrea AD. DNA damage responses and their many interactions with the replication fork. Carcinogenesis. 2006;27:883–892. doi: 10.1093/carcin/bgi319. [DOI] [PubMed] [Google Scholar]
- [18].Kennedy RD, D’Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005;19:2925–2940. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- [19].Wang W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nat Rev Genet. 2007;8:735–748. doi: 10.1038/nrg2159. [DOI] [PubMed] [Google Scholar]
- [20].Venkitaraman AR. Tracing the network connecting BRCA and Fanconi anaemia proteins. Nat Rev Cancer. 2004;4:266–276. doi: 10.1038/nrc1321. [DOI] [PubMed] [Google Scholar]
- [21].Andreassen PR, D’Andrea AD, Taniguchi T. ATR couples FANCD2 monoubiquitination to the DNA-damage response. Genes Dev. 2004;18:1958–1963. doi: 10.1101/gad.1196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bogliolo M, Lyakhovich A, Callen E, Castella M, Cappelli E, Ramirez MJ, Creus A, Marcos R, Kalb R, Neveling K, Schindler D, Surralles J. Histone H2AX and Fanconi anemia FANCD2 function in the same pathway to maintain chromosome stability. Embo J. 2007;26:1340–1351. doi: 10.1038/sj.emboj.7601574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang X, Kennedy RD, Ray K, Stuckert P, Ellenberger T, D’Andrea AD. Chk1-mediated phosphorylation of FANCE is required for the Fanconi anemia/BRCA pathway. Mol Cell Biol. 2007;27:3098–3108. doi: 10.1128/MCB.02357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA, Elledge SJ. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- [25].Xiao Z, Chen Z, Gunasekera AH, Sowin TJ, Rosenberg SH, Fesik S, Zhang H. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J Biol Chem. 2003;278:21767–21773. doi: 10.1074/jbc.M300229200. [DOI] [PubMed] [Google Scholar]
- [26].Kennedy RD, D’Andrea AD. DNA repair pathways in clinical practice: lessons from pediatric cancer susceptibility syndromes. J Clin Oncol. 2006;24:3799–3808. doi: 10.1200/JCO.2005.05.4171. [DOI] [PubMed] [Google Scholar]
- [27].Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- [28].Nghiem P, Park PK, Kim Y, Vaziri C, Schreiber SL. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9092–9097. doi: 10.1073/pnas.161281798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, Foà R, Santoni A. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2008;113:3503–3511. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- [30].Raderschall E, Golub EI, Haaf T. Nuclear foci of mammalian recombination proteins are located at single-stranded DNA regions formed after DNA damage. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1921–1926. doi: 10.1073/pnas.96.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Heffernan TP, Simpson DA, Frank AR, Heinloth AN, Paules RS, Cordeiro-Stone M, Kaufmann WK. An ATR- and Chk1-dependent S checkpoint inhibits replicon initiation following UVC-induced DNA damage. Mol Cell Biol. 2002;22:8552–8561. doi: 10.1128/MCB.22.24.8552-8561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rodriguez-Bravo V, Guaita-Esteruelas S, Florensa R, Bachs O, Agell N. Chk1- and Claspin-Dependent but ATR/ATM- and Rad17-Independent DNA Replication Checkpoint Response in HeLa Cells. Cancer Res. 2006;66:8672–8679. doi: 10.1158/0008-5472.CAN-05-4443. [DOI] [PubMed] [Google Scholar]
- [33].Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J Cell Sci. 1992;103:857–862. doi: 10.1242/jcs.103.3.857. [DOI] [PubMed] [Google Scholar]
- [34].Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G. LOckett, Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86:3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Howlett NG, Taniguchi T, Durkin SG, D’Andrea AD, Glover TW. The Fanconi anemia pathway is required for the DNA replication stress response and for the regulation of common fragile site stability. Hum Mol Genet. 2005;14:693–701. doi: 10.1093/hmg/ddi065. [DOI] [PubMed] [Google Scholar]
- [36].Redon CE, Dickey JS, Nakamura AJ, Kareva IG, Naf D, Nowsheen S, Kryston TB, Bonner WM, Georgakilas AG, Sedelnikova OA. Tumors induce complex DNA damage in distant proliferative tissues in vivo. Proc Natl Acad Sci U S A. 2010;107:17992–17997. doi: 10.1073/pnas.1008260107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gaddameedhi S, Sancar A. Melanoma and DNA damage from a distance (farstander effect) Pigment Cell Melanoma Res. 2010;24:3–20. doi: 10.1111/j.1755-148X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- [38].Facoetti A, Mariotti L, Ballarini F, Bertolotti A, Nano R, Parsi F, Ranza E, Ottolenghi A. Experimental and theoretical analysis of cytokine release for the study of radiation-induced bystander effect. Int J Radiat Biol. 2009;85:690–699. doi: 10.1080/09553000903020016. [DOI] [PubMed] [Google Scholar]
- [39].Zhang Y, Zhou J, Baldwin J, Held KD, Prise KM, Redmond RW, Liber HL. Ionizing radiation-induced bystander mutagenesis and adaptation: quantitative and temporal aspects. Mutat Res. 2009;671:20–25. doi: 10.1016/j.mrfmmm.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nagasawa H, Little JB. Bystander effect for chromosomal aberrations induced in wild-type and repair deficient CHO cells by low fluences of alpha particles. Mutat Res. 2002;508:121–129. doi: 10.1016/s0027-5107(02)00193-8. [DOI] [PubMed] [Google Scholar]
- [41].Lyng FM, Seymour CB, Mothersill C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br. J. Cancer. 2000;83:1223–1230. doi: 10.1054/bjoc.2000.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rothfuss A, Grompe M. Repair kinetics of genomic interstrand DNA cross-links: evidence for DNA double-strand break-dependent activation of the Fanconi anemia/BRCA pathway. Mol Cell Biol. 2004;24:123–134. doi: 10.1128/MCB.24.1.123-134.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dunn J, Potter M, Rees A, Runger TM. Activation of the Fanconi anemia/BRCA pathway and recombination repair in the cellular response to solar ultraviolet light. Cancer Res. 2006;66:11140–11147. doi: 10.1158/0008-5472.CAN-06-0563. [DOI] [PubMed] [Google Scholar]
- [44].Spardy N, Duensing A, Charles D, Haines N, Nakahara T, Lambert PF, Duensing S. The human papillomavirus type 16 E7 oncoprotein activates the Fanconi anemia (FA) pathway and causes accelerated chromosomal instability in FA cells. J Virol. 2007;81:13265–13270. doi: 10.1128/JVI.01121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- [46].Nagasawa H, Wilson PF, Chen DJ, Thompson LH, Bedford JS, Little JB. Low doses of alpha particles do not induce sister chromatid exchanges in bystander Chinese hamster cells defective in homologous recombination. DNA Repair (Amst) 2008;7:515–522. doi: 10.1016/j.dnarep.2007.11.014. [DOI] [PubMed] [Google Scholar]
- [47].Neveling K, Kalb R, Florl AR, Herterich S, Friedl R, Hoehn H, Hader C, Hartmann FH, Nanda I, Steinlein C, Schmid M, Tonnies H, Hurst CD, Knowles MA, Hanenberg H, Schulz WA, Schindler D. Disruption of the FA/BRCA pathway in bladder cancer. Cytogenet Genome Res. 2007;118:166–176. doi: 10.1159/000108297. [DOI] [PubMed] [Google Scholar]
- [48].van der Groep P, Hoelzel M, Buerger H, Joenje H, de Winter JP, van Diest PJ. Loss of expression of FANCD2 protein in sporadic and hereditary breast cancer. Breast Cancer Res Treat. 2008;107:41–47. doi: 10.1007/s10549-007-9534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Narayan G, Arias-Pulido H, Nandula SV, Basso K, Sugirtharaj DD, Vargas H, Mansukhani M, Villella J, Meyer L, Schneider A, Gissmann L, Durst M, Pothuri B, Murty VV. Promoter hypermethylation of FANCF: disruption of Fanconi Anemia-BRCA pathway in cervical cancer. Cancer Res. 2004;64:2994–2997. doi: 10.1158/0008-5472.can-04-0245. [DOI] [PubMed] [Google Scholar]
- [50].Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- [51].Burdak-Rothkamm S, Prise K. New molecular targets in radiotherapy: DNA damage signalling and repair in targeted and non-targeted cells. Eur J Pharmacol. 2009;625:151–155. doi: 10.1016/j.ejphar.2009.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gluckman E, Devergie A, Dutreix J. Radiosensitivity in Fanconi anaemia: application to the conditioning regimen for bone marrow transplantation. Br. J. Haematol. 1983;54:431–440. doi: 10.1111/j.1365-2141.1983.tb02117.x. [DOI] [PubMed] [Google Scholar]
- [53].Djuzenova CS, Rothfuss A, Oppitz U, Spelt G, Schindler D, Hoehn H, Flentje M. Response to X-irradiation of Fanconi anemia homozygous and heterozygous cells assessed by the single-cell gel electrophoresis (comet) assay. Lab. Invest. 2001;81:185–192. doi: 10.1038/labinvest.3780226. [DOI] [PubMed] [Google Scholar]
- [54].Alter BP. Radiosensitivity in Fanconi’s anemia patients. Radiother Oncol. 2002;62:345–347. doi: 10.1016/s0167-8140(01)00474-1. [DOI] [PubMed] [Google Scholar]
- [55].Djuzenova CS, Flentje M. Characterization of Fanconi anemia fibroblasts in terms of clonogenic survival and DNA damage assessed by the Comet assay. Med. Sci. Monit. 2002;8:BR421–30. [PubMed] [Google Scholar]
- [56].Godthelp BC, van Buul PPW, Jaspers NGJ, Elghalbzouri-Maghrani E, van Duijn-Goedhart A, Arwert F, Joenje H, Zdzienicka MZ. Cellular characterization of cells from the Fanconi anemia complementation group, FA-D1/BRCA2. Mutat. Res. 2006;601:191–201. doi: 10.1016/j.mrfmmm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- [57].Mohseni-Meybodi A, Mozdarani H, Mozdarani S. DNA damage and repair of leukocytes from Fanconi anaemia patients, carriers and healthy individuals as measured by the alkaline comet assay. Mutagenesis. 2009;24:67–73. doi: 10.1093/mutage/gen052. [DOI] [PubMed] [Google Scholar]
- [58].D’Andrea AD. Susceptibility pathways in Fanconi’s anemia and breast cancer. N. Engl. J. Med. 2010;362:1909–1919. doi: 10.1056/NEJMra0809889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Birkeland AC, Auerbach AD, Sanborn E, Parashar B, Kuhel WI, Chandrasekharappa SC, Smogorzewska A, Kutler DI. Postoperative clinical radiosensitivity in patients with fanconi anemia and head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2011;137:930–934. doi: 10.1001/archoto.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.