Abstract
The visual system has often been thought of as a parallel processor because distinct regions of the brain process different features of visual information. However, increasing evidence for convergence and divergence of circuit connections, even at the level of the retina where visual information is first processed, chips away at a model of dedicated and distinct pathways for parallel information flow. Instead, our current understanding is that parallel channels may emerge, not from exclusive microcircuits for each channel, but from unique combinations of microcircuits. This review depicts diagrammatically the current knowledge and remaining puzzles about the retinal circuit with a focus on the mouse retina. Advances in techniques for labelling cells and genetic manipulations have popularized the use of transgenic mice. We summarize evidence gained from serial electron microscopy, electrophysiology and light microscopy to illustrate the wiring patterns in mouse retina. We emphasize the need to explore proposed retinal connectivity using multiple methods to verify circuits both structurally and functionally.
Introduction
The retina is composed of five major neuronal classes: photoreceptors, horizontal cells, bipolar cells, amacrine cells and ganglion cells (Fig.1A). Each cell class comprises multiple cell types: rod and cone photoreceptors, 1–3 horizontal cell types, at least 12 types of bipolar cells, at least 29 types of amacrine cells and at least 12 types of ganglion cells (reviewed in Field & Chichilnisky, 2007; Masland, 2012). When photons strike the retina, photoreceptors transduce light energy into a change in glutamate release, which is sensed by bipolar cells. Each bipolar cell type receives input from either single or multiple photoreceptor types and from variable numbers of photoreceptors (Wässle 2009; Breuninger et al. 2011). Bipolar cells, in turn, alter their glutamate release onto ganglion cells. This vertical pathway is modulated by lateral interactions instigated by horizontal and amacrine cells.
Figure 1. Basic retinal wiring diagram colouring book and palette.
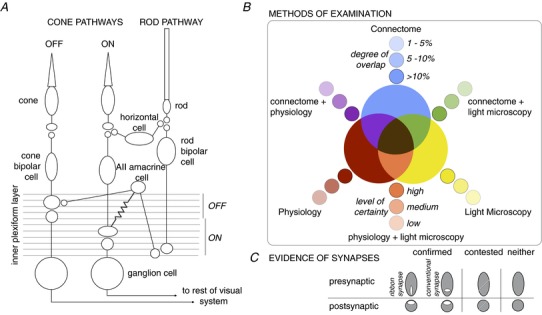
A, basic wiring diagram of the mouse retina. Signals from cones traverse the retina either through ON or OFF cone bipolar cells and ganglion cells. Synaptic connections between OFF bipolar and ganglion cells are predominantly found in the upper 0–40% of the inner plexiform layer, whereas synaptic connections between ON bipolar and ganglion cells are predominantly found in the lower 41–100% of the inner plexiform layer. Horizontal cells provide feedback to photoreceptors. Signals from rods traverse the retina through the rod bipolar pathway, which involves the rod bipolar, AII amacrine cell, electrical synapses with ON cone bipolar cells (symbolized by a resistor), chemical synapses with OFF cone bipolar cells (symbolized by 2 circles in close proximity), and respective connections with ON and OFF ganglion cells. B, colour code of methods used to examine connections. The primary methods include: the connectome, which is the serial reconstruction of electron micrographs (blue); physiological evidence (red); and light microscopy used to determine appositions (yellow). Combinations of methods are indicated by secondary colours: connectome + physiology (purple), physiology + light microscopy (orange), and connectome + light microscopy (green). Finally, in the case of all three methods of examination, connectome + physiology + light microscopy, the terminal is coloured brown. The saturation of colours indicates either the degree of overlap between cells, as determined in the connectome, or the level of certainty for the other methods of examination. C, evidence for the presence of synaptic marker proteins, either by ultrastructure or light microscopy, is indicated by white ovals within the pre- and/or postsynaptic terminals. Shape of the presynaptic oval represents either a ribbon synapse (vertical) or conventional synapse (horizontal). Contested synapses are indicated by a diagonal line through the terminal. If synaptic markers have not been examined, the terminal is left a solid colour to indicate that only pre- and postynaptic cell fills were used. These colours and symbols are consistently used throughout the figures.
The presence of what appear to be distinct and dedicated microcircuits in the retina has frequently led to the impression that the retina partitions visual information into channels that are processed separately and in parallel. Circuits often cast in this light include cases in which each presynaptic cell type synapses primarily or exclusively with one postsynaptic cell type. For example, the most numerous ganglion cell types in primate retina, midgets and parasol, receive input from distinct bipolar cell types, thus suggesting information could be processed by separate pathways (Boycott & Wassle, 1991; Jacoby et al. 2000; Kolb & Marshak, 2003; Wässle, 2004). Further examination of this assumption has recently led to the dissolution of the idea that the retina processes information in parallel without cross communication (Masland, 2012). Prominent instances have emerged, for example, of convergence and divergence of connectivity in the retina. In primate retina, parasol ganglion cells receive input from multiple cone bipolar cell types (Boycott & Wassle, 1991; Jacoby et al. 2000). Furthermore, the two most numerous ganglion cell types in cat, α and β cells, both receive input primarily from the same cone bipolar cell type (Sterling et al. 1988; Freed & Sterling, 1988). The mouse retina has become the central subject of large-scale connectomic efforts, and advances in transgenic techniques have facilitated the anatomical and physiological exploration of the visual system. Here we assess the advances made in the last decade on the wiring patterns in the mouse retina. We highlight how recent work in this species has contributed to dispelling the notion that information traverses the retina in segregated parallel pathways, and has instead helped substantiate that the retina relays information through many convergent and divergent connections crossing between conventional parallel channels.
Our review will centre around a paradigm for describing connections between retinal neurons, a key pertinent throughout the review. We begin with a blank template of the types of neurons described in the literature – a colouring book if you will (Fig.1A). We offer a system of indicating which connections have been examined using one method or combinatorially by three main methods: (1) connectomic reconstructions of serial electron micrographs, (2) physiological recordings, and (3) light microscopy used to determine potential synapses (Fig.1B). These methods of examination and their combinations are indicated by a specific hue.
Recent large-scale connectomic reconstructions of retina have relied on appositions to define contact as synaptic specializations were not preserved (Helmstaedter et al. 2013). Thus the study quantified connectivity as the area of membrane appositions of neighbouring cells normalized by the total membrane area contacts of either the pre- or postsynaptic cell types. In a separate dataset where membranes and synaptic specializations were preserved, Helmstaedter et al. (2013) estimated that 1 μm2 area contact is associated with a 95% probability of a synapse. Criteria for drawing connectomic connections include the following caveats: (1) not all of the membrane appositions in the electron micrographs have been confirmed by synaptic structures, and (2) percentage contact depends on whether all cells within the volume of retina were reconstructed. Incomplete large-field amacrine and ganglion cells could not be accurately typed and evaluated, thus lowering the total number of cells assessed within the volume. Thus, in assessing the connectome for this review, we have chosen 1% contact between the membranes of neighbouring cells as the threshold for reporting connectivity (‘Supplementary Information 4’ from Helmstaedter et al. 2013). At this threshold, the smallest contact area is 3 μm2 and is indicated by the least saturated hue. Connections between pairs of cell types exhibiting >10% connectivity are indicated by a fully saturated hue. Similarly, the degree of certainty for connections established by physiology and light microscopy is also indicated by saturation of hue. In addition to the colour scheme, we define symbols to indicate the presence and/or absence of synaptic markers at these putative connections (Fig.1C). Evidence for synaptic markers can come from electron and/or light microscopy. Throughout this review we use this colouring book and system of symbols to illustrate what we have learned and what remains unknown about wiring patterns in the mouse retina.
Cone pathways
To follow the connectome's emphasis on the inner plexiform layer, we focus on bipolar-to-ganglion cell connections and two amacrine cell types. We begin with an examination of convergence between different bipolar cell types and each ganglion cell type described in the connectome (Fig.2; Helmstaedter et al. 2013) as well as ganglion cells not described in the connectome (Fig.3). We then view these connections from the point of view of divergence between each bipolar cell type and its output to ganglion cells (Fig.4). For simplicity we use the nomenclature defined by Helmstaedter et al. (2013), and refer to bipolar cell types by type number to be consistent with previous classifications (Wässle et al. 2009). Helmstaeder noted that bipolar cell types 1 and 2 may have been flipped with respect to previous classification, which we correct here, and that the type 5R comprises more than one bipolar cell type (M. Helmstaeder, personal communication). We refer to ganglion cell types by GC number, which are more difficult to match with previous classifications (Sun et al. 2002; Kong et al. 2005; Coombs et al. 2006; Völgyi et al. 2009). Speculations about how ganglion cells might be matched across the literature are shown in Table1. We acknowledge the tenuousness of these matches, as agreements about ganglion cell classification across studies have not yet been reached.
Figure 2. Convergence of bipolar cell types onto individual ganglion cells.
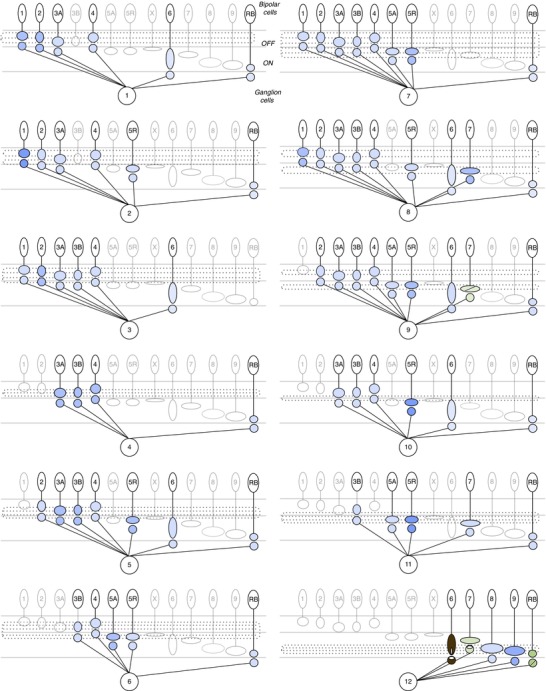
Putative connections between bipolar cell types and each ganglion cell described in the connectome. Presynaptic bipolar cell axonal stratification level represented by location of oval in the inner plexiform layer, and approximate axonal size represented by different sized ovals. Potential contacts of postsynaptic ganglion cells are represented by a circle near the bipolar presynaptic terminal. Circles representing contacts may fall outside the dendritic stratification level of the ganglion cells. The identity of the ganglion cells (GC) 1–12 corresponds to the nomenclature used in Helmstaedter et al. (2013). Refer to Table1 for the potential correlates of this ganglion cell typing scheme to those of other studies. Dotted lines in the inner plexiform layer correspond to the dendritic stratification of each ganglion cell. Connections between the type 7 bipolar cells and GC 9, which corresponds to the ON–OFF direction selective ganglion cell, have been examined by presynaptic markers (Lin & Masland, 2005) and the connectome (Helmstaedter et al. 2013). Connections with the A-type ON sustained ganglion cell (GC 12) have been examined by all methods for the type 6 bipolar cell (Schwartz et al. 2012), by synaptic markers and the connectome for the type 7 cone bipolar cells and rod bipolar cells (Morgan et al. 2011). For individual pairs of A-type ON ganglion cells and rod bipolar cells, synaptic markers were present during development but disappeared by postnatal day 21. For all other ganglion cell types, the connectome is the only method so far that has suggested connections between bipolar cell and ganglion cell types. The amount of overlap between each pair is normalized by each ganglion cell's total connectivity.
Figure 3. Circuit diagrams of ganglion cells not identified in the connectome.
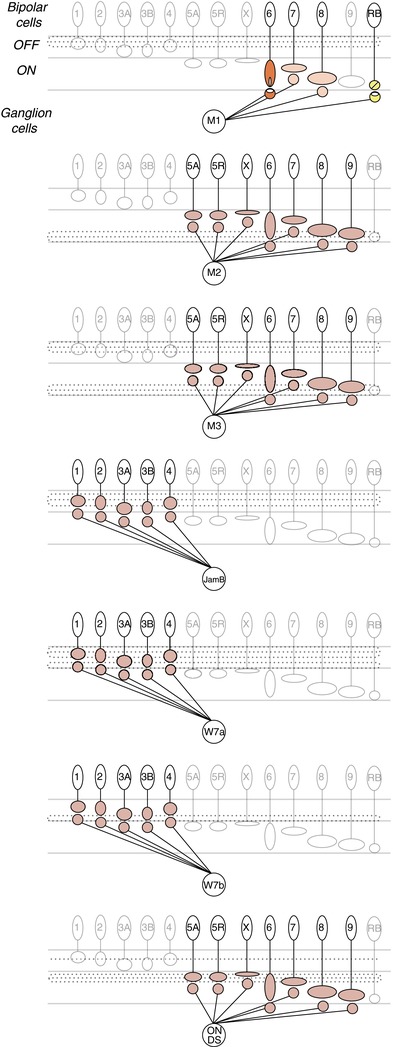
Ganglion cell types missing from the connectome include the melanopsin-expressing ganglion cells, M1, M2, M3; JamB; W7a; W7b; and the ON direction selective (ON DS) ganglion cells. Evidence from physiology and synaptic markers indicates inputs from ON cone bipolar cells to the M1 melanopsin ganglion cell (orange). En passant synapses from either the type 6, 7, or 8 ON cone bipolar cells have been speculated by Dumitrescu et al. (2009). Both the M2 and M3 melanopsin ganglion cells receive excitatory ON input (Schmidt & Kofuji, 2010; Schmidt et al. 2011a,b2011b); however, the bipolar cell type providing input remains unknown, hence we indicate a low level of certainty from physiology (red). The JamB, W7a and W7b ganglion cells described by Kim et al. (2010) depolarize to light decrements in their receptive field centres, suggesting that these cells may receive inputs from OFF cone bipolar cells. These synapses are coloured by the lowest level of certainty for the method of physiology (red). The majority of ON direction selective ganglion cell dendrites stratify in the ON sublamina of the inner plexiform layer and their membrane potential depolarizes to light increments, suggesting inputs from ON cone bipolar cells (Dhande et al. 2013). However, a particular type has not been implicated, thus the level of certainty for each type is low.
Figure 4. Divergence of bipolar cell output to ganglion cell types.
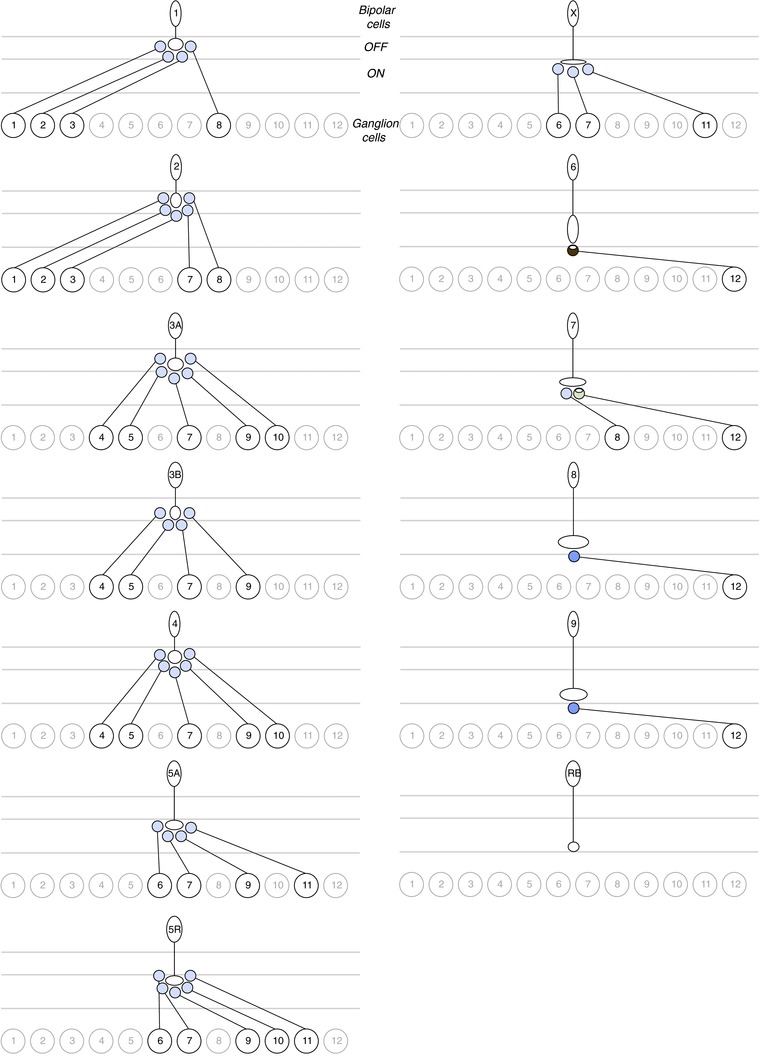
Putative connections between each bipolar cell type and the ganglion cell types described in the connectome (Helmstaedter et al. 2013). Type 6, 7, 8 and 9 ON cone bipolar cells have major connections with the GC 12 A-type ON ganglion cell. All other bipolar cell types diverge to multiple ganglion cell types. The rod bipolar cell does not have connections exceeding 1% with any of the ganglion cells. The amount of overlap between each pair is normalized by each bipolar cell type's total connectivity.
Table 1.
Ganglion cell classification
| Ganglion cell type | Nomenclature with stratification levels in the inner plexiform layer | Putative correlate to Völgyi et al. (2009) classification as determined by Helmstaedter et al. (2013) | Other potential matches or presence in transgenic lines |
|---|---|---|---|
| GC 1 | gc14–30 | G11 | A-type OFF sustained (Bleckert et al. 2014) |
| GC 2 | gc10–40 | — | — |
| GC 3 | gc15–42 | G7 | — |
| GC 4 | gc35–41 | G4 | — |
| GC 5 | gc37–46 | G4 | — |
| GC 6 | gc31–56 | G5 | — |
| GC 7 | gc36–51 | G8, 13 | W3a in TYW3 (Kim et al. 2010); local edge detector (Helmstaedter et al. 2013) |
| GC 8 | gc21–69 | G12 | — |
| GC 9 | gc30–63 | G17 | ON–OFF direction selective; DRD4-GFP (Huberman et al. 2009); BD, FSTL4-Cre-ER (Kim et al. 2010); W9 (Kay et al. 2011); Cdh6-CreER (Kay et al. 2011); TRHR-GFP (Rivlin-Etzion et al. 2011); Hb9-eGFP (Trenholm et al. 2011) |
| GC 10 | gc44–52 | G4, 5, 9 and/or 14 | W3b in TYW3 (Kim et al. 2010) |
| GC 11 | gc47–57 | G14 | — |
| GC 12 | gc76–86 | G1, 2 and/or 10 | A-type ON sustained, M4, M5 (Margolis & Detwiler, 2007; van Wyk et al. 2009; Estevez et al. 2012; Bleckert et al. 2014); inner α (Tagawa et al. 1999); ON Y-cell (Borghuis et al. 2013) |
| Melanopsin M1 | 0–20% | G19 | Opn4Cre/+; Z/EG (Ecker et al. 2010); Opn4-EGFP (Schmidt et al. 2008) |
| Melanopsin M2 | 80–100% | — | Opn4Cre/+; Z/EG (Ecker et al. 2010); Opn4-EGFP (Schmidt et al. 2008) |
| Melanopsin M3 | 0–20%, 80–100% | — | Opn4-EGFP (Schmidt et al. 2008) |
| JamB | 0–30% | G15 | JamB-Cre-ER (Kim et al. 2008) |
| W7a | 0–20%, 30–40% | — | TYW7 (Kim et al. 2010); A-type OFF sustained (Margolis & Detwiler, 2007; van Wyk et al. 2009; Bleckert et al. 2014); outer δ (Tagawa et al. 1999) |
| W7b | 30–40% | — | TYW7 (Kim et al. 2010); A-type OFF transient (Margolis & Detwiler, 2007; van Wyk et al. 2009); CB2-GFP (Huberman et al. 2008; but see Sümbül et al. 2014); outer α (Tagawa et al. 1999); OFF Y-cell (Borghuis et al. 2013); PV-5 (Münch et al. 2009) |
| ON DS (Hoxd10) | 55–70%, 15–20% | — | ON direction selective, Hoxd10-GFP (Dhande et al. 2013); SPIG1 (Yonehara et al. 2008) |
Classification of ganglion cell types (first column). Ganglion cell dendritic stratification levels in the inner plexiform layer where 0 indicates the outer border and 100 indicates the inner border (second column). Numbers that start with GC come directly from the nomenclature defined by Helmstaedter et al. (2013). Putative correlates with the Völgyi et al. (2009) classification of ganglion cells, as determined by Helmstaedter et al. (2013) (third column). Other potential matches or transgenic lines (fourth column). We note how speculative these correlations still are. Helmstaedter et al. (2013) assigned a level of correspondence to each of the Völgyi et al. (2009) types in their Supplemental Fig. 7.
When the rich data set of Helmstaedter et al. (2013) is displayed in our simple pictorial form, we find multiple themes. We start to see common patterns across the connections between bipolar cells and ganglion cells. The connectome diagrams and comparisons with other methods reveal which contacts may not be true connections, as well as point out the dearth of information about most connections in the retina. The depiction of bipolar-to-ganglion cell contacts also uncovers clues about how photoreceptor type-specific signals traverse the retinal circuit.
Convergence of bipolar cell types onto individual ganglion cell types
First, in seeking patterns of connectivity in convergence, each of the 12 ganglion cell types contacts multiple bipolar cell types, ranging from 4 to 9 of the total 13 bipolar cell types (Fig.2). Second, each ganglion cell contacts a unique combination of bipolar cell types. Third, each ganglion cell is contacted by a combination of major and minor inputs. For example, GC 2, 3, 6, 7, 9, 10 and 11 have major contacts with a single bipolar cell type and minor contact with a variety of other bipolar cell types. In contrast, GC 1, 4, 5, 8 and 12 have major contacts with more than one bipolar cell type. In the case of the GC 12, Schwartz et al. (2012) showed that 70% of its postsynaptic puncta were apposed to type 6 ON cone bipolar cells. Such variation across ganglion cells could reflect a true difference in connectivity patterns or could be skewed by normalizing by an incomplete set of cell types within the reconstructed retinal block. If true, these points support the idea that distinct pathways in the retina are formed by unique patterns of inputs.
Next, we speculate which contacts may not constitute true synapses. Eleven of the 12 ganglion cells described have minor connections with the rod bipolar cell. However, since all ganglion cell dendrites must enter the inner plexiform layer through the gauntlet of dense rod bipolar axon terminals, apposition of membranes seems inevitable, even if no synapses are made. In the case of the GC 12, likely to be the A-type ON sustained ganglion cell (Bleckert et al. 2014), apposition between rod bipolar cell terminals and ganglion cell dendrites did not guarantee the presence of postsynaptic markers. Examining pairs of rod bipolar cells and A-type ganglion cells, Morgan et al. (2011) found synaptic connections during development that were, however, eliminated by postnatal day 21. Thus the rod bipolar cell and GC 12 pair is depicted with contentious evidence for postsynaptic markers. Such results from light microscopy are consistent with evidence from electron microscopy showing no direct input between rod bipolar cells and ganglion cells in rat retina (Chun et al. 1993). However, such findings cannot rule out the possibility that a subset of rod bipolar cells within the ganglion cell's dendritic field make synaptic contacts.
Finally, we point out the striking paucity of methods used to examine connections between bipolar and ganglion cell types. Only two ganglion cell types have evidence beyond the connectome for or against connections with specific bipolar cell types. The GC 9 ON–OFF direction-selective ganglion cell has synaptic and connectomic evidence for synapses with the type 7 ON cone bipolar cell (Lin & Masland, 2005; but see Helmstaedter et al. 2013). Lin & Masland (2005) examined overlap between the type 7 ON cone bipolar cell and a variety of ganglion cell types; however, one can only speculate whether these ganglion cells match those described in the connectome. The A-type ON ganglion cell (GC 12) has been studied most extensively, specifically with regard to its connections with the type 6 and 7 cone bipolar cells (Morgan et al. 2011; Schwartz et al. 2012). Interestingly, the type 9 ON cone bipolar cell, known as the short-wavelength-sensitive (S) cone bipolar (Haverkamp et al. 2005; Breuninger et al. 2011), has a major contact with the A-type ganglion cell; this suggests that in dorsal retina, where cones expressing middle-wavelength (M) opsin dominate (Wang et al. 2011), pure S signals carried by the type 9 bipolar cell may mix with M signals conveyed by the other bipolar cell types that provide input to this ganglion cell. These connectome data leave open the possibility that the type 9 cone bipolar cell provides major input to another ganglion cell type not described in the connectome, thus undiscovered circuitry could preserve S cone signals through the output of the retina.
Circuit diagrams of ganglion cells not described in the connectome
The limited size of the reconstructed block of retina (114 μm × 80 μm) precluded the analysis of large-field ganglion cells that extended beyond the block (Helmstaedter et al. 2013). We noted a subset of ganglion cell types that appears to be missing: melanopsin-expressing ganglion cells M1, M2, M3 (Schmidt et al. 2008; Ecker et al. 2010), JamB (Kim et al. 2008, 2010), W7a, W7b (Kim et al. 2010), and the direction selective ON ganglion cells found in the Hoxd10 (Dhande et al. 2013) and SPIG1 (Yonehara et al. 2008) transgenic lines (Fig.3). For the majority of these ganglion cells, physiological responses to light stimuli suggest either inputs from ON (Schmidt & Kofuji, 2010, 2011; Dhande et al. 2013) or OFF (Kim et al. 2010) cone bipolar cells; however, the types have not been specified nor the circuitry determined. To be specific, in the case where voltages and spikes, as opposed to input currents, were measured, we cannot distinguish between direct input from an OFF cone bipolar cell or an OFF light response mediated by an amacrine cell inverting the responses from an ON cone bipolar cell. The inputs to the M1 melanopsin ganglion cell have been examined with both synaptic markers and physiological measures of input currents (Dumitrescu et al. 2009). A bipolar cell with resemblance to the type 6 cone bipolar cell was shown to have ectopic synapses in the OFF sublamina coinciding with the dopaminergic amacrine cells, which costratify with the M1 (Dumitrescu et al. 2009), thus making this bipolar cell type a likely candidate for providing direct synaptic input to the M1 melanopsin ganglion cell. As for the rod bipolar-to-M1 melanopsin ganglion cell contact, Dumitrescu et al. (2009) found evidence against presynaptic markers in mice, consistent with primates (Grünert et al. 2011), whereas Østergaard et al. (2007) found evidence for both pre- and postsynaptic markers in rats. Each of these non-connectomic ganglion cell types can be targeted in an existing transgenic line, which should facilitate further studies to determine connectivity.
Divergence of bipolar cell types to diverse ganglion cell types
In addition to the convergence of inputs from bipolar cell types to each ganglion cell type, the connectome data allow us to explore the possible divergence between each bipolar cell type and multiple ganglion cell types (Fig.4). In viewing divergence we find different patterns of connection compared with viewing convergence and focusing on the postsynaptic ganglion cell. First, from the perspective of each bipolar cell, connectivity takes on two patterns. Potential contacts with ganglion cells are (i) minor contacts spread among several ganglion cell types, in the cases of the cone bipolar cell types 1, 2, 3A, 3B, 4, 5A, 5R and X, or (ii) major contacts with few ganglion cells, in the cases of cone bipolar cell types 6, 7, 8 and 9. Second, from the perspective of the rod bipolar cell, no ganglion cell provides connections equal to or greater than 1% of the rod bipolar cell's total connections. In contrast to convergence, this suggests that the rod bipolar cell provides major output to other bipolar cells and amacrine cells rather than to ganglion cells.
Third, each bipolar cell diverges to a lower range of 1–5 ganglion cell types compared to convergence. A lower number of divergent outputs could be biased by the incomplete population of ganglion cells in the limited block of retina. For example, the type 6, 7, 8 and 9 ON cone bipolar cells provide major output to the GC 12, but these bipolar cells could potentially contact the M2, M3 and ON direction selective ganglion cells which have dendrites stratifying in the ON sublamina. Connections between the type 9 S cone bipolar cell and the M2 melanopsin ganglion cell seem highly plausible based on common stratification and evidence from primate retina that melanopsin-positive ganglion cells receive input from the S cone pathway (Dacey et al. 2005).
Finally, divergence reiterates the observation that pure cone signals carried by selective bipolar cells, e.g. the M cone selective type 1 and S cone selective type 9 cone bipolar cells (Breuninger et al. 2011) will spread to ganglion cells receiving input from other bipolar cells that do not discriminate cone input types. Therefore these circuit diagrams imply that cone-specific pathways are not preserved at the level of ganglion cells across the entire gradient of cone types in mouse retina (Chang et al. 2013).
Direction selective pathways
Several transgenic lines and focused connectome efforts have created an extensive body of work on the ON–OFF direction selective ganglion cells (GC 9), one of the few ganglion cells for which we know its preferred stimulus feature is movement of an object in a particular direction (Huberman et al. 2009; Briggman et al. 2011; Kay et al. 2011; Rivlin-Etzion et al. 2011; Trenholm et al. 2011). Wei & Feller (2011) recently provided a comprehensive description of GC 9 and its circuits. Here, we highlight findings in the connectome related to direction selectivity. As extensively as the direction selective circuit has been examined, the bipolar cell types which provide input to the starburst amacrine cells, the central mechanism of direction selectivity, remain undetermined. Figure5A shows that all the OFF cone bipolar cell types are potential partners of the OFF starburst amacrine cell. In contrast, 5 of the 8 ON bipolar cell types are potential partners of the ON starburst amacrine cell (Fig.5B; Helmstaedter et al. 2013). According to recent functional imaging and physiological recordings, the bipolar cell inputs to starburst amacrine cells exhibit no direction selective tuning (Yonehara et al. 2013; Park et al. 2014).
Figure 5. Direction selective circuits.
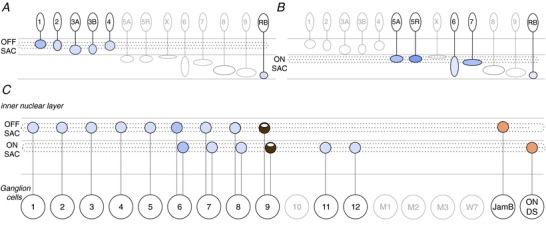
Bipolar cell types which have ≥1% overlap with the OFF (A) and ON (B) starburst amacrine cells’ (SAC) total connectivity. C, ganglion cells which receive ≥1% of total connections from the starburst amacrine cells or which have physiological evidence for direction selectivity, in the cases of the JamB and ON DS ganglion cells (Kim et al. 2008; Dhande et al. 2013). The GC 9 is the ON–OFF direction selective ganglion cell, whose connections with starburst amacrine cells have been confirmed by all methods (Wei et al. 2011; Briggman et al. 2011). The GC 6 is hypothesized to be motion or direction sensitive according to the connectome (Helmstaedter et al. 2013). Dotted lines in the inner plexiform layer represent the dendritic stratification of the starburst amacrine cells. The amount of overlap between each pair is normalized by each ganglion cell type's total connectivity.
In addition to showing the starburst amacrine cell inputs, we also illustrate the potential starburst amacrine cell outputs to ganglion cells (Fig.5C). We were surprised to see that 11 of the 12 ganglion cells described in the connectome have ≥1% connectivity with one or both of the starburst amacrine cells. Helmstaedter et al. (2013) highlighted the discovery of potential motion or direction sensitivity in the GC 6, which has the second-most connectivity with the starburst amacrine cells. Further, the other ganglion cell types could tap into the starburst amacrine cells for other reasons if connections are not specialized to confer direction selectivity (Briggman et al. 2011). In Fig.5C we also depict the potential connectivity of the JamB and ON direction selective ganglion cells with the starburst amacrine cells because of the physiological evidence for direction selectivity of these ganglion cell types (Kim et al. 2008; Dhande et al. 2013) and the evidence for proximity to starburst amacrine cell dendrites. Trans-synaptic viral tracing also revealed starburst amacrine connections with the ON–OFF (GC 9), ON and OFF direction selective ganglion cells, as well as the ON (GC 12) and OFF A-type ganglion cells (Beier et al. 2013).
Rod pathways
The illustrations of convergence and divergence in the cone pathways demonstrate that information flow in the mammalian retina may not be restricted along the classically defined parallel pathways. Furthermore, recent work on rod pathways confirms that rod signals traverse the retina through multiple conduits that do not remain segregated (Fig.6A). The primary rod bipolar pathway piggybacks onto cone bipolar cell output signals and has been examined by all methods (Sterling et al. 1988; Feigenspan et al. 2001; Tsukamoto et al. 2001; Deans et al. 2002; Veruki & Hartveit, 2002 in rat; Haverkamp et al. 2003; reviewed in Field et al. 2005). The secondary pathway, involving gap junctions between rods and cones, relies on rod signals travelling through cones to cone bipolar cells; signal crossing occurs at a level earlier than in the primary rod pathway (Tsukamoto et al. 2001; Deans et al. 2002; Feigenspan, 2004). The tertiary pathway involving direct connections between rods and cone bipolar cells has been found by methods of electron microscopy (Tsukamoto et al. 2001, 2007; Mataruga et al. 2007; Haverkamp et al. 2008), light microscopy (Hack et al. 1999; Mataruga et al. 2007; Haverkamp et al. 2008), as well as by physiology (Soucy et al. 1998; Feigenspan et al. 2001; Pang et al. 2010; Arman & Sampath, 2012). Recent work challenges the notion that these rod pathways operate under distinct conditions (Ke et al. 2014), thus rod signals could be carried by cone pathways at multiple stages of processing.
Figure 6. Basic rod pathways wiring diagram and AII amacrine connectivity.
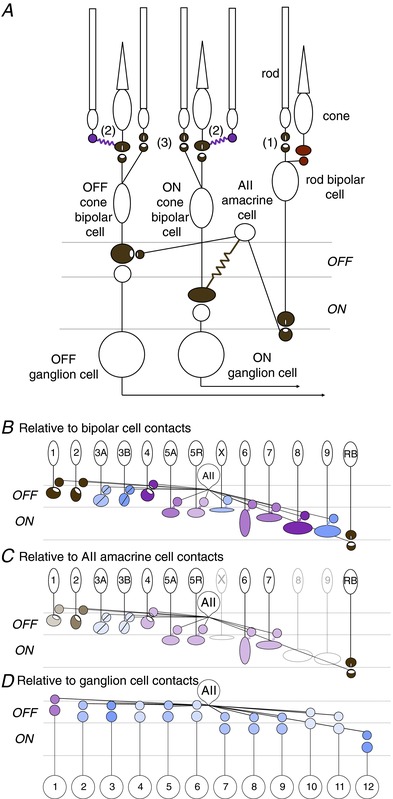
A, circuit diagram illustrating the multiple pathways for rod signals to traverse the retina. The primary rod pathway (1) involves a glutamatergic synapse between the rod and rod bipolar cell, glutamatergic synapse between rod bipolar cell and AII amacrine cell, electrical synapse between the AII amacrine cell and ON cone bipolar cell, glycinergic synapse between AII amacrine cell and OFF cone bipolar cell, and glutamatergic synapses between the ON cone bipolar and ON ganglion cell as well as between the OFF cone bipolar cell and OFF ganglion cell (Feigenspan et al. 2001; Tsukamoto et al. 2001; Deans et al. 2002; Veruki & Hartveit, 2002 in rat; Haverkamp et al. 2003). The secondary rod pathway (2) involves electrical synapses between rods and cones and subsequent reliance on cone pathways (Tsukamoto et al. 2001; Deans et al. 2002; but see Pang et al. 2010). The tertiary rod pathway (3) begins with either direct input from rods to OFF cone bipolar cells (Soucy et al. 1998; Hack et al. 1999; Tsukamoto et al. 2001; Mataruga et al. 2007; Haverkamp et al. 2008) or to ON cone bipolar cells (Tsukamoto et al. 2007; Pang et al. 2010). Finally, Pang et al. (2010) reported physiological evidence for direct inputs from cones to rod bipolar cells. B and C, connections between the AII amacrine cell and each bipolar cell type either normalized by the connections of all bipolar cell contacts (B), or by the connections of all AII amacrine cell contacts (C). Physiological evidence bolsters a subset of connections (Veruki & Hartveit, 2002; Mazade & Eggers, 2013), and fluorescence of pre- and postsynaptic markers bolsters connections of type 1 and 2 OFF cone bipolar cells with the AII amacrine cell (Sassoè-Pognetto et al. 1994 in rat; Haverkamp et al. 2003). Ultrastructural evidence for synaptic proteins provides evidence for AII amacrine cell input to the type 4, but not to the type 3 OFF cone bipolar cells (Tsukamoto et al. 2001). In the case of physiological evidence for electrical coupling between the AII amacrine and type 5 cone bipolar cells in rat (Veruki & Hartveit, 2002), we show that either of the subtypes (5A and 5R) could be coupled with the AII amacrine cell. D, putative direct connections between the AII amacrine cell and ganglion cell types described in the connectome. Several studies have supported direct input between AII amacrine cells and the A-type OFF ganglion cells (GC 1). The amount of overlap with the AII amacrine cell is normalized by each ganglion cell's total connectivity.
Bipolar cell connections with the AII amacrine cell
The connectome provides insight on unanswered questions about the primary rod bipolar pathway, such as which bipolar cells receive input from the AII amacrine cell. In considering this question, we find evidence from cat suggesting that the AII amacrine cell is selective in terms of the OFF cone bipolar cell types to which it provides glycinergic input and the ON cone bipolar cell types to which it is electrically coupled (McGuire et al. 1984; Cohen and Sterling 1990; reviewed in Demb & Singer, 2012). When we examine the mouse connectome between each bipolar cell type and the AII amacrine cell normalizing by all the connections of the bipolar cell (Fig.6B) or normalizing by all the connections of the AII amacrine cell (Fig.6C; Helmstaedter et al. 2013), we find different answers. From the perspective of the bipolar cells, every bipolar cell makes ≥1% contacts with the AII amacrine cell. From the perspective of the AII amacrine cells, only a subset of cone bipolar cell types makes ≥1% contacts, suggesting that the AII amacrine cell connects with cell types other than the bipolar cells, e.g. amacrine and ganglion cells. Connectivity with specific OFF cone bipolar cell types has been corroborated by bipolar cell recordings which demonstrate glycinergic inputs (Mazade & Eggers, 2013), but whether this input originates from AII amacrine cells remains unsettled. Connectivity with ON cone bipolar cells has been supported by paired recordings between AII amacrine cells and identified ON cone bipolar cell types in the rat (Veruki & Hartveit, 2002). In the future, direct measurements of electrical connections between ON cone bipolar cells and AII amacrine cells and synaptic markers between OFF cone bipolar cells and AII amacrine cells will support the proposed connections with AII amacrine cells.
AII amacrine cell connections with ganglion cells
The connectome also allows us to answer a second question: which ganglion cells receive direct input from the AII amacrine cell (Kolb, 1979; Sassoè-Pognetto et al. 1994)? In considering this question, we were surprised to find ubiquity of the AII amacrine-to-ganglion cell contact for the subset of ganglion cells described in the connectome (Fig.6D). Physiological evidence for direct glycinergic input to A-type OFF ganglion cells (potentially GC 1) (Manookin et al. 2008; van Wyk et al. 2009; Münch et al. 2009; Murphy & Rieke, 2011) at low light levels suggests that glycinergic input comes via AII amacrine cells. The connectome reveals that not only do the putative A-type OFF ganglion cells receive direct input from the AII amacrine cell, but all of the ganglion cells examined have ≥1% of their connectivity with the AII amacrine cell. The connectome even proposed a connection between the AII amacrine cell and A-type ON ganglion cell (GC 12), for which there is no prior evidence. Further examination by physiology, pharmacological blockade and synaptic markers is necessary to confirm or refute the potential connections proposed by the connectome.
Ganglion cells involved in the primary rod bipolar cell pathway
Yet another outstanding question in the literature is which ganglion cells convey information from the primary, secondary and tertiary rod pathways (Völgyi et al. 2009). We look to the connectome for potential clues. If we start with the assumption that not all cone bipolar cell types receive input from the AII amacrine cell (Fig.6D), then the possibility remains that some ganglion cells would not receive input from the primary rod bipolar pathway. We have depicted the connections from Fig.2 in a matrix highlighting the bipolar cell types connected to the AII amacrine cell (Table2). Convergence is indicated when multiple boxes are filled within a single column. The table shows that even if a particular cone bipolar cell type may not receive major input from the AII amacrine cell, convergence ensures that each ganglion cell receives input from another cone bipolar cell type which does receive AII amacrine cell inputs. Thus every ganglion cell described in the connectome should receive input from the primary rod bipolar cell pathway. Given that the secondary and tertiary pathways originate in earlier stages of processing, it is likely that every ganglion cell in the connectome should receive input from the other rod pathways. The contributions of specific rod pathways and cone pathways to each ganglion cell type in the mouse require further elucidation by physiological methods.
Table 2.
Matrix of bipolar-to-ganglion cell connectivity and relationship to the primary rod bipolar cell pathway
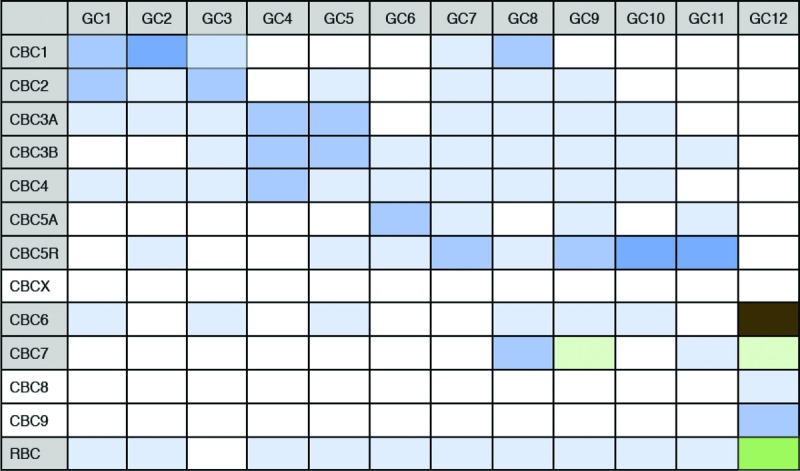 |
Table of connections between ganglion cell types (GC) and cone bipolar cell types (CBC) and the rod bipolar cell (RBC). Matrix form of same data shown in Fig.2 to illustrate that each ganglion cell described in the connectome would receive input from the rod bipolar cell pathway. Hue and saturation of the boxes correspond to the same key explained in Fig.1. Multiple connections in each column indicate convergence between multiple bipolar cell types to each ganglion cell type. Multiple connections in each row indicate divergence from one bipolar cell type to multiple bipolar cell types. If the bipolar cell type has ≥1% connectivity with the AII amacrine cell, then the bipolar cell label is coloured grey (first column). Connectivity between the bipolar cell and AII amacrine cell is normalized by the AII amacrine cell's total connectivity.
Summary
Rather than posing a list of unanswered questions about wiring patterns in mouse retina, we have depicted the discoveries and outstanding mysteries in a series of diagrams. Any connection not coloured brown and without symbols of synaptic markers can be considered an unsolved puzzle. Each connection remains a hypothesis until multiple methods of examination have confirmed or refuted its existence. As a field we have delved deeper into the complexity of the retinal wiring diagram. Evidence across methods allows us to appreciate the multiplexing of signals from rods and cones and of signals carried by different bipolar types, i.e. pathways. Furthermore, we have ignored the lateral connections formed by horizontal and most amacrine cells, which could commingle circuits further than we have described here. Parallel processing in the visual system, then, must arise from the unique combinations of inputs onto a ganglion cell (Masland, 2012). Perhaps a unique microcircuit serving each ganglion cell confers distinct properties to each channel exiting the retina (Asari & Meister, 2012).
‘Because of the increasing availability of mutant mice, the mouse retina will become the most important tool for studying the synaptic and molecular details of mammalian retinal organization’ (Wässle, 2004). Indeed, Wässle's prediction foretold how much we could learn about mammalian retina using the mouse. Over the next decade, we will continue drawing these circuit diagrams and colouring in the evidence for connections. The next generation of serial electron micrographs with preservation of synaptic structures and collection of larger volumes, more transgenic mouse lines where specific cell types can be reliably targeted, correlative fluorescence and electron microscopy to reconstruct specific cell types (Bleckert et al. 2013), super-resolution fluorescence microscopy to localize pre- and postsynaptic proteins, and trans-synaptic viral tracing to map connections (Cruz-Martín et al. 2014) will allow us to refine these circuit diagrams. Eventually these diagrams will represent all potential pathways for information flow; however, information flow could be modulated by stimuli (Manookin et al. 2008; Ke et al. 2014; reviewed by Demb, 2008), circadian rhythms (reviewed by Völgyi et al. 2013; McMahon et al. 2014), and other pathways which are simultaneously activated (Olveczky et al. 2007). Thus even after the static diagrams have been solved, much remains to be discovered about the dynamics of information flow through these pathways, as well as about modulation of these pathways by signalling mechanisms invisible to the methods of identifying physical connections between neurons. Together, the static circuits and dynamic information flow diagrams constitute the functional output of the retina.
Acknowledgments
We are grateful to the following individuals for their thoughtful and astute contributions to the manuscript: Adam Bleckert, Rana El-Danaf, Onkar Dhande, Moritz Helmstaeder, Mrinalini Hoon, Andrew Huberman, Sidney Kuo, Haruhisa Okawa, Fred Rieke and Timm Schubert. We thank Philip Mardoum for insightful contributions at each stage of writing this review.
Biography
Felice Dunn received her ScB and AB from Brown University while working with David Berson, and her PhD from the University of Washington in Fred Rieke's lab. She completed postdoctoral work with Mark Stopfer at the NIH and with Rachel Wong at the University of Washington. In her own laboratory in the Department of Ophthalmology at UCSF, she plans to continue the quest to understand the anatomy and function of retinal circuits.Rachel Wong is Professor of Biological Structure at the University of Washington. She began her studies on the development of the retina as a PhD student with Abbie Hughes at the Australian National University. She trained with Carla Shatz as a postdoctoral fellow at Stanford University, and then joined the Vision, Touch and Hearing Research Centre, University of Queensland as an R. D. Wright Fellow. Her first faculty position was at the Department of Anatomy and Neurobiology, Washington University in St Louis. Her research programme focuses on the development, degeneration and regeneration of retinal circuits. 
Additional information
Competing interests
None declared.
Funding
The authors’ work is supported by the Helen Hay Whitney Foundation (F.A.D.), and NIH grants EY-022910 (F.A.D.) and EY-017101 (R.O.L.W.).
References
- Arman AC, Sampath AP. Dark-adapted response threshold of OFF ganglion cells is not set by OFF bipolar cells in the mouse retina. J Neurophysiol. 2012;107:2649–2659. doi: 10.1152/jn.01202.2011. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asari H, Meister M. Divergence of visual channels in the inner retina. Nat Neurosci. 2012;15:1581–1589. doi: 10.1038/nn.3241. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Borghuis BG, El-Danaf RN, Huberman AD, Demb JB, Cepko CL. Transsynaptic tracing with vesicular stomatitis virus reveals novel retinal circuitry. J Neurosci. 2013;33:35–51. doi: 10.1523/JNEUROSCI.0245-12.2013. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckert A, Parker ED, Kang Y, Pancaroglu R, Soto F, Lewis R, Craig AM, Wong ROL. Spatial relationships between gabaergic and glutamatergic synapses on the dendrites of distinct types of mouse retinal ganglion cells across development. PLoS One. 2013;8:e69612. doi: 10.1371/journal.pone.0069612. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckert A, Schwartz GW, Turner MH, Rieke F, Wong ROL. Visual space is represented by nonmatching topographies of distinct mouse retinal ganglion cell types. Curr Biol. 2014;24:310–315. doi: 10.1016/j.cub.2013.12.020. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghuis BG, Marvin JS, Looger LL, Demb JB. Two-photon imaging of nonlinear glutamate release dynamics at bipolar cell synapses in the mouse retina. J Neurosci. 2013;33:10972–10985. doi: 10.1523/JNEUROSCI.1241-13.2013. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott BB, Wassle H. Morphological classification of bipolar cells of the primate retina. Eur J Neurosci. 1991;3:1069–1088. doi: 10.1111/j.1460-9568.1991.tb00043.x. &. [DOI] [PubMed] [Google Scholar]
- Breuninger T, Puller C, Haverkamp S, Euler T. Chromatic bipolar cell pathways in the mouse retina. J Neurosci. 2011;31:6504–6517. doi: 10.1523/JNEUROSCI.0616-11.2011. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188. doi: 10.1038/nature09818. &. [DOI] [PubMed] [Google Scholar]
- Chang L, Breuninger T, Euler T. Chromatic coding from cone-type unselective circuits in the mouse retina. Neuron. 2013;77:559–571. doi: 10.1016/j.neuron.2012.12.012. &. [DOI] [PubMed] [Google Scholar]
- Chun MH, Han SH, Chung JW, Wassle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993;332:421–432. doi: 10.1002/cne.903320404. &. [DOI] [PubMed] [Google Scholar]
- Cohen E, Sterling P. Demonstration of cell types among cone bipolar neurons of cat retina. Philos Trans R Soc Lond, B, Biol Sci. 1990;330:305–321. doi: 10.1098/rstb.1990.0201. &. [DOI] [PubMed] [Google Scholar]
- Coombs J, van der List D, Wang G-Y, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140:123–136. doi: 10.1016/j.neuroscience.2006.02.079. &. [DOI] [PubMed] [Google Scholar]
- Cruz-Martín A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, Nguyen PL, Callaway EM, Ghosh A, Huberman AD. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature. 2014;507:358–361. doi: 10.1038/nature12989. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Liao H-W, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau K-W, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. &. [DOI] [PubMed] [Google Scholar]
- Deans MR, Völgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB. Functional circuitry of visual adaptation in the retina. J Physiol. 2008;586:4377–4384. doi: 10.1113/jphysiol.2008.156638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Singer JH. Intrinsic properties and functional circuitry of the AII amacrine cell. Vis Neurosci. 2012;29:51–60. doi: 10.1017/S0952523811000368. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Estevez ME, Quattrochi LE, El-Danaf RN, Nguyen PL, Berson DM, Huberman AD. Genetic dissection of retinal inputs to brainstem nuclei controlling image stabilization. J Neurosci. 2013;33:17797–17813. doi: 10.1523/JNEUROSCI.2778-13.2013. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu ON, Pucci FG, Wong KY, Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009;517:226–244. doi: 10.1002/cne.22158. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen S-K, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez ME, Fogerson PM, Ilardi MC, Borghuis BG, Chan E, Weng S, Auferkorte ON, Demb JB, Berson DM. Form and function of the M4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. J Neurosci. 2012;32:13608–13620. doi: 10.1523/JNEUROSCI.1422-12.2012. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Teubner B, Willecke K, Weiler R. Expression of neuronal connexin36 in AII amacrine cells of the mammalian retina. J Neurosci. 2001;21:230–239. doi: 10.1523/JNEUROSCI.21-01-00230.2001. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A. Expression of Connexin36 in Cone Pedicles and OFF-Cone Bipolar Cells of the Mouse Retina. Journal of Neuroscience. 2004;24:3325–3334. doi: 10.1523/JNEUROSCI.5598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Chichilnisky EJ. Information processing in the primate retina: circuitry and coding. Annu Rev Neurosci. 2007;30:1–30. doi: 10.1146/annurev.neuro.30.051606.094252. &. [DOI] [PubMed] [Google Scholar]
- Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behavior to mechanism. Annu Rev Physiol. 2005;67:491–514. doi: 10.1146/annurev.physiol.67.031103.151256. &. [DOI] [PubMed] [Google Scholar]
- Freed MA, Sterling P. The ON-alpha ganglion cell of the cat retina and its presynaptic cell types. J Neurosci. 1988;8:2303–2320. doi: 10.1523/JNEUROSCI.08-07-02303.1988. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünert U, Jusuf PR, Lee SCS, Nguyen DT. Bipolar input to melanopsin containing ganglion cells in primate retina. Vis Neurosci. 2011;28:39–50. doi: 10.1017/S095252381000026X. &. [DOI] [PubMed] [Google Scholar]
- Hack I, Peichl L, Brandstätter JH. An alternative pathway for rod signals in the rodent retina: rod photoreceptors, cone bipolar cells, and the localization of glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14130–14135. doi: 10.1073/pnas.96.24.14130. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Müller U, Harvey K, Harvey RJ, Betz H, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the α3 subunit. J Comp Neurol. 2003;465:524–539. doi: 10.1002/cne.10852. &. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Specht D, Majumdar S, Zaidi NF, Brandstätter JH, Wasco W, Wässle H, tom Dieck S. Type 4 OFF cone bipolar cells of the mouse retina express calsenilin and contact cones as well as rods. J Comp Neurol. 2008;507:1087–1101. doi: 10.1002/cne.21612. &. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, Denk W. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature. 2013;500:168–174. doi: 10.1038/nature12346. &. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA, Barres BA. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59:425–438. doi: 10.1016/j.neuron.2008.07.018. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–334. doi: 10.1016/j.neuron.2009.04.014. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby RA, Wiechmann AF, Amara SG, Leighton BH, Marshak DW. Diffuse bipolar cells provide input to OFF parasol ganglion cells in the macaque retina. J Comp Neurol. 2000;416:6–18. doi: 10.1002/(sici)1096-9861(20000103)416:1<6::aid-cne2>3.0.co;2-x. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, De la Huerta I, Kim I-J, Zhang Y, Yamagata M, Chu MW, Meister M, Sanes JR. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci. 2011;31:7753–7762. doi: 10.1523/JNEUROSCI.0907-11.2011. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke J-B, Wang YV, Borghuis BG, Cembrowski MS, Riecke H, Kath WL, Demb JB, Singer JH. Adaptation to background light enables contrast coding at rod bipolar cell synapses. Neuron. 2014;81:388–401. doi: 10.1016/j.neuron.2013.10.054. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I-J, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci. 2010;30:1452–1462. doi: 10.1523/JNEUROSCI.4779-09.2010. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I-J, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. &. [DOI] [PubMed] [Google Scholar]
- Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979;8:295–329. doi: 10.1007/BF01236124. [DOI] [PubMed] [Google Scholar]
- Kolb H, Marshak D. The midget pathways of the primate retina. Doc Ophthalmol. 2003;106:67–81. doi: 10.1023/a:1022469002511. &. [DOI] [PubMed] [Google Scholar]
- Kong J-H, Fish DR, Rockhill RL, Masland RH. Diversity of ganglion cells in the mouse retina: unsupervised morphological classification and its limits. J Comp Neurol. 2005;489:293–310. doi: 10.1002/cne.20631. &. [DOI] [PubMed] [Google Scholar]
- Lin B, Masland RH. Synaptic contacts between an identified type of ON cone bipolar cell and ganglion cells in the mouse retina. Eur J Neurosci. 2005;21:1257–1270. doi: 10.1111/j.1460-9568.2005.03967.x. &. [DOI] [PubMed] [Google Scholar]
- McGuire BA, Stevens JK, Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984;4:2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res. 2014;39:58–76. doi: 10.1016/j.preteyeres.2013.12.001. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, Detwiler PB. Different mechanisms generate maintained activity in ON and OFF retinal ganglion cells. J Neurosci. 2007;27:5994–6005. doi: 10.1523/JNEUROSCI.0130-07.2007. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–280. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataruga A, Kremmer E, Müller F. Type 3a and type 3b OFF cone bipolar cells provide for the alternative rod pathway in the mouse retina. J Comp Neurol. 2007;502:1123–1137. doi: 10.1002/cne.21367. &. [DOI] [PubMed] [Google Scholar]
- Mazade RE, Eggers ED. Light adaptation alters the source of inhibition to the mouse retinal OFF pathway. J Neurophysiol. 2013;110:2113–2128. doi: 10.1152/jn.00384.2013. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, Soto F, Wong ROL, Kerschensteiner D. Development of cell type-specific connectivity patterns of converging excitatory axons in the retina. Neuron. 2011;71:1014–1021. doi: 10.1016/j.neuron.2011.08.025. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci. 2009;12:1308–1316. doi: 10.1038/nn.2389. &. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Electrical synaptic input to ganglion cells underlies differences in the output and absolute sensitivity of parallel retinal circuits. J Neurosci. 2011;31:12218–12228. doi: 10.1523/JNEUROSCI.3241-11.2011. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olveczky BP, Baccus SA, Meister M. Retinal adaptation to object motion. Neuron. 2007;56:689–700. doi: 10.1016/j.neuron.2007.09.030. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard J, Hannibal J, Fahrenkrug J. Synaptic contact between melanopsin-containing retinal ganglion cells and rod bipolar cells. Invest Ophthalmol Vis Sci. 2007;48:3812–3820. doi: 10.1167/iovs.06-1322. &. [DOI] [PubMed] [Google Scholar]
- Pang J-J, Gao F, Lem J, Bramblett DE, Paul DL, Wu SM. Direct rod input to cone BCs and direct cone input to rod BCs challenge the traditional view of mammalian BC circuitry. Proc Natl Acad Sci U S A. 2010;107:395–400. doi: 10.1073/pnas.0907178107. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJH, Kim I-J, Looger LL, Demb JB, Borghuis BG. Excitatory synaptic inputs to mouse on-off direction-selective retinal ganglion cells lack direction tuning. J Neurosci. 2014;34:3976–3981. doi: 10.1523/JNEUROSCI.5017-13.2014. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Zhou K, Wei W, Elstrott J, Nguyen PL, Barres BA, Huberman AD, Feller MB. Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. J Neurosci. 2011;31:8760–8769. doi: 10.1523/JNEUROSCI.0564-11.2011. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoè-Pognetto M, Wassle H, Grünert U. Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the α1 subunit of the glycine receptor. J Neurosci. 1994;14:5131–5146. doi: 10.1523/JNEUROSCI.14-08-05131.1994. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Chen S-K, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011a;34:572–580. doi: 10.1016/j.tins.2011.07.001. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Do MTH, Dacey D, Lucas R, Hattar S, Matynia A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci. 2011b;31:16094–16101. doi: 10.1523/JNEUROSCI.4132-11.2011. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J Neurosci. 2010;30:16262–16271. doi: 10.1523/JNEUROSCI.3656-10.2010. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Structure and function of bistratified intrinsically photosensitive retinal ganglion cells in the mouse. J Comp Neurol. 2011;519:1492–1504. doi: 10.1002/cne.22579. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J Neurophysiol. 2008;100:371–384. doi: 10.1152/jn.00062.2008. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GW, Okawa H, Dunn FA, Morgan JL, Kerschensteiner D, Wong RO, Rieke F. The spatial structure of a nonlinear receptive field. Nat Neurosci. 2012;15:1572–1580. doi: 10.1038/nn.3225. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. &. [DOI] [PubMed] [Google Scholar]
- Sterling P, Freed MA, Smith RG. Architecture of rod and cone circuits to the on-beta ganglion cell. J Neurosci. 1988;8:623–642. doi: 10.1523/JNEUROSCI.08-02-00623.1988. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sümbül U, Song S, McCulloch K, Becker M, Lin B, Sanes JR, Masland RH, Seung HS. A genetic and computational approach to structurally classify neuronal types. Nat Commun. 2014;5:3512. doi: 10.1038/ncomms4512. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002;451:115–126. doi: 10.1002/cne.10323. &. [DOI] [PubMed] [Google Scholar]
- Tagawa Y, Sawai H, Ueda Y, Tauchi M, Nakanishi S. Immunohistological studies of metabotropic glutamate receptor subtype 6-deficient mice show no abnormality of retinal cell organization and ganglion cell maturation. J Neurosci. 1999;19:2568–2579. doi: 10.1523/JNEUROSCI.19-07-02568.1999. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, Johnson K, Li X, Smith RG, Awatramani GB. Parallel mechanisms encode direction in the retina. Neuron. 2011;71:683–694. doi: 10.1016/j.neuron.2011.06.020. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ishii M, Takao M, Iwatsuki K, Nakanishi S, Fukuda Y. A novel connection between rods and ON cone bipolar cells revealed by ectopic metabotropic glutamate receptor 7 (mGluR7) in mGluR6-deficient mouse retinas. J Neurosci. 2007;27:6261–6267. doi: 10.1523/JNEUROSCI.5646-06.2007. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk M, Wässle H, Taylor WR. Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Vis Neurosci. 2009;26:297–308. doi: 10.1017/S0952523809990137. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci. 2002;22:10558–10566. doi: 10.1523/JNEUROSCI.22-24-10558.2002. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol. 2009;512:664–687. doi: 10.1002/cne.21912. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi B, Deans MR, Paul DL, Bloomfield SA. Convergence and segregation of the multiple rod pathways in mammalian retina. J Neurosci. 2009;24:11182–11192. doi: 10.1523/JNEUROSCI.3096-04.2004. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi B, Kovács-Oller T, Atlasz T, Wilhelm M, Gábriel R. Gap junctional coupling in the vertebrate retina: variations on one theme. Prog Retin Eye Res. 2013;34:1–18. doi: 10.1016/j.preteyeres.2012.12.002. &. [DOI] [PubMed] [Google Scholar]
- Wang YV, Weick M, Demb JB. Spectral and temporal sensitivity of cone-mediated responses in mouse retinal ganglion cells. J Neurosci. 2011;31:7670–7681. doi: 10.1523/JNEUROSCI.0629-11.2011. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Feller MB. Organization and development of direction-selective circuits in the retina. Trends Neurosci. 2011;34:638–645. doi: 10.1016/j.tins.2011.08.002. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Hamby AM, Zhou K, Feller MB. Development of asymmetric inhibition underlying direction selectivity in the retina. Nature. 2011;469:402–406. doi: 10.1038/nature09600. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara K, Shintani T, Suzuki R, Sakuta H, Takeuchi Y, Nakamura-Yonehara K, Noda M. Expression of SPIG1 reveals development of a retinal ganglion cell subtype projecting to the medial terminal nucleus in the mouse. PLoS One. 2008;3:e1533. doi: 10.1371/journal.pone.0001533. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara K, Farrow K, Ghanem A, Hillier D, Balint K, Teixeira M, Jüttner J, Noda M, Neve RL, Conzelmann K-K, Roska B. The First Stage of Cardinal Direction Selectivity Is Localized to the Dendrites of Retinal Ganglion Cells. Neuron. 2013 doi: 10.1016/j.neuron.2013.08.005. &; DOI: 10.1016/j.neuron.2013.08.005. [DOI] [PubMed] [Google Scholar]


