Abstract
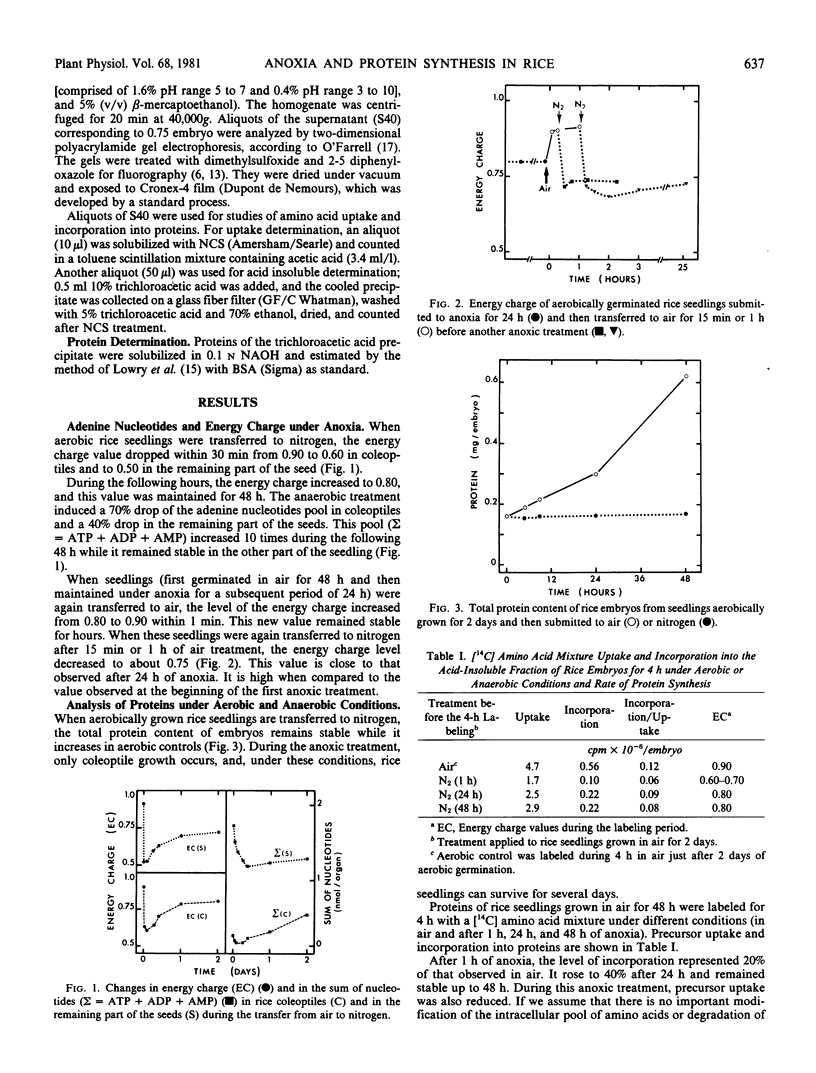
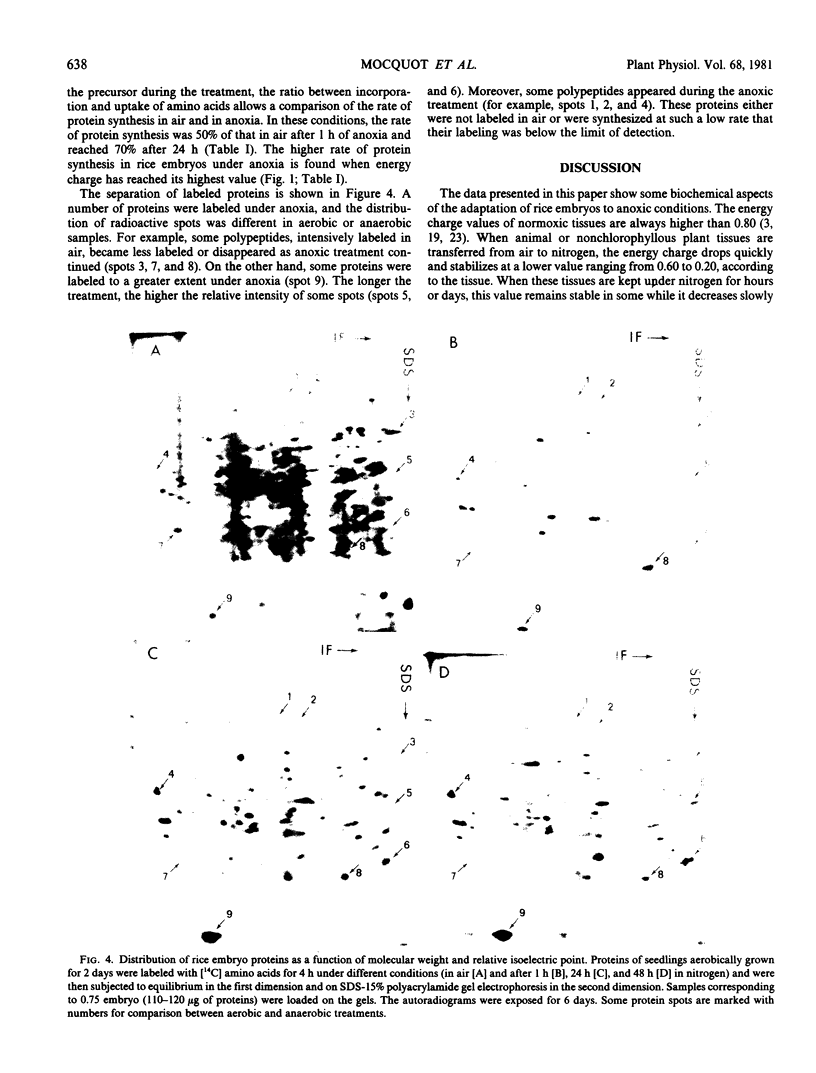
Energy charge, adenine nucleotide levels, and protein synthesis were studied during the transfer of rice seedlings from air to anoxia. Within minutes, the energy charge value dropped from 0.90 in air to 0.50 in the seed and 0.60 in the coleoptile after the transfer to a nitrogen atmosphere, and then increased to a value of 0.80 during the subsequent hours. The sum of nucleotides also dropped to 60% of the value in air in the seeds and to 30% in the coleoptiles. However, during the anaerobic growth of coleoptiles, a considerable increase in the nucleotide pool occurred.
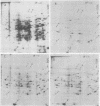
The incorporation of amino acids into proteins was measured at different stages in anoxic treatment. In rice embryo, we observed a considerable protein synthesis correlated with a high value of energy charge under anoxia. The analysis of labeled proteins by two-dimensional polyacrylamide gel electrophoresis showed a modified pattern of polypeptides synthesized during anoxic treatment. Some of these proteins were intensively labeled and appeared to be induced by anaerobic treatment.
Our data indicate that high metabolic activity occurs in rice embryo under anoxia, which can be correlated with a high energy charge value. These phenomena may be part of the mechanisms which permit the adaptation of rice embryos to anaerobiosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akkerman J. W., Gorter G. Relation between energy production and adenine nucleotide metabolism in human blood platelets. Biochim Biophys Acta. 1980 Mar 7;590(1):107–116. doi: 10.1016/0005-2728(80)90150-4. [DOI] [PubMed] [Google Scholar]
- Ball W. J., Jr, Atkinson D. E. Adenylate energy charge in Saccharomyces cerevisiae during starvation. J Bacteriol. 1975 Mar;121(3):975–982. doi: 10.1128/jb.121.3.975-982.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley J. D., Gwódź E. A. Plant Desiccation and Protein Synthesis: II. On the Relationship between Endogenous Adenosine Triphosphate Levels and Protein-synthesizing Capacity. Plant Physiol. 1975 Jun;55(6):1110–1114. doi: 10.1104/pp.55.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Freudenberg H., Mager J. Studies on the mechanism of the inhibition of protein synthesis induced by intracellular ATP depletion. Biochim Biophys Acta. 1971 Mar 25;232(3):537–555. doi: 10.1016/0005-2787(71)90608-3. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W. Design of metabolic and enzymic machinery to fit lifestyle and environment. Biochem Soc Symp. 1976;(41):3–31. [PubMed] [Google Scholar]
- Jefferson L. S., Wolpert E. B., Giger K. E., Morgan H. E. Regulation of protein synthesis in heart muscle. 3. Effect of anoxia on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2171–2178. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Key J. L. Dissocation and reassembly of polyribosomes in relation to protein synthesis in the soybean root. J Mol Biol. 1967 Jun 14;26(2):237–247. doi: 10.1016/0022-2836(67)90294-x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., De Michelis M. I. Correlation between Oxygen Availability, Energy Charge, and Protein Synthesis in Squash Cotyledons Isolated from Germinating Seeds. Plant Physiol. 1978 Jan;61(1):85–88. doi: 10.1104/pp.61.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond P., Pradet A. Stabilization of adenine nucleotide ratios at various values by an oxygen limitation of respiration in germinating lettuce (Lactuca sativa) seeds. Biochem J. 1980 Jul 15;190(1):39–44. doi: 10.1042/bj1900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Saglio P. H., Raymond P., Pradet A. Metabolic Activity and Energy Charge of Excised Maize Root Tips under Anoxia: CONTROL BY SOLUBLE SUGARS. Plant Physiol. 1980 Dec;66(6):1053–1057. doi: 10.1104/pp.66.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Marcus A. Oxygen availability as a control factor in the density-dependent regulation of protein synthesis in cell culture. J Cell Sci. 1974 Mar;14(2):331–337. doi: 10.1242/jcs.14.2.331. [DOI] [PubMed] [Google Scholar]