Abstract
In mammals, cellular swelling activates release of small organic osmolytes, including the excitatory amino acids (EAA) glutamate and aspartate, via a ubiquitously expressed volume-regulated chloride/anion channel (VRAC). Pharmacological evidence suggests that VRAC plays plural physiological and pathological roles, including excitotoxic release of glutamate in stroke. However, the molecular identity of this pathway was unknown. Two recent studies discovered that LRRC8 gene family members encode heteromeric VRAC composed of LRRC8A plus LRRC8B-E, which mediate swelling-activated Cl− currents and taurine release in human non-neural cells (Z. Qiu et al. Cell 157: 447, 2014; F.K. Voss et al. Science 344: 634, 2014). Here, we tested the contribution of LRRC8A to the EAA release in brain glia. We detected and quantified expression levels of LRRC8A-E in primary rat astrocytes with quantitative RT-PCR and then downregulated LRRC8A with gene-specific siRNAs. In astrocytes exposed to hypo-osmotic media, LRRC8A knockdown dramatically reduced swelling-activated release of the EAA tracer d-[3H]aspartate. In parallel HPLC assays, LRRC8A siRNA prevented hypo-osmotic media-induced loss of the endogenous intracellular l-glutamate and taurine. Furthermore, downregulation of LRRC8A completely ablated the ATP-stimulated release of d-[3H]aspartate and [14C]taurine from non-swollen astrocytes. Overall, these data indicate that LRRC8A is an indispensable component of a permeability pathway that mediates both swelling-activated and agonist-induced amino acid release in brain glial cells.
Introduction
Animal cells endure constant changes in their volume due to alterations in osmolarity of the extracellular and intracellular milieu, or changes in the transmembrane transport of osmotically active molecules (osmolytes). The majority of cell types, including those in the CNS, homeostatically regulate their volume by releasing or accumulating osmolytes via plasmalemmal channels and transporters (reviewed in Lang et al. 1998; Hoffmann et al. 2009). In response to swelling, cell volume is typically adjusted with a net loss of K+ ions via K+ channels and simultaneous release of negatively charged molecules through the ubiquitous volume-regulated anion channel (VRAC) (Lang et al. 1998; Hoffmann et al. 2009). In addition to conducting Cl− and other inorganic anions, VRAC is permeable to small organic molecules, including the excitatory amino acids (EAA), glutamate and aspartate, but also taurine, inositol and others (Kimelberg et al. 1990; Banderali & Roy, 1992; Manolopoulos et al. 1997).
VRAC, also known as volume-sensitive organic osmolyte–anion channel or volume-sensitive outwardly rectifying Cl− channel can be readily detected in virtually every vertebrate cell type (reviewed in Strange et al. 1996; Nilius et al. 1997; Okada, 1997). Yet, the molecular identity of this channel remained an enigma for nearly three decades. Recently, two studies have identified the leucine-rich repeat containing protein 8A (LRRC8A) as an essential component of VRAC, and four other members of the same family (LRRC8B-E) as complementary VRAC subunits in non-neural cells (Qiu et al. 2014; Voss et al. 2014).
Besides its fundamental role in cell volume regulation, VRAC is thought to be involved in numerous other physiological processes, including proliferation, apoptosis, cell migration and release of biologically active molecules (Lang et al. 1998; Okada et al. 2001; Hoffmann et al. 2009). In the CNS, VRAC activity is probably important for systemic osmosensing and for intercellular signalling via release of physiologically active compounds (Deleuze et al. 1998; Mongin & Kimelberg, 2002). Moreover, this channel is thought to play a major pathological role in brain disorders that are associated with cellular swelling (reviewed in Mongin & Kimelberg, 2005a). In stroke, hyponatraemia and epilepsy, VRAC is hypothesized to mediate release of EAA from swollen astrocytes and in this way contribute to the excitotoxic damage to neurons. In rodent models of stroke, the VRAC blockers tamoxifen and 4-[(2-butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl)oxy]butanoic acid (DCPIB), reduce ischaemic brain damage by as much as 80% (Kimelberg et al. 2000; Zhang et al. 2008).
Although pharmacological evidence for VRAC involvement in stroke pathogenesis is thought to be fairly conclusive, recent studies established that VRAC blockers also interfere with other pathologically relevant glutamate transporters (Ye et al. 2009; Bowens et al. 2013). Furthermore, several previous studies suggested that swelling-activated release of organic molecules, including EAA, may be mediated by a separate pathway (Lambert & Hoffmann, 1994; Mongin et al. 1999; Stutzin et al. 1999). The discovery that VRAC is comprised of LRRC8 gene family members in non-CNS cells opened up an opportunity to test whether the same proteins are also responsible for EAA release in the CNS.
Methods
Ethics statement
All animal procedures in this study were approved by the Institutional Animal Care and Use Committee of the Albany Medical College, and conformed to the NIH Guidelines for Care and Use of Laboratory Animals.
Astrocyte culture
Primary astrocytes were prepared from cortices of neonatal Sprague–Dawley rats as recently described (Hyzinski-Garcia et al. 2011). Briefly, postnatal day 0–1 pups were killed by rapid decapitation. Aseptically isolated cortical tissue was dissociated with recombinant protease TrypLE. The resulting cell suspension was plated at low density into T75 flasks and grown to confluency in minimal essential medium containing 10% heat inactivated horse serum and antibiotics (all from Life Technologies/Invitrogen, Carlsbad, CA, USA). The purity of astrocyte cultures was ≥98% as routinely checked with the anti-GFAP antibody (St. Louis, MO, USA, Sigma-Aldrich).
Gene expression analysis
Expression levels of the LRRC8 family members were measured by quantitative RT-PCR. mRNA specimens were isolated from cells grown on 60 mm dishes using RNAqeous-4PCR kit (Life Technologies/Ambion, Austin, TX, USA) and converted to cDNA with iScript cDNA synthesis kit (BioRad, Hercules, CA, USA). The expression levels were determined by quantitative PCR using a CFX96 Real Time PCR setup (BioRad) and quantitative primers from Qiagen (Valencia, CA, USA), which are listed in Table1. The expression levels were normalized within each sample to the transcripts of three housekeeping genes.
Table 1.
List of qPCR primers and siRNA constructs
| Target | qPCR | siRNAs name/cat. no. |
|---|---|---|
| gene | primer cat. no. | (manufacturer) |
| LRRC8A | QT01575483 | siLRRC8A_1/SI01725339 (Q) |
| QT00390978 | siLRRC8A_3/SI01725353 (Q) | |
| siLRRC8A_4/SI01725360 (Q) | ||
| siLRRC8A_mix/J090547–02–0005 (GE) | ||
| LRRC8B | QT00434805 | N/A |
| LRRC8C | QT01583897 | N/A |
| LRRC8D | QT00370111 | N/A |
| LRRC8E | QT01591352 | N/A |
| GAPDH | QT00199633 | N/A |
| RPL13a | QT00178675 | N/A |
| RPS20 | QT00431333 | N/A |
Abbreviation: N/A, not applicable. All primers for qPCR were from Qiagen. siRNA constructs were from either Qiagen (Q) or GE Health Care/Dharmacon (GE).
siRNA transfections
Gene expression knockdowns were performed using commercially available gene-specific siRNA constructs listed in Table1. Cells grown to ∼50–70% confluency were transfected with one or several siRNAs and Lipofectamine® RNAiMAX reagent as previously optimized for primary astrocytes (Bowens et al. 2013). As a negative control we used Alexa-488-labelled Allstars scrambled siRNA from Qiagen. After 4–8 h of incubation with siRNA/Lipofectamine complexes, transfection media were replaced with fresh culture media. This transfection technique produces negligible cell toxicity. Changes in the mRNA expression levels were quantified at 48 h post-transfection, and all functional assays were performed at 72 h post-transfection. Based on the Alexa-488 fluorescence analysis in negative controls, the efficacy of transfections routinely reached 95%.
Amino acid release assays
Glutamate transport was traced using a non-metabolizable glutamate analogue, d-[3H]aspartate, and [14C]taurine, as previously described (Hyzinski-Garcia et al. 2011; Bowens et al. 2013). Cells grown on either 18×18 mm coverslips or in 12-well plates were preloaded in serum-containing medium with radiolabelled amino acids overnight. For the majority of assays, we used a Lucite perfusion chamber, in which cells were superfused with Hepes-buffered media at a rate of 1.2 ml min−1. Basal iso-osmotic medium contained (in mm): 135 NaCl, 3.8 KCl, 1.2 MgSO4, 1.3 CaCl2, 1.2, KH2PO4, 10 d-glucose, 10 Hepes (pH 7.4, osmolarity ≈290 mosmol l–1). To activate the VRAC pathway, cells were exposed to hypo-osmotic medium, in which [NaCl] was reduced by 50 mm, while all other components remained the same (osmolarity ≈200 mosmol l–1). One minute superfusate fractions were collected and analysed for the presence of released d-[3H]aspartate and [14C]taurine in a liquid scintillation counter TriCarb 2900TR (Perkin Elmer, Waltham, MA, USA). To increase the throughput, some of the experiments were done in a multiwell plate format, in which media in individual wells were changed once every 10 min and the integral d-[3H]aspartate release was compared between basal iso-osmotic conditions and during exposure to hypo-osmotic medium.
HPLC analysis of intracellular amino acid content
Endogenous amino acid contents were determined using a reverse-phase HPLC as described in detail elsewhere (Hyzinski-Garcia et al. 2011). Briefly, cells were lysed in medium containing 5 mm Hepes and 1 mm EDTA (pH 7.4), and amino acid levels were quantified after derivatization with o-phthalaldehyde and 2-mercaptoethanol using an Agilent 1200 HPLC setup (Agilent Technologies, Santa Clara, CA, USA) and Eclipse XBD-C18 column. Calculated concentrations were further normalized to protein content in each individual well.
Statistical analysis
All data are presented as means ± s.e.m. Statistical significance of differences between experimental groups was determined using one-way or two-way ANOVA, as appropriate, and post hoc corrections for multiple comparisons. When several experimental groups were normalized to controls, we used Student's one-sample t test with Bonferroni correction for multiple comparisons. Origin 8.1 (OriginLab, Northampton, MA, USA) and Prism 5.0 (GraphPad Software, San Diego, CA, USA) were used in all statistical analyses.
Results
LRRC8 family expression levels in primary rat astrocytes
We first quantified mRNA expression levels for all five members of the LRRC8 family. The expression was normalized within each sample to three housekeeping genes, GAPDH, RPL13a and RPS20, with highly reproducible results regardless of using one or several genes for normalization. As shown in Fig.1, mRNA levels for LRRC8A, which forms the indispensable VRAC subunit in human non-CNS cells (Qiu et al. 2014), were ∼0.2% of the levels of the highly abundant glycolytic enzyme GAPDH. Three other LRRC8 species, LRRC8B, LRRC8C and LRRC8D showed approximately 2-fold higher abundance as compared to LRRC8A, while LRRC8E levels were ∼10-fold lower. There was very little variation in the mRNA expression levels between three independently tested astrocyte cultures.
Figure 1. mRNA expression levels for five members of the LRRC8 protein family in primary rat astrocytes.
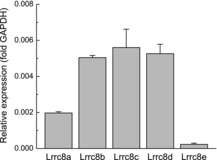
mRNA expression levels for LRRC8A-E were detected using quantitative RT-PCR and normalized to GAPDH levels within the same samples. Similar normalization was performed for RPL13a and RPS20 (not shown). Data are the means ± s.e.m. for three independently prepared astrocytic cultures.
LRRC8A siRNA knockdown suppresses hypo-osmotic medium-induced amino acid release and preserves intracellular amino acid content
We next knocked down astrocytic LRRC8A using several siRNA constructs. As seen in Fig.2A, the most effective downregulation (≥80%) was seen in cells treated with either the LRRC8A siRNA mix (siLRRC8A_mix; GE Health Care/Dharmacon, Lafayette, CO, USA), or the siRNA construct siLRRC8A_4. The subsequent screening in a multiwell format established that siLRRC8A_4 and siLRRC8A_mix reduced swelling-activated d-[3H]aspartate release by 65% (P < 0.001) and 73% (P < 0.001), respectively (Fig.2B). Consistent with the mRNA expression data, siLRRC8A_1 and siLRRC8A_3 also caused reductions in the amino acid efflux, but with an intermediate efficacy (Fig.2B). We then looked at the kinetics of EAA release in the siLRRC8A_mix-treated cells. As seen in Fig.3A, the LRRC8A protein knockdown decreased the maximum rate of d-[3H]aspartate efflux under hypo-osmotic condition by 78% (P < 0.001) as compared to negative control siRNA, and the integral 10 min release values by 68% (P = 0.002). To assure that hypo-osmotic medium stimulated EAA release was due to changes in medium osmolarity (cell swelling) rather than reduction in the extracellular [NaCl], we performed additional controls in which NaCl was replaced with equiosmolar mannitol. In these latter experiments no changes in d-[3H]aspartate release were found as compared to iso-osmotic baseline (data not shown, n = 4, P < 0.001 hypo-osmotic vs. low [NaCl] medium). Interestingly, in cells treated with siLRRC8A_mix we additionally found significant decrease in the basal EAA release rates (P = 0.03; Fig.3A), suggesting that VRAC is at least partially responsible for amino acid ‘leak’ from non-swollen cells.
Figure 2. Effect of LRRC8A knockdown on astrocytic mRNA expression levels and swelling-activated glutamate (d-[3H]aspartate) release.
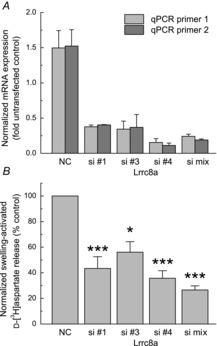
A, quantification of the LRRC8A mRNA expression levels in cells treated with three individual LRRC8A siRNAs (Qiagen), or the LRRC8A siRNA mix (GE Health Care/Dharmacon). Data are the means ± s.e.m. of six independent qRT-PCR assays in two independently prepared astrocyte cultures. Expression levels were normalized to mRNA levels in non-transfected cells in the same experiment. B, multiwell assays of the swelling-activated d-[3H]aspartate release from cells treated with LRRC8A siRNAs or the siRNA mix. Data are the means ± s.e.m. of six independent measurements in two astrocyte cultures. *P < 0.05, ***P < 0.001 vs. NC siRNA-treated cells (Student's one population t test after Bonferroni correction). NC, negative control.
Figure 3. Effect of LRRC8A knockdown on kinetics of glutamate (d-[3H]aspartate) release and the intracellular levels of l-glutamate and taurine in astrocyte exposed to hypo-osmotic medium.
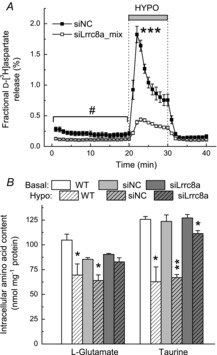
A, kinetics of preloaded d-[3H]aspartate release from primary rat astrocytes exposed to 30% reduction in medium osmolarity measured 72 h after treatment with either siNC or siLRRC8A_mix. Data are the means ± s.e.m. of five to six independent experiments performed in two different cell cultures. ***P < 0.001, maximal release rates in siNC vs. siLRRC8A_mix (Student's paired t test). #P < 0.05, basal release values in siNC vs. siLRRC8A_mix (two-way ANOVA). B, HPLC analysis of the endogenous l-glutamate and taurine levels in untransfected cells (WT) and cells treated with siNC, or the siLRRC8A_mix. Data are the means ± s.e.m. for three different cell cultures. *P < 0.05; **P < 0.01, hypo-osmotic vs. iso-osmotic conditions for the same treatment (Student's paired t test). hypo, hypo-osmotic; siNC, negative control siRNA; WT, wild-type.
To validate and extend these results using an independent approach, we also measured the effect of RNAi on intracellular content of endogenous amino acids using an HPLC analysis. For brevity, we present the data for two major cytosolic osmolytes, l-glutamate and taurine, only. In untransfected cells, exposure to hypo-osmotic medium reduced the intracellular taurine content by 50% (Fig.3B; P = 0.015), and very similar 46% reduction was found in cells transfected with the negative control siRNA (Fig.3B; P = 0.002). These results resemble previously published findings (Hyzinski-Garcia et al. 2011 and references therein). LRRC8A knockdown largely prevented this effect: only a small 12% reduction in taurine content was found in swollen cells (P = 0.035; Fig.3B). In a very similar fashion, the LRRC8A knockdown prevented loss of the endogenous l-glutamate from the hypo-osmotically swollen cells (Fig.3B). It is important to note that decreases in the intracellular amino acid content are determined exclusively by changes in medium osmolarity (cellular swelling). In our previous study (Hyzinski-Garcia et al. 2011) iso-osmotic replacement of NaCl with mannitol produced no effect on endogenous taurine and l-glutamate levels.
LRRC8A is critical for the agonist-stimulated excitatory amino acid release
There is a significant interest in the agonist-induced astrocytic glutamate release in non-swollen cells due to its proposed role in astrocyte-to-neuron communication (reviewed in Haydon & Carmignoto, 2006). Therefore, we also tested if the LRRC8A function is important for the ATP-stimulated amino acid release in cultured glia. To this end, we exposed astrocytes to 50 μm of ATP, and measured the release of the glutamate analogue d-[3H]aspartate, and simultaneously the release of another major osmolyte [14C]taurine. Numerous previous studies demonstrated that purinergic signalling transiently stimulates release of several small organic osmolytes, including glutamate, aspartate and taurine, in non-swollen glial cells and in cells subjected to physiological levels of swelling (see for example Mongin & Kimelberg, 2002; Cheema et al. 2005). In cells transfected with the negative control siRNA, ATP triggered a transient ∼2.5-fold increase in the release of d-[3H]aspartate (Fig.4A) and the same phenomenon was observed for the concomitant [14C]taurine efflux (Fig.4B). Previous studies established that the effect of ATP is mediated by activation of metabotropic P2Y1 receptor with additional contribution of the UTP/UDP-sensitive P2Y2/4/6 (Mongin & Kimelberg, 2002). Importantly, transfection with the siLRRC8A_4 completely abolished the ATP-induced efflux of both amino acids (P = 0.002 and P < 0.001 for d-[3H]aspartate and [14C]taurine, respectively), and showed a trend for reduction in their basal release levels (P = 0.09 and P = 0.02 for d-[3H]aspartate and [14C]taurine, respectively).
Figure 4. Effect of LRRC8A knockdown on the ATP induced of d-[3H]aspartate and [14C]taurine release from astrocytes.
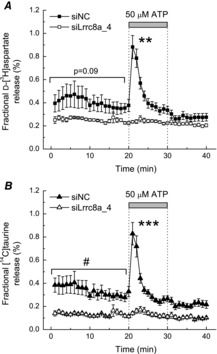
A, primary rat astrocytes were loaded with radiolabelled d-aspartate and taurine, and simultaneous d-[3H]aspartate (A) and [14C]taurine (B) efflux rates were measured before and after addition of 50 μm ATP in cells treated with either siNC, or the LRRC8A_4 siRNA (siLRRC8A_4). Data are the means ± s.e.m. for five experiments performed in two different cell cultures. ***P < 0.01, siNC vs. siLRRC8A_4 under hypo-osmotic conditions; #P < 0.05, siNC vs. siLRRC8A_4 during the first 20 min of exposure to basal medium (two-way ANOVA). siNC, negative control siRNA.
Discussion
The critical finding of this work is that LRRC8A is an essential component of VRAC in the CNS, and plays an indispensable role in swelling-activated and the agonist-induced EAA release from rat glial cells. This discovery has strong physiological and pathological implications for the CNS as outlined below.
The idea that VRAC plays an indispensable role in swelling-activated release of organic osmolytes in intact cells and in situ has a long history, but it has never been decisively tested because of unknown molecular identity of VRAC channel. This concept was developed based on strong pharmacological similarity between VRAC currents recorded in electrophysiological experiments, radiotracer release of 36Cl− and 125I−, and efflux of [3H]-labelled small organic osmolytes, including EAA, across several cell types (Jackson & Strange, 1993; Manolopoulos et al. 1997; Abdullaev et al. 2006; reviewed in Strange et al. 1996; Nilius et al. 1997). Additionally, several laboratories directly demonstrated electrophysiological VRAC currents carried by intracellular l-aspartate, l-glutamate or taurine (Banderali & Roy, 1992; Jackson & Strange, 1993; Manolopoulos et al. 1997). Nevertheless, the picture remained far from clear, because other studies identified substantial differences in either pharmacology or regulation of the swelling-activated transmembrane transport of inorganic anions, d-[3H]aspartate, and [3H]taurine (Lambert & Hoffmann, 1994; Mongin et al. 1999; Stutzin et al. 1999). This led to the hypothesis that multiple swelling-activated pathways for inorganic anions and organic molecules exist in mammalian cells (reviewed in Hoffmann et al. 2009). Our study resolves this controversy and provides proof that, at least in primary glial cells, the LRRC8A protein is an indispensable component of the pathway for swelling-activated EAA release. This is consistent with the findings of two recent studies that uncovered the obligatory role for the LRRC8A for swelling-activated VRAC Cl− currents and taurine release in non-CNS cells (Qiu et al. 2014; Voss et al. 2014).
In the CNS, VRAC is frequently discussed in the context of brain pathology because of the early discovery that swelling of primary astrocytes triggers substantial release of the excitotoxic glutamate via a VRAC-like pathway (Kimelberg et al. 1990). This latter finding resonated with numerous independent in vivo observations of astrocytic swelling in traumatic brain injury, stroke, hyponatraemia and other pathologies (reviewed in Mongin & Kimelberg, 2005a). Systemic application of the poorly selective VRAC blocker, tamoxifen or intracerebroventricular injection of the more selective inhibitor, DCPIB, dramatically reduces brain damage in rodent models of stroke (Kimelberg et al. 2000; Zhang et al. 2008). Furthermore, several groups found that structurally unrelated VRAC blockers, including DCPIB, strongly diminish pathological EAA release during cerebral ischaemia (Phillis et al. 1997; Feustel et al. 2004; Zhang et al. 2008). Even though the evidence for VRAC involvement in stroke appears to be quite conclusive, there are inherent problems associated with a strictly pharmacological analysis. VRAC blockers poorly discriminate between several transport pathways that have been suggested to contributing to glutamate release in stroke (Malarkey & Parpura, 2008; Ye et al. 2009; Bowens et al. 2013). Our current findings open an opportunity to use the RNAi-based tools and gene knockout animals for directly testing VRAC involvement in stroke and other neural disorders, and for further development of more refined pharmacological agents.
An additional point of interest is the potential contribution of VRAC to the physiological glutamate release in glia. In the past two decades, our views on physiological functions of astrocytes have undergone a dramatic transformation. These cells are capable of modulating activity of individual neurons and neuronal networks via release of glutamate and other physiologically active ‘gliotransmitters’ (see for example Parpura et al. 1994; Newman, 2003, and review of Haydon & Carmignoto, 2006). Such gliotransmitters are commonly assumed to be released via a Ca2+-dependent vesicular mechanism (Haydon & Carmignoto, 2006) but alternative hypotheses have also been proposed, including contribution of VRAC (reviewed in Hamilton & Attwell, 2010). Although VRAC has no direct Ca2+ dependency, the activity of this channel is potently modulated by a variety of GPCR-coupled receptors and downstream signalling cascades involving Ca2+-dependent protein kinases (see for example Mongin & Kimelberg, 2002, 2005b; and review of Fisher et al. 2008). Because of the limited selectivity of pharmacological tools and the unknown molecular identity, the contribution of VRAC to physiological gliotransmitter release has been difficult to corroborate or rule out, with the notable exception of the physiological release of taurine in the hypothalamus (Deleuze et al. 1998). In our study, the LRRC8A knockdown completely eliminated the ATP-induced release of preloaded d-[3H]aspartate and [14C]taurine, indicating the critical involvement of VRAC in the agonist-induced release of EAA and other cytosolic gliotransmitters. This finding opens a door for testing the relative contribution of the LRRC8A and other members of the same family to bidirectional astrocyte–neuron communication using an RNAi approach.
Based on the highly conserved nature of VRAC and strong similarity of VRAC currents among various cell types and in situ, it is possible that composition of astrocytic VRAC in vivo will be similar to that discovered in cultured cells. However, the definitive physiological and pathological contributions of the LRRC8A and other members of this new protein family will have to be further determined in vivo.
Acknowledgments
We thank Dr Mark W. Fleck for very helpful advice on the study and the manuscript, and Steven P. Obrzut for critical reading of the manuscript.
Glossary
- CNS
central nervous system
- DCPIB
4-[(2-butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl)oxy]butanoic acid
- EAA
excitatory amino acids
- LRRC8
leucine-rich repeat containing protein 8
- VRAC
volume-regulated anion channel
Key points
Swelling-activated release of amino acids in the CNS is thought to be mediated by an unidentified volume-regulated anion channel.
Two recent studies discovered that LRRC8 family members form a volume-regulated anion channel in non-neural cells.
In this work we established a critical contribution of the LRRC8A gene product to swelling-activated glutamate and taurine release from primary rat astrocytes.
We also found that LRRC8A is indispensable for glutamate and taurine release from non-swollen astrocytes when they are stimulated with ATP.
These findings suggest that LRRC8A may play a role in physiological release of gliotransmitters, and mediate pathological glutamate release in the CNS disorders associated with cellular swelling.
Additional information
Competing interests
The authors declare no competing financial interests.
Author contributions
Conception and design of experiments: M.C.H-G. and A.A.M. Collection and analysis of data: M.C.H-G., A.R. and A.A.M. Writing the paper: M.C.H-G., A.R. and A.A.M.
Funding
This study was supported by grant from the National Institutes of Health (R01 NS061953) to A.A.M.
References
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl− currents in rat cultured astrocytes. J Physiol. 2006;572:677–689. doi: 10.1113/jphysiol.2005.103820. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banderali U, Roy G. Anion channels for amino-acids in Mdck cells. Am J Physiol Cell Physiol. 1992;263:C1200–C1207. doi: 10.1152/ajpcell.1992.263.6.C1200. &. [DOI] [PubMed] [Google Scholar]
- Bowens NH, Dohare P, Kuo YH, Mongin AA. DCPIB, the proposed selective blocker of volume-regulated anion channels, inhibits several glutamate transport pathways in glial cells. Mol Pharmacol. 2013;83:22–32. doi: 10.1124/mol.112.080457. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema TA, Ward CE, Fisher SK. Subnanomolar concentrations of thrombin enhance the volume-sensitive efflux of taurine from human 1321N1 astrocytoma cells. J Pharmacol Exp Ther. 2005;315:755–763. doi: 10.1124/jpet.105.090787. &. [DOI] [PubMed] [Google Scholar]
- Deleuze C, Duvoid A, Hussy N. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J Physiol. 1998;507:463–471. doi: 10.1111/j.1469-7793.1998.463bt.x. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feustel PJ, Jin Y, Kimelberg HK. Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. Stroke. 2004;35:1164–1168. doi: 10.1161/01.STR.0000124127.57946.a1. &. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Cheema TA, Foster DJ, Heacock AM. Volume-dependent osmolyte efflux from neural tissues: regulation by G-protein-coupled receptors. J Neurochem. 2008;106:1998–2014. doi: 10.1111/j.1471-4159.2008.05510.x. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters. Nat Rev Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. &. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. &. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. &. [DOI] [PubMed] [Google Scholar]
- Hyzinski-Garcia MC, Vincent MY, Haskew-Layton RE, Dohare P, Keller RW, Jr, Mongin AA. Hypoosmotic swelling modifies glutamate-glutamine cycle in the cerebral cortex and in astrocyte cultures. J Neurochem. 2011;118:140–152. doi: 10.1111/j.1471-4159.2011.07289.x. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PS, Strange K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am J Physiol Cell Physiol. 1993;265:C1489–C1500. doi: 10.1152/ajpcell.1993.265.6.C1489. &. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Feustel PJ, Jin Y, Paquette J, Boulos A, Keller RW, Jr, Tranmer BI. Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport. 2000;11:2675–2679. doi: 10.1097/00001756-200008210-00014. &. [DOI] [PubMed] [Google Scholar]
- Lambert IH, Hoffmann EK. Cell swelling activates separate taurine and chloride channels in Ehrlich mouse ascites tumor cells. J Membr Biol. 1994;142:289–298. doi: 10.1007/BF00233436. &. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. &. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52:142–154. doi: 10.1016/j.neuint.2007.06.005. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolopoulos VG, Voets T, Declercq PE, Droogmans G, Nilius B. Swelling-activated efflux of taurine and other organic osmolytes in endothelial cells. Am J Physiol Cell Physiol. 1997;273:C214–C222. doi: 10.1152/ajpcell.1997.273.1.C214. &. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes. Am J Physiol Cell Physiol. 2002;283:C569–C578. doi: 10.1152/ajpcell.00438.2001. &. [DOI] [PubMed] [Google Scholar]
- Mongin AA. Astrocytic swelling in neuropathology. In: Ransom BR, Kimelberg HK, editors; Kettenmann H, editor. Neuroglia. Oxford: Oxford University Press; 2005a. pp. 550–562. & &, eds. [Google Scholar]
- Mongin AA, Kimelberg HK. ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am J Physiol Cell Physiol. 2005b;288:C204–C213. doi: 10.1152/ajpcell.00330.2004. &. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Reddi JM, Charniga C, Kimelberg HK. [3H]Taurine and D-[3H]aspartate release from astrocyte cultures are differently regulated by tyrosine kinases. Am J Physiol Cell Physiol. 1999;276:C1226–C1230. doi: 10.1152/ajpcell.1999.276.5.C1226. &. [DOI] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. &. [DOI] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol Cell Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD) J Physiol. 2001;532:3–16. doi: 10.1111/j.1469-7793.2001.0003g.x. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling [see comments] Nature. 1994;369:744–747. doi: 10.1038/369744a0. &. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Song D, O'Regan MH. Inhibition by anion channel blockers of ischemia-evoked release of excitotoxic and other amino acids from rat cerebral cortex. Brain Res. 1997;758:9–16. doi: 10.1016/s0006-8993(97)00155-8. &. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157:447–458. doi: 10.1016/j.cell.2014.03.024. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol Cell Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. &. [DOI] [PubMed] [Google Scholar]
- Stutzin A, Torres R, Oporto M, Pacheco P, Eguiguren AL, Cid LP, Sepulveda FV. Separate taurine and chloride efflux pathways activated during regulatory volume decrease. Am J Physiol Cell Physiol. 1999;277:C392–C402. doi: 10.1152/ajpcell.1999.277.3.C392. &. [DOI] [PubMed] [Google Scholar]
- Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344:634–638. doi: 10.1126/science.1252826. &. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Oberheim N, Kettenmann H, Ransom BR. Pharmacological ‘cross-inhibition’ of connexin hemichannels and swelling activated anion channels. Glia. 2009;57:258–269. doi: 10.1002/glia.20754. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Feustel PJ, Kimelberg HK. DCPIB, a specific inhibitor of volume regulated anion channels (VRACs), reduces infarct size in MCAo and the release of glutamate in the ischemic cortical penumbra. Exp Neurol. 2008;210:514–520. doi: 10.1016/j.expneurol.2007.11.027. &. [DOI] [PMC free article] [PubMed] [Google Scholar]


