Abstract
We investigated the influence of group III/IV lower limb muscle afferents on the development of supraspinal fatigue and the responsiveness of corticospinal projections to an arm muscle. Eight males performed constant-load leg cycling exercise (80% peak power output) for 30 s (non-fatiguing) and to exhaustion (∼9 min; fatiguing) both under control conditions and with lumbar intrathecal fentanyl impairing feedback from μ-opioid receptor-sensitive lower limb muscle afferents. Voluntary activation (VA) of elbow flexors was assessed via transcranial magnetic stimulation (TMS) during maximum voluntary contraction (MVC) and corticospinal responsiveness was monitored via TMS-evoked potentials (MEPs) during a 25% MVC. Accompanied by a significant 5 ± 1% reduction in VA from pre- to post-exercise, elbow flexor MVC progressively decreased during the fatiguing trial (P < 0.05). By contrast, with attenuated feedback from locomotor muscle afferents, MVC and VA remained unchanged during fatiguing exercise (P > 0.3). MEPs decreased by 36 ± 6% (P < 0.05) from the start of exercise to exhaustion under control conditions, but this reduction was prevented with fentanyl blockade. Furthermore, fentanyl blockade prevented the significant increase in elbow flexor MEP observed from rest to non-fatiguing exercise under control conditions and resulted in a 14% lower corticospinal responsiveness during this short bout (P < 0.05). Taken together, in the absence of locomotor muscle fatigue, group III/IV-mediated leg muscle afferents facilitate responsiveness of the motor pathway to upper limb flexor muscles. By contrast, in the presence of cycling-induced leg fatigue, group III/IV locomotor muscle afferents facilitate supraspinal fatigue in remote muscle not involved in the exercise and disfacilitate, or inhibit, the responsiveness of corticospinal projections to upper limb muscles.
Introduction
Locomotor exercises involving multiple limb muscles evoke alterations in the central nervous system (CNS) which can also affect muscles not directly involved in the task. Although there is no unanimous agreement on the consistency of this phenomenon (Millet et al. 2003; Racinais et al. 2007), these so-called ‘spill-over’ effects from rhythmically active muscle to a non-exercised muscle group have been documented to include decreases in cortical voluntary activation (VA) (Rasmussen et al. 2010). A reduction in cortical VA, which reflects the development of supraspinal fatigue, occurs as the result of a deficit in neural drive from at or above the motor cortical output cell, and is quantified via the twitch interpolation technique using transcranial magnetic stimulation (TMS) (Gandevia et al. 1996; Todd et al. 2003). Although the exact mechanisms of the spill-over effect are not resolved, group III/IV afferent feedback originating in the exercising muscles may be a contributor. This hypothesis is based on studies which found that group III/IV-mediated neural feedback from lower limb muscle during locomotor exercise impairs central motor drive to the muscle group of its origin (Amann et al. 2009, 2011). Although single joint contraction studies involving post-exercise circulatory occlusion (PECO) to maintain afferent discharge after exercise have shown that neural feedback from a fatigued muscle to the CNS may spill over and reduce cortical VA in an unexercised muscle (Kennedy et al. 2013, 2014), the evidence in the context of locomotor exercise remains equivocal to date.
The influence of group III/IV locomotor muscle afferents on the responsiveness of corticospinal projections to a remote muscle is even more elusive. Based on PECO experiments following maximal isometric elbow flexor contractions, neural feedback from the fatigued muscle has been dissociated from changes in the responsiveness of corticospinal projections (Gandevia et al. 1996). By contrast, studies utilizing hypertonic saline infusion in a rested muscle have documented that the responsiveness of corticospinal projections of a muscle is compromised by nociceptive group III/IV muscle afferents (Martin et al. 2008). This controversy is further fuelled by the fact that the type of muscle afferents activated via PECO and hypertonic saline is likely to represent a subset of group III/IV fibres which are functionally and potentially anatomically different from those activated during normal, freely perfused voluntary exercise (Graven-Nielsen et al. 2003; Light et al. 2008; Birdsong et al. 2010; Jankowski et al. 2013).
Taken together, the effects of voluntary, fatiguing locomotor exercise and associated afferent feedback on cortical VA and the corticospinal responsiveness of a remote muscle remain unknown. It was therefore the goal of the current study to investigate the mechanistic link between locomotor exercise and the development of central fatigue and alterations in the corticospinal responsiveness of a remote muscle not directly involved in cycling exercise. Specifically, a novel pharmacological method was utilized whereby lumbar intrathecal fentanyl was administered to partially attenuate feedback from the group III/IV afferents in the exercising muscle of an intact human. It was hypothesized that group III/IV locomotor muscle afferent feedback associated with high-intensity leg cycling exercise to exhaustion causes a deficit in cortical VA and changes in the responsiveness of corticospinal neurons projecting to the non-exercised elbow flexor.
Methods
Subjects
Eight healthy, recreationally active males (maximal O2 consumption: 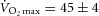 ml; peak power output: Wpeak = 300 ± 12 W), with a mean ± s.d. age of 27 ± 1 years, body mass of 82 ± 5 kg and height of 177 ± 2 cm volunteered to participate in the study. Written informed consent was obtained from each participant. All experimental procedures were approved by the University of Utah and Salt Lake City Veteran Affairs Medical Center Institutional Review Board and conformed to the Declaration of Helsinki.
ml; peak power output: Wpeak = 300 ± 12 W), with a mean ± s.d. age of 27 ± 1 years, body mass of 82 ± 5 kg and height of 177 ± 2 cm volunteered to participate in the study. Written informed consent was obtained from each participant. All experimental procedures were approved by the University of Utah and Salt Lake City Veteran Affairs Medical Center Institutional Review Board and conformed to the Declaration of Helsinki.
Experimental protocol
Subjects participated in a total of five sessions and were thoroughly familiarized with the experimental procedures during a preliminary visit. In this preliminary session, subjects performed a maximal incremental exercise test (20 + 25 W min−1) on a bicycle ergometer (Velotron, Elite Model; RacerMate, Inc., Seattle, WA, USA) to determine Wpeak and 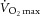 . Subjects were instructed to maintain their preferred pedal frequency, as determined in the preliminary session, throughout the study (80 ± 3 rpm). In a follow-up practice session, subjects sustained a constant-workload (80% Wpeak = 240 ± 9 W) bicycle exercise to task failure, which was defined as a drop in pedal frequency below 80% of individual target cadence for >10 s despite vocal encouragement. Subjects practised performing both the cycling task and elbow flexion of various intensities during the cycling exercise. In a third session, subjects repeated the entire protocol and exercise-induced peripheral locomotor muscle fatigue was assessed by evaluating pre- to post-exercise changes in quadriceps twitch torque. The purpose of this session was to quantify the magnitude of both central and peripheral locomotor muscle fatigue induced by the exhaustive constant-load bike exercise. Mean time from task failure to the first quadriceps fatigue assessment post-exercise was 40 ± 2 s. In two separate study sessions, in a counter-balanced order, subjects sustained the same exercise under either control conditions (CTRL) or experimental conditions during which intrathecal fentanyl (FENT) was applied (Amann et al. 2009). The sustained exercise intensity, as well as the pedal frequency, was kept constant in all sessions. Exercise sessions were separated by at least 48 h (7 ± 2 days) and subjects were instructed to refrain from exercise for 48 h before each trial.
. Subjects were instructed to maintain their preferred pedal frequency, as determined in the preliminary session, throughout the study (80 ± 3 rpm). In a follow-up practice session, subjects sustained a constant-workload (80% Wpeak = 240 ± 9 W) bicycle exercise to task failure, which was defined as a drop in pedal frequency below 80% of individual target cadence for >10 s despite vocal encouragement. Subjects practised performing both the cycling task and elbow flexion of various intensities during the cycling exercise. In a third session, subjects repeated the entire protocol and exercise-induced peripheral locomotor muscle fatigue was assessed by evaluating pre- to post-exercise changes in quadriceps twitch torque. The purpose of this session was to quantify the magnitude of both central and peripheral locomotor muscle fatigue induced by the exhaustive constant-load bike exercise. Mean time from task failure to the first quadriceps fatigue assessment post-exercise was 40 ± 2 s. In two separate study sessions, in a counter-balanced order, subjects sustained the same exercise under either control conditions (CTRL) or experimental conditions during which intrathecal fentanyl (FENT) was applied (Amann et al. 2009). The sustained exercise intensity, as well as the pedal frequency, was kept constant in all sessions. Exercise sessions were separated by at least 48 h (7 ± 2 days) and subjects were instructed to refrain from exercise for 48 h before each trial.
Study sessions CTRL and FENT began with the performance of two elbow flexor maximum voluntary contractions (MVCs) while either resting on the bike or during brief cycling trials at 80% Wpeak. Thereafter, assessment of neuromuscular function of the elbow flexor muscle was carried out while the subject rested on the bike (no-leg exercise), while cycling (‘non-fatiguing exercise’ and ‘fatiguing exercise’) and immediately after fatiguing exercise. In FENT, the same protocol was repeated with intrathecal fentanyl (Fig.1B).
Figure 1. Experimental set-up, schematic of the protocol and raw traces of twitches and evoked potentials from a representative subject.

A, diagram of the experimental set-up. Throughout the study, the transcranial magnetic stimulation (TMS; T) coil was held stable at the optimal position on the motor cortex by an experimenter. Motor nerve stimulations (MNS; M) were elicited at the brachial plexus and responses were measured from the biceps brachii and triceps brachii. An isometric myograph was used to measure mechanical evoked twitches. B, experimental protocol for sessions in which intrathecal fentanyl (FENT) was administered and neurophysiological assessment of the elbow flexor muscle was carried out during no leg exercise, non-fatiguing exercise and fatiguing exercise sustained to task failure, and immediately post-failure. Subjects repeated ‘no leg exercise’ and ‘non-fatiguing exercise’ trials following the administration of intrathecal fentanyl. C, raw traces of twitch forces and electromyographic (EMG) evoked responses to TMS and MNS from a single subject, representative of group data during control (CTRL) and FENT sessions, respectively. Background voluntary force during contractions has been offset to allow for direct comparisons. Data are taken from the elbow flexion contractions set at the start and failure of exercise in both CTRL and FENT sessions. The data show an increase in superimposed twitch, no change in Mmax and a reduction in motor evoked potentials during CTRL. None of these variables were altered during FENT. Mmax, maximum compound muscle action potential; MVC, maximal voluntary contraction.
Elbow flexions with no leg exercise
Subjects performed three baseline sets of contractions, separated by at least 2 min, while they were seated on the bike with the right leg resting at the dead centre of the bottom of the crank cycle. During each set, three single TMS pulses (lower-intensity stimulation) and one electrical motor nerve stimulation (MNS) were elicited during a sustained 25% MVC elbow flexion in order to measure corticospinal responsiveness. Note that throughout the study, torque during the submaximal contraction was fixed as a percentage of initial MVC torque. The three TMS pulses were randomized with the MNS. After 5 s, subjects performed an MVC followed by contractions of 75% and 50% of MVC (each contraction separated by 2–3 s) during which TMS (higher-intensity stimulation) was elicited. This series of contractions was used to calculate cortical VA (Todd et al. 2003). In order to quantify pre- to post-exercise changes in the elbow flexor neuromuscular function, the same procedure (i.e. three contraction sets) was repeated immediately after task failure was reached. Mean time from task failure to the first elbow flexor assessment was 26 ± 1 s.
Non-fatiguing exercise
Subjects performed three non-fatiguing trials (separated by 3 min) during which they cycled at 80% Wmax for approximately 30 s. In a preliminary study, no measurable degree of fatigue, as evidenced by a lack of reduction in quadriceps MVC torque and potentiated twitch torque, was found following these short bouts of exercise (data not shown). To measure the corticospinal responsiveness of the elbow flexors during each of these cycling trials, subjects were asked to perform a 25% MVC elbow flexion, and three lower-intensity TMS pulses and one MNS were elicited in random order and separated by 2–3 s. After 5 s, subjects performed an MVC (3 s) during which higher-intensity TMS was elicited in order to estimate supraspinal fatigue and measure the corticospinal responsiveness of the higher-threshold motoneurons.
Fatiguing exercise
Subjects sustained 80% Wpeak cycling exercise to task failure. A set of contractions identical to that performed during non-fatiguing exercise was performed at the start of exercise once the desired workload and cadence were reached (within approximately 10 s), and subsequently every minute throughout the task. The final set was performed when the subjects were close to (or at) task failure (mean time from last stimulation to complete termination of cycling exercise: 5 ± 1 s).
Force and electromyogram recordings
Isometric elbow flexion and knee extension force was measured using a calibrated linear strain gauge (MLP 300; Transducer Techniques, Inc., Temecula, CA, USA). Lever arm (from the wrist to lateral epicondyle) was measured in each subject to allow the calculation of torque (lever arm × force). Electromyogram (EMG) recordings were made with surface electrodes (Ag-AgCl, 10 mm diameter) placed over the muscle belly of the biceps brachii, triceps brachii and vastus lateralis in a bipolar configuration. EMG signals were amplified (×500), bandpass-filtered (50–2000 Hz) and converted from analogue to digital form at a sampling rate of 2000 Hz (Acknowledge; Biopac Systems, Inc., Goleta, CA, USA).
Cycle ergometer, arm and quadriceps fatigue assessment set-up
Subjects performed cycling exercise while the right arm was fixed in an isometric custom-made myograph. The right shoulder and elbow were flexed at 90 deg with the forearm vertical and fully supinated (Fig.1A). A mouthpiece was mounted to a horizontal bar above the myograph for the measurement of pulmonary ventilation and gas exchange. This set-up also ensured that the upper body and head were kept stable in space during stimulations to allow consistent TMS application. The crank angle on the bicycle ergometer was monitored continuously by a calibrated linear encoder. In the session in which locomotor muscle fatigue was assessed by pre- to post-exercise changes in quadriceps function, subjects were seated comfortably on a custom-built chair with full back support, such that the hip and knee were at 90 deg flexion. A cuff attached to the strain gauge (MLP 300; Transducer Techniques, Inc.) was fixed ∼2 cm above the lateral malleolus of the right leg using a Velcro strap. Leg position was marked at the ankle and knee to ensure identical positioning at pre- and post-exercise.
Stimulations
Two forms of stimulation were used during each session, consisting of MNS and TMS. Evoked muscle action potentials were recorded using surface EMG and evoked twitches were recorded via a torque transducer. Arm-related stimulations during cycling were delivered at a fixed crank angle (i.e. 45 deg after top dead centre). Optimal stimulation intensities were determined while subjects cycled at a submaximal workload (i.e. 25% Wpeak) to account for any lower limb dynamic activity and phase-dependent effects on responses elicited in the upper limbs.
Motor nerve stimulation
The brachial plexus was stimulated to determine the sizes of the maximal compound muscle action potential (Mmax) of, respectively, the biceps brachii and triceps brachii. Single stimuli (200 μs duration) were delivered to the brachial plexus with the cathode in the supraclavicular fossa and an anode on the acromion (Model DS7AH; Digitimer Ltd, Welwyn Garden City, UK). The stimulation intensity was increased in 20 mA increments until the size of the compound muscle action potential demonstrated no further increase [i.e. maximal M-wave (Mmax)] at rest and during a 50% MVC contraction. The stimulation intensity was set at 130% of Mmax intensity (225 ± 8 mA). For quadriceps fatigue assessment (session 3), the same procedure was carried out except that the femoral nerve was electrically stimulated (Mmax intensity: 346 ± 24 mA). The optimal position of the stimulating electrode over the femoral nerve was determined by fixing the anode between the greater trochanter and iliac crest and delivering low-intensity, single-pulse stimuli (200 μs pulse width, 100–150 mA) via a movable cathode probe and a constant voltage stimulator (Model DS7AH; Digitimer Ltd). The optimal position of the stimulating cathode was defined as the spot associated with the greatest torque output in response to the low-intensity stimulation. Once the optimal position had been located, the cathode electrode was fixed. Stimulations were delivered during the 3 s MVCs to measure superimposed twitches and at rest immediately following the MVCs to measure potentiated resting twitches. This allowed the measurement of conventional VA and peripheral fatigue of the quadriceps.
Transcranial magnetic stimulation
Transcranial magnetic stimulation (Magstim 200; Magstim Co. Ltd, Whitland, UK) was delivered via a figure-of-eight coil (diameter: 70 mm). The optimal coil position (posterior to anterior direction of current flow in the motor cortex) to preferentially activate the left motor cortex was determined (position relative to vertex: ∼5–6 cm) at the start of the session. The position was marked directly on the scalp for accurate placement throughout the session. For cortical VA measurements, TMS intensity was increased stepwise to produce the largest possible motor evoked potential (MEP) (approximately 50–60% Mmax) in the biceps muscle during brief 50% MVCs and only a small MEP (<20% Mmax) in the triceps muscle. The group mean stimulation intensity was 66 ± 1% of maximum stimulator output. For corticospinal responsiveness measurements, TMS intensity was set to 120% of active motor threshold (AMT) obtained during a 25% MVC contraction (group mean stimulation intensity: 44 ± 1% of maximum stimulator output). The AMT was defined as the lowest TMS intensity that elicited a clearly visible response in at least three of five stimulations. The rationale for setting the stimulus intensity for responsiveness measures relative to AMT obtained during a 25% MVC contraction (∼25% Mmax response) was to ensure that the response was not close to saturation on the stimulus–response curve (Todd et al. 2003); this not only allows a feasible window for changes to occur, but also permits the monitoring of small motoneurons that are active during submaximal contractions (McNeil et al. 2011).
Cardiorespiratory responses
Pulmonary ventilation and gas exchange were measured continuously at rest and during all cycling trials using an open-circuit calorimetry system (True Max 2400; Parvo Medics, Inc., Salt Lake City, UT, USA). Heart rate (HR) was measured from the R–R interval of an electrocardiogram with a three-lead arrangement (Acknowledge; Biopac Systems, Inc.).
Intrathecal fentanyl
Subjects were seated in a flexed position and 1 ml of fentanyl (0.025 mg ml−1) was delivered intrathecally at the vertebral interspace L3–L4, as previously reported (Amann et al. 2009).
Steady-state CO2 response test
Any migration of fentanyl sufficient to reach the brain would negate the significance of our findings. To exclude this potential scenario, we utilized the knowledge that the binding of fentanyl on medullary opioid receptors attenuates the ventilatory responsiveness to hypercapnia (Lalley, 2008). Therefore, to exclude the potential cephalad movement of fentanyl to and beyond the brainstem, steady-state CO2 response tests were performed before and ∼10 min after the fentanyl injection using an open-circuit technique. Participants were seated comfortably in a chair while breathing through a mouthpiece. In addition to eupnoeic air breathing, ventilatory responses to two different concentrations of CO2 (70% O2, 3% and 6% CO2, balance N2) were measured. Subjects breathed each gas mixture for 4 min and breathing frequency (fR) and tidal volume (VT) were assessed and averaged over the final minute of each condition.
Data analysis
In both CTRL and FENT sessions, cortical VA at rest, both before and after exercise, was quantified by expressing the amplitude of the superimposed twitch (SIT) in Nm elicited from TMS as a fraction of the estimated resting twitch (ERT); thus, cortical VA = (1 − SIT/ERT) × 100 (Todd et al. 2004). Resting twitch was estimated by extrapolating the linear relation between the amplitude of the SIT and voluntary torque. Regressions with r2 < 0.9 were excluded from the data analysis (4% of total trials were excluded; group mean r2 of included trials: 0.97 ± 0.02). For estimation of cortical VA during cycling exercise, the amplitude of the SIT during MVCs is reported as a percentage of the voluntary torque measured immediately before stimulation. In the session in which quadriceps fatigue was assessed, the SIT evoked via peripheral MNS elicited during the six MVCs was expressed as a percentage of the resting twitch (RT) elicited immediately after each MVC; thus, VA = (1 − SIT/RT) × 100.
Peak to peak amplitude and area of MEPs and Mmax were measured. However, because amplitude and area showed similar changes, only area is reported. The area of each MEP was normalized to that of Mmax. The background root mean squared (rms) EMG and torque during 25% elbow flexion contractions was taken over a period of 100 ms prior to the point of stimulation. Evoked responses elicited in each set were averaged as single time-points during non-fatiguing exercise and fatiguing exercise. For quadriceps fatigue assessment, the first two measurements were discarded to avoid the effects of potentiation on peripheral fatigue measurements and averages of the last four measurements were used. The duration of the silent period (SP, in ms) was determined as the interval from the stimulus to the return of the continuous EMG by visual inspection during MVCs.
Note that only six of eight subjects performed the non-fatiguing and pre–post responsiveness aspect of the protocol. Data from sets performed at rest (i.e. no cycling and pre–post exercise comparisons) and during non-fatiguing exercise were pooled and averaged. During fatiguing exercise, data from sets that corresponded to the start, and 25%, 50% and 75% of endurance time and task failure were identified and pooled for individual time-points.
Statistical analysis
Normality of the data was confirmed by Shapiro–Wilk W test. During fatiguing exercise, variables were analysed using two-way ANOVA with repeated measures on the factors ‘exercise time’ (start, 25%, 50% and 75% of endurance time, failure) and ‘session’ (FENT, CTRL). For pre–post exercise comparisons, two-way ANOVA with repeated measures on the factors ‘time’ (pre- and post-exercise) and ‘session’ (FENT, CTRL) was performed. Finally, for non-fatiguing exercise, two-way ANOVA with repeated measures on the factors ‘locomotor activity’ (no cycling, cycling) and ‘afferent feedback’ (intact, block) was performed. If the data did not conform to the assumption of sphericity, the P-value was Greenhouse–Geisser corrected. When ANOVA revealed significant main effects or an interaction, planned contrasts were used to test for changes in: (i) values at the start of fatiguing exercise (or pre-exercise) and at task failure (or post-exercise) within a session; (ii) values across sessions within a time-point; (iii) values for no cycling and cycling during non-fatiguing trials, and (iv) values during non-fatiguing cycling for intact and blocked feedback conditions. Paired t tests were used to test for differences in cardioventilatory responses between conditions. Finally, Pearson's correlation coefficients were calculated to determine the relationship between the following: changes in MEP (%Mmax) and EMGrms during fatiguing exercise from start of exercise to task failure for both FENT and CTRL sessions, and changes in MEP (%Mmax) and EMGrms pre–post exercise for both FENT and CTRL sessions. All data are reported as the mean ± s.e.m. Statistical significance was set at P ≤ 0.05.
Results
Resting ventilatory responses to CO2
As reflected by similar breathing patterns, eupnoeic air breathing was not altered in any of the subjects following fentanyl injection (t7 < 1.4, P > 0.2). Further exposure to two levels of increased CO2 resulted in similar hypercapnic ventilatory responses in all subjects post-fentanyl injection (3% CO2: t7 < 1.2, P > 0.1; 6% CO2: t3 < 0.7, P > 0.5) (Table1).
Table 1.
Resting ventilatory responses during the last minute of exposure to CO2 before and after fentanyl (FENT) blockade. All experiments were performed at barometric pressure of 639 ± 1 mmHg
|
fR, breaths min−1 |
VT, l |
|||
|---|---|---|---|---|
| Pre-FENT | Post-FENT | Pre-FENT | Post-FENT | |
| Room (n = 8) | 12.9 ± 1.5 | 11.5 ± 1.3 | 1.1 ± 0.1 | 1.0 ± 0.1 |
| 3% CO2 (n = 8) | 14.2 ± 1.5 | 12.8 ± 1.3 | 1.7 ± 0.1 | 1.6 ± 0.2 |
| 6% CO2 (n = 4) | 12.6 ± 1.4 | 11.8 ± 2.4 | 1.6 ± 0.2 | 1.4 ± 0.2 |
Effects of sustained cycling exercise on locomotor muscle fatigue
Constant-load cycling exercise to exhaustion caused a substantial degree of locomotor muscle fatigue. This was reflected in the 16% decrease in quadriceps MVC (from 239.5 ± 16.2 N m to 204.2 ± 20.7 N m; t7 = 3.4, P = 0.01), a 46% decrease in potentiated resting muscle twitch force (from 82.8 ± 4.8 N m to 44.8 ± 5.3 N m; t7 = 10.7, P < 0.001), and a 10% reduction in VA (from 87.8 ± 2.6% to 78.8 ± 2.4%; t7 = 3.4, P = 0.01) from pre- to immediately post-exercise. EMG during MVC (∼0.7 mV; t7 = 1.6, P = 0.15), Mmax elicited during MVC (∼6 mV; t7 = 1.9, P = 0.09) and Mmax elicited at rest (∼6 mV; t7 = 0, P = 0.9) did not differ from pre- to immediately post-exercise.
Effect of group III/IV locomotor muscle afferents on cycling performance, ventilation, HR responses and rating of perceived exertion
Time to exhaustion did not differ between the CTRL and FENT conditions (9.8 ± 1.3 min and 8.5 ± 1.1 min, respectively; t7 = 1.7, P = 0.14). At rest, fentanyl had no effect on VE, fR, VT, VT/TI or HR (t7 < 1.8, P > 0.1). There was a main effect of fentanyl on VE, 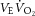 ,
, 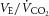 , fR and HR during exercise (F1,7 > 6.4, P < 0.05) with significant differences between those at 25% and 75% of cycling time to exhaustion (t7 > 3.2, P < 0.05) (Table2). The observed hypoventilation during FENT was accounted for by a reduction in fR as VT was not affected by fentanyl blockade. The impact of fentanyl blockade on various cardioventilatory variables diminished towards the end of exercise and only
, fR and HR during exercise (F1,7 > 6.4, P < 0.05) with significant differences between those at 25% and 75% of cycling time to exhaustion (t7 > 3.2, P < 0.05) (Table2). The observed hypoventilation during FENT was accounted for by a reduction in fR as VT was not affected by fentanyl blockade. The impact of fentanyl blockade on various cardioventilatory variables diminished towards the end of exercise and only 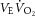 remained significantly lower than in the CTRL condition (t7 = 3.2, P = 0.02).
remained significantly lower than in the CTRL condition (t7 = 3.2, P = 0.02).
Table 2.
Mean power output, exercise time and cardioventilatory responses at start, at 25%, 50% and 75% of endurance time and at task failure of sustained cycling exercise
| CTRL |
FENT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | 25% | 50% | 75% | Failure | Start | 25% | 50% | 75% | Failure | ANOVA P-value | |
| Power output (W) | — | — | — | — | 220 ± 11 | — | — | — | — | 220 ± 11 | — |
| Exercise time (min) | — | — | — | — | 9.8 ± 1.3 | — | — | — | — | 8.5 ± 1.1 | — |
| Heart rate (beats min−1) | 77 ± 3 | 161 ± 3 | 171 ± 2 | 175 ± 1 | 176 ± 2 | 68 ± 4 | 147 ± 5* | 161 ± 5* | 169 ± 2 | 177 ± 1 | < 0.05 |
| VE (l min−1) | 12.0 ± 0.8 | 50.3 ± 5.5 | 73.5 ± 4.0 | 85.9 ± 4.2 | 90.6 ± 5.3 | 13.7 ± 0.7 | 48.4 ± 2.8 | 66.2 ± 3.9 | 77.1 ± 3.7* | 85.9 ± 4.3 | < 0.05 |
| fR (breaths min−1) | 15.0 ± 1.4 | 31.6 ± 2.1 | 39.8 ± 2.2 | 47.3 ± 2.7 | 50.9 ± 3.8 | 16.5 ± 0.6 | 29.2 ± 1.5 | 36.4 ± 2.6* | 42.6 ± 2.9* | 49.6 ± 4.2 | < 0.05 |
| VT (l) | 1.2 ± 0.1 | 2.3 ± 0.2 | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.6 ± 0.1 | 1.3 ± 0.0 | 2.4 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.6 ± 0.1 | 0.80 |
| TI (s) | 3.8 ± 0.3 | 2.0 ± 0.1 | 1.6 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | 3.7 ± 0.1 | 2.1 ± 0.1 | 1.7 ± 0.1* | 1.5 ± 0.1* | 1.3 ± 0.1 | 0.08 |
| VT/TI (l s−1) | 0.4 ± 0.1 | 1.2 ± 0.1 | 1.8 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 0.4 ± 0.02 | 1.2 ± 0.1 | 1.6 ± 0.1 | 1.9 ± 0.1* | 2.1 ± 0.1 | 0.15 |
| TE (s) | 1.8 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 2.0 ± 0.1 | 1.1 ± 0.0 | 0.9 ± 0.0* | 0.8 ± 0.0* | 0.7 ± 0.1 | 0.06 |
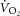 (l min−1) (l min−1) |
0.5 ± 0.03 | 2.5 ± 0.2 | 3.1 ± 0.1 | 3.3 ± 0.1 | 3.4 ± 0.2 | 0.5 ± 0.03 | 2.8 ± 0.1 | 3.2 ± 0.1 | 3.5 ± 0.1 | 3.6 ± 0.2 | 0.16 |
 (l min−1) (l min−1) |
0.4 ± 0.03 | 2.4 ± 0.3 | 3.3 ± 0.1 | 3.5 ± 0.1 | 3.5 ± 0.2 | 0.5 ± 0.04 | 2.6 ± 0.1 | 3.3 ± 0.1 | 3.6 ± 0.1 | 3.6 ± 0.1 | 0.60 |
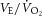 |
34.2 ± 0.9 | 30.3 ± 1.3 | 34.5 ± 1.1 | 37.8 ± 1.3 | 39.4 ± 2.0 | 37.6 ± 1.2 | 25.4 ± 0.7* | 30.1 ± 0.7* | 32.3 ± 0.7* | 35.2 ± 1.3* | < 0.05 |
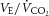 |
39.3 ± 1.2 | 31.5 ± 1.3 | 32.0 ± 0.9 | 35.3 ± 1.2 | 37.5 ± 1.7 | 35.3 ± 0.7 | 27.4 ± 0.6* | 29.1 ± 1.0* | 31.5 ± 1.0* | 34.8 ± 1.5 | < 0.05 |
| RPE | — | 5.9 ± 0.4 | 8.3 ± 0.3 | 9.4 ± 0.2 | 9.9 ± 0.1 | — | 4.9 ± 0.4* | 7.4 ± 0.5 | 8.5 ± 0.3 | 10.0 ± 0 | 0.10 |
P < 0.05 vs. CTRL. ANOVA P-value indicates a main effect of session. Abbreviations: CTRL, control condition; FENT, fentanyl blockade; RPE, rating of perceived exertion.
Effect of group III/IV locomotor muscle afferents on elbow flexor central fatigue
Fatiguing exercise
There was an interaction between exercise time and session (F4,28 = 2.9, P = 0.04) for MVC. MVC at task failure of CTRL was less than that at start of exercise (108.6 ± 3.5 N m and 116.6 ± 4.5 N m, respectively; P = 0.006), but remained unchanged during FENT (P = 0.6). For SIT, there was a main effect of session (F1,7 = 5.3, P = 0.05). SIT (%MVC torque) increased from the start of exercise to task failure in the CTRL session (from 1.1 ± 0.5% to 2.2 ± 0.6%; P = 0.03), but remained unchanged (P = 0.9) with fentanyl blockade (Fig.2).
Figure 2. Group mean maximal voluntary contraction (MVC) torque and superimposed twitches (SIT) measured with transcranial magnetic stimulation (TMS) during cycling.
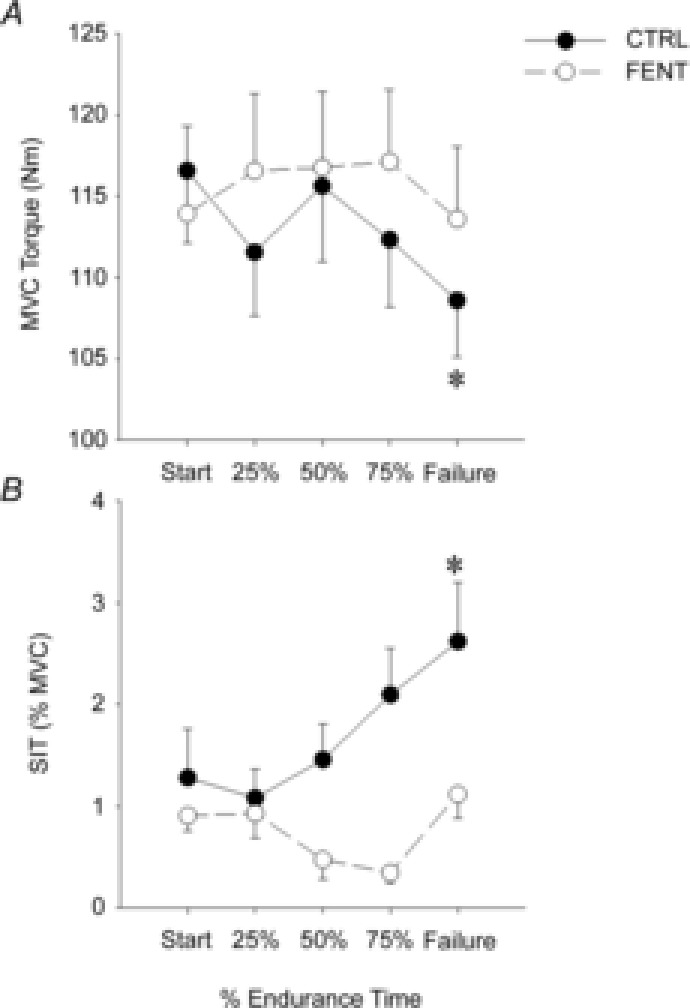
Group mean maximum (MVC) elbow flexor torque (A) and SIT evoked by TMS (B). Measurements were taken in the control (CTRL, •) and fentanyl (FENT, ○) conditions at the start of exercise, at 25%, 50% and 75% of endurance time, and at task failure. *Significant difference from the start of exercise.
Pre- to post-cycling comparison
Fentanyl injection had no effect on pre-exercise baseline elbow flexor MVC (∼112 N m; t7 = 0.3, P = 0.8) or cortical VA (∼94%; t7 = 0.9, P = 0.4). There was significant interaction between session and time (F1,7 = 7.5, P = 0.03) for cortical VA and a significant main effect of session (F1,7 = 5.5, P = 0.05) for MVC. Both elbow flexor MVC torque (from 107.6 ± 4.1 N m to 102.8 ± 3.7 N m) and cortical VA (from 94.2 ± 2.2% to 89.1 ± 1.8%) decreased from pre- to post-exercise in the CTRL session (P < 0.05). However, neither of these variables changed during the FENT session (P > 0.1). In addition, both elbow flexor MVC torque and cortical VA differed across sessions post-exercise (P < 0.03). There were no main effects of session and time or interaction for ERT (F1,7 = 1.1, P = 0.3) (Fig.3).
Figure 3. Group mean maximal voluntary contraction (MVC) torque, cortical voluntary activation (VA) and estimated resting twitches (ERT).
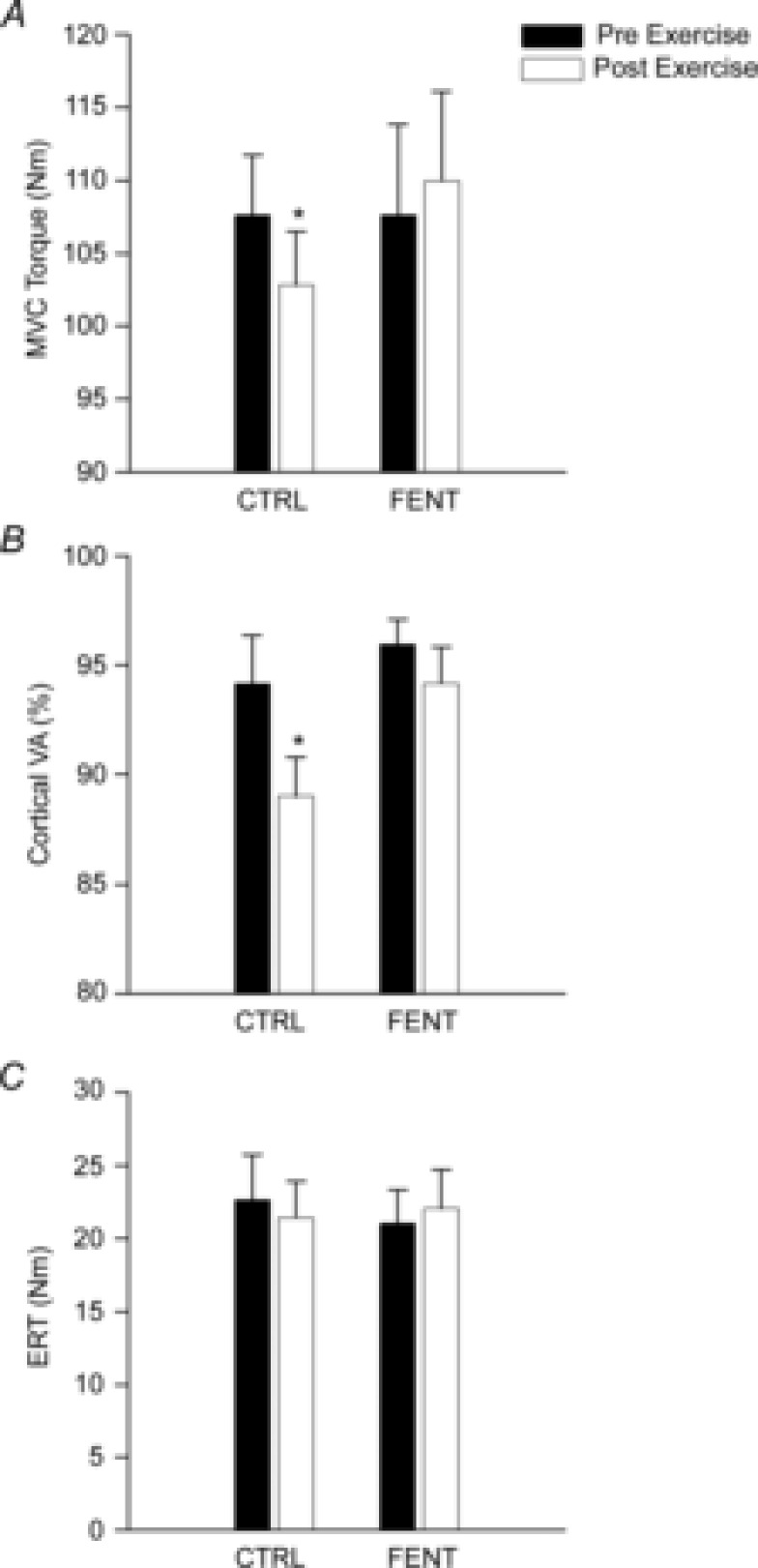
Group mean MVC torque (A), cortical VA (B) and ERT (C) measured with transcranial magnetic stimulation (TMS) from pre- to post-exercise in the control (CTRL) and fentanyl (FENT) conditions. *Significant difference from pre-exercise.
Effect of group III/IV locomotor muscle afferents on the responsiveness of corticospinal projections to elbow flexor muscle during non-fatiguing exercise
During the short non-fatiguing exercise, cycling per se had no effect on elbow flexion torque (∼28 N m during 25% MVC and ∼104 N m during MVC; F1,5 < 0.1, P > 0.7), EMGrms in biceps (∼0.1 mV during 25% MVC and ∼0.6 mV during MVC; F1,5 < 3.4, P > 0.1) and Mmax in biceps (∼14 mV during 25% MVC; F1,5 = 0.3, P = 0.6). Furthermore, fentanyl blockade had no effect on these observations (F1,5 < 3.4, P > 0.1) (Fig.4A).
Figure 4. Group mean root mean squared electromyogram (EMGrms) and transcranial magnetic stimulation (TMS)-evoked electromyogram (EMG) responses in biceps brachii.
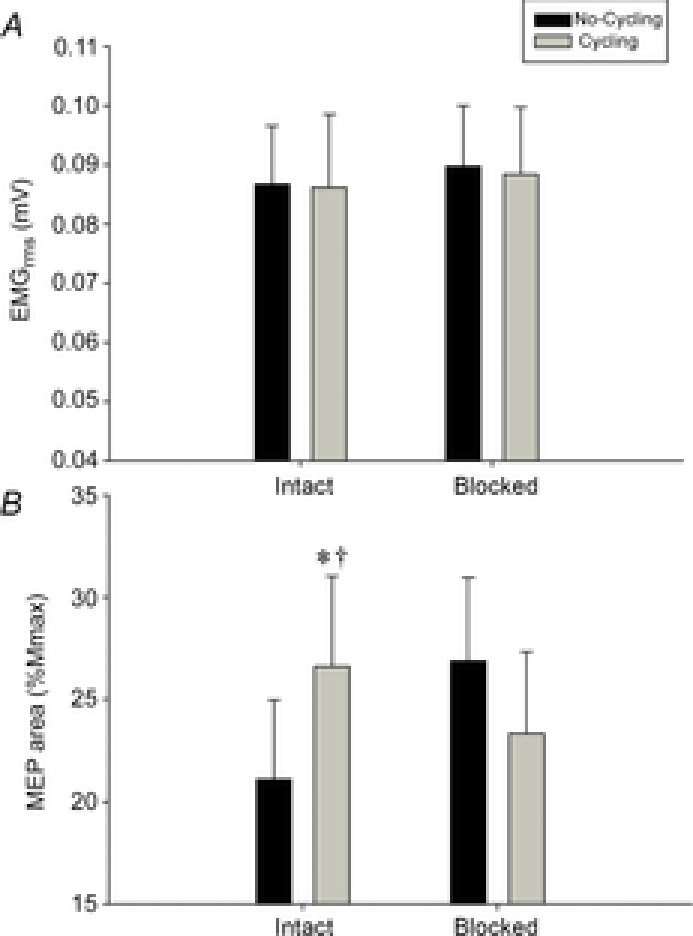
Group EMGrms in biceps brachii (A) and TMS motor evoked potential (MEP) EMG responses in biceps brachii during 25% maximal voluntary contraction of the elbow flexor muscle (B) during the fentanyl (FENT) session. *Significant difference from no-cycling condition; †significant difference from blocked cycling.
There was an interaction between locomotor activity and afferent feedback for MEP (%Mmax) when measured during 25% MVC contraction in biceps (F1,5 = 14.0, P = 0.01) (Fig.4B). By contrast, during MVC, although no main effects or interaction between locomotor activity and afferent feedback were seen (F1,5 = 2.7, P = 0.2), MEP increased from 55.5 ± 8.0%Mmax to 70.3 ± 10.3%Mmax with cycling. During non-fatiguing cycling, afferent blockade reduced MEP (%Mmax) during 25% MVC (P = 0.049). Furthermore, MEP (%Mmax) increased from no cycling to cycling during 25% MVC with intact feedback (P = 0.02); however, this increase was not evident after fentanyl blockade (P = 0.2).
Effect of group III/IV locomotor muscle afferents on the responsiveness of corticospinal projections to elbow flexor muscle as a consequence of fatiguing exercise
MVCs while cycling
There were no main effects or interactions between session and time (F4,28 < 1.0, P > 0.4) for EMGrms, MEP (%Mmax) in both the biceps and triceps and SP in the biceps. The MEP evoked was 80.2 ± 9.8% Mmax in the biceps and 19.9 ± 1.3% Mmax in the triceps at the start of exercise, suggesting that the motor cortex stimulation activated a high proportion of agonist motor units while minimizing activation of the antagonist motor units. SP was 116.7 ± 6.2 ms in the biceps at the start of exercise.
25% MVC while cycling
There were no main effects or interactions (F4,28 < 1.0, P > 0.4) between session and time for torque during the 25% MVC (∼28 N m) and Mmax in the biceps (Fig.5A). However, there was significant interaction between session and time (F4,28 > 4.8, P < 0.05) for both MEP (%Mmax) and EMGrms. MEP (%Mmax) at 75% endurance time (P = 0.04) and task failure (P = 0.006) in CTRL were less than at the start of exercise. MEP (%Mmax) did not change throughout exercise during FENT (P > 0.9). EMGrms at 75% endurance time (P = 0.03) and task failure (P < 0.001) in FENT were greater than at the start of exercise. EMGrms did not change throughout the CTRL session (P > 0.6). Alterations in MEP (%Mmax) were positively correlated to alterations in EMGrms during FENT (r = 0.68, P < 0.01) but not during CTRL (P = 0.4).
Figure 5. Group mean changes from start of exercise.
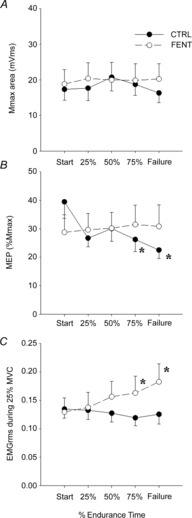
Group mean changes from start of exercise in maximal M-wave (Mmax) (A), motor evoked potential (MEP) (B) and root mean squared electromyogram (EMGrms) (C) data obtained from biceps brachii during 25% maximal voluntary contraction (MVC) elbow flexion during fatiguing exercise in control (CTRL, •) and fentanyl (FENT, ○) sessions. *Significant difference from start of exercise.
25% MVCs while resting (pre–post-cycling comparison)
There were no significant differences in main effects or interaction between time and session for Mmax (F1,5 = 0.06, P = 0.8) (Fig.6A). There was, however, a significant interaction between session and time for MEP (%Mmax) (F1,5 = 39.8, P = 0.001) (Fig.6B). MEP (%Mmax) decreased from pre- to post-exercise in the CTRL session (from 24.1 ± 4.6% to 17.6 ± 3.0%; P = 0.04), but remained unchanged (P = 0.8) with fentanyl blockade (Fig.6B). There were no main effects or interaction between session and time for EMGrms (F1,5 = 4.5, P = 0.08) (Fig.6C). Finally, changes in MEP (%Mmax) were not correlated to changes in EMGrms in either the FENT (P = 0.4) or CTRL (P = 0.1) conditions.
Figure 6. Group mean pre- to post-exercise changes.

Group mean changes from pre- to post-exercise in maximal M-wave (Mmax) (A), motor evoked potential (MEP) (B) and root mean squared electromyogram (EMGrms) (C) from biceps brachii during 25% maximal voluntary contraction (MVC) elbow flexion in control (CTRL) and fentanyl (FENT) sessions. *Significant difference from pre-exercise.
Discussion
The current study investigated the effects of group III/IV lower limb muscle afferent feedback during leg cycling exercise on VA and the responsiveness of corticospinal projections to an upper limb muscle, in both the absence and presence of locomotor muscle fatigue. The experiments provide two major findings and identify the mechanistic link mediating these observations. Firstly, the effect of exhaustive endurance exercise on central fatigue is not exclusively limited to the working muscle: group III/IV afferent feedback originating from the exercising muscle accounts for this ‘spread’. Secondly, these sensory neurons also influence the corticospinal responsiveness of upper limb muscles during leg cycling and the overall net effect depends on the severity of locomotor muscle fatigue and the associated magnitude of ensemble neural feedback. Specifically, in the absence of locomotor muscle fatigue, group III/IV leg muscle afferents facilitate the responsiveness of the elbow flexor corticospinal tract. By contrast, in the presence of locomotor muscle fatigue, sensory feedback from the legs might inhibit or withdraw the facilitating effect on the corticospinal tract responsiveness of upper limb muscle.
Evaluation of fentanyl site of action
Opioid receptors are widely distributed throughout the brain, including in various areas known to be involved in the regulation of motor function and behaviour (Bruijnzeel, 2009). A cephalad movement of fentanyl within the cerebrospinal fluid to directly affect these receptors would negate the implications of our findings. Of critical importance for evaluating such potential brain effects of fentanyl is the fact that this drug can directly bind to medullary opioid receptors, attenuating the ventilatory response to a given CO2 stimulus (Lalley, 2008). We excluded the migration of fentanyl beyond cervical levels in each participant based upon on the lack of an effect of fentanyl administration on CO2 sensitivity (Table1), resting MVC and cortical VA. Furthermore, a direct effect of fentanyl on the corticospinal tract at the cervical level or below may also be excluded based on the fact that there was no influence of fentanyl administration on MEPs evoked during voluntary elbow flexion contractions (no cycling).
Group III/IV locomotor muscle afferents compromise force-generating capacity and facilitate central fatigue in a remote muscle
Cycling exercise to exhaustion caused a progressive reduction in the maximum force-generating capacity of the non-exercised elbow flexor (Fig.3). As elbow flexor ERT remained similar throughout the trial, a contribution of peripheral fatigue can be excluded and the reduction in MVC is likely to be attributable to central fatigue. Indeed, the central contribution to this force loss was evident by the progressively increasing TMS-evoked twitches during elbow flexor MVCs and the significant exercise-induced reduction in VA. To evaluate the central projection of locomotor muscle afferents as the potential mechanism accounting for this phenomenon, subjects repeated the same cycling exercise with attenuated group III/IV locomotor muscle afferent feedback. Under these conditions, elbow flexor MVC and VA remained unchanged during the exercise (i.e. the development of central fatigue was abolished). This represents direct evidence that the reduction in neural drive from at or above the motor cortical output cells to an unfatigued muscle is, at least in part, a consequence of inhibitory feedback effects – or a disfacilitation – mediated by group III/IV afferents originating in fatigued muscle.
Limited studies have recently implicated small-diameter muscle afferents as the potential mechanism responsible for the spill-over of central fatigue to unfatigued muscles. The common, but not unproblematic, approach utilized in these previous investigations has been to increase afferent feedback from one muscle while quantifying central fatigue-related changes in an unfatigued muscle. The unanimous conclusion was that these sensory neurons may mediate the spill-over of central fatigue and compromise the performance of a remote muscle during static maximal contractions (Kennedy et al. 2013, 2014) and submaximal dynamic endurance exercise (Amann et al. 2013). However, potential limitations associated with various previous approaches may preclude an explicit conclusion (see Introduction). The present investigation used a novel approach that differed entirely from that of earlier studies (specific blockade of naturally evoked sensory feedback versus ‘artificial’ facilitation of afferent feedback) and provides the first direct evidence of the central fatigue-related impact of these afferents on the maximal force-generating capacity of a remote non-exercised muscle. This contradicts a previous correlative study which suggested exercise-induced cerebral deoxygenation (at sea level) as the key factor for the spill-over of central fatigue to remote muscle (Rasmussen et al. 2010) and isolates group III/IV muscle afferents as representing the major determinant of this phenomenon.
In the absence of locomotor muscle fatigue, group III/IV muscle afferents facilitate corticospinal projections to remote muscle
In the absence of leg fatigue, feedback from group III/IV lower limb muscle afferents facilitates corticospinal pathways to upper limb muscle. Specifically, during non-fatiguing exercise (i.e. short cycling bouts), the responsiveness of the lower-threshold corticospinal projections to the elbow flexor was attenuated when feedback from group III/IV locomotor muscle afferents was blocked (Fig.4B). Furthermore, the increase in lower-threshold corticospinal responsiveness associated with the transition from rest to cycling (despite a constant descending motor drive during the 25% elbow flexor MVC), was diminished when leg afferent feedback was blocked (Fig.4B). In combination, these findings suggest that, in the absence of fatigue, feedback from the group III/IV locomotor muscle afferents increases responsiveness of corticospinal projections to non-exercised muscles. The observed increase in upper limb responsiveness during leg cycling also provides evidence for the proposed existence of neural coupling between arms and legs during rhythmic movements in humans (Zehr & Duysens, 2004; Balter & Zehr, 2007; Zehr et al. 2007a).
In the presence of locomotor muscle fatigue, group III/IV muscle afferents disfacilitate or inhibit corticospinal projections to remote muscle
Exhaustive leg cycling under control conditions was associated with a progressive reduction in the responsiveness of the motor cortical cells and/or spinal motoneurons projecting to elbow flexors (Fig.5). This decrease occurred despite constant effort and motoneuronal output in the upper limb. The critical finding was that this impact on elbow flexor corticospinal responsiveness was abolished when the same exercise was performed with blocked lower limb muscle afferents. This suggests that, in the presence of significant locomotor muscle fatigue, group III/IV lower limb muscle afferents may exert inhibitory influences on the responsiveness of lower-threshold corticospinal motor pathways of the upper limb muscles. Alternatively, the group III/IV-mediated influence during fatigue may not be inhibitory, but may instead lead to a withdrawal of the facilitation of the corticospinal pathway as observed herein during the transition from rest to exercise.
It is important to recognize that the bigger MEPs (i.e. those evoked via a higher TMS stimulator power output during MVCs) were not altered during the brief MVCs performed during cycling exercise. As the responsiveness of both small and large motoneurons influences the size of large MEPs during MVCs, a potentially increased responsiveness of the higher-threshold corticospinal projections may have masked the influences from the disfacilitated lower-threshold motoneurons, with a net effect of no change. The observed lack of an overall change may indicate that lower limb neural feedback associated with peripheral fatigue either increases or does not affect the responsiveness of the relatively higher-threshold neurons in the pathway from the motor cortex to the segmental motoneurons innervating upper limb flexor muscles.
It is not clear why, despite the absence of peripheral fatigue, elbow flexor EMG associated with the 25% MVCs increased during FENT. Speculatively, a blockade-related increase in antagonistic coactivation, which would require additional elbow flexor activation to maintain the required load (i.e. 25% MVC), may explain this observation. Regardless of the underlying mechanism, based on the correlation between EMG and MEP, it could be argued that the increase in EMG, per se, ‘facilitated’ the MEP size in the FENT condition (Fuglevand et al. 1993; McNeil et al. 2011) and therefore masked the fall seen in the CTRL condition (Fig.5). Alternatively, because fentanyl only partially blocks feedback from μ-opioid receptor-sensitive muscle afferents, it is likely that some inhibitory input remained unaffected. The associated central inhibition, or the withdrawal of facilitation, may potentially have counteracted the EMG-induced ‘increase’ in MEP with a net effect of no change during FENT.
Potential effects of muscle afferents on cortical versus spinal responsiveness
Although the current study does not allow for differentiation between cortical and spinal contributions to the changes in elbow flexor MEPs, the observed facilitating effect of group III/IV leg muscle afferents on upper limb corticospinal responsiveness during cycling in the absence of fatigue is, based on previous evidence, likely to be mediated at the spinal level (Zehr et al. 2007b). By contrast, the group III/IV-mediated inhibition, or disfacilitation, of the elbow flexor corticospinal pathway during cycling in the presence of locomotor muscle fatigue may be secondary to decreased responsiveness of the motor cortical cells (Martin et al. 2008) and/or increased intracortical inhibition (Hilty et al. 2011; Schabrun & Hodges, 2012; Sidhu et al. 2013). The net effect for overall corticospinal responsiveness may depend on how the afferent influence on one compartment outweighs that on the other (i.e. spinal versus brain effects). Specifically, if afferent feedback is low (absence of fatigue), minimal or absent, cortical inhibition may be outweighed by facilitation at the spinal level. With high afferent feedback (presence of fatigue), cortical inhibition (or disfacilitation) is likely to dominate, outweighing a potential spinal facilitation.
Methodological considerations
The 5% pre- to post-exercise decrease in elbow flexor VA induced via locomotor exercise is small compared with the 20% or more decrease that is frequently observed in muscles at the end of a 2 min MVC (Kennedy et al. 2013). However, the 5% decrease in elbow flexor VA is similar to that observed in knee extensors following exhaustive cycling exercise (Sidhu et al. 2009; Amann et al. 2011). Furthermore, the unavoidable delay associated with our post-exercise assessment of VA is likely to have allowed for some recovery, potentially leading to an underestimation of the true degree of exercise-induced central fatigue. Indeed, end-exercise central fatigue has previously been documented to recover within the first 5 min following exercise (Bigland-Ritchie et al. 1986; Amann et al. 2009, 2011). Finally, based on previous findings suggesting that various elbow flexor muscles behave similarly during fatiguing paradigms (Yoon et al. 2012), we assessed only the biceps brachii and have no data from other agonists in the current study.
Conclusion
This study provides direct evidence that feedback from group III/IV lower limb muscle afferents causes supraspinal fatigue in upper limb muscle. The findings also suggest a twofold effect of these leg muscle afferents on the corticospinal responsiveness of an upper limb muscle. Namely, in the absence of locomotor muscle fatigue, these lower limb sensory neurons appear to facilitate corticospinal projections to an upper limb muscle, whereas in the presence of locomotor muscle fatigue, the facilitating effect seems to be outweighed by an overall inhibitory influence on the motor pathway or, alternatively, a withdrawal of facilitation. These findings emphasize the critical contribution of group III/IV muscle afferents to determining the functional exercise capacity of humans, recognition of which is particularly relevant in clinical populations characterized by an overactive sensory feedback mechanism, such as in patients with heart failure (Amann et al. 2014).
Acknowledgments
This work was performed at the Salt Lake City Veterans Affairs Medical Center, Salt Lake City, UT, USA.
Glossary
- AMT
active motor threshold
- CNS
central nervous system
- CTRL
control
- EMGrms
root mean squared electromyogram
- ERT
estimated resting twitch
- FENT
fentanyl
- fR
breathing frequency
- HR
heart rate
- Mmax
maximum compound muscle action potential
- MEP
motor evoked potential
- MNS
motor nerve stimulation
- MVC
maximal voluntary contraction
- PECO
post-exercise circulatory occlusion
- RPE
rating of perceived exertion
- RT
resting twitch
- SIT
superimposed twitch
- SP
silent period
- TMS
transcranial magnetic stimulation
- VA
voluntary activation
- Wpeak
peak power output
Additional information
Competing interests
None declared.
Author contributions
S.K.S. and M.A. conceived and designed the study. All authors executed the study. S.K.S. analysed the data and prepared the figures. S.K.S. and M.A. interpreted the data and prepared the manuscript. All authors edited, revised and approved the final version of the manuscript.
Funding
This study was supported by the National Heart, Lung and Blood Institute (HL-103786 and HL-116579) and a Veterans Affairs Merit Grant (E6910R).
Key points
We aimed to elucidate the role of group III/IV locomotor muscle afferents in the development of central fatigue and the responsiveness of the corticospinal tract in relation to an unexercised arm muscle.
Intrathecal fentanyl, a μ-opioid receptor agonist, was employed to attenuate afferent feedback from the leg muscles during intense cycling exercise characterized by either no or severe peripheral locomotor muscle fatigue.
In the absence of locomotor muscle fatigue, group III/IV-mediated leg afferent feedback facilitates the responsiveness of the motor pathway to upper limb flexor muscles.
By contrast, in the presence of leg fatigue, group III/IV locomotor muscle afferents facilitate supraspinal fatigue in a remote muscle not involved in the exercise and disfacilitate the responsiveness of associated corticospinal projections.
References
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF. Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol. 2011;589:5299–5309. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF. Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Venturelli M, Ives SJ, McDaniel J, Layec G, Rossman MJ. Richardson RS. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J Appl Physiol (1985) 2013;115:355–364. doi: 10.1152/japplphysiol.00049.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Jonathan Groot H, Walter Wray D, Stehlik J. Richardson RS. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol. 2014;174:368–375. doi: 10.1016/j.ijcard.2014.04.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balter JE. Zehr EP. Neural coupling between the arms and legs during rhythmic locomotor-like cycling movement. J Neurophysiol. 2007;97:1809–1818. doi: 10.1152/jn.01038.2006. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Dawson NJ, Johansson RS. Lippold OC. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP. McCleskey EW. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–749. doi: 10.1016/j.neuron.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW. κ-Opioid receptor signalling and brain reward function. Brain Res Rev. 2009;62:127–146. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglevand AJ, Winter DA. Patla AE. Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol. 1993;70:2470–2488. doi: 10.1152/jn.1993.70.6.2470. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE. Taylor JM. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graven-Nielsen T, Jansson Y, Segerdahl M, Kristensen JD, Mense S, Arendt-Nielsen L. Sollevi A. Experimental pain by ischaemic contractions compared with pain by intramuscular infusions of adenosine and hypertonic saline. Eur J Pain. 2003;7:93–102. doi: 10.1016/s1090-3801(02)00069-1. [DOI] [PubMed] [Google Scholar]
- Hilty L, Lutz K, Maurer K, Rodenkirch T, Spengler CM, Boutellier U, Jancke L. Amann M. Spinal opioid receptor-sensitive muscle afferents contribute to the fatigue-induced increase in intracortical inhibition in healthy humans. Exp Physiol. 2011;96:505–517. doi: 10.1113/expphysiol.2010.056226. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Ekmann KM, Anderson CE. Koerber HR. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol. 2013;109:2374–2381. doi: 10.1152/jn.01067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DS, McNeil CJ, Gandevia SC. Taylor JL. Firing of antagonist small-diameter muscle afferents reduces voluntary activation and torque of elbow flexors. J Physiol. 2013;591:3591–3604. doi: 10.1113/jphysiol.2012.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DS, McNeil CJ, Gandevia SC. Taylor JL. Fatigue-related firing of distal muscle nociceptors reduces voluntary activation of proximal muscles of the same limb. J Appl Physiol (1985) 2014;116:385–394. doi: 10.1152/japplphysiol.01166.2013. [DOI] [PubMed] [Google Scholar]
- Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol. 2008;164:160–167. doi: 10.1016/j.resp.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z. Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC. Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol. 2008;586:1277–1289. doi: 10.1113/jphysiol.2007.140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Giesebrecht S, Gandevia SC. Taylor JL. Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol. 2011;589:3533–3544. doi: 10.1113/jphysiol.2011.207191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet GY, Martin V, Lattier G. Ballay Y. Mechanisms contributing to knee extensor strength loss after prolonged running exercise. J Appl Physiol (1985) 2003;94:193–198. doi: 10.1152/japplphysiol.00600.2002. [DOI] [PubMed] [Google Scholar]
- Racinais S, Bishop D, Denis R. Lattier G. Muscle deoxygenation and neural drive to the muscle during repeated sprint cycling. Med Sci Sports Exerc. 2007;39:268–274. doi: 10.1249/01.mss.0000251775.46460.cb. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Nielsen J, Overgaard M, Krogh-Madsen R, Gjedde A, Secher NH. Petersen NC. Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. J Physiol. 2010;588:1985–1995. doi: 10.1113/jphysiol.2009.186767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabrun SM. Hodges PW. Muscle pain differentially modulates short interval intracortical inhibition and intracortical facilitation in primary motor cortex. J Pain. 2012;13:187–194. doi: 10.1016/j.jpain.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Bentley DJ. Carroll TJ. Locomotor exercise induces long-lasting impairments in the capacity of the human motor cortex to voluntarily activate knee extensor muscles. J Appl Physiol. 2009;106:556–565. doi: 10.1152/japplphysiol.90911.2008. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Lauber B, Cresswell AG. Carroll TJ. Sustained cycling exercise increases intracortical inhibition. Med Sci Sports Exerc. 2013;45:654–662. doi: 10.1249/MSS.0b013e31827b119c. [DOI] [PubMed] [Google Scholar]
- Todd G, Gorman RB. Gandevia SC. Measurement and reproducibility of strength and voluntary activation of lower-limb muscles. Muscle Nerve. 2004;29:834–842. doi: 10.1002/mus.20027. [DOI] [PubMed] [Google Scholar]
- Todd G, Taylor JL. Gandevia SC. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol. 2003;551:661–671. doi: 10.1113/jphysiol.2003.044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Schlinder-Delap B, Keller ML. Hunter SK. Supraspinal fatigue impedes recovery from a low-intensity sustained contraction in old adults. J Appl Physiol (1985) 2012;112:849–858. doi: 10.1152/japplphysiol.00799.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Balter JE, Ferris DP, Hundza SR, Loadman PM. Stoloff RH. Neural regulation of rhythmic arm and leg movement is conserved across human locomotor tasks. J Physiol. 2007a;582:209–227. doi: 10.1113/jphysiol.2007.133843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP. Duysens J. Regulation of arm and leg movement during human locomotion. Neuroscientist. 2004;10:347–361. doi: 10.1177/1073858404264680. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Klimstra M, Johnson EA. Carroll TJ. Rhythmic leg cycling modulates forearm muscle H-reflex amplitude and corticospinal tract excitability. Neurosci Lett. 2007b;419:10–14. doi: 10.1016/j.neulet.2007.03.045. [DOI] [PubMed] [Google Scholar]


