Abstract
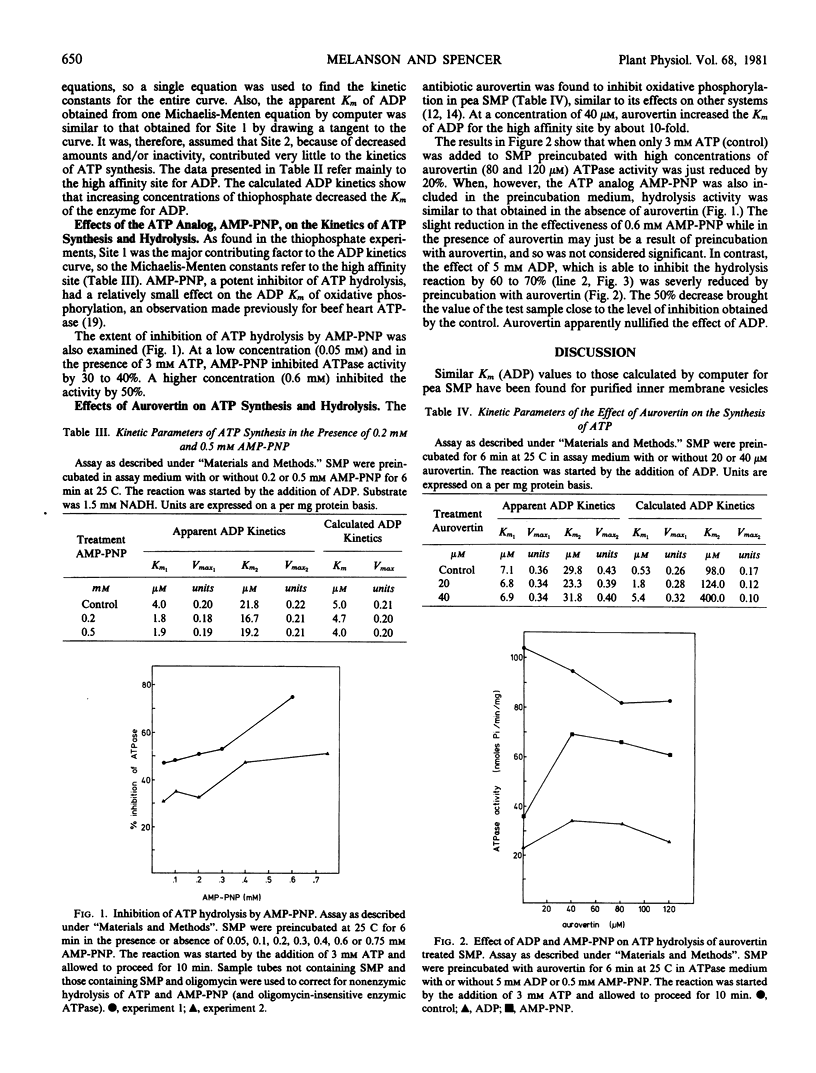
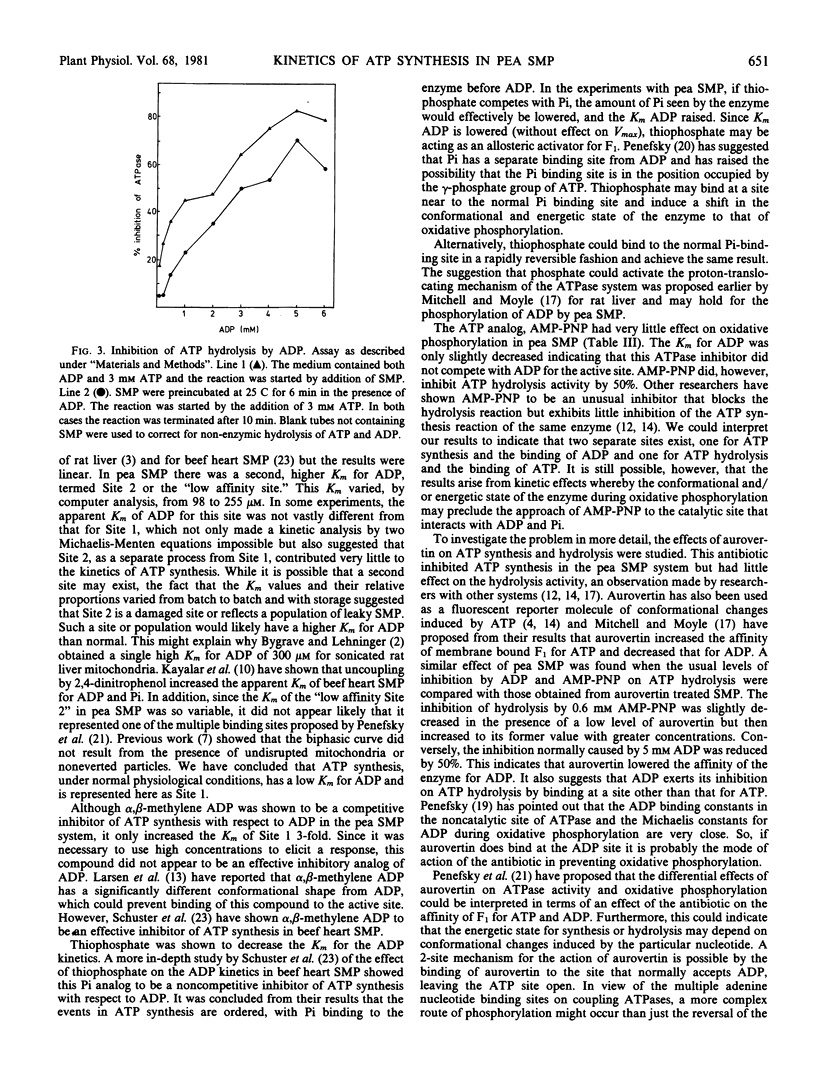
A kinetic study of oxidative phosphorylation by pea submitochondrial particles gave two Km values for ADP, one low, the other high. The high value probably reflected a damaged site or a population of leaky mitochondria. Only the high affinity site with a low Km for ADP was involved in ATP synthesis. α,β-Methylene ADP was found to be a competitive inhibitor of ATP synthesis. The inorganic phosphate analog, thiophosphate, decreased the apparent Km of ADP while the rate of the reaction remained approximately the same. Adenyl imidodiphosphate, a specific inhibitor of ATP hydrolysis activity, had little effect on oxidative phosphorylation. A slight decrease in the Km of the high affinity binding site for ADP was noted. Aurovertin was found to be a potent inhibitor of oxidative phosphorylation in pea submitochondrial particles. The Km of the high affinity site was increased 10-fold. Also, the inhibition normally exerted by ADP on ATPase activity was severely reduced by aurovertin. In contrast, increasing the concentration of aurovertin only slightly affected the level of inhibition caused by adenyl imidodiphosphate on ATP hydrolysis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer P. D., Cross R. L., Momsen W. A new concept for energy coupling in oxidative phosphorylation based on a molecular explanation of the oxygen exchange reactions. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2837–2839. doi: 10.1073/pnas.70.10.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F. L., Lehninger A. L. The affinity of mitochondrial oxidative phosphorylation mechanisms for phosphate and adenosine diphosphate. Proc Natl Acad Sci U S A. 1967 May;57(5):1409–1415. doi: 10.1073/pnas.57.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. II. Interaction with adenosine diphosphate. J Biol Chem. 1972 Dec 25;247(24):7969–7976. [PubMed] [Google Scholar]
- Chang T., Penefsky H. S. Aurovertin, a fluorescent probe of conformational change in beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1973 Apr 25;248(8):2746–2754. [PubMed] [Google Scholar]
- Grubmeyer C., Duncan I., Spencer M. Partial characterization of a soluble ATPase from pea cotyledon mitochondria. Can J Biochem. 1977 Aug;55(8):812–818. doi: 10.1139/o77-120. [DOI] [PubMed] [Google Scholar]
- Grubmeyer C., Melanson D., Duncan I., Spencer M. Oxidative phosphorylation in pea cotyledon submitochondrial particles. Plant Physiol. 1979 Nov;64(5):757–762. doi: 10.1104/pp.64.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. A. The interactions of coupling ATPases with nucleotides. Biochim Biophys Acta. 1978 Mar 10;463(3-4):245–273. doi: 10.1016/0304-4173(78)90002-2. [DOI] [PubMed] [Google Scholar]
- Hilborn D. A., Hammes G. G. Equilibrium binding of nucleotides to beef heart mitochondrial adenosine triphosphatase. Biochemistry. 1973 Feb 27;12(5):983–990. doi: 10.1021/bi00729a030. [DOI] [PubMed] [Google Scholar]
- Kozlov I. A., Skulachev V. P. H+-Adenosine triphosphatase and membrane energy coupling. Biochim Biophys Acta. 1977 Jun 21;463(1):29–89. doi: 10.1016/0304-4173(77)90003-9. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., CONNELLY J. L., JOHNSON D. ANTIBIOTIC STUDIES. II. INHIBITION OF PHOSPHORYL TRANSFER IN MITOCHONDRIA BY OLIGOMYCIN AND AUROVERTIN. Biochemistry. 1964 Dec;3:1961–1968. doi: 10.1021/bi00900a030. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen M., Willett R., Yount R. G. Imidodiphosphate and pyrophosphate: possible biological significance of similar structures. Science. 1969 Dec 19;166(3912):1510–1511. doi: 10.1126/science.166.3912.1510. [DOI] [PubMed] [Google Scholar]
- Lee C., Ernster L. Studies of the energy-transfer system of submitochondrial particles. 2. Effects of oligomycin and aurovertin. Eur J Biochem. 1968 Feb;3(4):391–400. doi: 10.1111/j.1432-1033.1967.tb19542.x. [DOI] [PubMed] [Google Scholar]
- Malhotra S. S., Spencer M. Preparation and properties of purified (Na+ plus K+)-stimulated mitochondrial ATPase from germinating pea seeds. Can J Biochem. 1974 Jun;52(6):491–499. doi: 10.1139/o74-073. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Aurovertin-sensitive phosphate activation of mitochondrial adenosine triphosphatase. FEBS Lett. 1970 Sep 7;9(6):305–308. doi: 10.1016/0014-5793(70)80385-4. [DOI] [PubMed] [Google Scholar]
- Neal J. L. Analysis of Michaelis kinetics for two independent, saturable membrane transport functions. J Theor Biol. 1972 Apr;35(1):113–118. doi: 10.1016/0022-5193(72)90196-8. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Differential effects of adenylyl imidodiphosphate on adenosine triphosphate synthesis and the partial reactions of oxidative phosphorylation. J Biol Chem. 1974 Jun 10;249(11):3579–3585. [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Reid K. G., Utech N. M., Holden J. T. Multiple transport components for dicarboxylic amino acids in Streptococcus faecalis. J Biol Chem. 1970 Oct 25;245(20):5261–5272. [PubMed] [Google Scholar]
- Schuster S. M., Reinhart G. D., Lardy H. A. Studies on the kinetic mechanism of oxidative phosphorylation. J Biol Chem. 1977 Jan 25;252(2):427–432. [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Solomos T., Malhotra S. S., Prasad S., Malhotra S. K., Spencer M. Biochemical and structural changes in mitochondria and other cellular components of pea cotyledons during germination. Can J Biochem. 1972 Jul;50(7):725–737. doi: 10.1139/o72-101. [DOI] [PubMed] [Google Scholar]



