Abstract
Background
Local anesthetics are commonly used for the treatment of a variety of tendinopathies in combination with corticosteroids injection. The goal of this study was to evaluate the effects of lidocaine and triamcinolone acetonide (TA) on cultured rat tenocytes and to determine whether there is a synergistic effect.
Material/Methods
Rat patellar tendon-derived tenocytes were cultured with or without TA and lidocaine, and the culture without any additive served as the control. Cell morphology and cell viability were evaluated. Expressions of tenocyte-related genes were measured by qRT-PCR.
Results
TA, when exposed to tenocytes in vitro, significantly decreased cell viability. The cells cultured with TA had a flattened shape. Moreover, the expressions of tenocyte-related genes in tenocytes were markedly decreased in the TA-treated group. We found that 1% lidocaine synergistically increased the deleterious effects of TA.
Conclusions
Our data provide evidence of the detrimental effects of these drugs on tendon tissues. Injection of TA in combination with 1% lidocaine should be used with caution.
MeSH Keywords: Cell Survival, Lidocaine, Patellar Ligament, Triamcinolone Acetonide
Background
Tendinopathy, a common tendon disorder, often impairs joint function. It is a spectrum of pathological conditions ranging from chronic inflammation to degeneration of tendons. In recent years, extensive research has been conducted to discover effective treatments for these conditions. Clinical research suggests corticosteroid injection could improve tendon function by alleviating inflammation and pain [1,2]. Triamcinolone acetonide (TA) injections are often used to treat tendinopathy, but it has substantial adverse effects, such as impaired function of supraspinatus tendon cells and high rate of re-rupture [1,3]. Previous studies also reported the deleterious effects of TA on histological and biomechanical properties of the injured tendons [4,5].
Local anesthetics are used for adjunctive pain control in combination with corticosteroids injection in tendinopathies. However, recent studies suggest that local anesthetics of amide derivatives, such as bupivacaine and lidocaine, have deleterious effects on various cell types [6–11]. It was shown that the cytotoxicity of bupivacaine was dose-dependent in chondrocytes in vitro [12]. Lidocaine has been found to significantly reduce the viability of mesenchymal stem cells [13]. A more recent study showed that lidocaine could significantly decrease positive effects of platelet-rich plasma on human tendon cells [14]. While local anesthetics are commonly given with corticosteroids injection in treating tendinopathies, the combined effect of steroids and local anesthetics on tenocytes is largely unknown.
Based on these studies, we hypothesized that local anesthetics might potentiate the deleterious effects of TA on tenocytes. The aim of this study was to determine the effects of lidocaine and TA on cultured rat tenocytes and whether there is a synergistic effect.
Material and Methods
Ethics statement
Animal experiments were carried out under the Rules and Regulations of the Animal Care and Use Committee at Harbin Medical University.
Isolation and culture of rat tenocytes
Sprague-Dawley male rats (weighing 200–250 mg) were used for isolation of tenocytes. The procedures for the isolation and culture of tenocytes have been established [15]. Harvested patellar tendons were used as explants for primary cell cultures. Each patellar tendon was cut into small sections in aseptic conditions, and then placed and cultured in 6-well culture plates. After 5 min of air-drying for better adherence, Dulbecco’s Modified Eagle Medium (DMEM), 10% fetal bovine serum (FBS), and 1% penicillin and streptomycin were supplemented to each well. The explants were incubated at 37°C in a humidified culture chamber supplemented with 5% CO2. Cells began to emerge from the tissue pieces after 3–4 days in culture and began to attach to the surface of the culture dishes. After the cells reached 80% confluence, they were passaged by washing with phosphate-buffered saline (PBS) and detached with trypsin/ethylene deaminate triacetic acid (EDTA). Tenocytes between passages 2 and 4 were used in the following experiments.
Exposure of tenocytes to TA and lidocaine in vitro
Rat patellar tendon-derived tenocytes were plated in culture plates. After 24 h of culture, attached tenocytes were treated with TA in culture media (regular medium with 0.1 mg/ml of TA). Lidocaine was administered using the method previously described [12,16,17]. Tenocytes were exposed to 1% lidocaine for 15 min. Samples were then washed with PBS solution, and returned to regular culture media with or without TA at 37°C in a humidified 5% CO2 incubator. There were 4 groups, including the control group, TA group, lidocaine group, and TA + lidocaine group. Control tenocytes were those grown in regular culture media. Each group was cultured in triplicate, and the culture medium was changed every 3 days. Cell viability, cell morphology, and expression of tenocyte-related genes were assayed at Day 7.
Cell morphology
We exposed 5×104 cells to TA and/or lidocaine in 6-well plates. Cell morphology was observed under a confocal microscope at Day 7.
Cell viability assay
Cell viability was determined using the Cell-Counting Kit-8 (CCK-8) (Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions. Tenocytes were seeded in 96-well plates at a density of 5×103 cells per well for 24 h. Cells were exposed to 1% lidocaine for 15 min, followed by removal of each medium and returning to regular culture media with or without TA. Then, tenocytes were allowed to recover in regular medium for 24 h prior to assessing their viability. For the cell viability assay, each well was supplemented with 10 μl CCK-8 and incubated at 37°C for 2 h. The optical density was spectrophotometrically measured at 450 nm. Total cell viability for each group is expressed as percentage of the untreated control.
Reverse transcription-polymerase chain reaction
Tenocyte characterization was performed by the molecular analysis of typical tenocyte markers. Scleraxis (SCX) and tenomodulin (TNMD) are closely related to tendon regeneration. SCX is the best-characterized early differentiation marker of tendon tissue. It positively regulates tendon-associated genes such as collagen I and TNMD expression in tendon fibroblasts. As a later differentiation marker of tendon cells, TNMD is a regulator of tenocyte proliferation and is involved in collagen fibril maturation. Therefore, Collagen I, TNMD, and SCX were evaluated using RT-PCR in this study. Total RNA was extracted using the RNeasy mini kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. cDNA was synthesized using the First-strand kit (Invitrogen, Carlsbad, California). The qRT-PCR was carried out with QuantiTect SYBR Green RT-PCR kit (Qiagen, Hilden, Germany). We amplified 2 μl total cDNA of each sample in a final volume of 50 μl reaction mixture. The cycling conditions were: held at 65°C for 5 min, snap cooling at 4°C for 1 min, 42°C for 50 min, and at 72°C for 15 min. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Relative gene expression levels were calculated with the 2ΔCT formula.
Rat-specific primers were used for Collagen I, TNMD, SCX, and GAPDH as follows: 5′-CCGGACTGTGAGGTTAGGAT-3′ (forward) and 5′-AACCCAAAGGACCCAAATAC-3′ (reverse) for Collagen I; 5′-CCATGCTGGATGAGAGAGGTTAC-3′ (forward) and 5′CACAGACCCTGCGGCAGTA-3′ (reverse) for TNMD; 5′-AACACGGCCTTCACTGCGCTG-3′ (forward) and 5′-CAGTAGCACGTTGCCCAGGTG-3′ (reverse) for SCX; 5′-TGACTCTACCCACGGCAAGTTCAA-3′ (forward) and 5′-ACGACATACTCAGCACCAGCATCA-3′ (reverse) for GAPDH.
Statistical analysis
The values are expressed as mean ± standard deviation (SD). One-way ANOVA with the Student-Newman-Kuels test was used for multiple comparisons. Statistical analyses were carried out with the SPSS 11.0 statistical package. All values of p<0.05 were accepted as statistically significant.
Results
Cell morphology
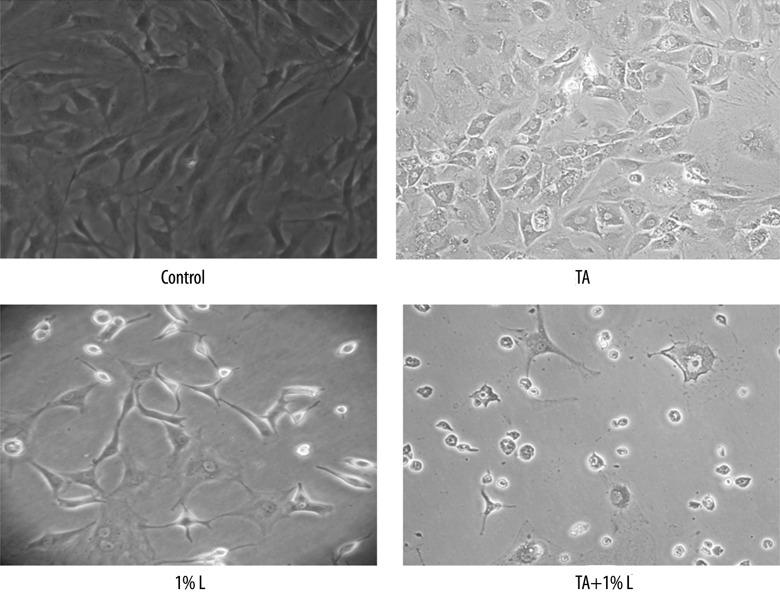
The effect of TA and lidocaine on the morphology of tenocytes was evaluated. The tenocytes in the control group showed spindle fibroblast-like shape cells. However, cells lost orientation and became flattened after exposed to TA or lidocaine. The cell density was lower for cells cultured with TA or lidocaine compared to control. The combination of TA and 1% lidocaine induce more obvious morphologic change (Figure 1).
Figure 1.
Exposure to TA or lidocaine yielded flattened and polygonal cells and reduced cell numbers. The combination of TA and 1% lidocaine induce more obvious morphologic change.
Cell viability
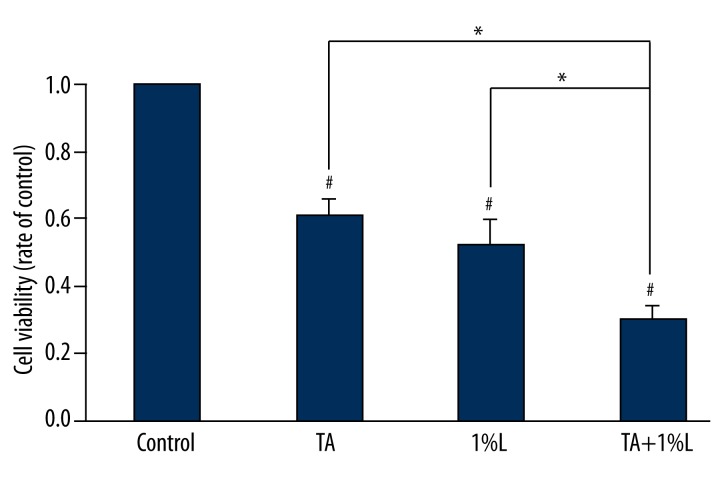
When the tenocytes were exposed to TA or lidocaine, the viability of the tenocytes was lower than that of the control group. Moreover, we found that viability of the tenocytes cultured with TA and 1% lidocaine was significantly lower than with TA or lidocaine alone (Figure 2).
Figure 2.
Cell viability in the TA or lidocaine group was significantly lower than that in the control group. Cell viability in the TA plus lidocaine group was significantly lower than that in the TA or lidocaine group. * p<0.05. # p<0.05 compared with the values for the control group.
Tenocyte-related genes expression
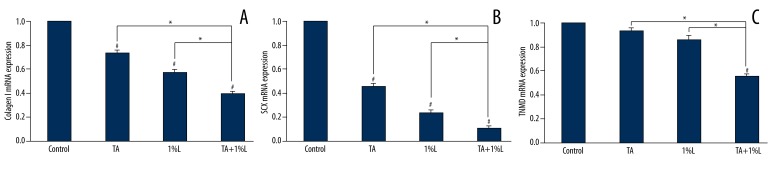
RT-PCR analysis on the cultures exposed to TA and lidocaine was performed as above. We found that the expressions of tenocyte-related genes, Collagen I and SCX, were decreased by the treatment of TA or 1% lidocaine (Figure 3), whereas no significant difference has been detected for the expression of TNMD. When the tenocytes were incubated with the combination of TA and lidocaine, the expressions of tenocyte-related genes were significantly decreased when compared with TA or 1% lidocaine administration alone.
Figure 3.
The expression of tenocyte-related genes, Collagen I, TNMD, and SCX, were evaluated by qRT-PCR. * p< 0.05. # p<0.05 compared with the values for the control group.
Discussion
Current literature reports indicate that either TA or local anesthetics can have significant detrimental effects on tendon cells [3,6,8,11,19,20]. However, the combined effects of TA and local anesthetics were unknown. This study shows that TA has cytotoxic effect on tenocytes in vitro, which is synergistically increased when used in conjunction with 1% lidocaine.
Many studies have been performed to investigate the effects of glucocorticoids on cultured tendon cells [18,21,22]. They showed that TA could decrease cell viability, induce apoptosis, block cell migration, and reduce collagen secretion of tendon cells [3,19]. Our study shows that tenocytes cultured with TA have a flattened shape. TA, when exposed to tenocytes in vitro, also significantly decreased cell viability. The expressions of tenocyte-related genes in tenocytes were assessed in our study. Tenocytes showed significantly lower mRNA levels of collagen I and SCX in the TA-treated group than in the control group. Collagen I and SCX are important for tendon stability and early tenogenic differentiation [23,24]. These results might account for the poor healing in tendons after injection of TA. TA is commonly used to treat a wide range of tendon tissue disorders; therefore, we selected it for use in our study. Effects of other glucocorticoids on tenocytes warrant additional investigation.
Several other investigators have studied the effects of local anesthetics on tendon cells in vitro and in vivo [6,8]. Piper et al. observed a dose-dependent toxicity of lidocaine to tenocytes [8]. Lehner et al. discovered a severe, reactive oxygen species-mediated effect of bupivacaine on tendon cells [6]. Moreover, functional damage of tendon tissue was also elicited by bupivacaine. Because local anesthetics are commonly used with corticosteroids injection, our study attempted to show the combined effect of lidocaine and TA on tenocytes. This study showed that lidocaine affects not only morphology, but also functional activities of tenocytes. We showed that the deleterious effects of TA were enhanced by the administration of lidocaine. Therefore, it we suggest that the combination of TA and lidocaine has greater adverse effects on tendon tissues than TA alone. These results are consistent with previous reports describing the toxicity of lidocaine in other cell types [17]. Future in vivo studies are necessary to determine whether the combination of TA and lidocaine could induce the increased risk for tendon pathology. TA injection in combination with lidocaine should be used with caution due to the deleterious effects.
One limitation of our study is that the underlying molecular mechanisms that are responsible for the deleterious effects of TA and lidocaine in this study are yet to be determined and need to be elucidated in further investigations. Moreover, we chose lidocaine because it is widely used. It was reported that ropivacaine could carry a lower risk for tenocyte toxicity [8]. Therefore, future studies should include other local anaesthetics and TA combinations.
Conclusions
Our findings indicate that TA affects the properties of tenocyte by blocking the migration, decreasing the cell viability, and inhibiting the functional activities of the tenocytes. Moreover, 1% lidocaine synergistically increased the deleterious effects of TA. This in vitro study provides information on the detrimental effects of these drugs on tendon tissues. The use of TA with lidocaine in humans should be done with caution given the outcomes of this study.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Source of support: This work was supported by grants from the National Natural Sciences Foundation of China (No. 30901516, 81272049, 81150024), Reserve Talents of Universities Overseas Research Program of Heilongjiang, Foundation for University Key Teacher of Heilongjiang Province of China (1253G042), Natural Science Foundation of Heilongjiang (QC2011C049), Natural Science Foundation of Wuliande (WLD-QN1408), and Scientific Research Foundation for Young People of the Second Hospital of Harbin Medical University (QN-2012-02)
References
- 1.Nichols AW. Complications associated with the use of corticosteroids in the treatment of athletic injuries. Clin J Sport Med. 2005;15:370–75. doi: 10.1097/01.jsm.0000179233.17885.18. [DOI] [PubMed] [Google Scholar]
- 2.Blair B, Rokito AS, Cuomo F, et al. Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg Am. 1996;78:1685–89. doi: 10.2106/00004623-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Tempfer H, Gehwolf R, Lehner C, et al. Effects of crystalline glucocorticoid triamcinolone acetonide on cultered human supraspinatus tendon cells. Acta Orthop. 2009;80:357–62. doi: 10.3109/17453670902988360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapetanos G. The effect of the local corticosteroids on the healing and biomechanical properties of the partially injured tendon. Clin Orthop Relat Res. 1982;163:170–79. [PubMed] [Google Scholar]
- 5.Hugate R, Pennypacker J, Saunders M, et al. The effects of intratendinous and retrocalcaneal intrabursal injections of corticosteroid on the biomechanical properties of rabbit Achilles tendons. J Bone Joint Surg Am. 2004;86:794–801. doi: 10.2106/00004623-200404000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Lehner C, Gehwolf R, Hirzinger C, et al. Bupivacaine induces short-term alterations and impairment in rat tendons. Am J Sports Med. 2013;41:1411–18. doi: 10.1177/0363546513485406. [DOI] [PubMed] [Google Scholar]
- 7.Girard AC, Atlan M, Bencharif K, et al. New insights into lidocaine and adrenaline effects on human adipose stem cells. Aesthetic Plast Surg. 2013;37:144–52. doi: 10.1007/s00266-012-9988-9. [DOI] [PubMed] [Google Scholar]
- 8.Piper SL, Laron D, Manzano G, et al. A comparison of lidocaine, ropivacaine and dexamethasone toxicity on bovine tenocytes in culture. J Bone Joint Surg Br. 2012;94:856–62. doi: 10.1302/0301-620X.94B6.29063. [DOI] [PubMed] [Google Scholar]
- 9.Dragoo JL, Braun HJ, Kim HJ, et al. The in vitro chondrotoxicity of single-dose local anesthetics. Am J Sports Med. 2012;40:794–99. doi: 10.1177/0363546511434571. [DOI] [PubMed] [Google Scholar]
- 10.Syed HM, Green L, Bianski B, et al. Bupivacaine and triamcinolone may be toxic to human chondrocytes: a pilot study. Clin Orthop Relat Res. 2011;469:2941–47. doi: 10.1007/s11999-011-1834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scherb MB, Han SH, Courneya JP, et al. Effect of bupivacaine on cultured tenocytes. Orthopedics. 2009;32:26. doi: 10.3928/01477447-20090101-19. [DOI] [PubMed] [Google Scholar]
- 12.Breu A, Rosenmeier K, Kujat R, et al. The Cytotoxicity of Bupivacaine, Ropivacaine, and Mepivacaine on Human Chondrocytes and Cartilage. Anesth Analg. 2013;117:514–22. doi: 10.1213/ANE.0b013e31829481ed. [DOI] [PubMed] [Google Scholar]
- 13.Rahnama R, Wang M, Dang AC, et al. Cytotoxicity of local anesthetics on human mesenchymal stem cells. J Bone Joint Surg Am. 2013;95:132–37. doi: 10.2106/JBJS.K.01291. [DOI] [PubMed] [Google Scholar]
- 14.Carofino B, Chowaniec DM, McCarthy MB, et al. Corticosteroids and local anesthetics decrease positive effects of platelet-rich plasma: an in vitro study on human tendon cells. Arthroscopy. 2013;28:711–19. doi: 10.1016/j.arthro.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Tsai WC, Hsu CC, Pang JH, et al. Low-level laser irradiation stimulates tenocyte migration with up-regulation of dynamin II expression. PLoS One. 2012;7:e38235. doi: 10.1371/journal.pone.0038235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karpie JC, Chu CR. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med. 2007;35:1621–27. doi: 10.1177/0363546507304719. [DOI] [PubMed] [Google Scholar]
- 17.Seshadri V, Coyle CH, Chu CR. Lidocaine potentiates the chondrotoxicity of methylprednisolone. Arthroscopy. 2009;25:337–47. doi: 10.1016/j.arthro.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–33. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 19.Muto T, Kokubu T, Mifune Y, et al. Platelet-rich plasma protects rotator cuff-derived cells from the deleterious effects of triamcinolone acetonide. J Orthop Res. 2013;31:976–82. doi: 10.1002/jor.22301. [DOI] [PubMed] [Google Scholar]
- 20.Haasters F, Polzer H, Prall WC, et al. Bupivacaine, ropivacaine, and morphine: comparison of toxicity on human hamstring-derived stem/progenitor cells. Knee Surg Sports Traumatol Arthrosc. 2011;19:2138–44. doi: 10.1007/s00167-011-1564-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Keenan C, Wang JH. The effects of dexamethasone on human patellar tendon stem cells: implications for dexamethasone treatment of tendon injury. J Orthop Res. 2013;31:105–10. doi: 10.1002/jor.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zargar Baboldashti N, Poulsen RC, Franklin SL, et al. Platelet-rich plasma protects tenocytes from adverse side effects of dexamethasone and ciprofloxacin. Am J Sports Med. 2011;39:1929–35. doi: 10.1177/0363546511407283. [DOI] [PubMed] [Google Scholar]
- 23.Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–20. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- 24.Shukunami C, Takimoto A, Oro M, et al. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–47. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]