Abstract
The post-Communist countries of Central and Eastern Europe and Central Asia are at various stages of development with respect to their capacity to protect human research participants. We examined the impact of two Fogarty-funded programs in this region, the Union Graduate College–Vilnius University Advanced Certificate Program and the Case Western Reserve University Master’s Degree Program, by surveying these programs’ graduates and by examining alumni activities. Alumni have served in leadership roles on research ethics committees, developed and taught new courses in research ethics, and contributed to scholarship. However, political, social, and economic challenges impede the ability of graduates to maximize their effectiveness. Additional curricular attention is needed in research methodology, policy development and implementation, and the interplay between research ethics and human rights.
Keywords: research ethics, capacity building, training
Research is an increasingly global endeavor, and the number of clinical trials conducted in the post-Communist countries of Central and Eastern Europe and Central Asia is growing rapidly. Pharmaceutical companies and contract research organizations continue to move their research into low- and middle-income countries where the cost of conducting clinical trials is lower and patient recruitment is easier. Of the research studies listed in ClinicalTrials.gov, a registry and results database of all public and privately funded clinical trials subject to U.S. Food and Drug Administration (U.S. FDA) regulation, over 14,000 list trial locations in one or more post-Communist countries at the time of writing (U.S. Department of Health and Human Services, 2012). Of these, nearly 3,500 were actively recruiting research subjects. Moreover, this likely underestimates the true amount of research occurring in this region of the world. Not all clinical trials are subject to U.S. registration requirements, and some studies suggest that pharmaceutical and biotech companies may not be registering every trial that occurs in countries like Russia (Patrone, 2010). Moreover, not all research studies involving human subjects are clinical trials of new drugs or devices.
Accompanying the growth in the number of clinical trials in this region is a concomitant demand for increasing numbers of human subjects; the more trials that are being conducted in a particular region of the world, the more volunteers that are needed. However, it is unclear whether the current system for oversight of human subjects research in the post-Communist countries of Central and Eastern Europe and Central Asia provides adequate review of research protocols. These countries are at various stages of development in terms of their capacity to protect research participants, and information about potential procedural and structural deficiencies in human subjects review and oversight is lacking. There are also little data on the efficiency and effectiveness of existing regulatory systems in protecting the rights and safety of research participants. For example, a brief survey in 2000 sent to researchers in Albania, Bulgaria, Croatia, Czech Republic, Estonia, Hungary, Lithuania, Poland, Romania, Russia, and Ukraine revealed that most countries had structures and review committees (such as research ethics committees [RECs] or institutional review boards [IRBs]) in place to oversee clinical research, but provided little information on the effectiveness of those committees (Coker & McKee, 2001). A more recent analysis of human subjects protections in the Baltic countries found well-established procedural mechanisms for the review of human subjects research, but startling differences in the level of review for clinical drug trials and other types of research with human subjects (Gefenas et al., 2010). Similar asymmetries in review were seen in Belarus where RECs suffer from a lack of independence, training, and material and political support (Famenka, 2011).
Despite the lack of information, particularly for the countries of the Commonwealth of Independent States, the U.S. federal government actively funds efforts to support and improve review and oversight of clinical research in post-Communist countries, including several projects funded by the U.S. National Institutes of Health (NIH) Fogarty International Center (FIC) to provide training in research ethics in Eastern Europe and Central Asia. Two such training programs are headed by the authors of this paper, the Union Graduate College–Vilnius University Advanced Certificate Program in Research Ethics for Central and Eastern Europe and the Case Western Reserve University International Research Ethics Training Program (hereafter referred to as the UGC-Vilnius and CWRU programs, respectively). Two additional programs have been established to provide research ethics education in the region—one serving the Balkans and Black Sea and the other serving Turkey and the Central Asian Republics—but at the time of writing neither program had yet graduated a class of alumni. Table 1 lists all FIC bioethics programs accepting trainees from post-Soviet countries, and those grant abstracts can be found on the FIC website at http://www.fic.nih.gov/Grants/Search/Pages/Bioethics-2R25TW007085-05.aspx.
TABLE 1.
FIC Bioethics Programs Accepting Trainees from Post-Soviet Countries.
| Name of Program | Years Funded | Awardee Institutions | Degree or Non-degree | Length of Training Program | Locations of Teaching | Nationalities of Trainees |
|---|---|---|---|---|---|---|
| E-Education in Research Ethics: Central and Eastern Europe | 2004-present | Union Graduate College | Non-degree | 16 month | Online and onsite in Vilnius, Lithuania | Belarus Croatia Czech Republic Estonia FYR Macedonia Georgia Kazakhstan Kyrgyzstan Latvia Lithuania Moldova Poland Romania Russia Serbia Slovakia Ukraine |
| Fogarty nternational Research Ethics Initiative-Turkey/Central Asia | 2012-present | Children’s Hospital Boston | M.A. | 12 month | Boston, MA | Azerbaijan Kazakhstan Kyrgyzstan Tajikistan Turkey Uzbekistan |
| Research Ethics Education Program for the Balkans and Black Sea | 2012-present | Icahn School of Medicine at Mount Sinai | Non-degree | 24 month | Online and onsite in Belgrade, Serbia | Albania Bosnia-Herzegovina Bulgaria FYR Macedonia Montenegro Romania Serbia |
| Training Program in International Research Ethics | 2000-present | Case Western Reserve University | M.A. | 9 months plus time for re-entry project which varies from 3 months to 1 year | Cleveland, OH; Short term trainees in Russia and Romania | Romania Russia Tajikistan |
In this paper, we report on our efforts to: (1) assess research ethics needs/capacity and impediments to human subjects protection in post-Communist countries; (2) examine ways in which trainees of the UGC-Vilnius and CWRU programs contribute to capacity building in Central and Eastern Europe and Central Asia; and (3) develop recommendations and best practices with respect to recruitment, training, placement, and support of trainees and alumni of these and other training programs in the region.
Program Descriptions and Goals
Union Graduate College–Vilnius University: Advanced Certificate Program in Research Ethics for Central and Eastern Europe
Based on the same distance-learning model used for its Masters of Science in Bioethics program, for the past eight years Union Graduate College has offered a graduate level, hybrid online and onsite Advanced Certificate Program in Research Ethics in partnership with the Department of Medical History and Ethics of Vilnius University (Lithuania). The goals of the UGC-Vilnius program are three-fold: to provide trainees with the knowledge and skills necessary to function as independent research ethicists in their home countries; to prepare trainees to act as research ethics educators; and to enable trainees to facilitate institutional and national change to improve research practices and human subjects protections. The program also supports the continued development of a cadre of graduate-level teachers and researchers in research ethics by providing additional pedagogical training and research project support. Included in this support is funding to translate English language materials into Russian, Lithuanian, Georgian, Romanian-Moldovan, and Serbian and Croatian, and develop online courses. Translated materials and course syllabi, developed by faculty and alumni of the UGC-Vilnius program, are publicly available on the program’s website (http://researchethicseurope.com). The program makes its distance-learning platform available to fellows and alumni teaching university courses in local languages. Additionally, the program provides material support to sustain a center of excellence in bio-ethics and research ethics at Vilnius University, and to build and sustain a network of research ethicists in Central and Eastern Europe and Central Asia.
Fellows in the UGC-Vilnius program are clinicians, scientists, academics, lawyers, and administrators recruited from countries of the former Soviet Bloc. They must complete seven graduate-level courses in research ethics, taught in English by European and American lecturers. The program includes two intensive onsite courses held at Vilnius University and other European locations, three online courses, and two hybrid onsite/online practica and project courses. The use of a distance-learning approach means that fellows can be enrolled in the program without the need to leave their homes or jobs for long periods of time. Scholarships are available for promising trainees interested in completing the hybrid online/onsite Masters of Science in Bioethics from the Union Graduate College–Mount Sinai School of Medicine Bioethics Program. To date, 49 trainees from three cohorts have completed the program. Six trainees have completed a Masters of Science in Bioethics from Union Graduate College. By the end of 2013, it is anticipated that 53 fellows will have received the Advanced Certificate.
Case Western Reserve University (CWRU): Training Program in International Research Ethics
Established in 2000, the CWRU training program is conducted in collaboration with two institutional partners: Colegiul Medicilor (equivalent to the American Medical Association) in Romania and PRIZMA, a nongovernmental organization (NGO) focusing on HIV research that is dually headquartered in Moscow and Dushanbe, Tajikistan. Secondary collaborators include the Russian Academy of Sciences and the Academy of Sciences of Tajikistan. Its goals are: to develop a critical mass of professionals in each of the three collaborating countries (Romania, Russia, and Tajikistan) who are trained in international research ethics; to increase the research ethics capacity of institutions and personnel in each of the partner countries; and to provide assistance to collaborators in the development, implementation, and enhancement of research ethics activities, and local training and support networks.
Every year one trainee from Romania, Russia, and Tajikistan is recruited to complete a Masters of Arts in research ethics at Case Western Reserve University in Cleveland, Ohio, followed by a three-month reentry project in their home country. The program also supports, in collaboration with partner institutions in each country, one in-country short course per year, as well as in-country consultations and faculty exchanges that enable scholars from each collaborating country to participate in research ethics–related activities at CWRU. To date, 17 trainees from Russia and Romania have completed the Masters of Arts program. In addition, nearly 1,500 Romanian and 225 Russian trainees have completed one of the in-country short courses that are offered yearly.
Political, Social, and Economic Context
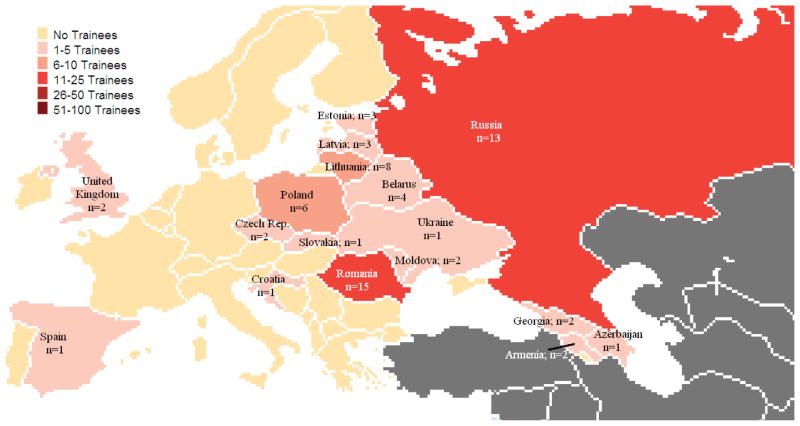
The UGC-Vilnius and CWRU programs train fellows from post-Communist countries that are currently or have in the recent past been considered World Bank–designated low- or middle-income countries (Figure 1). Current fellows and alumni of the UGC-Vilnius program come from 17 different countries: Belarus, Croatia, the Czech Republic, Estonia, the Former Yugoslav Republic of Macedonia (FYROM), Georgia, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Moldova, Poland, Serbia, Slovakia, Romania, Russia, and the Ukraine. CWRU fellows and alumni are from Romania, Russia, and Tajikistan. Most remain in or return to their home country following completion of the UGC-Vilnius or CWRU training programs. To date, only three trainees have immigrated to other countries: two to the United Kingdom and one to Spain.
FIG. 1.
Central and Eastern Europe long-term trainees by country.*
Aside from being a region where countries vary considerably in terms of land mass and population, a key characteristic of the region in which these two programs provide training and support is the considerable political, social, and economic variation among countries. Consider, for instance, the wide variation in the Corruption Perceptions Index (CPI) for countries in the region.1 The CPI provides a rough estimate of perceived corruption in the public sector on a scale of zero to 100, with zero being the most corrupt and 100 being the least corrupt. In 2012, Transparency International ranked Finland as the least corrupt country with a CPI of 90, while Somalia and Afghanistan were ranked the most corrupt with a CPI of 8. Table 2 shows the wide regional variation among the countries served by the UGC-Vilnius and CWRU programs, from the Russian Federation, which has a large landmass and population (143.1 million) and ranks as relatively corrupt (CPI = 28), to adjacent Estonia, which is relatively small in size and population (1.3 million) and is one of the less corrupt countries in the world (CPI = 64).
TABLE 2.
Demographic and Political Characteristics of UGC-Vilnius and CWRU Trainee Home Countries.
| Country | 2012 Population (millions) | Area (km2) | GDP (millions of USD) | Human Development Index [UNDP] | % Below poverty line [CIA] | Health expenditure
|
Corruption Perceptions Index [Transparency International]1* | Gender Inequality Index [UNDP] | Registered clinical trials (active/listed) [ClinicalTrials.gov]§ | |
|---|---|---|---|---|---|---|---|---|---|---|
| %GDP [CIA] | Per capita [WHO] | |||||||||
| Belarus | 9.4 | 207,600 | 63,000 | 50 | 6 | 5.8 | $ 782 | 31 | N.A. | 511 / 1986 |
| Croatia | 4.3 | 56,542 | 57,000 | 47 | 18 | 7.8 | $ 1,556 | 46 | 0.179 | 11 / 472 |
| Czech Republic | 10.5 | 78,866 | 196,000 | 28 | 9 | 7.6 | $ 2,107 | 54 | 0.122 | 474 / 1864 |
| Estonia | 1.3 | 45,226 | 22,000 | 33 | 18 | 4.3 | $ 1,338 | 64 | 0.158 | 86 / 436 |
| FYR Macedonia | 2.1 | 25,713 | 10,000 | 78 | 31 | 6.9 | $ 763 | 43 | 0.162 | 29 / 90 |
| Georgia | 4.5 | 69,700 | 16,000 | 72 | 10 | 11.3 | $ 487 | 52 | 0.436 | 18 / 64 |
| Kazakhstan | 16.8 | 2,724,900 | 196,000 | 69 | 8 | 4.3 | $ 514 | 28 | 0.312 | 6 / 21 |
| Kyrgyzstan | 5.5 | 199,900 | 7,000 | 125 | 34 | 4.1 | $ 148 | 24 | 0.357 | 1 / 3 |
| Latvia | 2.0 | 64,589 | 28,000 | 44 | 6 | 8.1 | $ 1,066 | 49 | 0.216 | 92 / 396 |
| Lithuania | 3.2 | 65,200 | 42,000 | 41 | 4 | 7.8 | $ 1,292 | 54 | 0.157 | 118 / 438 |
| Moldova | 3.6 | 33,843 | 7,000 | 113 | 22 | 11.9 | $ 357 | 36 | 0.303 | 7 / 29 |
| Poland | 38.5 | 312,685 | 488,000 | 39 | 11 | 7.1 | $ 1,391 | 58 | 0.140 | 681 / 2699 |
| Romania | 19.1 | 238,391 | 169,000 | 56 | 21 | 5.4 | $ 818 | 44 | 0.327 | 306 / 1167 |
| Russia | 143.1 | 17,075,400 | 2,022,000 | 55 | 13 | 5.4 | $ 1,043 | 28 | 0.312 | 511 / 1986 |
| Serbia | 7.1 | 88,361 | 92,000 | 64 | 9 | 9.9 | $ 1,162 | 39 | N.A. | 106 / 434 |
| Slovakia | 5.5 | 48,845 | 37,000 | 35 | 21 | 8.5 | $ 2,084 | 46 | 0.171 | 208 / 859 |
| Tajikistan | 7.8 | 143,100 | 7,500 | 125 | 47 | 5.3 | $ 123 | 22 | 0.338 | 1 / 1 |
| Ukraine | 45.7 | 603,628 | 176,000 | 78 | 35 | 7.0 | $ 485 | 26 | 0.338 | none recorded |
Corruption Perception Index (CPI) and CPI World Ranking as reported by Transparency International for 2012. The CPI is an estimate of perceived corruption in the public sector (0–100, with 0 being the most corrupt and 100 being the least corrupt. For the 176 countries for which Transparency International has data, Finland is the least corrupt (CPI = 90) and Somalia and Afghanistan the most corrupt (CPI = 8 for both).
As listed on April 12, 2012 in the NIH-run ClinicalTrials.gov database.
Comparative analysis of neighboring Lithuania and Belarus (Famenka, 2013) offers an example of the contrast in the enabling and developmental conditions that influence research ethics programs and protections in the various countries. Lithuania (population 3.5 million) and Belarus (population 9.5 million) share a similar culture and a similar fate of being incorporated into the Soviet Union. They have diverged considerably with respect to social, political, and economic developments since achieving independence in the 1990s. For example, Lithuania embraced market reform and privatization. In 1993 it joined the Council of Europe and in 2004 joined the European Union, indicating that it achieved acceptable progress in the development of democratic and economic institutions. By contrast, Belarus is neither a member of the EU nor the Council of Europe, and is considered by organizations like Freedom House to be one of “the world’s most repressive societies” (Freedom House, 2011).
Lithuania and Belarus took markedly different paths in structuring their regulatory frameworks for research ethics review (Famenka, 2013). The Lithuanian model consists of a national body—the Lithuanian Bioethics Committee (LBC)—and two regional research ethics committees, whereas Belarus relies on a network of 50 RECs associated with teaching hospitals, research institutions, and universities. Not only are the challenges of overseeing and managing 50 RECs considerable in comparison to a single national and two regional committees, but Belarus also lacks the statutory and regulatory framework to support and assure ethics committee performance. Moreover, there appears to be a disproportionate number of Belorussian RECs related to need. According to the Center for Expertise and Testing in Health Care of the Republic of Belarus, in 2008 there were only 28 clinical drug trials (9 of these being multi-center trials) in a country of less than 10 million people (Famenka, 2011). By contrast, a recent report of the Lithuanian Bioethics Committee (available at http://bioetika.sam.lt/index.php?526052694) found that there were 88 clinical drug trials and 88 other biomedical research projects approved by the LBC or one of the two regional RECs in 2012.
Assessment of Programmatic Achievements
We used the framework developed by Hyder et al. (2009) to define research ethics capacity. That conceptualization of the research ethics system identifies four system-linked determinants of human subjects protection: (1) research ethics review function; (2) institutional commitments, e.g., organizational structures and procedures, and conformity with national and regional laws and guidance; (3) researcher conduct, e.g., respect for government, institutional, and ethics committee policies, procedures, and recommendations; and (4) national and regional capacity, e.g., legal and regulatory authority for RECs, national guidelines, budget priorities for research ethics, investment in training and capacity building. Surrounding this system are enabling conditions, including strong civil society, public accountability, and trust in basic transactional processes. In turn these are surrounded by development conditions including political freedoms, economic facilities, social opportunities, and transparency guarantees.
For this analysis, we gathered information from three sources: an Internet-based survey of UGC-Vilnius and CWRU alumni, case studies of individual alumni accomplishments and achievements since completing their respective training programs, and a comparative analysis of research ethics structures in two post-Communist countries conducted by a UGC-Vilnius alumnus. The contributions that UGC-Vilnius and CWRU alumni made in building research ethics capacity in post-Communist countries were categorized according to the Hyder et al. systems model. Examples of this categorization include: (1) serving on institutional, regional, or national RECs (research ethics review); (2) building institutional capacity by establishing RECs and training REC members (institutional commitments); (3) teaching courses on research ethics and integrity to future researchers (researcher conduct); and (4) teaching and scholarship aimed at policy and program change, establishing, supporting, and serving on oversight and regulatory agencies and on advocacy, educational, and professional bodies dedicated to human subjects protection (national and regional capacity).
Program success in building research ethics capacity is measured by the extent to which alumni participate in activities corresponding to the above four categories.
ALUMNI SURVEY
Alumni of the UGC-Vilnius and CWRU training programs were asked to complete a brief survey (up to 40 multiple-choice questions and short-answer questions, with selection of some questions being dynamic based on prior responses). Questions focused on contributions to building research ethics capacity in their home countries and institutions, identifying impediments to human subjects protection, and suggestions as to additional skills and competencies that should be given greater emphasis in training curricula. A copy of the survey questions is available for download on the UGC-Vilnius program website (http://researchethicseurope.com).
The survey was developed and administered using the web-based tool Survey Monkey, and distributed to the current list of UGC-Vilnius and CWRU program alumni. Alumni were sent a cover letter describing the project, asking for their participation, and containing a link to the online survey. No e-mails were returned as undeliverable, suggesting that the survey was distributed to 100% of potential respondents. Two e-mail reminders were also sent.
Results
Of the 37 alumni of the UGC-Vilnius program, 34 completed the survey, giving an overall response rate of 92%. Of the 17 trainees who have completed the CWRU Master of Arts program, 13 completed the survey, giving an overall response rate of 76.5%.
Table 3 summarizes program alumni contributions. Of particular note are the significant roles that UGC-Vilnius alumni in particular played in policy research, development, and evaluation at the local, national, and international levels. Alumni established eight RECs, including the first research ethics committee in the Republic of Moldova. Twelve UGC-Vilnius and CWRU alumni serve on committees or commissions that are directly involved in public policy making, including the Estonian Council on Bioethics, the Bioethics Committee of the Polish Academy of Sciences, the Bioethics Committee of the Council of Europe (DH-BIO), the UNESCO executive group on bioethics education in Belarus, the Ethics Section of the Romanian College of Physicians, and the Central Asian Bioethics Association. Other notable policy-related accomplishments by alumni include: the establishment of the Center of Bioethics within the Polish National Chamber of Physicians and Dentists; establishing new rules and codes of ethics on research at universities in the Czech Republic and Poland; drafting policy recommendations on regulation of genetic research that were adopted by the Inter-Parliament Assembly of the Confederation of Independent States (CIS); drafting policy recommendations on healthcare ethics that were adopted by the Parliament of the Czech Republic; and serving as Counselor on bioethical problems for the Moldovan Minister of Health.
TABLE 3.
Trainee Achievements as Determined in Response to Select Alumni Survey Questions.
| Research Ethics–Related Activities | % (N) of Respondents
|
|
|---|---|---|
| UGC-Vilnius | CWRU | |
| REC Activities | 34.4% (12/35) | 28.6% (4/10) |
| REC Member | 58.3% (7/12) | 100.0% (4/4) |
| REC Chair | 33.3% (4/12) | 50.0% (2/4) |
| REC Administrator | 16.7% (2/12) | 50.0% (2/4) |
| Training of Members | 58.3% (7/12) | 25.0% (1/4) |
| Established REC | 41.7% (5/12) | 75.0% (3/4) |
| Appointed After Completing Program | 50.0% (6/12) | 75.0% (3/4) |
| Policy-Making Committees/Commissions | 25.7% (9/35) | 21.4% (3/14) |
| Research/Needs Assessment | 11.1% (1/9) | 0.00% (0/3) |
| Preparation of Position Papers | 55.6% (5/9) | 33.3% (1/3) |
| Drafting of Policies or Laws | 66.7% (6/9) | 0.00% (0/3) |
| Implementation of Policies/Laws | 0.00% (0/9) | 0.00% (0/3) |
| Monitoring and Oversight | 11.1% (1/9) | 0.00% (0/3) |
| Evaluation of Policies and Laws | 66.7% (6/9) | 33.3% (1/3) |
| Education | 22.2% (2/9) | 33.3% (1/3) |
| Appointed After Completing Program | 66.7% (6/9) | 100.0% (3/3) |
| Education and Teaching | — | — |
| Credit-Granting Courses | 62.9% (22/35) | 38.5% (5/13) |
| Short Courses and Workshops | 65.7% (23/35) | 61.5% (8/13) |
| Conferences and Symposia | 45.7% (16/35) | 41.7% (5/13) |
| Public Lectures | 51.4% (18/35) | 66.7% (8/12) |
| Advocacy/Activism | 40.0% (18/35) | 66.7% (8/12) |
| Media Interviews | 28.6% (10/35) | 58.3% (7/12) |
| Websites | 37.1% (13/25) | 66.7% (8/12) |
| Independent Research and Scholarship | — | — |
| Peer-Reviewed Articles | 67.6% (23/35) | 50.0% (6/12) |
| Invited Presentations/Lectures | 70.6% (24/35) | 41.7% (5/12) |
| External Awards/Grants | 32.4% (11/35) | 16.7% (2/12) |
In addition to collecting information on alumni achievements, the survey explored perceived barriers to building research ethics capacity in the post-Communist countries of Central and Eastern Europe and Central Asia. For example, alumni were asked the following question: “Given your knowledge of conditions in your home country, what do you consider to be the major impediments to protecting the rights and well-being of human research subjects?” Answers were grouped into categories adapted from Hyder’s component model: (1) environmental conditions (combining enabling and developmental conditions; (2) national and institutional commitment (combining national and regional strategies and institutional commitment; (3) clinical researcher conduct; and (4) REC expertise and commitment (research ethics review). These results, summarized in Table 4, include widespread corruption, lack of human rights for stigmatized and vulnerable populations, gaps in research ethics legislation and regulation, uncontrolled conflicts of interest, and inadequate training for researchers and REC members.
Similarly, alumni of the UGC-Vilnius and CWRU programs were asked to indicate which research ethics skills and competencies should be given more emphasis in the curriculum. Interestingly, the provision of training in the ethical review and oversight of human subjects protocols was ranked the lowest. Rather, they thought trainees should also be taught other skills, of which the most important were pedagogy (e.g., developing and teaching courses in ethics) and policy formulation, implementation, and evaluation. There was also a general sentiment that additional training in qualitative and quantitative research methodology was needed. This training is important not only to enable trainees to establish themselves as independent research ethicists, but also to equip those who are serving as members of research ethics review committees with the skills necessary to review protocols. These data indicate that research ethics training initiatives like UGC-Vilnius and CWRU programs need to do more to help trainees develop new educational offerings (including providing material support by way of curricula, translated materials, and online educational platforms) and provide trainees with the resources and tools to conduct independent research aimed at identifying and rectifying systemic and regulatory gaps.
Case Studies
The following vignettes illustrate the ways in which trainees of the UGC-Vilnius and CWRU programs contributed to research ethics capacity building in Central and Eastern Europe and Central Asia. Vignettes were selected to highlight four categories of contributions described in the alumni survey results above, including: leadership on research ethics committees, development and teaching of new courses in research ethics, publication and presentation of original papers and research, and service on other committees and commissions. The selected alumni from the UGC-Vilnius and CWRU programs represent a variety of different disciplines: science, medicine, philosophy, and law. Permission to publish these case studies was obtained from all trainees identified.
LEADERSHIP ON RESEARCH ETHICS COMMITTEES
Joanna Rozynska, PhD (Poland; UGC-Vilnius)
Dr. Rozynska developed and implemented policies establishing an REC at the Warsaw University of Physical Education. One of the first graduates of the UGC-Vilnius program, Dr. Rozynska is currently a faculty member in the Advanced Certificate Program. She also helped to develop the first Masters of Bioethics Program in Poland, first offered at the University of Warsaw in 2013. She is also the Head of the Polish Unit of the UNESCO Chair in Bioethics, member and Secretary of the Bioethics Committee of the Polish Academy of Sciences, and a member of the National Committee for Cooperation with the European Network of Research Integrity Offices.
Christina Gavrilovici, MD (Romania; CWRU)
Since Dr. Gavrilovici’s completion of the CWRU training program, she served in the following roles: Chair of the ethical review committee for scientific grants at the Grigore T. Popa University of Medicine and Pharmacy in Iaşi, member of the European Commission’s Descartes Great Jury for Excellence in European Research, subject matter expert for the ethical review of scientific grants financed by the European Commission, Chair of the Grigore T. Popa University of Medicine and Pharmacy IRB, and Chair of the Pediatric Hospital Ethics Committee.
DEVELOPMENT AND TEACHING OF NEW COURSES IN RESEARCH ETHICS
Renata Veselska, PhD (Czech Republic; UGC-Vilnius)
Dr. Veselska is currently Associate Professor of Molecular Biology and Head of the Laboratory of Tumor Biology at Masaryk University in Brno. She is also a researcher in the Department of Pediatric Oncology at the University Hospital, where she serves as a mentor and role model for young scientists and clinicians. Recently, she developed several ethics courses that were officially incorporated into the academic curriculum at Masaryk, which she teaches in addition to her other courses on molecular and cell biology.
Catalin Cazacu (Romania; CWRU)
Mr. Cazacu played an important role as a member of a team that developed a course focused on discrimination against Roma in Romania and ethical issues related to their access to health care and participation in research. This course and the accompanying text received national attention from the Romanian government.
PUBLICATION AND PRESENTATION OF ORIGINAL PAPERS AND RESEARCH
Vents Silis, PhD (Latvia; UGC-Vilnius)
As part of his training project, Dr. Silis conducted an analysis of the Latvian research ethics system. He found that the Central Medical Ethics Committee of Latvia, formally assigned the responsibility of coordinating and supervising RECs, was inadequately funded and trained. Following publication of his findings in English- and Latvian-language journals, he was invited by the Chairman of the Central Medical Ethics Committee to become the Executive Secretary. His responsibilities, in addition to coordinating the work of the committee, include: assessment of compliance with ethical norms on national and international biomedical research, including those related to new medical technologies; assessment of the documents produced by the Council of Europe Committee on Bioethics; and serving as liaison to national and international institutions interested in biomedical ethics.
Alexandra Kurlenkova (Russia) and Ana Gabriela Benghiac (Romania; CWRU)
These two CWRU trainees increased the visibility and credibility of research ethics as a legitimate field of inquiry in their countries by establishing materials devoted to bioethics and research ethics. Alexandra Kurlenkova established and serves as managing editor of an e-journal, Medical Anthropology and Bioethics, published in Russian and English, which is the one of the first Russian journals to explore issues arising at the intersection of anthropology and bioethics. Dr. Benghiac developed a series of videos, available via the Internet, that address the rights of research participants. This resource is accessible and educational for nonprofessionals as well as members of research ethics committees. It is the first time that such resources have been made available to the general Romanian population.
SERVICE ON OTHER COMMITTEES AND COMMISSIONS
Marek Czarkowski, MD, PhD (Poland; UGC-Vilnius)
In 2007, Dr. Czarkowski, a practicing physician and researcher with Department of Endocrinology and Internal Medicine of the Medical University of Warsaw, lobbied successfully for the Polish Chamber of Physicians and Dentists to form a Center for Bioethics of the Supreme Medical Council of Poland. Functions of that Center, which Dr. Czarkowski directs, are to investigate bioethical issues in Poland, focus attention on gaps in laws, regulations, and practices, and make recommendations to the Polish Government.
Beatrice Ioan, MD, JD (Romania; CWRU)
As a forensic pathologist and lawyer by training, since completing the CWRU program Dr. Ioan has served as President of the Bioethics Commission of the Romanian College of Physicians (2006–present); President of the Superior Discipline Commission of the Romanian College of Physicians (2007–present), Vice Dean of the Faculty of Medicine of Iasi, Romania (2007–present), and Vice Editor in Chief of the Romanian Journal of Bioethics (2003–present).
Discussion
Past Challenges and Future Needs
CURRICULAR CHALLENGES
Current curricula (for the training programs described here and similar programs in other countries and regions) tend to place considerable emphasis on the theory and practice of ethical review of research protocols in preparing its fellows to serve on research ethics committees. The process of preparing trainees to serve on RECs is well understood. Specially designed case studies, role-playing exercises that simulate REC decision making, and the ready availability of other curricular materials make competency building in research ethics review a straightforward task.
However, as found through surveys of current trainees and alumni as well as through country-level analyses such as Silis (2011) and Famenka (2011), in many post-Communist countries in Central and Eastern Europe and Central Asia these committees are often floundering in an indifferent or hostile environment. Accordingly, to accomplish their capacity-building aims, training programs must also prepare their fellows to be “agents of change,” when serving as educators, researchers, and advocates. Preparing trainees to serve as ethics educators, researchers, and advocates is complex. While fellows can and do learn certain specific skills through graduate-level courses (e.g., adult pedagogy and grant writing), developing the competencies necessary to serve in these roles depends to a certain extent on the knowledge and skills trainees bring with them when they enter the program. Thus, it is important that program recruitment and selection criteria be carefully designed to reflect this (e.g., by recruiting fellows who have some prior experience as educators or as advocates).
POLITICAL, SOCIAL, AND ECONOMIC VARIATION
Given the considerable political, social, and economic variation seen among the countries of Central and Eastern Europe and Central Asia, transnational training programs face structural challenges in recruiting and retaining fellows, designing the curricula, and calibrating expectations for alumni to match the reality in individual countries. In Estonia, for example, alumni of the UGC-Vilnius program made significant contributions by serving and leading institutional and national RECs and in formulating ethical review policy. This is due in part to both the small size of Estonia and its well-developed regulatory structures for research ethics review. All of the Estonian alumni know each other, all are associated with a major university, and all are institutionally connected. Thus, this small group of trainees was able to form a critical mass for change. By contrast, the four Russian alumni of the UGC-Vilnius program were less successful in promoting and achieving institutional change. None of these alumni, who are in different, distant cities, were involved in REC activity. Even if they were, their impact would be limited. In Russia, ethical review of protocols is mainly required for clinical drug trials, although other biomedical research projects are, in principle, reviewed by local RECs. The Board of Ethics of the Ministry of Health centrally reviews all clinical drug protocols, with regional and institutional RECs playing a minimal role. Given this restricted role, the ability of alumni to facilitate change by serving on local ethics committees is limited.
Finally, ongoing political and economic instability in some countries can disrupt the professional and personal lives of trainees and alumni.
ADMINISTRATIVE CHALLENGES
The eligibility of trainees to participate in programs funded by the Fogarty International Center is linked to the World Bank’s definition of their home country’s income level: only those from low- and middle-income countries can participate. As described previously, particularly for large countries like Russia, it may take years to train enough alumni and to build the institutional capacity necessary to create a “critical mass” for change. Over the past nine years, countries like Croatia, the Czech Republic, Estonia, Latvia, Lithuania, Poland, Russia, and Slovakia became ineligible when reclassified as high-income countries. These are countries where the programs invested considerable time and effort in building institutional connections with the anticipation of further advances in developing research ethics capacity. While the UGC-Vilnius and CWRU programs achieved a critical mass of trained ethicists in some of these countries, for other countries this was not accomplished in the limited time available. Uncertainty over whether a country will progress to the upper-income level and become ineligible to participate in the Fogarty training programs presents an administrative challenge to programs as they decide how to manage resources.
Current Needs and Recommendations
Current needs differ across the various countries because of variations in the degree to which research ethics infrastructure has been developed and the availability of in-country resources focused on research ethics. Consequently, the task of capacity building will differ, which poses a challenge for programs attempting to design transnational curricula in support of research ethics capacity building. Despite this, our analysis found several common themes that resonate across all of the countries in the region.
For example, recent graduates of both the UGC-Vilnius and CWRU programs noted the need for additional training in the skills necessary to formulate, implement, and evaluate policy, and to influence policy development. Respondents also underscored the need to change the institutional climate to garner more support for research ethics. The integration of a policy focus into the current training programs will require additional knowledge of in-country mechanisms for policy formulation on the part of the program directors and likely require the initiation or enhancement of program participation by policy makers in each of the participating countries.
Survey respondents felt that there was less necessity for training in the review of research protocols involving human participants. However, this perspective—which likely reflects the fact that many trainees already have experience in human subjects protocol review prior to entering the UGC-Vilnius or CWRU programs—conflicts with their frequent observation that few individuals in their respective countries have knowledge of research ethics. This suggests that training of research ethicists in protocol review and oversight is necessary, but insufficient. In order to address the general lack of knowledge related to research ethics, program directors may wish to work with past and current trainees on the development of curricular materials that can be widely disseminated within their home countries through diverse mechanisms to a broad research audience.
Responses to a question about current impediments also provided insight into the current needs in the region. Many graduates of both the certificate and degree programs noted the lack of oversight of research outside of biomedical fields or clinical trials, such as social science research. The rights of stigmatized and vulnerable populations such as minorities and mentally ill persons, and the lack of understanding of these populations by members of the RECs point to the need to focus training on understanding the concept of vulnerability within the context of each country and the relationship between human rights and research ethics (Eckenwiler et al., 2008; Hurst, 2008). Graduates also noted the general lack of understanding of research within the general population and the need to better inform members of the public about their rights as research participants. These concerns suggest the need to provide additional programmatic focus on communication and public engagement skills.
Educational Implications
Training programs in research ethics should be expanded to include increased emphasis on four areas: first, to support the efforts of past and current trainees to develop additional curricular materials in research ethics that can be disseminated in their home countries, with the goal of increasing researcher knowledge and understanding of research ethics; second, to provide training in policy development, implementation, and evaluation at the institutional, local, and national levels, including practical training in how to use a variety of mechanisms to effect policy change; third, to train and support trainees in developing and disseminating lay-language materials designed to increase the level of understanding of the general population of research and research ethics; and finally, to teach trainees how to recognize and leverage the interplay between research ethics, particularly in regard to vulnerable populations (see, e.g., Loue, 2013), and human rights and associated international and national obligations.
TABLE 4.
UGC-Vilnius and CWRU Alumni Perceptions of Key Barriers to Building Research Ethics Capacity in Post-Communist Countries.
| Component Model Category | Perceived Barrier |
|---|---|
| Enabling and Developmental Conditions | Decision making driven by economics |
| Legal nihilism | |
| Lack of public transparency | |
| Medical paternalism | |
| Political, judicial, and institutional corruption | |
| Lack of human rights for stigmatized and vulnerable populations | |
| National and Institutional Commitment | Lack of research ethics legislation and policies |
| Lack of regulatory enforcement | |
| Lack of institutional support for RECs | |
| Lack of an organized structure to collect information on REC effectiveness | |
| Clinical Researcher Conduct | Inadequate knowledge and training |
| Lack of funding for research ethics training programs | |
| Uncontrolled conflicts of interest | |
| REC Expertise and Commitment | Lack of transparency in appointments and review |
| Lack of procedural and regulatory clarity | |
| Lack of training of REC members |
Acknowledgments
The projects described herein were made possible by grants R25 TW007085 (E-Education in Research Ethics: Central and Eastern Europe; M. Strosberg, E. Gefenas, and S. Philpott) and Grant R25 R25TW001603 (International Research Ethics Training Program; S. Loue) from the U.S. National Institutes of Health (NIH) Fogarty International Center.
Biographies
Martin Strosberg is Professor of Bioethics and Healthcare Policy at the Center for Bioethics and Clinical Leadership of Union Graduate College. His interests are in policy public and management. He had overall responsibility for the writing of the manuscript.
Eugenijus Gefenas is Associate Profess or and Director of the Department of Medical History and Ethics at the Medical Faculty of Vilnius University. The areas of his professional interest include the ethical, philosophical, and public policy–making issues related to human research. His role in this paper was to edit the paper in collaboration with the other authors.
Sana Loue is Professor of Bioethics of Case Western Reserve University. She has directed the Fogarty-funded training program in research ethics since 2000. Her research interests include HIV risk and prevention, severe mental illness, family violence, research ethics, and forensic epidemiology. She was responsible for the development of the online survey of Case Western Reserve University trainees, and also contributed to the writing of the manuscript.
Sean Philpott is Associate Professor of Bioethics and Director of the C enter for Bioethics and Clinical Leadership of Union Graduate College. Before joining Union Graduate College he was Science and Ethics Officer at the international nonprofit organization PATH, where his work focused on building the capacity of developing country researchers and stakeholders to design, conduct, and monitor clinical trials. He designed, conducted, and analyzed the survey, and wrote and edited portions of the manuscript.
Footnotes
Developed by the NGO Transparency International (http://www.transparencyinternational.org), the CPI is commonly used as a comparator for government performance and is strongly correlated with markers of economic development such as real gross domestic product per capita; one study found that the Corruption Perceptions Index could explain over three fourths of the variance in real gross domestic product per capita between countries (Wilhelm 2002).
Not shown: 2 long-term trainees from Kazakhstan, 1 from Krygyzstan, and 4 from Tajikistan.
The contents are solely the responsibility of the authors and do not necessarily represent the official view of the Fogarty International Center or the NIH.
Contributor Information
Martin A. Strosberg, Union Graduate College (USA)
Eugenijus Gefenas, Vilnius University (Lithuania).
Sana Loue, Case Western Reserve University (USA).
Sean Philpott, Union Graduate College (USA).
References
- Coker R, McKee M. Ethical approval for health research in Central and Eastern Europe: An international survey. Clinical Medicine. 2001;1:197–199. doi: 10.7861/clinmedicine.1-3-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenwiler LA, Ells C, Feinholz D, Schonfeld T. Hopes for Helsinki: Reconsidering “vulnerability. Journal of Medical Ethics. 2008;34:765–766. doi: 10.1136/jme.2007.023481. [DOI] [PubMed] [Google Scholar]
- Famenka A. Ethical review of biomedical research in Belarus: Current status, problems and perspectives. Romanian Journal of Bioethics. 2011;2:74–83. [PMC free article] [PubMed] [Google Scholar]
- Famenka A. Assessing research ethics capacity of the post-Soviet transition societies from a public policy perspective: The case of Lithuania and Belarus. 2013. In preparation. [Google Scholar]
- Freedom House. Freedom in the world: The authoritarian challenge to democracy. 2011 Retrieved from www.freedomhouse.org/report/freedom-world-2011/essay-freedom-world-2011-authoritarian-challenge-democracy.
- Gefenas E, Dranseika V, Cekanauskaite A, Hug K, Mezinska S, Peicius E, et al. Non-equivalent stringency of ethical review in the Baltic states: A sign of a systematic problem in Europe? Journal of Medical Ethics. 2010;36:435–439. doi: 10.1136/jme.2009.035030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst S. Vulnerability in research and health care: Describing the elephant in the room? Bioethics. 2008;22:191–202. doi: 10.1111/j.1467-8519.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- Hyder AA, Dawson L, Bachani AM, Lavery J. Moving from research ethics review to research ethics systems in low-income and middle-income countries. Lancet. 2009;373:862–865. doi: 10.1016/S0140-6736(09)60488-8. [DOI] [PubMed] [Google Scholar]
- Loue S. Is there a universal understanding of vulnerability: Experiences with Russian and Romanian trainees in research ethics. Journal of Empirical Research in Human Research Ethics. 2013 doi: 10.1525/jer.2013.8.5.17. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization for Security and Cooperation in Europe (OSCE), Office for Democratic Institutions Human Rights. Belarus, Presidential Election, December. 2010;19:2010. Final report, OSCS/ODIHR election observation mission report. Retrieved from www.osce.org/odihr/elections/75713. [Google Scholar]
- Patrone D. Discrepancies between research advertisements and disclosure of study locations in trial registrations for U.S.-sponsored research in Russia. Journal of Medical Ethics. 2010;36:431–434. doi: 10.1136/jme.2010.035378. [DOI] [PubMed] [Google Scholar]
- Silis V. Research ethics system in Latvia: Structure, functioning and problems. DILEMATA–International Journal of Applied Ethics. 2011;2:55–59. [Google Scholar]
- United States Department of Health and Human Services (U.S. DHHS), National Institutes of Health (NIH) 2012 ClinicalTrials.gov.
- Wilhelm PG. International validation of the Corruption Perceptions Index: Implications for business ethics and entrepreneurship education. Journal of Business Ethics. 2002;35:177–189. [Google Scholar]