Abstract
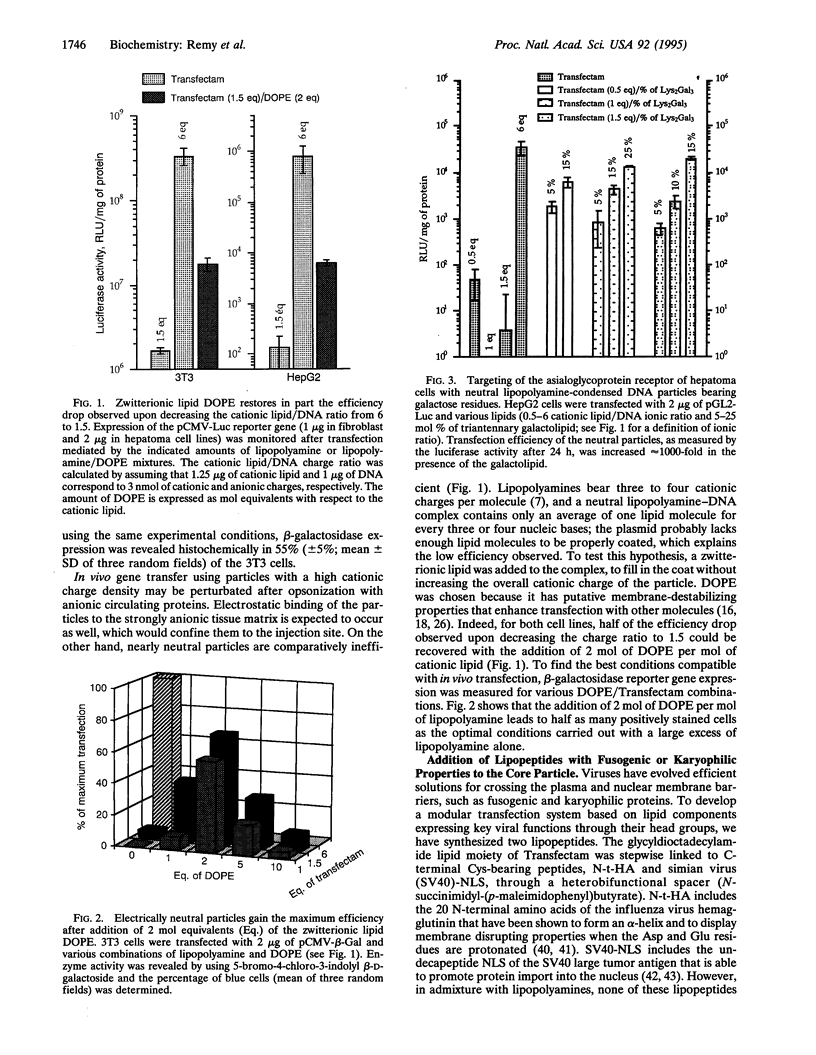
Optimal in vitro gene delivery with cationic lipids requires an excess of cationic charges with respect to DNA phosphates. In these conditions, in vivo delivery will be hampered by interference from cationic lipid-binding macromolecules either circulating or in the extracellular matrix. To overcome this problem, we are developing a modular transfection system based on lipid-coated DNA particles reminiscent of enveloped viruses. The particle core consists of the lipopolyamine-condensed nucleic acid in an electrically neutral ratio to which other synthetic lipids with key viral properties are hydrophobically adsorbed. As a first result, we have found that a good transfection level can be achieved simply with the neutral core particle, provided a zwitterionic lipid (dioleoyl phosphatidylethanolamine) is added to completely coat the DNA. Addition of lipids bearing a fusogenic or a nuclear localization peptide head group to the particles does not significantly improve an already efficient system, in contrast to polylysine-based gene transfer methods that rely on lysosomotropic or fusogenic agents to be effective. This emphasizes the distinctive properties of the lipopolyamines, including cell membrane destabilization, endosome buffering capacity, and possibly nuclear tropism. Most importantly, addition of lipids with a triantennary galactosyl residue drives the neutral nucleolipidic particles to the asialoglycoprotein receptor of human hepatoma HepG2 cells: Transfection increases approximately 1000-fold with 25% galactolipid. This receptor-mediated process is saturable and slightly less efficient than receptor-independent transfection obtained in vitro with a large excess of cationic lipid alone. Yet, electrically silent particles may provide an attractive solution for gene transfer in vivo where their external saccharide coat should allow them to diffuse within the organism and reach their target cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliño S. F., Bobadilla M., Garcia-Sanz M., Lejarreta M., Unda F., Hilario E. In vivo delivery of human alpha 1-antitrypsin gene to mouse hepatocytes by liposomes. Biochem Biophys Res Commun. 1993 Apr 15;192(1):174–181. doi: 10.1006/bbrc.1993.1397. [DOI] [PubMed] [Google Scholar]
- Alton E. W., Middleton P. G., Caplen N. J., Smith S. N., Steel D. M., Munkonge F. M., Jeffery P. K., Geddes D. M., Hart S. L., Williamson R. Non-invasive liposome-mediated gene delivery can correct the ion transport defect in cystic fibrosis mutant mice. Nat Genet. 1993 Oct;5(2):135–142. doi: 10.1038/ng1093-135. [DOI] [PubMed] [Google Scholar]
- Anderson W. F. Human gene therapy. Science. 1992 May 8;256(5058):808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- Barthel F., Remy J. S., Loeffler J. P., Behr J. P. Gene transfer optimization with lipospermine-coated DNA. DNA Cell Biol. 1993 Jul-Aug;12(6):553–560. doi: 10.1089/dna.1993.12.553. [DOI] [PubMed] [Google Scholar]
- Behr J. P., Demeneix B., Loeffler J. P., Perez-Mutul J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr J. P. Gene transfer with synthetic cationic amphiphiles: prospects for gene therapy. Bioconjug Chem. 1994 Sep-Oct;5(5):382–389. doi: 10.1021/bc00029a002. [DOI] [PubMed] [Google Scholar]
- Burger K. N., Wharton S. A., Demel R. A., Verkleij A. J. The interaction of synthetic analogs of the N-terminal fusion sequence of influenza virus with a lipid monolayer. Comparison of fusion-active and fusion-defective analogs. Biochim Biophys Acta. 1991 Jun 18;1065(2):121–129. doi: 10.1016/0005-2736(91)90221-s. [DOI] [PubMed] [Google Scholar]
- Cotten M., Wagner E. Non-viral approaches to gene therapy. Curr Opin Biotechnol. 1993 Dec;4(6):705–710. doi: 10.1016/0958-1669(93)90053-y. [DOI] [PubMed] [Google Scholar]
- Cotten M., Wagner E., Zatloukal K., Phillips S., Curiel D. T., Birnstiel M. L. High-efficiency receptor-mediated delivery of small and large (48 kilobase gene constructs using the endosome-disruption activity of defective or chemically inactivated adenovirus particles. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6094–6098. doi: 10.1073/pnas.89.13.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Huang L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem Biophys Res Commun. 1991 Aug 30;179(1):280–285. doi: 10.1016/0006-291x(91)91366-k. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Gariépy J., Schoolnik G., Kornberg R. D. Synthetic peptides as nuclear localization signals. Nature. 1986 Aug 14;322(6080):641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- Haensler J., Schuber F. Influence of the galactosyl ligand structure on the interaction of galactosylated liposomes with mouse peritoneal macrophages. Glycoconj J. 1991 Apr;8(2):116–124. doi: 10.1007/BF00731021. [DOI] [PubMed] [Google Scholar]
- Haensler J., Szoka F. C., Jr Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem. 1993 Sep-Oct;4(5):372–379. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- Haensler J., Szoka F. C., Jr Synthesis and characterization of a trigalactosylated bisacridine compound to target DNA to hepatocytes. Bioconjug Chem. 1993 Jan-Feb;4(1):85–93. doi: 10.1021/bc00019a012. [DOI] [PubMed] [Google Scholar]
- Huckett B., Ariatti M., Hawtrey A. O. Evidence for targeted gene transfer by receptor-mediated endocytosis. Stable expression following insulin-directed entry of NEO into HepG2 cells. Biochem Pharmacol. 1990 Jul 15;40(2):253–263. doi: 10.1016/0006-2952(90)90686-f. [DOI] [PubMed] [Google Scholar]
- Hyde S. C., Gill D. R., Higgins C. F., Trezise A. E., MacVinish L. J., Cuthbert A. W., Ratcliff R., Evans M. J., Colledge W. H. Correction of the ion transport defect in cystic fibrosis transgenic mice by gene therapy. Nature. 1993 Mar 18;362(6417):250–255. doi: 10.1038/362250a0. [DOI] [PubMed] [Google Scholar]
- Ito A., Miyazoe R., Mitoma J., Akao T., Osaki T., Kunitake T. Synthetic cationic amphiphiles for liposome-mediated DNA transfection. Biochem Int. 1990 Oct;22(2):235–241. [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Lear J. D., DeGrado W. F. Membrane binding and conformational properties of peptides representing the NH2 terminus of influenza HA-2. J Biol Chem. 1987 May 15;262(14):6500–6505. [PubMed] [Google Scholar]
- Ledley F. D. Hepatic gene therapy: present and future. Hepatology. 1993 Nov;18(5):1263–1273. [PubMed] [Google Scholar]
- Lee Y. C. Binding modes of mammalian hepatic Gal/GalNAc receptors. Ciba Found Symp. 1989;145:80-93, discussion 93-5. doi: 10.1002/9780470513828.ch6. [DOI] [PubMed] [Google Scholar]
- Legendre J. Y., Szoka F. C., Jr Cyclic amphipathic peptide-DNA complexes mediate high-efficiency transfection of adherent mammalian cells. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):893–897. doi: 10.1073/pnas.90.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventis R., Silvius J. R. Interactions of mammalian cells with lipid dispersions containing novel metabolizable cationic amphiphiles. Biochim Biophys Acta. 1990 Mar 30;1023(1):124–132. doi: 10.1016/0005-2736(90)90017-i. [DOI] [PubMed] [Google Scholar]
- Loughrey H. C., Choi L. S., Cullis P. R., Bally M. B. Optimized procedures for the coupling of proteins to liposomes. J Immunol Methods. 1990 Aug 28;132(1):25–35. doi: 10.1016/0022-1759(90)90394-b. [DOI] [PubMed] [Google Scholar]
- Midoux P., Mendes C., Legrand A., Raimond J., Mayer R., Monsigny M., Roche A. C. Specific gene transfer mediated by lactosylated poly-L-lysine into hepatoma cells. Nucleic Acids Res. 1993 Feb 25;21(4):871–878. doi: 10.1093/nar/21.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D. Human gene therapy comes of age. Nature. 1992 Jun 11;357(6378):455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- Muller C. D., Schuber F. Neo-mannosylated liposomes: synthesis and interaction with mouse Kupffer cells and resident peritoneal macrophages. Biochim Biophys Acta. 1989 Nov 17;986(1):97–105. doi: 10.1016/0005-2736(89)90277-0. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Nabel E. G., Plautz G., Nabel G. J. Transduction of a foreign histocompatibility gene into the arterial wall induces vasculitis. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5157–5161. doi: 10.1073/pnas.89.11.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnaduwage P., Schmitt L., Huang L. Use of a quaternary ammonium detergent in liposome mediated DNA transfection of mouse L-cells. Biochim Biophys Acta. 1989 Oct 2;985(1):33–37. doi: 10.1016/0005-2736(89)90099-0. [DOI] [PubMed] [Google Scholar]
- Plank C., Zatloukal K., Cotten M., Mechtler K., Wagner E. Gene transfer into hepatocytes using asialoglycoprotein receptor mediated endocytosis of DNA complexed with an artificial tetra-antennary galactose ligand. Bioconjug Chem. 1992 Nov-Dec;3(6):533–539. doi: 10.1021/bc00018a012. [DOI] [PubMed] [Google Scholar]
- Plautz G. E., Yang Z. Y., Wu B. Y., Gao X., Huang L., Nabel G. J. Immunotherapy of malignancy by in vivo gene transfer into tumors. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4645–4649. doi: 10.1073/pnas.90.10.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Buonocore L., Whitt M. A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. Biotechniques. 1991 Apr;10(4):520–525. [PubMed] [Google Scholar]
- Schwartz A. L. The hepatic asialoglycoprotein receptor. CRC Crit Rev Biochem. 1984;16(3):207–233. doi: 10.3109/10409238409108716. [DOI] [PubMed] [Google Scholar]
- Staedel C., Remy J. S., Hua Z., Broker T. R., Chow L. T., Behr J. P. High-efficiency transfection of primary human keratinocytes with positively charged lipopolyamine:DNA complexes. J Invest Dermatol. 1994 May;102(5):768–772. doi: 10.1111/1523-1747.ep12377673. [DOI] [PubMed] [Google Scholar]
- Stribling R., Brunette E., Liggitt D., Gaensler K., Debs R. Aerosol gene delivery in vivo. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11277–11281. doi: 10.1073/pnas.89.23.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Plank C., Zatloukal K., Cotten M., Birnstiel M. L. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7934–7938. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Zatloukal K., Cotten M., Kirlappos H., Mechtler K., Curiel D. T., Birnstiel M. L. Coupling of adenovirus to transferrin-polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6099–6103. doi: 10.1073/pnas.89.13.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Zenke M., Cotten M., Beug H., Birnstiel M. L. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Y., Wilson J. M., Shalaby F., Grossman M., Shafritz D. A., Wu C. H. Receptor-mediated gene delivery in vivo. Partial correction of genetic analbuminemia in Nagase rats. J Biol Chem. 1991 Aug 5;266(22):14338–14342. [PubMed] [Google Scholar]
- Wu G. Y., Wu C. H. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J Biol Chem. 1987 Apr 5;262(10):4429–4432. [PubMed] [Google Scholar]
- Zhou X. H., Klibanov A. L., Huang L. Lipophilic polylysines mediate efficient DNA transfection in mammalian cells. Biochim Biophys Acta. 1991 May 31;1065(1):8–14. doi: 10.1016/0005-2736(91)90003-q. [DOI] [PubMed] [Google Scholar]
- Zhu N., Liggitt D., Liu Y., Debs R. Systemic gene expression after intravenous DNA delivery into adult mice. Science. 1993 Jul 9;261(5118):209–211. doi: 10.1126/science.7687073. [DOI] [PubMed] [Google Scholar]