Abstract
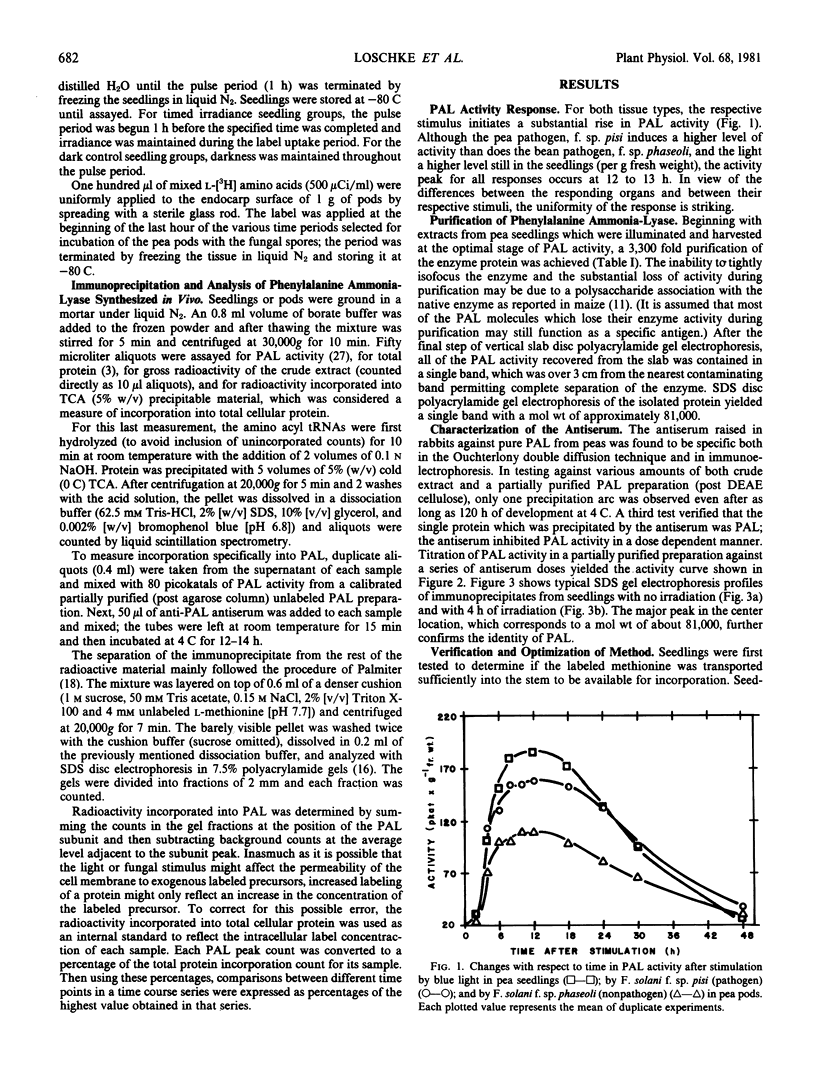
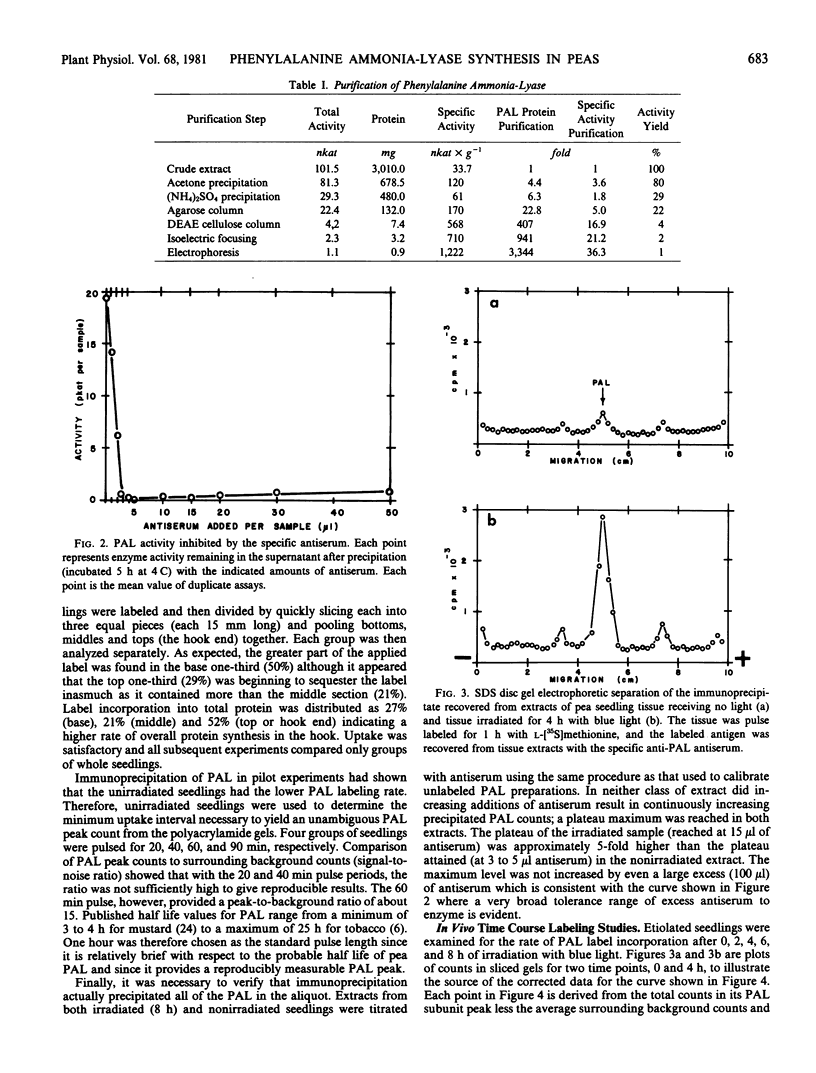
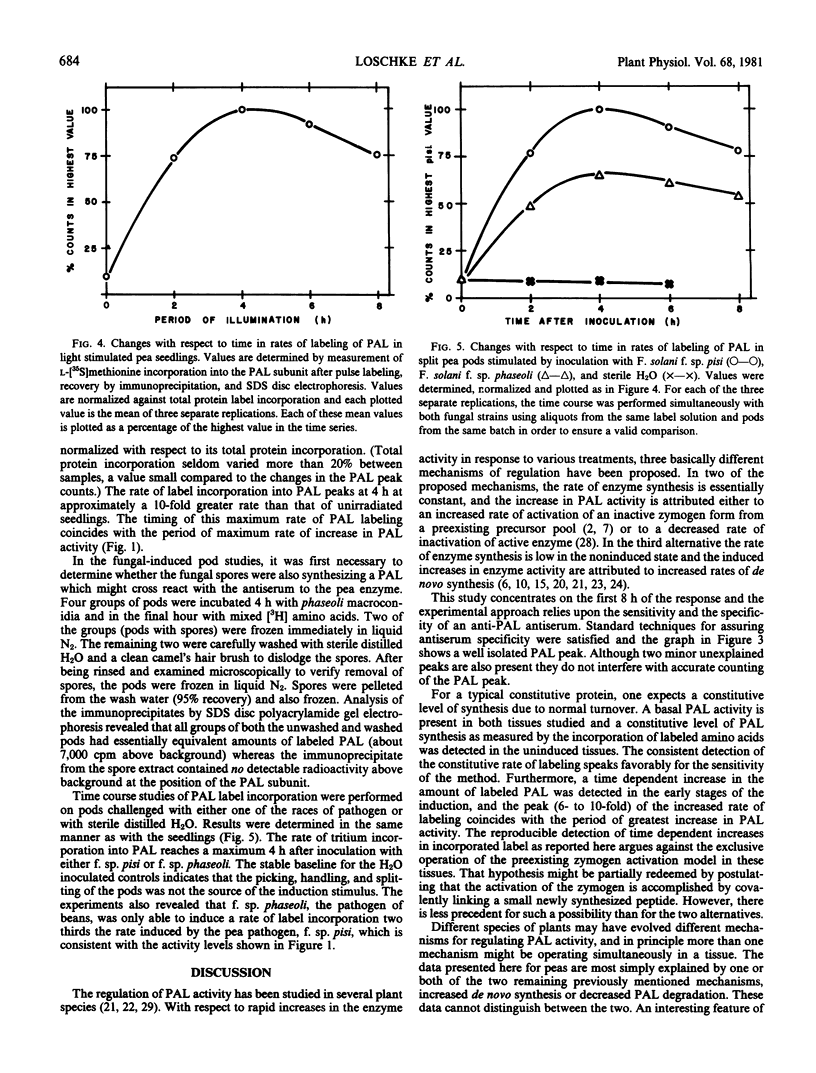
Phenylalanine ammonia-lyase was purified from peas, and a specific antiserum against the enzyme was produced in rabbits. The antiserum was used to study the first 8 hours of the phenylalanine ammonia-lyase activity response in two different organs of the pea from different developmental stages and in response to two different stimuli. Etiolated seedlings were pulse-labeled with l-[35S]methionine after either no light exposure or after specific periods of irradiation with blue light. Immature pods were pulse labeled with mixed l-[3H]amino acids after specific time periods following inoculation of the pod endocarp surfaces with macroconidia of Fusarium solani. Immunoprecipitates isolated from extracts of each group were analyzed with sodium dodecyl sulfate disc gel electrophoresis and were found to contain a radioactive protein with an electrophoretic mobility identical to that of the phenylalanine ammonia-lyase subunit (Mr 81,000). The radioactivity contained in the subunit band was interpreted as being due to de novo synthesis of the enzyme. The net rate of phenylalanine ammonia-lyase labeling, found to be initially low in both tissue types, rose dramatically, peaking at approximately a six- to ten-fold greater level at 4 hours after the beginning of the stimulus. Thereafter, the rate of labeling declined slowly. Inoculation with F. solani f. sp. pisi, a true pathogen of peas, caused a fifty per cent greater rate of peak labeling than did inoculation with a nonpathogen, F. solani f. sp. phaseoli. The time profile of the changing rate of labeling correlates with the changing activity level of the enzyme which peaks at 12 hours after the onset of the stimulus. The data presented favor a model which explains the changing activity of phenylalanine ammonia-lyase as being due to a changing rate of synthesis or degradation (or both) of the enzyme rather than due to the activation of a preformed zymogen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge T. H., Smith H. Density-labelling evidence for the blue-light-mediated activation of phenylalanine ammonia lyase in Cucumis sativus seedlings. Biochim Biophys Acta. 1974 May 24;343(3):452–464. doi: 10.1016/0304-4165(74)90262-1. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Duchesne M., Fritig B., Hirth L. Phenylalanine ammonia-lyase in tobacco mosaic virus-infected hypersensitive tobacco. Density-labelling evidence of de novo synthesis. Biochim Biophys Acta. 1977 Dec 8;485(2):465–481. doi: 10.1016/0005-2744(77)90182-6. [DOI] [PubMed] [Google Scholar]
- Engelsma G. On the Mechanism of the Changes in Phenylalanine Ammonia-lyase Activity Induced by Ultraviolet and Blue Light in Gherkin Hypocotyls. Plant Physiol. 1974 Nov;54(5):702–705. doi: 10.1104/pp.54.5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCHFELD J. Immune-electrophoretic demonstration of qualitative differences in human sera and their relation to the haptoglobins. Acta Pathol Microbiol Scand. 1959;47:160–168. doi: 10.1111/j.1699-0463.1959.tb04844.x. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K. Regulation of phenylalanine ammonia-lyase activity in cell-suspension cultures of Petroselinum hortense. Apparent rates of enzyme synthesis and degradation. Eur J Biochem. 1976 Mar 16;63(1):137–145. doi: 10.1111/j.1432-1033.1976.tb10216.x. [DOI] [PubMed] [Google Scholar]
- KOUKOL J., CONN E. E. The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J Biol Chem. 1961 Oct;236:2692–2698. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb C. J., Merritt T. K. Density labelling studies of the photocontrol of L-phenylalanine ammonia-lyase in discs of potato (Solanum tuberosum) tuber parenchyme. Biochim Biophys Acta. 1979 Nov 15;588(1):1–11. doi: 10.1016/0304-4165(79)90364-7. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Ovalbumin messenger ribonucleic acid translation. Comparable rates of polypeptide initiation and elongation on ovalbumin and globin messenger ribonucleic acid in a rabbit reticulocyte lysate. J Biol Chem. 1973 Mar 25;248(6):2095–2106. [PubMed] [Google Scholar]
- Schröder J., Kreuzaler F., Schäfer E., Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979 Jan 10;254(1):57–65. [PubMed] [Google Scholar]
- Schröder J. Light-induced increase of messenger RNA for phenylalanine ammonia-lyase in cell suspension cultures of Petroselinum hortense. Arch Biochem Biophys. 1977 Aug;182(2):488–496. doi: 10.1016/0003-9861(77)90529-x. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Uritani I. Immunochemical studies on fluctuation of phenylalanine ammonia-lyase activity in sweet potato in response to cut injury. J Biochem. 1976 Jan;79(1):217–219. doi: 10.1093/oxfordjournals.jbchem.a131049. [DOI] [PubMed] [Google Scholar]
- Tong W. F., Schopfer P. Phytochrome-mediated de novo synthesis of phenylalanine ammonia-lyase: An approach using pre-induced mustard seedlings. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4017–4021. doi: 10.1073/pnas.73.11.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Induction of Phenylalanine Deaminase by Light and its Relation to Chlorogenic Acid Synthesis in Potato Tuber Tissue. Plant Physiol. 1965 Sep;40(5):779–784. doi: 10.1104/pp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Induction of phenylalanine ammonia-lyase in xanthium leaf discs: increased inactivation in darkness. Plant Physiol. 1971 Mar;47(3):442–444. doi: 10.1104/pp.47.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]


