Abstract
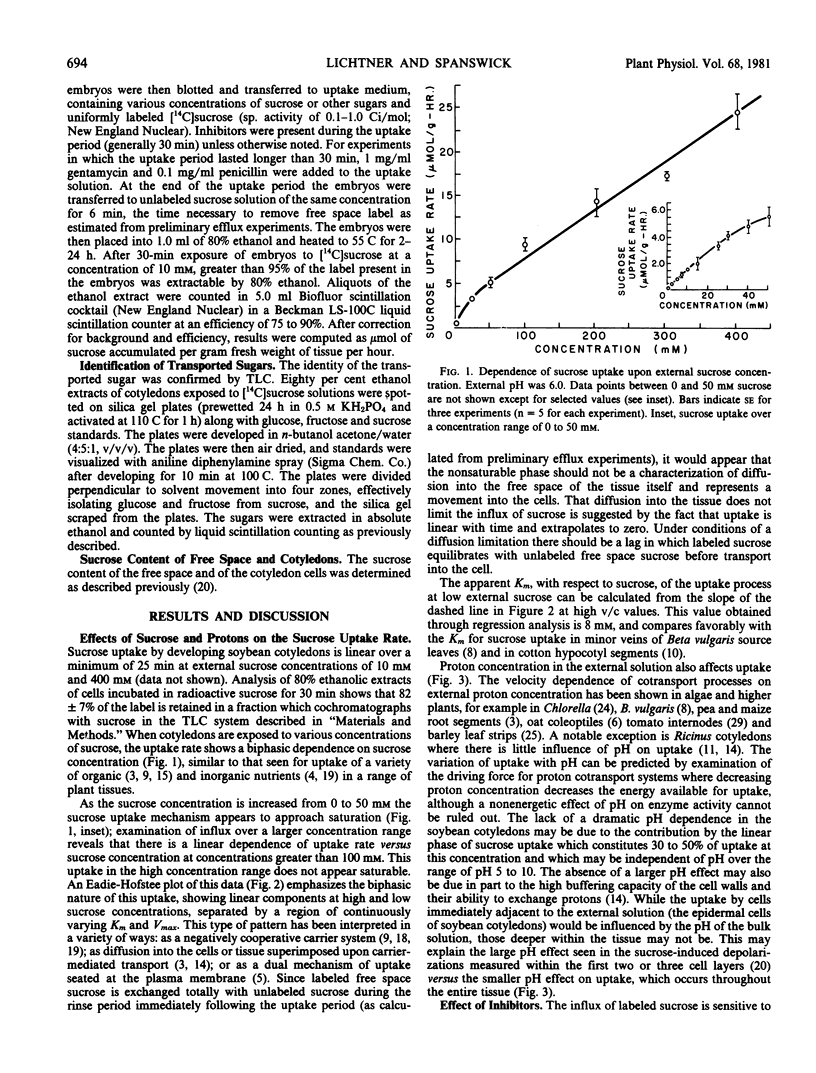
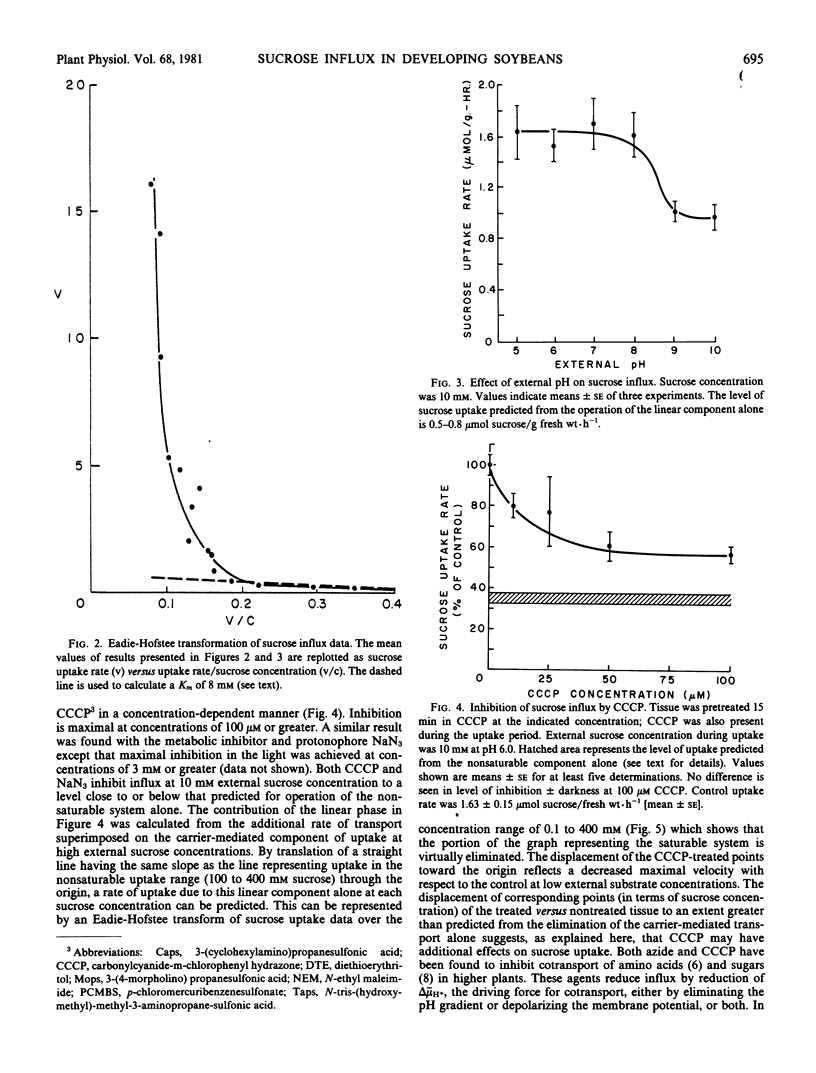
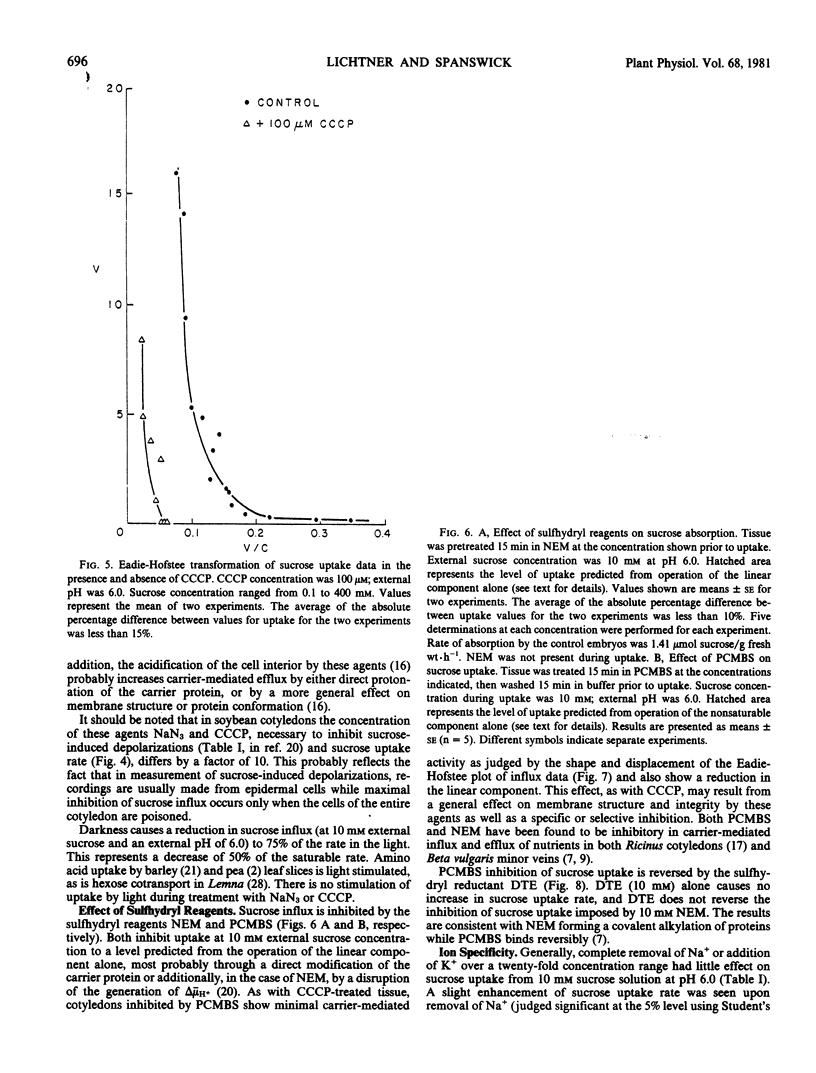
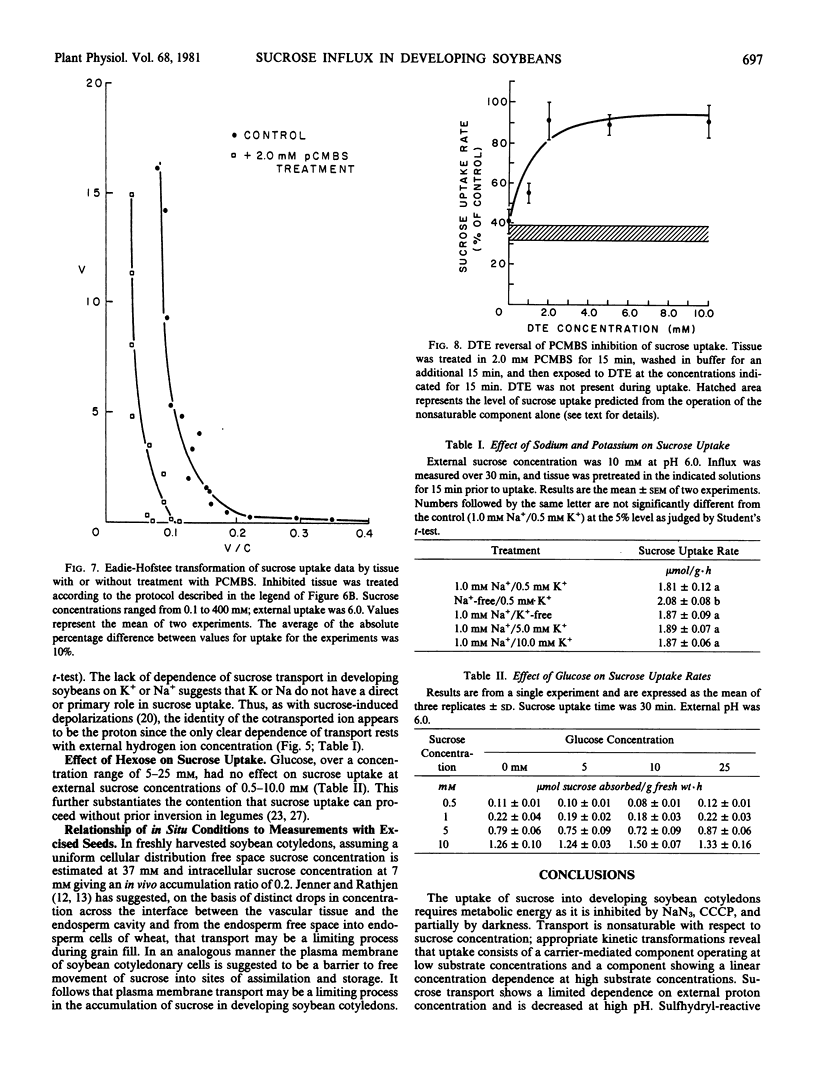
Sucrose uptake by excised developing soybean cotyledons shows a biphasic dependence on sucrose concentration. At concentrations less than about 50 millimolar external sucrose, uptake can be described as a carrier-mediated process, with a Km of 8 millimolar. At higher external sucrose concentrations, a linear dependence becomes apparent, which suggests the participation of a nonsaturable component in total uptake. Sucrose absorption is dependent on the presence of an electrochemical potential gradient for protons since agents interfering with the generation or maintenance of this gradient (NaN3 or carbonylcyanide-m-chlorophenyl hydrazone) decrease sucrose transport to a level at or below that predicted from the operation of the noncarrier-mediated process alone. The saturable component of sucrose uptake is also sensitive to the sulfhydryl-modifying compounds N-ethylmaleimide and p-chloro-mercuribenzenesulfonate. The thiol-reducing agent diethioerythritol reverses fully the p-chloro-mercuri-benzenesulfonate inhibition, but not that of N-ethyl maleim de. Sucrose transport is sensitive to external pH, being decreased at high pH0. Since sucrose-induced depolarization of the membrane potential and carrier-mediated sucrose influx show similar pH-dependence, inhibitor sensitivity, and values of Km for sucrose, a sucrose/proton contransport process appears to operate in developing soybean cotyledon cells. Measurement of free space and intracellular sucrose concentrations in vivo suggests that the carrier-mediated process is fully saturated and that sucrose transport may be limiting for sucrose accumulation by the developing seed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheung Y. N., Nobel P. S. Amino Acid uptake by pea leaf fragments: specificity, energy sources, and mechanism. Plant Physiol. 1973 Dec;52(6):633–637. doi: 10.1104/pp.52.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Hagen C. E. A KINETIC STUDY OF THE ABSORPTION OF ALKALI CATIONS BY BARLEY ROOTS. Plant Physiol. 1952 Jul;27(3):457–474. doi: 10.1104/pp.27.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Evidence for amino Acid-h co-transport in oat coleoptiles. Plant Physiol. 1978 Jun;61(6):933–937. doi: 10.1104/pp.61.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Phloem Loading of Sucrose: pH Dependence and Selectivity. Plant Physiol. 1977 Apr;59(4):750–755. doi: 10.1104/pp.59.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson S. E., Loomis R. S., Rains D. W. Characteristics of sugar uptake in hypocotyls of cotton. Plant Physiol. 1978 Dec;62(6):846–850. doi: 10.1104/pp.62.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Schwab W. G., Tanner W. The effect of intracellular pH on the rate of hexose uptake in Chlorella. Biochim Biophys Acta. 1979 Aug 23;555(3):524–530. doi: 10.1016/0005-2736(79)90406-1. [DOI] [PubMed] [Google Scholar]
- Komor E., Weber H., Tanner W. Essential Sulfhydryl Group in the Transport-catalyzing Protein of the Hexose-Proton Cotransport System of Chlorella. Plant Physiol. 1978 May;61(5):785–786. doi: 10.1104/pp.61.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Cation-stimulated Adenosine Triphosphatase Activity and Cation Transport in Corn Roots. Plant Physiol. 1976 Sep;58(3):331–335. doi: 10.1104/pp.58.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The driving force for proton(s) metabolites cotransport in bacterial cells. FEBS Lett. 1976 Jul 15;66(2):159–163. doi: 10.1016/0014-5793(76)80493-0. [DOI] [PubMed] [Google Scholar]
- Sacher J. A. The regulation of sugar uptake and accumulation in bean pod tissue. Plant Physiol. 1966 Jan;41(1):181–189. doi: 10.1104/pp.41.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab W. G., Komor E. A possible mechanistic role of the membrane potential in proton-sugar cotransport of Chlorella. FEBS Lett. 1978 Mar 1;87(1):157–160. doi: 10.1016/0014-5793(78)80156-2. [DOI] [PubMed] [Google Scholar]
- Smith J. G. Embryo Development in Phaseolus vulgaris: II. Analysis of Selected Inorganic Ions, Ammonia, Organic Acids, Amino Acids, and Sugars in the Endosperm Liquid. Plant Physiol. 1973 Mar;51(3):454–458. doi: 10.1104/pp.51.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H. Kinetics of C-photosynthate uptake by developing soybean fruit. Plant Physiol. 1980 May;65(5):975–979. doi: 10.1104/pp.65.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]




