Abstract
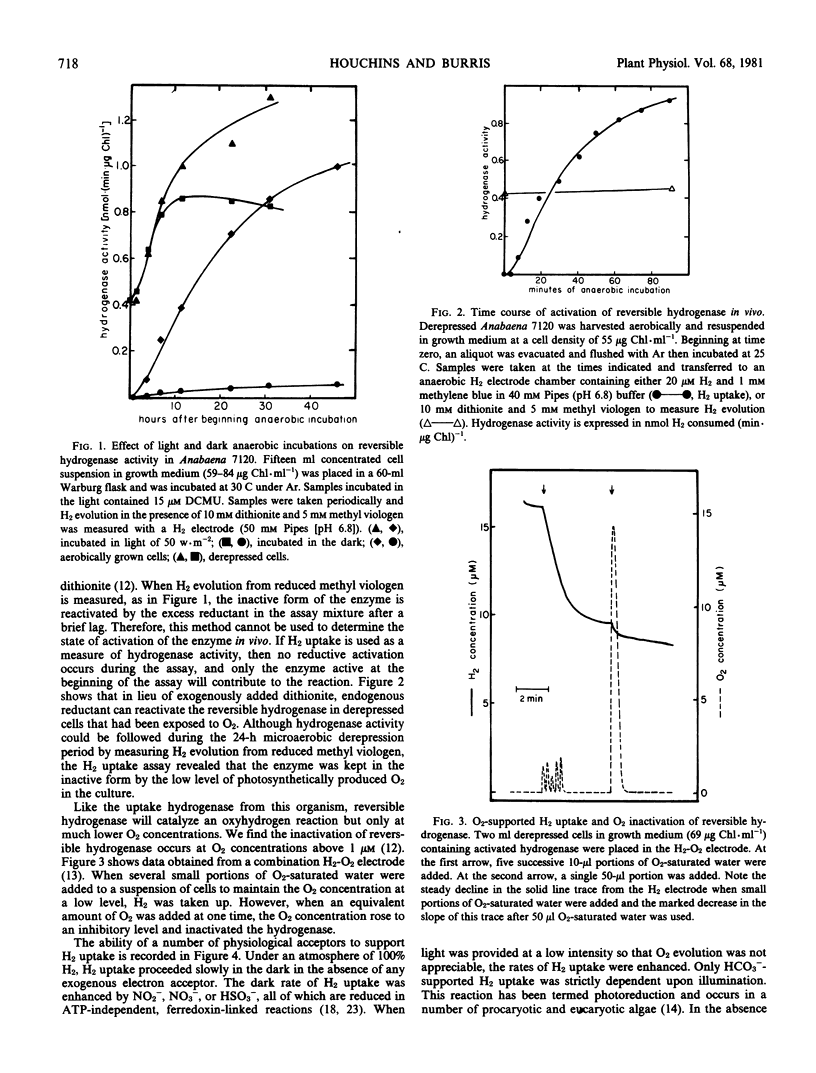
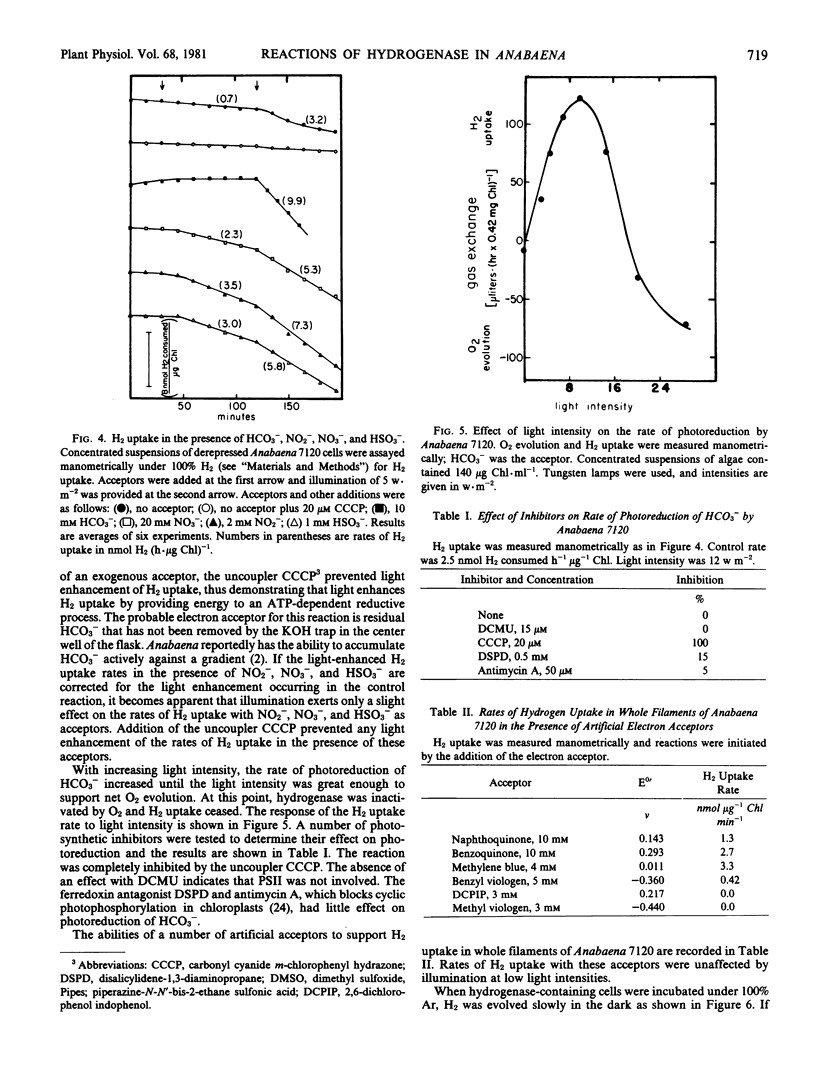
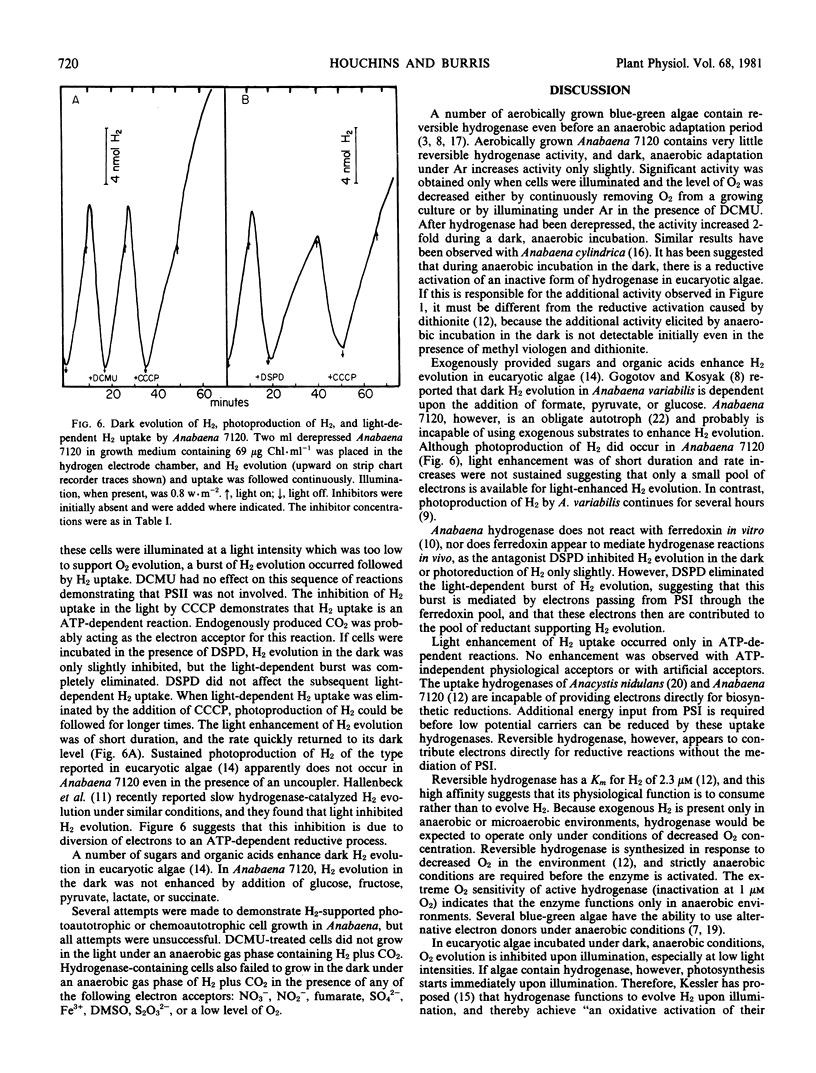
The reversible hydrogenase from Anabaena 7120 appeared when O2 was continuously removed from a growing culture. Activity increased further when cells were incubated under argon in the dark or in the light plus 3-(3,4-dichlorophenyl)-1,1-dimethylurea. Hydrogenase existed in an inactive state during periods of O2 evolution. It could be reductively activated by exposure to reduced methyl viologen or by dark, anaerobic incubation. Hydrogenase-containing cells evolved H2 slowly during dark anaerobic incubations, and the rate of H2 evolution was increased by illumination with low intensity light. Light enhancement of H2 evolution was of short duration and was eliminated by the ferredoxin antagonist disalicylidene diaminopropane. Physiological acceptors that supported H2 uptake included NO3−, NO2−, and HSO3−, and light had a slight influence on the rate of H2 uptake with these acceptors. Low levels of O2 supported H2 uptake, but higher concentrations of O2 inactivated the hydrogenase. Hydrogen uptake with HCO3− as acceptor was the most rapid reaction measured, and it was strictly light-dependent. It occurred only at low light intensities, and higher light intensities restored normal O2-evolving photosynthesis. It is suggested that hydrogenase is present to capture exogenous H2 as a source of reducing equivalents during growth in anaerobic environments.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick S., Oren A., Padan E. Occurrence of facultative anoxygenic photosynthesis among filamentous and unicellular cyanobacteria. J Bacteriol. 1977 Feb;129(2):623–629. doi: 10.1128/jb.129.2.623-629.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchins J. P., Burris R. H. Occurrence and localization of two distinct hydrogenases in the heterocystous cyanobacterium Anabaena sp. strain 7120. J Bacteriol. 1981 Apr;146(1):209–214. doi: 10.1128/jb.146.1.209-214.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Bishop N. I. Simultaneous measurement of oxygen and hydrogen exchange from the blue-green alga anabaena. Plant Physiol. 1976 Apr;57(4):659–665. doi: 10.1104/pp.57.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llama M. J., Serra J. L., Rao K. K., Hall D. O. Isolation and characterization of the hydrogenase activity from the non-heterocystous cyanobacterium Spirulina maxima. FEBS Lett. 1979 Feb 15;98(2):342–346. doi: 10.1016/0014-5793(79)80213-6. [DOI] [PubMed] [Google Scholar]
- Manzano C., Candau P., Gomez-Moreno C., Relimpio A. M., Losada M. Ferredoxin-dependent photosynthetic reduction of nitrate and nitrite by particles of Anacystis nidulans. Mol Cell Biochem. 1976 Feb 25;10(3):161–169. doi: 10.1007/BF01731687. [DOI] [PubMed] [Google Scholar]
- Peschek G. A. Anaerobic hydrogenase activity in Anacystis nidulans. H2-dependent photoreduction and related reactions. Biochim Biophys Acta. 1979 Nov 8;548(2):187–202. doi: 10.1016/0005-2728(79)90128-2. [DOI] [PubMed] [Google Scholar]
- Slovacek R. E., Hind G. Influence of antimycin a and uncouplers on anaerobic photosynthesis in isolated chloroplasts. Plant Physiol. 1977 Oct;60(4):538–542. doi: 10.1104/pp.60.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tel-Or E., Luijk L. W., Packer L. Hydrogenase in N2-fixing cyanobacteria. Arch Biochem Biophys. 1978 Jan 15;185(1):185–194. doi: 10.1016/0003-9861(78)90158-3. [DOI] [PubMed] [Google Scholar]


