Abstract
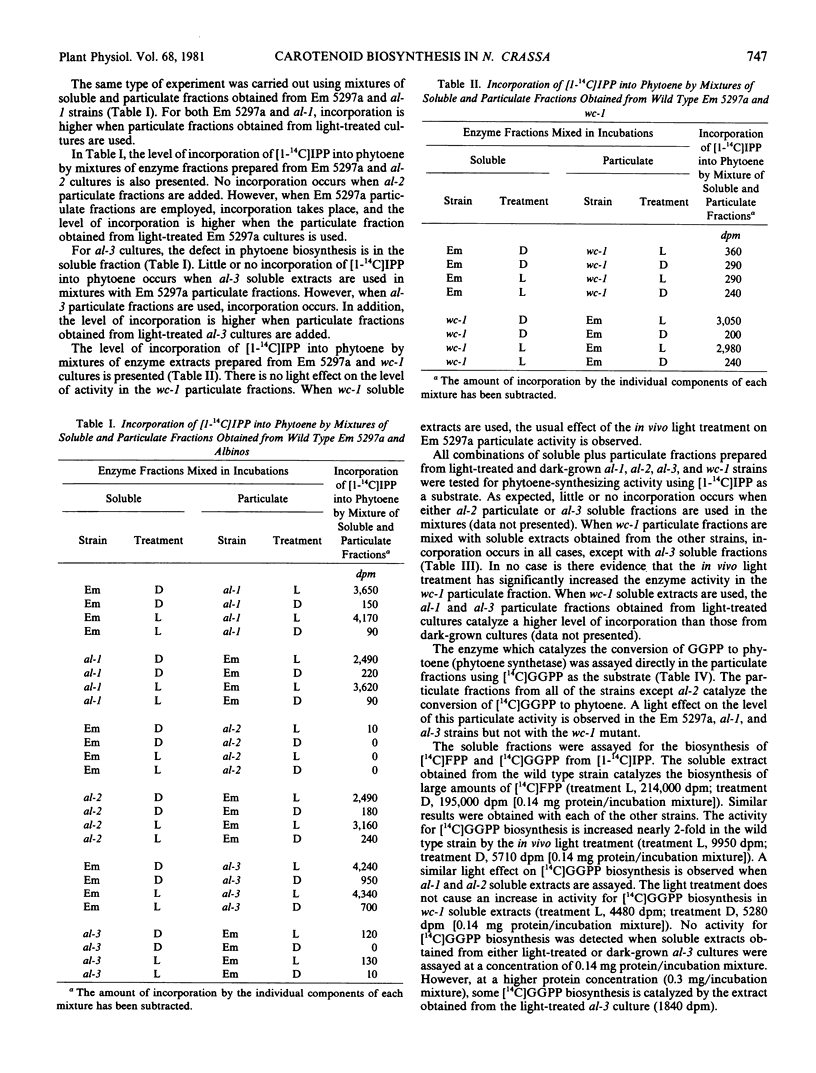
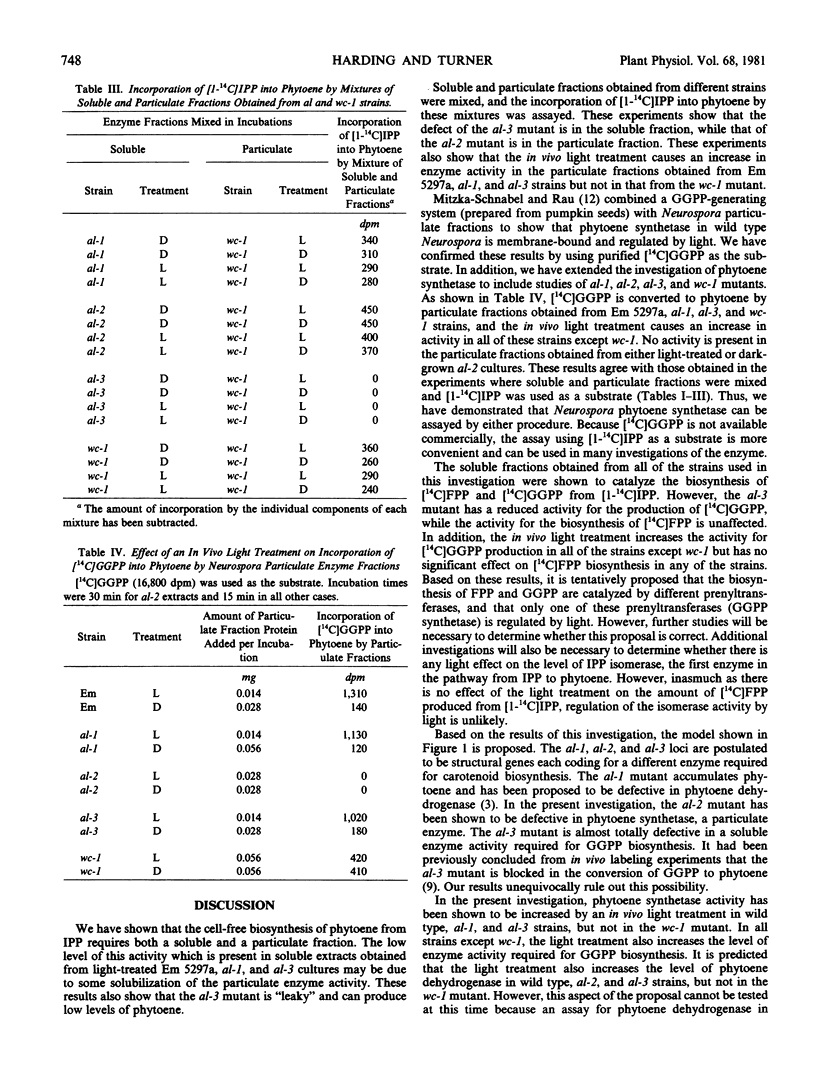
The conversion of isopentenyl pyrophosphate to phytoene in Neurospora crassa requires both a soluble and a particulate fraction. Soluble and particulate enzyme fractions obtained from light-treated and dark-grown wild type, albino-1, albino-2, albino-3, and white collar-1 strains were mixed in various combinations, and the activity for conversion of [1-14C]isopentenyl pyrophosphate to phytoene was assayed. From such experiments it can be concluded that: (a) albino-3 is defective in the soluble fraction; (b) albino-2 is defective in the particulate fraction; (c) the in vivo light treatment increases the enzyme activity in the particulate fraction; (d) this light effect occurs in wild type, albino-1, and albino-3 strains; and (e) enzyme activity is present in the particulate fraction obtained from the white collar-1 mutant, but the in vivo light treatment does not cause an increase in this activity. To measure directly the level of particulate enzyme activity, [14C]geranylgeranyl pyrophosphate was used as a substrate. This compound, which is not available commercially, was synthesized enzymically using extracts of pea cotyledons. Particulate enzyme fractions obtained from wild type, albino-1, and albino-3 strains incorporate [14C]geranylgeranyl pyrophosphate into phytoene, and this activity is higher in extracts obtained from light-treated cultures. The particulate fraction obtained from the white collar-1 mutant also incorporates [14C]geranylgeranyl pyrophosphate into phytoene, but the in vivo light treatment does not cause an increase in this activity. No incorporation occurs when particulate fractions obtained from either dark-grown or light-treated albino-2 cultures are assayed. The soluble enzyme fraction obtained from the albino-3 mutant was shown to be almost totally defective in enzyme activity required for the biosynthesis of [14C]geranylgeranyl pyrophosphate from [1-14C]isopentenyl pyrophosphate. An in vivo light treatment increases the level of this activity in wild type, albino-1, albino-2, and albino-3 strains, but not in the white collar-1 mutant. A model is presented to account for all of the results obtained in this investigation. It is proposed that the white collar-1 strain is a regulatory mutant blocked in the light induction process, whereas the albino-1, albino-2, and albino-3 strains are each defective for a different enzyme in the carotenoid biosynthetic pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Fabo E. C., Harding R. W., Shropshire W. Action Spectrum between 260 and 800 Nanometers for the Photoinduction of Carotenoid Biosynthesis in Neurospora crassa. Plant Physiol. 1976 Mar;57(3):440–445. doi: 10.1104/pp.57.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie A. H., Subden R. E. The neutral carotenoids of wild-type and mutant strains of Neurospora crassa. Biochem Genet. 1973 Nov;10(3):275–284. doi: 10.1007/BF00485705. [DOI] [PubMed] [Google Scholar]
- Gregonis D. E., Rilling H. C. Photoinduced prephytoene pyrophosphate synthesis in a mycobacterium sp. Biochem Biophys Res Commun. 1973 Sep 5;54(1):449–454. doi: 10.1016/0006-291x(73)90942-x. [DOI] [PubMed] [Google Scholar]
- Harding R. W., Huang P. C., Mitchell H. K. Photochemical studies of the carotenoid biosynthetic pathway in Neurospora crassa. Arch Biochem Biophys. 1969 Feb;129(2):696–707. doi: 10.1016/0003-9861(69)90230-6. [DOI] [PubMed] [Google Scholar]
- Howes C. D., Batra P. P. Mechanism of photoinduced carotenoid synthesis. Further studies on the action spectrum and other aspects of carotenogenesis. Arch Biochem Biophys. 1970 Mar;137(1):175–180. doi: 10.1016/0003-9861(70)90424-8. [DOI] [PubMed] [Google Scholar]
- Johnson J. H., Reed B. C., Rilling H. C. Early photoinduced enzymes of photoinduced carotenogenesis in a Mycobacterium species. J Biol Chem. 1974 Jan 25;249(2):402–406. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oster M. O., West C. A. Biosynthesis of trans-geranylgeranyl pyrophosphate in endosperm of Echinocystis macrocarpa Greene. Arch Biochem Biophys. 1968 Sep 20;127(1):112–123. doi: 10.1016/0003-9861(68)90207-5. [DOI] [PubMed] [Google Scholar]
- RILLING H. C. ON THE MECHANISM OF PHOTOINDUCTION OF CAROTENOID SYNTHESIS. Biochim Biophys Acta. 1964 May 25;79:464–475. doi: 10.1016/0926-6577(64)90212-8. [DOI] [PubMed] [Google Scholar]
- Sagami H., Ogura K., Seto S. Solanesyl pyrophosphate synthetase from Micrococcus lysodeikticus. Biochemistry. 1977 Oct 18;16(21):4616–4622. doi: 10.1021/bi00640a014. [DOI] [PubMed] [Google Scholar]
- Spurgeon S. L., Turner R. V., Harding R. W. Biosynthesis of phytoene from isopentenyl pyrophosphate by a Neurospora enzyme system. Arch Biochem Biophys. 1979 Jun;195(1):23–29. doi: 10.1016/0003-9861(79)90323-0. [DOI] [PubMed] [Google Scholar]
- Weeks O. B., Saleh F. K., Wirahadikusumah M., Berry R. A. Photoregulated carotenoid biosynthesis in non-photosynthetic microorganisms. Pure Appl Chem. 1973;35(1):63–80. doi: 10.1351/pac197335010063. [DOI] [PubMed] [Google Scholar]
- ZALOKAR M. Biosynthesis of carotenoids in Neurospora; action spectrum of photoactivation. Arch Biochem Biophys. 1955 Jun;56(2):318–325. doi: 10.1016/0003-9861(55)90252-6. [DOI] [PubMed] [Google Scholar]



