Abstract
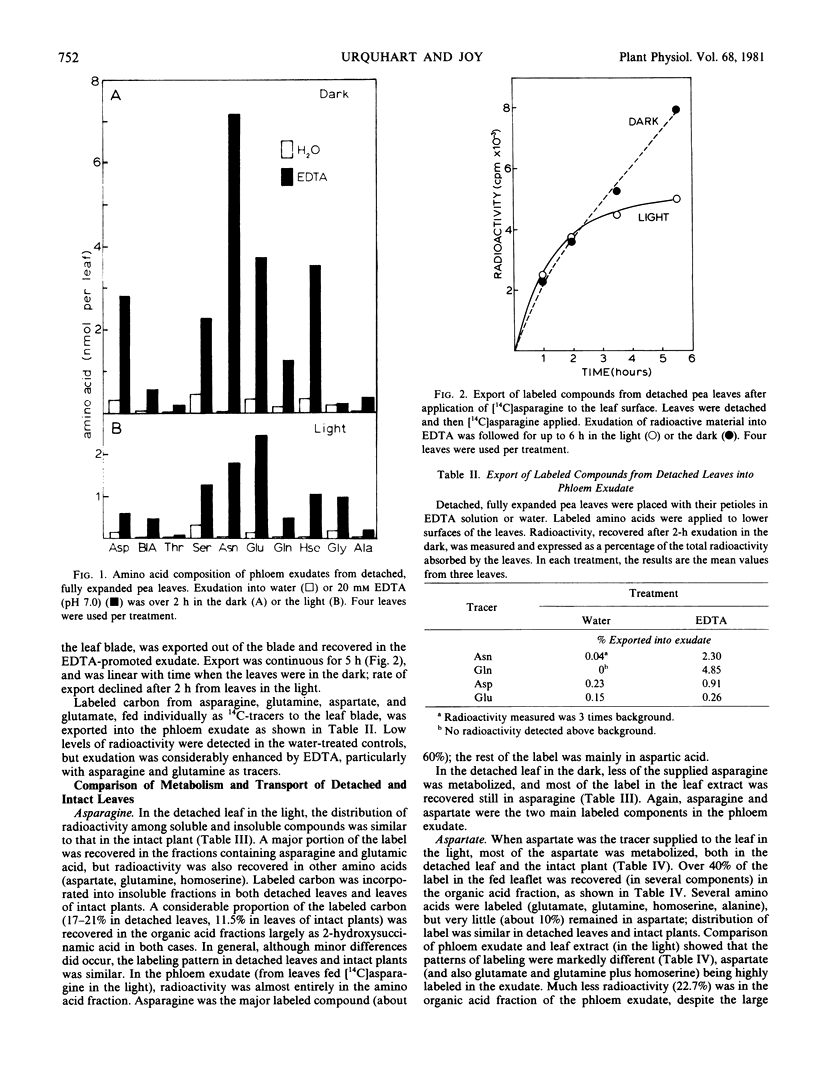
The phloem exudation technique using ethylenediaminetetraacetic acid (EDTA) was evaluated in studies of amino acid translocation in Pisum sativum L. seedlings. Exudation of phloem sap from cut petioles of fully expanded leaves was enhanced by EDTA (20 millimolar disodium salt [pH 7.0]). Amino acids (mainly asparagine, homoserine, glutamate, and also aspartate and serine) were present in petiole exudates from EDTA-treated leaves at levels which were commonly 5- to 10-fold (or more) higher compared with water-treated controls. Exudation was greater from darkened leaves, and the pattern of amino acids was markedly different from the more uniform mixture leaking from water-treated controls.
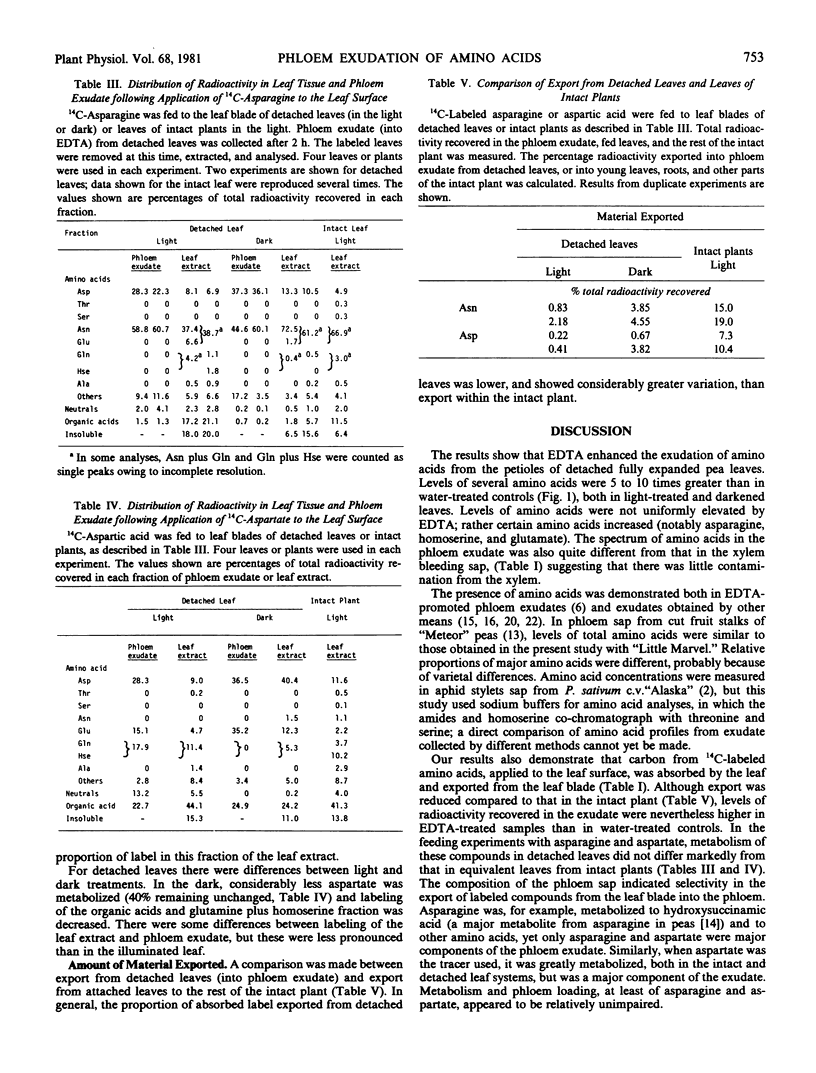
After feeding 14C-labeled amino acids to the leaf blade, distribution of radioactivity in components of the exudate differed from that of the leaf tissue, suggesting selectivity of amino acid loading. [14C]Asparagine was converted to 2-hydroxysuccinamic acid and to other amino acids by the leaf, but was recovered in exudate mainly as asparagine (60%) and aspartate (30%). Similarly, in the exudate, 65 to 70% of the label from [14C]-aspartate was in amino acids, although in the leaf tissue 50% was in the organic acid fraction and only 11% remained as aspartate. Metabolism of asparagine and aspartate was essentially the same in intact leaf blades as in EDTA-treated leaves. Despite the possibility of EDTA damage in the petiole, phloem loading of amino acids appeared to be relatively unimpaired. Although the amount of labeled material appearing in the exudate is less than the amount translocated in the intact plant, the technique is useful in the study of amino acid transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Sharkey P. J. Asparagine metabolism-key to the nitrogen nutrition of developing legume seeds. Plant Physiol. 1975 Dec;56(6):807–812. doi: 10.1104/pp.56.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., Urquhart A. A., Joy K. W. Amino Acid metabolism of pea leaves: diurnal changes and amino Acid synthesis from N-nitrate. Plant Physiol. 1977 May;59(5):915–919. doi: 10.1104/pp.59.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Fellows R. J., Egli D. B., Leggett J. E. A Pod Leakage Technique for Phloem Translocation Studies in Soybean (Glycine max [L.] Merr.). Plant Physiol. 1978 Nov;62(5):812–814. doi: 10.1104/pp.62.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Zeevaart J. A. Enhancement of Phloem exudation from cut petioles by chelating agents. Plant Physiol. 1974 Jan;53(1):96–103. doi: 10.1104/pp.53.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard O. A., Glenn R. K. Translocation of assimilates and phosphate in detached bean leaves. Plant Physiol. 1968 Sep;43(9):1380–1388. doi: 10.1104/pp.43.9.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd N. D., Joy K. W. 2-Hydroxysuccinamic acid: a product of asparagine metabolis in plants. Biochem Biophys Res Commun. 1978 Mar 15;81(1):186–192. doi: 10.1016/0006-291x(78)91647-9. [DOI] [PubMed] [Google Scholar]
- Tully R. E., Hanson A. D. Amino Acids Translocated from Turgid and Water-stressed Barley Leaves: I. Phloem Exudation Studies. Plant Physiol. 1979 Sep;64(3):460–466. doi: 10.1104/pp.64.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGGE L. E., MITTLER T. Shell regeneration in some British molluscs. Nature. 1953 Mar 21;171(4351):528–529. doi: 10.1038/171528b0. [DOI] [PubMed] [Google Scholar]



