Abstract
Background:
The guideline-recommended elements to include in discussions about goals of care with patients with serious illness are mostly based on expert opinion. We sought to identify which elements are most important to patients and their families.
Methods:
We used a cross-sectional study design involving patients from 9 Canadian hospitals. We asked older adult patients with serious illness and their family members about the occurrence and importance of 11 guideline-recommended elements of goals-of-care discussions. In addition, we assessed concordance between prescribed goals of care and patient preferences, and we measured patient satisfaction with goals-of-care discussions using the Canadian Health Care Evaluation Project (CANHELP) questionnaire.
Results:
Our study participants included 233 patients (mean age 81.2 yr) and 205 family members (mean age 60.2 yr). Participants reported that clinical teams had addressed individual elements of goals-of-care discussions infrequently (range 1.4%–31.7%). Patients and family members identified the same 5 elements as being the most important to address: preferences for care in the event of life-threatening illness, values, prognosis, fears or concerns, and questions about goals of care. Addressing more elements was associated with both greater concordance between patients’ preferences and prescribed goals of care, and greater patient satisfaction.
Interpretation:
We identified elements of goals-of-care discussions that are most important to older adult patients in hospital with serious illness and their family members. We found that guideline-recommended elements of goals-of-care discussions are not often addressed by health care providers. Our results can inform interventions to improve the determination of goals of care in the hospital setting.
In Canada, dying is often an in-hospital, technology-laden experience.1–4 Rates of cardiopulmonary resuscitation (CPR) before death continue to increase among older adult patients in hospital,5 and one-fifth of deaths in hospital occur in an intensive care unit.1,2,6,7 These observations contrast sharply with patient-reported preferences. A recent Canadian study found that 80% of older adult patients in hospital with a serious illness prefer a less aggressive and more comfort-oriented end-of-life care plan that does not include CPR.8
Such patients and their families have identified communication with health care providers and decision-making about goals of care as high priorites for improving end-of-life care in Canada.9,10 We define “decision-making about goals of care” as an end-of-life communication and decision-making process that occurs between a clinician and a patient (or a substitute decision-maker if the patient is incapable) in an institutional setting to establish a plan of care. Often, this process includes deciding whether to use life-sustaining treatments.11 Current guidelines recommend that health care providers address 11 key elements when discussing goals of care with patients and families (Box 1).12–14 However, these elements are mostly based on expert opinion and lack input from patients and their families.
Box 1: Key elements of goals-of-care discussions with patients in hospital with serious illness12–14.
Ask about previous discussions or written documentation about the use of life-sustaining treatments
Offer a time to meet to discuss goals of care
Provide information about advance care planning to review before conversations with the physician
Disclose prognosis
Ask about patients’ values (i.e., what is important to them when considering health care decisions)
Provide information about outcomes, benefits and risks of life-sustaining treatments
Provide information about outcomes, benefits and risks of comfort measures
Prompt for additional questions about goals of care
Provide an opportunity to express fears or concerns
Ask about preferences for care in the event of a life-threatening illness
Facilitate access to legal documents to record patients’ wishes
Our primary objective was to determine which of these elements are most important to patients and their families. In addition, we examined whether these discussions were associated with concordance between patients’ (or family members’) preferences and prescribed goals of care, and with satisfaction with end-of-life communication and decision-making.
Methods
Setting and design
Our research program includes periodic audits of end-of-life communication and decision-making.8 Between Jan. 16, 2013, and June 28, 2013, we interviewed eligible patients and their family members at 9 Canadian acute care hospitals in British Columbia, Alberta, Ontario and Quebec using a validated questionnaire.15 We have previously reported data from the first audit cycle pertaining to end-of-life discussions before admission to hospital.8 Here, we report data from the second audit cycle that pertain to goals-of-care discussions that took place during the index hospital stay.
This study was approved by the research ethics board at each of the participating institutions.
Participants
Our eligibility criteria have been published previously.8 Briefly, we enrolled patients in hospital who were at high risk of dying within the next 6 months and their family members. We defined high risk as either 55 years of age or older with an advanced stage of one or more specific conditions (chronic obstructive pulmonary disease, congestive heart failure, cirrhosis, cancer and dementia) or 80 years of age or older with admission to hospital for an acute condition. If these criteria were not met, we deemed patients eligible if a member of their clinical team would not be surprised if the patient died within the next 6 months.
All eligible patients were admitted to a medical service, not an intensive care unit, of a participating hospital. We excluded patients who were unable to communicate because of cognitive impairment and patients who spoke neither English nor French.
We defined family members as people who knew the patient best, including partners, significant others and close friends, and who had visited the patient in hospital at least once. We excluded paid caregivers from this definition. The same criteria were used to identify and invite family members of eligible but nonparticipating patients. To obtain a representative sample, we planned to enroll 30 patients and 30 family members at each of the 9 participating sites.
Eligible patients were identified by treating teams or by research staff who screened hospital records. When research staff were available, we approached consecutive, eligible patients and their family members for consent. We enrolled patients 2–5 days after their admission to hospital so that presenting symptoms would have abated enough to allow the patient to participate in an interview. All participants provided written informed consent.
Study questionnaire
The questionnaire we used to audit end-of-life communication and decision-making practices has previously been shown to have face and content validity, good ratings of clarity and low psychological burden.15 Research staff interviewed patients and family members separately. We asked participants whether, since admission, a member of the care team had discussed 11 key elements of determining goals of care (Box 1), and to rate how important it was for each of these elements to be discussed with a member of the care team. Response options were “not at all important,” “not very important,” “somewhat important,” “very important” or “extremely important.”
We determined the 11 elements of goals-of-care discussions from a literature search for clinical practice guidelines regarding end-of-life communication and decision-making and from responses given during a focus group.12–14 The focus group consisted of 25 experts in critical care, internal medicine, palliative care, nursing, research methods and psychometrics.12
To assess satisfaction with end-of-life care, we used the validated Canadian Health Care Evaluation Project (CANHELP) questionnaire.16 This questionnaire asks participants to rate satisfaction with specific aspects of care during the previous 4 weeks as follows: 1, not at all satisfied; 2, not very satisfied; 3, somewhat satisfied; 4, very satisfied; 5, completely satisfied. The CANHELP instrument has good correlation with global ratings of satisfaction (0.49 and 0.63 for patient and family versions, respectively), and the domains pertinent to end-of-life communication and decision-making used in our analyses have high internal consistency (Cronbach’s α 0.84–0.91).16
Finally, we asked participants to state their preferences as to whether life-sustaining treatments should be used if their condition (or their loved one’s condition) were to deteriorate to the point of becoming life threatening. Response options were as follows: “artificial life-sustaining treatments, including CPR, to keep me alive at all costs”; “full medical care, but no CPR in the event that my heart or breathing stops”; “physicians will be focused on my comfort by alleviating suffering and not on keeping me alive by artificial means or heroic measures, such as trying to prolong my life with CPR and other life-sustaining technologies”; “a combination of these options (e.g., try to fix problems, but if I am not getting better, switch to focusing only my comfort, even if it hastens death)”; and “unsure.” Immediately after the interview, the research staff abstracted data from the patients’ medical records about orders for use or nonuse of life-sustaining treatments and categorized them in the same way as participants’ preferences.
Statistical analysis
We used descriptive statistics (mean, standard deviation [SD] and range for continuous data; counts and proportions for categorical data) to report characteristics of the study participants. We used counts and proportions to report the frequency of discussions about each of the 11 elements of determining goals of care.
Our primary analysis describes participants’ ratings of the importance of the individual elements of goals-of-care discussions. Missing responses were excluded from these analyses (we did not impute data). We ranked each element in order of importance based on the frequency of “very important” or “extremely important” ratings. We used the Spearman correlation coefficient to assess the correlation between patients and family members of the ranking of the 11 elements.
We conducted a secondary analysis to measure the association between occurrence of the elements of goals-of-care discussions and (i) concordance between goals of care preferred by participants and those prescribed in the medical record and (ii) participants’ satisfaction with end-of-life communication and decision-making. When there was no prescription for goals of care in the medical record, we assumed that prescribed goals of care encompassed CPR, because this is the default option in most Canadian hospitals. We measured concordance between preferences and prescribed goals of care using crude and chance-corrected agreement (κ).17 To measure satisfaction with end-of-life communication and decision-making, we calculated an overall score from domains of the CANHELP instrument identified a priori as pertinent to end-of-life communication and decision-making, according to the expert opinion of the investigators. These domains were relationship with physicians, communication and decision-making (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.140673/-/DC1). This overall score is the unweighted average for all answered questions within these domains. The score was rescaled to range from 0 (worst possible value) to 100 (best possible value).
We sought to determine whether there was an association between the number of key elements included in goals-of-care discussions and both (i) concordance between patients’ (or family members’) preferences and prescribed goals of care and (ii) CANHELP satisfaction scores. We used logistic regression, with separate models for patient and family member data, to quantify the association between the number of elements that were discussed (predictor variable 0–11) and concordance (dependent variable). We used linear regression to quantify the association between the number of elements discussed (predictor variable) and CANHELP scores (dependent variable). We adjusted our models for age, sex and education, and incorporated hospital site as a random effect to account for clustering of data within hospitals. We used SAS version 9.3 for all analyses.
Results
Study population
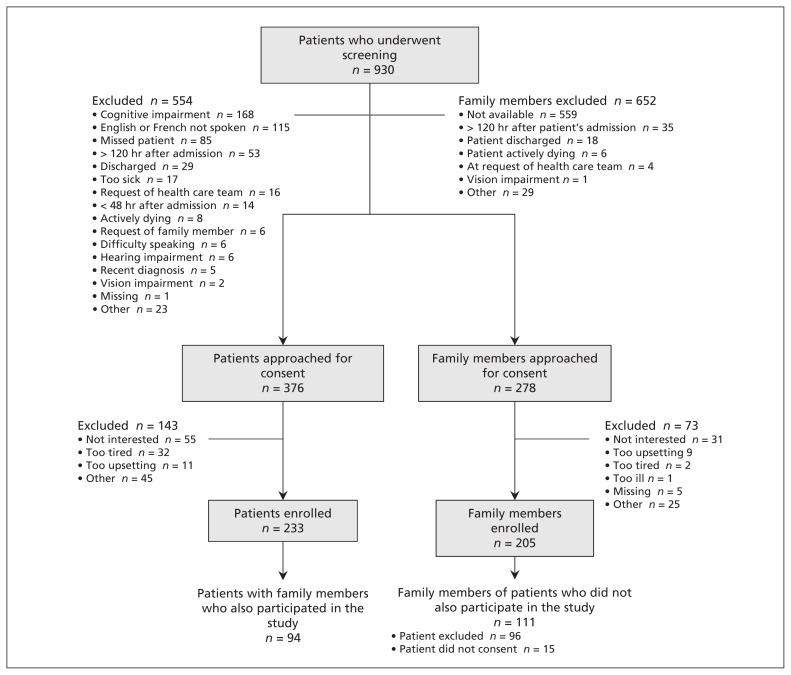
Of the 376 patients and 278 family members we approached to participate in the study, we enrolled 233 patients (62.0%) and 205 family members (73.7%). Of the 205 family members who participated, 94 (45.9%) corresponded to a participating patient; 111 (54.1%) of the family members did not correspond to a participating patient, either because the patient met exclusion criteria (n = 96) or because the patient chose not to participate (n = 15) (Figure 1). The average age of participating patients was 81.2 years, and 134 were women (57.5%). The average age of family members was 60.2 years, 158 were women (77.1%), and 125 were the son or daughter of a patient (61.0%) (Tables 1 and 2).
Figure 1:
Selection of study participants (patients and family members).
Table 1:
Characteristics of patients
| Characteristic | Participating patients, no. (%)* n = 233 | Nonparticipating patients, no. (%)* n = 111 | p value |
|---|---|---|---|
| Age, yr, mean ± SD (range) | 81.2 ± 8.9 (55.0–98.0) | 84.1 ± 7.5 (56.0–97.0) | 0.002 |
| Sex | 0.2 | ||
| Male | 99 (42.5) | 39 (35.1) | |
| Female | 134 (57.5) | 72 (64.9) | |
| Marital status | 0.1 | ||
| Married or living as married | 83 (35.6) | 45 (40.5) | |
| Widowed | 103 (44.2) | 55 (49.5) | |
| Never married | 16 (6.9) | 3 (2.7) | |
| Divorced or separated; not remarried | 31 (13.3) | 8 (7.2) | |
| Residence in last month before admission | 0.007 | ||
| Home | 178 (76.4) | 79 (71.2) | |
| Retirement residence | 47 (20.2) | 19 (17.1) | |
| Long-term care or nursing home | 4 (1.7) | 11 (9.9) | |
| Other | 4 (1.7) | 2 (1.8) | |
| Location of residence before admission | 0.9 | ||
| Rural | 11 (4.7) | 5 (4.5) | |
| Urban | 219 (94.0) | 105 (94.6) | |
| Missing | 2 (0.9) | 1 (0.9) | |
| Declined | 1 (0.4) | 0 (0.0) | |
| Level of education | 0.006 | ||
| Completed high school | 141 (60.5) | 46 (41.4) | |
| Did not complete high school | 92 (39.5) | 61 (55.0) | |
| Missing | 0 (0.0) | 1 (0.9) | |
| Declined | 0 (0.0) | 3 (2.7) | |
| Race; language | |||
| White | 222 (95.3) | 86 (77.5) | < 0.001 |
| White; speaks language other than English/French | 36 (15.5) | 30 (27.0) | 0.01 |
| Non-white; speaks language other than English/French | 4 (1.7) | 18 (16.2) | < 0.001 |
| Current fitness or frailty† | < 0.001 | ||
| Very fit (category 1) | 9 (3.9) | 0 (0.0) | |
| Well (category 2) | 25 (10.7) | 4 (3.6) | |
| Managing well (category 3) | 38 (16.3) | 14 (12.6) | |
| Vulnerable (category 4) | 68 (29.2) | 20 (18.0) | |
| Mildly frail (category 5) | 54 (23.2) | 16 (14.4) | |
| Moderately frail (category 6) | 27 (11.6) | 31 (27.9) | |
| Severely frail (category 7) | 5 (2.1) | 15 (13.5) | |
| Very severely frail (category 8) | 0 (0.0) | 10 (9.0) | |
| Missing | 4 (1.7) | 0 (0.0) | |
| Declined | 3 (1.3) | 1 (0.9) | |
| Inclusion criteria | 0.03 | ||
| Age ≥ 55 yr with COPD, CHF, cirrhosis, cancer or end-stage dementia | 70 (30.0) | 23 (20.7) | |
| Age ≥ 80 yr | 156 (67.0) | 88 (79.3) | |
| Care team assessment | 7 (3.0) | 0 (0.0) | |
| Length of hospital stay, d, mean ± SD (range) | 13.0 ± 15.6 (2.0–126.0) | 14.6 ± 11.4 (2.0–48.0) | 0.07 |
| Death in hospital | 7 (3.0) | 14 (12.6) | < 0.001 |
Note: CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease, SD = standard deviation.
Unless otherwise stated.
Rockwood et al.18
Table 2:
Characteristics of family members who participated in the study
| Characteristic | No. (%)* n = 205 |
|---|---|
| Age, yr, mean ± SD (range) | 60.2 (21.0–91.0) |
| Sex | |
| Male | 47 (22.9) |
| Female | 158 (77.1) |
| Relationship to patient | |
| Spouse or partner | 54 (26.3) |
| Daughter or son | 125 (61.0) |
| Sister or brother | 4 (2.0) |
| Other | 22 (10.7) |
| Education | |
| Completed high school | 170 (82.9) |
| Did not complete high school | 35 (17.1) |
| Race; language | |
| White | 180 (87.8) |
| White; speaks language other than English or French | 38 (18.5) |
| Non-white; speaks language other than English or French | 16 (7.8) |
| Surrogate decision-maker | 122 (59.5) |
| Family member of a participating patient† | 94 (45.9) |
Note: SD = standard deviation.
Unless otherwise stated.
Of the 205 family members who participated, 111 were family members of patients who did not participate, either because they were excluded or they chose not to consent. Reasons for patient exclusion: a spoken language other than English or French (n = 33), cognitive impairment (n = 49), a request by family members (n = 3), being too ill (n = 3), difficulty speaking (n = 2) or hearing (n = 1), the patient was missed (n = 1) or for other reasons (n = 4). Reasons for lack of consent: lack of interest (n = 5), participation was too upsetting (n = 3), the patient was too tired (n = 3), other (n = 4).
Discussions about and importance of key elements of determining goals of care
Between admission to hospital and the time of the interview (median 3.0 d), patients reported that 1.4 (SD 2.1, median 0) of our 11 key elements were discussed. Family members reported that 2.0 (SD 2.4, median 1) of the 11 elements were discussed (Table 3). Patients and family members ranked the same 5 elements as most important, based on the frequency of “very important” or “extremely important” ratings (Spearman correlation coefficient 0.84; p = 0.001) (Table 3). For patients, the top-ranked element was to be “asked about preferences for care in the event of a life-threatening illness.” For family members, the top-ranked element was discussing prognosis. Both patients and families rated the provision of information about advance care planning and related legal documents as the least important elements. Importance ratings were missing for 8%–16% of patients and 3%–15% of family members.
Table 3:
Discussions about and importance of individual elements in determining goals of care
| Element | Patients n = 233 |
Family members n = 205 |
||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Occurrence n/N (%) | Very or extremely important n/N (%) | Ranking by importance* | Occurrence n/N (%) | Very or extremely important n/N (%) | Ranking by importance* | |
| Asked the respondent about preferences for care in event of life-threatening illness | 46/214 (21.5) | 95/195 (48.7) | 1 | 45/186 (24.2) | 118/177 (66.7) | 4 |
|
| ||||||
| Inquired about the respondent’s values | 37/227 (16.3) | 98/215 (45.6) | 2 | 32/203 (15.8) | 126/194 (64.9) | 5 |
|
| ||||||
| Discussed prognosis | 22/224 (9.8) | 88/200 (44.0) | 3 | 35/202 (17.3) | 147/193 (76.2) | 1 |
|
| ||||||
| Gave an opportunity for the respondent to express fears or concerns | 50/222 (22.5) | 91/208 (43.8) | 4 | 52/182 (28.6) | 124/175 (70.9) | 2 |
|
| ||||||
| Asked the respondent if they had additional questions about goals of care | 44/221 (19.9) | 91/208 (43.8) | 5 | 63/199 (31.7) | 133/189 (70.4) | 3 |
|
| ||||||
| Provided information about outcomes, risks, benefits of comfort care | 22/223 (9.9) | 71/205 (34.6) | 6 | 35/201 (17.4) | 117/190 (61.6) | 6 |
|
| ||||||
| Asked about prior discussions or written documents | 54/227 (23.8) | 73/213 (34.3) | 7 | 59/203 (29.1) | 110/198 (55.6) | 9 |
|
| ||||||
| Offered a time to meet to discuss goals of care | 14/223 (6.3) | 61/202 (30.2) | 8 | 31/202 (15.3) | 113/193 (58.5) | 7 |
|
| ||||||
| Provided information about outcomes, risks, benefits of life sustaining treatments | 28/225 (12.4) | 58/207 (28.0) | 9 | 38/202 (18.8) | 107/192 (55.7) | 8 |
|
| ||||||
| Provided information to review about advance care planning before discussions | 8/228 (3.5) | 50/207 (24.2) | 10 | 8/203 (3.9) | 87/191 (45.5) | 10 |
|
| ||||||
| Helped access legal documents to record advance care plans | 3/219 (1.4) | 37/197 (18.8) | 11 | 3/200 (1.5) | 66/187 (35.3) | 11 |
According to percentage of participants who ranked the element as very or extremely important; Spearman correlation coefficient = 0.001.
Association between discussion of recommended elements of determining goals of care and outcomes
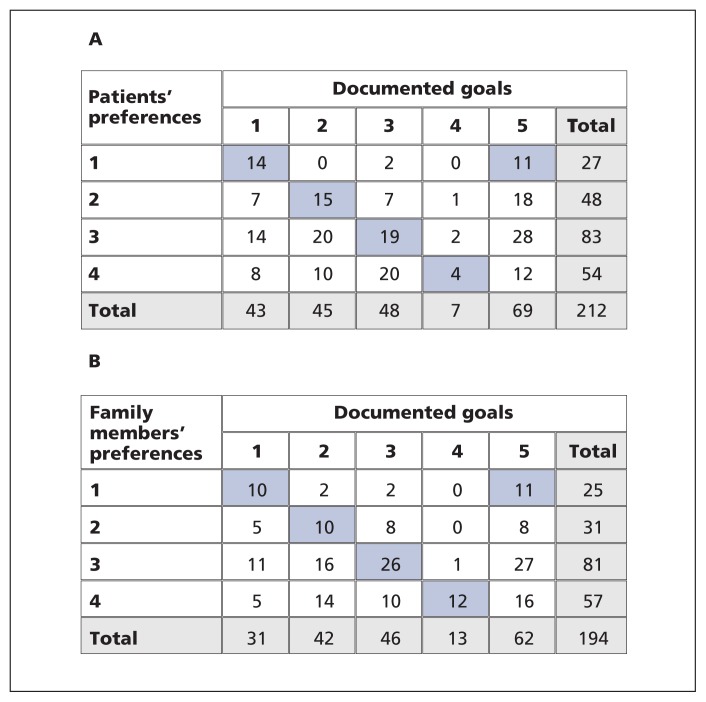
Concordance between prescribed goals of care and patient preferences for use (or non-use) of life sustaining treatments was 29.7% (κ = 0.11). Concordance between prescribed goals of care and family members’ preferences for their loved one was 35.6% (κ = 0.18) (Figure 2). Concordance could not be assessed for 21 (9.0%) patients and 11 (5.4%) family members because of missing preference data. Satisfaction with end-of-life communication and decision-making as represented by CANHELP scores was high for patients ( mean 70.9 ± SD 18.9) and family members (mean 64.7 ± SD 22.8). Scores were missing for 14 (6.0%) patients and 6 (2.9%) family members.
Figure 2:
(A) Concordance between patients’ stated preferences for life-sustaining treatment and prescribed orders for goals of care (agreement = 29.7%, κ = 0.11). (B) Concordance between family members’ stated preferences for life-sustaining treatment for the patient and prescribed orders for goals of care (agreement = 35.6%, κ =0.18). Blue highlighting shows concordance. A total of 21 patients and 11 family members were excluded because of missing preference data. Note: 1 = aggressive use of heroic measures and artificial life-sustaining treatments, including cardiopulmonary resuscitation (CPR), to keep patient alive at all costs; 2 = full medical care, but no CPR if the patient’s heart or breathing stops; 3 = a combination of the first 2 options (e.g., try to fix problems; if patient shows no improvement, switch to focusing only on patient comfort, even if it hastens death); 4 = doctors will focus on patient comfort and alleviating suffering, not on keeping patient alive by artificial means or heroic measures, such as trying to prolong life with CPR and other life-sustaining technologies; 5 = no documented goals of care.
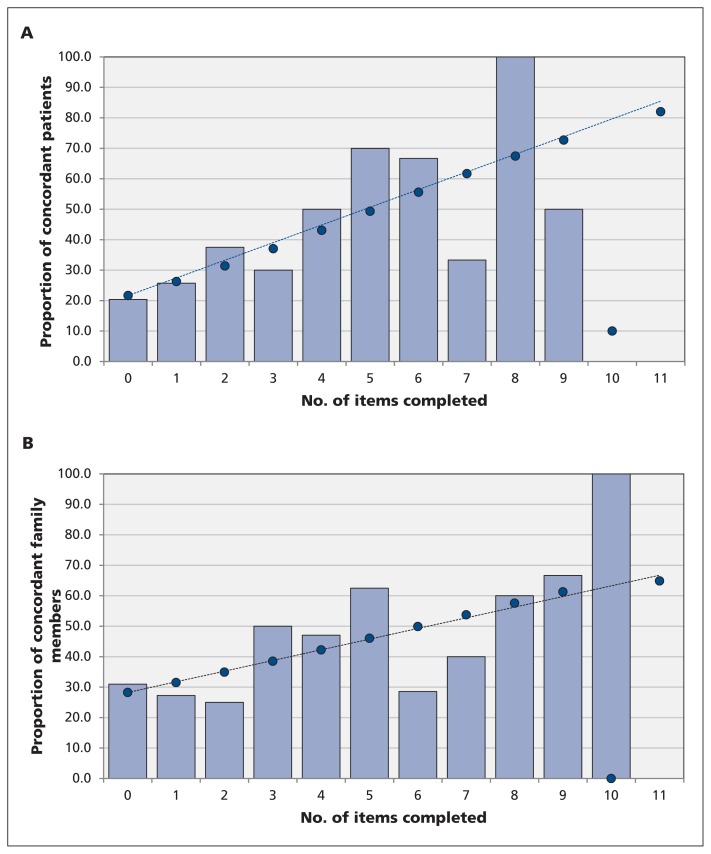
Discussion of a greater number of the 11 recommended elements of determining goals of care was significantly associated with concordance between participants’ preferences for use (or non-use) of life-sustaining treatments and prescribed goals of care (patient adjusted odds ratio [OR] 1.27, 95% confidence interval [CI] 1.09–1.47, p < 0.001; family member adjusted OR 1.20, 95% CI 1.03–1.39, p = 0.01) (Figure 3). Similarly, discussion of a greater number of the 11 recommended elements was significantly associated with greater satisfaction with end-of-life communication and decision-making, with a 1.4-point increase in CANHELP scores per element discussed among patients (p = 0.03), and a 2.3-point increase per element discussed among family members (p = 0.002).
Figure 3:
Concordance between (A) patients’ and (B) family members’ preferences for care at end of life and documented goals of care versus the number of components of determining goals of care that were reported to have been discussed. The line of best fit was calculated using logistic regression (odds ratios) and represents the predicted increase in concordance as more elements of goals-of-care discussions are addressed.
Interpretation
In this cross-sectional multicentre study, we have identified elements of goals of care discussions with health care providers that are most important to older adult patients admitted to hospital with serious illnesses and their family members. The 5 most important elements to discuss were preferences for care in the event of life-threatening illness, values, prognosis, fears or concerns, and additional questions about goals of care. We found that these elements are infrequently discussed and that concordance between preferred and prescribed goals of care is low. Moreover, our results suggest that concordance between preferences and prescribed goals of care, as well as satisfaction with end-of-life communication, increase with the number of elements discussed. Thus, our findings could be used to identify important opportunities to improve end-of-life communication and decision-making in the hospital setting.
Guidelines about end-of-life communication acknowledge that, because of limitations in the available evidence, the recommended elements of goals-of-care discussions are mostly based on expert opinion.12–14 To address this knowledge gap, we used content validation by experts to derive from these guidelines a set of 11 key elements of goals-of-care discussions. Our study provides empirical data to show that discussion of these 11 elements is associated with greater concordance between preferred and prescribed goals of care, and with greater satisfaction with end-of-life communication and decision-making. Furthermore, we have identified which of these elements are most important to patients and family members, an important perspective previously lacking from the guidelines.
We recently published a conversation guide that encourages clinicians to “Just Ask” patients about their goals of care.19 Our results build on that work by providing further direction on which specific elements should be discussed with patients and families in hospital. For example, both patients and family members rated prognostic disclosure as highly important. Indeed, prognostic disclosure is associated with greater satisfaction with end-of-life communication and decision-making.20 However, we found that prognosis was discussed with only 10% of patients and 17% of family members in our study. This is consistent with previous work that showed prognostic disclosure occurs infrequently among patients with serious illness8,20 and represents an important opportunity for improvement.
Our results highlight ongoing challenges in the alignment of prescribed goals of care with patient preferences for end-of-life care. More than 20 years ago, the landmark Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment (SUPPORT) identified important gaps between patient preferences for end-of-life care and the actual care received.21 In addition, a recent audit in Canadian hospitals found only 30% agreement between patient preferences and prescribed goals of care.8
Strengths and limitations
We used real-time face-to-face interviews, which would be expected to have a lower risk of recall bias than postdischarge questionnaires, and a validated questionnaire to gather our data.
Participants were required to speak either English or French, and most of our participants were white. As a result, the applicability of our results to more diverse populations who do not speak English or French as a first language and who may have different values related to end-of-life care22,23 remains uncertain.
Although we were able to enroll most of the eligible patients and family members (62% and 74%, respectively), thus minimizing selection bias, non-consenting patients or family members may have had different perspectives on what is important during end-of-life communication and decision-making.
We focused on goals-of-care discussions only during the early phase of the hospital stay. Although this is an important time to establish goals of care for patients at high risk of dying, our data may underestimate the frequency of these discussions, which may occur throughout the patient’s stay.
Conclusion
We have identified the guideline-recommended elements of goals-of-care discussions that are the most important to older adults patients in hospital with serious illness and their family members. However, these elements are infrequently addressed by health care providers in hospital, which may contribute to the identified gap between preferred and prescribed goals of care. Our results can inform interventions to improve end-of-life discussions in the hospital setting and the concordance between preferred and prescribed goals of care.
Supplementary Material
Acknowledgements
The authors thank the patients and family members who participated in the study, the research staff and co-investigators (Jessica Simon, Doris Barwich, Pat Porterfield and Carolyn Tayler) for their assistance with data collection, the staff at the Clinical Evaluation Research Unit, Kingston General Hospital, for support with the methods and analysis, and Amanda Roze des Ordons for feedback on an earlier draft of the manuscript.
Footnotes
See also CMAJ’s end-of-life care collection at www.cmaj.ca/site/misc/end-of-life-care.xhtml
Competing interests: None declared.
This article has been peer reviewed.
Contributors: John You and Daren Heyland designed the study and drafted the article. John You, Peter Dodek, Francois Lamontagne, James Downar and Daren Heyland acquired the data. All of the authors analyzed and interpreted the data, revised the article for important intellectual content, approved the final version submitted for publication, and agree to act as guarantor of the work.
Funding: John You is supported by a Research Early Career Award from Hamilton Health Sciences. This study was supported by funding from the Canadian Institutes of Health Research, the Michael Smith Health Services Research Foundation, Alberta Innovates, and the Ontario Academic Health Sciences Centre Alternate Funding Plan Innovation Fund. None of the funders had a role in the design or conduct of the study, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
References
- 1.Heyland DK, Lavery JV, Tranmer JE, et al. Dying in Canada: is it an institutionalized, technologically supported experience? J Palliat Care 2000;16(Suppl):S10–6. [PubMed] [Google Scholar]
- 2.Heyland DK, Lavery JV, Tranmer J, et al. The final days: an analysis of the dying experience in Ontario. Ann R Coll Physicians Surg Can 2000;33:356–61. [Google Scholar]
- 3.Health Care Use at the End of Life in Western Canada. Ottawa: Canadian Institute for Health Information; 2007. [Google Scholar]
- 4.Health Care Use at the End of Life in Atlantic Canada. Ottawa: Canadian Institute for Health Information; 2011. [Google Scholar]
- 5.Ehlenbach WJ, Barnato AE, Curtis JR, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med 2009;361:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruneir A, Mor V, Weitzen S, et al. Where people die: a multilevel approach to understanding influences on site of death in America. Med Care Res Rev 2007;64:351–78. [DOI] [PubMed] [Google Scholar]
- 7.Cook D, Rocker G, Marshall J, et al. ; Level of Care Study Investigators and the Canadian Critical Care Trials Group. Withdrawal of mechanical ventilation in anticipation of death in the intensive care unit. N Engl J Med 2003;349:1123–32. [DOI] [PubMed] [Google Scholar]
- 8.Heyland DK, Barwich D, Pichora D, et al. ; ACCEPT (Advance Care Planning Evaluation in Elderly Patients) Study Team; Canadian Researchers at the End of Life Network (CARENET). Failure to engage seriously ill hospitalized patients and their families in advance care planning: results of a multicenter prospective study. JAMA Intern Med 2013;173:778–87. [DOI] [PubMed] [Google Scholar]
- 9.Heyland DK, Cook DJ, Rocker GM, et al. ; Canadian Researchers at the End of Life Network (CARENET). Defining priorities for improving end-of-life care in Canada. CMAJ 2010;182: E747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heyland DK, Dodek P, Rocker G, et al. ; Canadian Researchers at the End of Life Network (CARENET). What matters most in end-of-life care: perceptions of seriously ill patients and their family members. CMAJ 2006;174:627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinuff T, Dodek P, You JJ, et al. Improving end-of-life communication and decision-making: the development of a conceptual framework and quality indicators [manuscript in submission] [DOI] [PubMed] [Google Scholar]
- 12.Advance Care Planning. Concise Guidance to Good Practice Series, No 12. London (UK): Royal College of Physicians; National Council for Palliative Care; British Society of Rehabilitation Medicine; British Geriatrics Society; Alzheimer’s Society; Royal College of Nursing; Royal College of Psychiatrists; Help the Aged; Royal College of General Practitioners; 2009. [Google Scholar]
- 13.Harle I, Johnston J, Mackay J, et al. Advance Care Planning with Cancer Patients: Evidentiary Base. Toronto: Program in Evidence-Based Care; Cancer Care Ontario; 2008. [Google Scholar]
- 14.Clayton JM, Hancock KM, Butow PN, et al. Clinical practice guidelines for communicating prognosis and end-of-life issues with adults in the advanced stages of a life-limiting illness, and their caregivers. Med J Aust 2007; 186:S77, S79,, S83–108. [DOI] [PubMed] [Google Scholar]
- 15.Heyland DK, Pichora D, Dodek P, et al. The development and validation of a questionnaire to audit advance care planning. J Palliat Care Med 2012;2:119. [Google Scholar]
- 16.Heyland DK, Cook DJ, Rocker GM, et al. ; Canadian Researchers at the End of Life Network. The development and validation of a novel questionnaire to measure patient and family satisfaction with end-of-life care: the Canadian Health Care Evaluation Project (CANHELP) Questionnaire. Palliat Med 2010;24:682–95. [DOI] [PubMed] [Google Scholar]
- 17.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37–46. [Google Scholar]
- 18.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You JJ, Fowler RA, Heyland DK; Canadian Researchers at the End of Life Network (CARENET). Just ask: discussing goals of care with patients in hospital with serious illness. CMAJ 2014; 186:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heyland DK, Allan DE, Rocker G, et al. ; Canadian Researchers at the End of Life Network. Discussing prognosis with patients and their families near the end of life: impact on satisfaction with end-of-life care. Open Med 2009;3:e101–10. [PMC free article] [PubMed] [Google Scholar]
- 21.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA 1995;274:1591–8. [PubMed] [Google Scholar]
- 22.Johnstone MJ, Kanitsaki O. Ethics and advance care planning in a culturally diverse society. J Transcult Nurs 2009;20:405–16. [DOI] [PubMed] [Google Scholar]
- 23.Con A. Cross-cultural considerations in promoting advance care planning in Canada. Vancouver: BC Cancer Agency; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.