Abstract
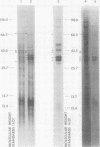
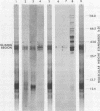
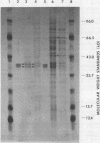
Free and membrane-associated polysomes were isolated in approximately equal amounts from endosperm of wheat kernels harvested 20 days after anthesis. The presence of heparin in the homogenizing buffer minimized polysome degradation. Ribonucleic acid from the isolated polysomes, when translated in vitro in a wheat germ system, yielded products ranging in size from about 12,000 to about 80,000 daltons, including at least two polypeptides that co-migrated with seed extract proteins in sodium dodecyl sulfate polyacrylamide gel electrophoresis. The nature of the translation products of free and membrane-associated RNA are distinctly different, with membrane-associated RNA yielding a higher proportion of polypeptides in the size range of 30,000 to 37,000 daltons. Analysis of membrane-associated 3′-terminal polyadenylyl-containing RNA in vitro translation products, by solubility in 70% ethanol and by immunoprecipitation, indicates that the 33,000- to 37,000-dalton polypeptides contain gliadins, and the analysis provides evidence that these proteins are synthesized in association with membranous cell organelles. Gliadin polypeptides synthesized in vitro are larger than authentic gliadins and probably are precursors which, in vivo, undergo modification to yield the smaller final products.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aragoncillo C., Rodríguez-Loperena M. A., Salcedo G., Carbonero P., García-Olmedo F. Influence of homoeologous chromosomes on gene-dosage effects in allohexaploid wheat (Triticum aestivum L.). Proc Natl Acad Sci U S A. 1978 Mar;75(3):1446–1450. doi: 10.1073/pnas.75.3.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste K., Wilczek J., Ruggieri A., Stern R. Translation of collagen messenger RNA in a cell-free system derived from wheat germ. Biochemistry. 1976 Feb 24;15(4):830–835. doi: 10.1021/bi00649a016. [DOI] [PubMed] [Google Scholar]
- Bernardin J. E., Kasarda D. D., Mecham D. K. Preparation and characterization of alpha-gliadin. J Biol Chem. 1967 Feb 10;242(3):445–450. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burr B., Burr F. A. Zein synthesis in maize endosperm by polyribosomes attached to protein bodies. Proc Natl Acad Sci U S A. 1976 Feb;73(2):515–519. doi: 10.1073/pnas.73.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crumpton M. J., Parkhouse R. M.E. Comparison of the effects of various detergents on antigen-antibody interaction. FEBS Lett. 1972 May 1;22(2):210–212. doi: 10.1016/0014-5793(72)80047-4. [DOI] [PubMed] [Google Scholar]
- Davies E., Larkins B. A., Knight R. H. Polyribosomes from peas: an improved method for their isolation in the absence of ribonuclease inhibitors. Plant Physiol. 1972 Nov;50(5):581–584. doi: 10.1104/pp.50.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. W., Kaesberg P. Translation of virus mRNA: synthesis of bacteriophage Q beta proteins in a cell-free extract from wheat embryo. J Virol. 1973 Dec;12(6):1434–1441. doi: 10.1128/jvi.12.6.1434-1441.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidlin P. J., Patterson R. J. Heparin releases monosomes and polysomes from rough endoplasmic reticulum. Biochem Biophys Res Commun. 1980 Mar 28;93(2):521–527. doi: 10.1016/0006-291x(80)91108-0. [DOI] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A., Baglioni C. Inhibition of initiation of protein synthesis by 7-methylguanosine-5'-monophosphate. Proc Natl Acad Sci U S A. 1976 Jan;73(1):19–23. doi: 10.1073/pnas.73.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilz H., Wiegers U., Adamietz P. Stimulation of proteinase K action by denaturing agents: application to the isolation of nucleic acids and the degradation of 'masked' proteins. Eur J Biochem. 1975 Aug 1;56(1):103–108. doi: 10.1111/j.1432-1033.1975.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Konzak C. F. Genetic control of the content, amino acid composition, and processing properties of proteins in wheat. Adv Genet. 1977;19:407–582. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Bracker C. E., Tsai C. Y. Storage Protein Synthesis in Maize: Isolation of Zein-synthesizing Polyribosomes. Plant Physiol. 1976 May;57(5):740–745. doi: 10.1104/pp.57.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Jones R. A., Tsai C. Y. Isolation and in vitro translation of zein messenger ribonucleic acid. Biochemistry. 1976 Dec 14;15(25):5506–5511. doi: 10.1021/bi00670a014. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Tsai C. Y. Dissociation of polysome aggregates by protease k. Plant Physiol. 1977 Oct;60(4):482–485. doi: 10.1104/pp.60.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe D. S., Peterson D. M. Cell-free Synthesis of Globulin by Developing Oat (Avena sativa L.) Seeds. Plant Physiol. 1977 May;59(5):836–841. doi: 10.1104/pp.59.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K., RAISON J. K. A COMPLETE INTRACELLULAR UNIT FOR INCORPORATION OF AMINO-ACID INTO STORAGE PROTEIN UTILIZING ADENOSINE TRIPHOSPHATE GENERATED FROM PHYTATE. Nature. 1963 Nov 2;200:429–433. doi: 10.1038/200429a0. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecham D. K., Kasarda D. D., Qualset C. O. Genetic aspects of wheat gliadin proteins. Biochem Genet. 1978 Aug;16(7-8):831–853. doi: 10.1007/BF00484739. [DOI] [PubMed] [Google Scholar]
- O'Donnell I. J., Frangione B., Porter R. R. The disulphide bonds of the heavy chain of rabbit immunoglobulin G. Biochem J. 1970 Jan;116(2):261–268. doi: 10.1042/bj1160261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Sullivan D., Summers N. M., Kiely M. L., Schimke R. T. Purification of ovalbumin messenger ribonucleic acid by specific immunoadsorption of ovalbumin-synthesizing polysomes and millipore partition of ribonucleic acid. J Biol Chem. 1973 Jan 25;248(2):540–548. [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Staynov D. Z., Gratzer W. B. Electrophoresis of RNA in formamide. Biochemistry. 1974 Dec 17;13(26):5373–5378. doi: 10.1021/bi00723a019. [DOI] [PubMed] [Google Scholar]
- Platt S. G., Kasarda D. D., Qualset C. O. Varietal relationships of the alpha-gliadin proteins in wheat. J Sci Food Agric. 1974 Dec;25(12):1555–1561. doi: 10.1002/jsfa.2740251217. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Shapiro D. J., Taylor J. M., McKnight G. S., Palacios R., Gonzalez C., Kiely M. L., Schimke R. T. Isolation of hen oviduct ovalbumin and rat live albumin polysomes by indirect immunoprecipitation. J Biol Chem. 1974 Jun 25;249(12):3665–3671. [PubMed] [Google Scholar]
- Solymosy F., Fedorcsák I., Gulyás A., Farkas G. L., Ehrenberg L. A new method based on the use of diethyl pyrocarbonate as a nuclease inhibitor for the extraction of undegraded nucleic acid from plant tissues. Eur J Biochem. 1968 Sep 24;5(4):520–527. doi: 10.1111/j.1432-1033.1968.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Tse T. P., Taylor J. M. Translation of albumin messenger RNA in a cell-free protein-synthesizing system derived from wheat germ. J Biol Chem. 1977 Feb 25;252(4):1272–1278. [PubMed] [Google Scholar]
- Wilson C. M. Plant Nucleases: VI. GENETIC AND DEVELOPMENTAL VARIABILITY IN RIBONUCLEASE ACTIVITY IN INBRED AND HYBRID CORN ENDOSPERMS. Plant Physiol. 1980 Jul;66(1):119–125. doi: 10.1104/pp.66.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley C. W., Shepherd K. W. Electrofocusing of grain proteins from wheat genotypes. Ann N Y Acad Sci. 1973 Jun 15;209:154–162. doi: 10.1111/j.1749-6632.1973.tb47526.x. [DOI] [PubMed] [Google Scholar]