Abstract
Effective oncolytic virus (OV) therapy is dependent on the ability of replication-competent viruses to kill infected cancer cells. We previously showed that human pancreatic ductal adenocarcinoma (PDAC) cell lines are highly heterogeneous in their permissiveness to vesicular stomatitis virus (VSV), in part due to differences in type I interferon (IFN) signaling. Here, using ten human PDAC cell lines and three different VSV recombinants (expressing ΔM51 or wild type matrix protein), we examined cellular and viral factors affecting VSV-mediated apoptosis activation in PDACs. In most cell lines VSVs activated both extrinsic and intrinsic apoptosis pathways, and VSV-ΔM51 primarily activated the type II extrinsic pathway. In cells with defective IFN signaling, all VSV recombinants induced robust apoptosis, whereas VSV-ΔM51 was a more effective apoptosis activator in PDACs with virus-inducible IFN signaling. Three cell lines constitutively expressing high levels of IFN-stimulated genes (ISGs) were resistant to apoptosis under most experimental conditions, even when VSV replication levels were dramatically increased by Jak inhibitor I treatment. Two of these cell lines also poorly activated apoptosis when treated with Fas activating antibody, suggesting a general defect in apoptosis.
INTRODUCTION
Oncolytic virus (OV) therapy is an innovative anticancer approach utilizing replication-competent viruses that preferentially infect and kill cancer cells [reviewed in (Russell et al., 2012)]. Vesicular stomatitis virus (VSV), a prototypic non-segmented negative-strand RNA virus (order Mononegavirales, family Rhabdoviridae), is a promising oncolytic virus against various malignancies [reviewed in (Barber, 2004; Hastie and Grdzelishvili, 2012)], and a phase I clinical trial using VSV against hepatocellular carcinoma is in progress (http://clinicaltrials.gov, trial NCT01628640). While wild type (wt) VSV cannot be utilized as an OV due to its unacceptable neurotoxicity, numerous VSV-based recombinants with significantly decreased neurotoxicity and improved oncoselectivity have been generated [reviewed in (Hastie and Grdzelishvili, 2012)]. One of the best performing oncolytic VSVs is VSV with replacement or deletion of the methionine at amino acid position 51 (M51) of the VSV matrix (M) protein. The oncoselectivity (and safety) of VSV M51 mutants is largely based on their inability to evade type I interferon (IFN) mediated antiviral responses in non-malignant cells (Ahmed et al., 2003; Brown et al., 2009; Ebert O et al., 2005; Stojdl DF et al., 2003; Trottier et al., 2007; Wollmann G et al., 2010). However, cancer cells often have defects in type I IFN signaling, which can provide a growth advantage to uninfected cells, but impairs their ability to inhibit VSV infection and replication [reviewed in (Barber, 2005; Hastie et al., 2013; Lichty BD et al., 2004)].
Pancreatic cancer is one of the most lethal abdominal malignancies with annual deaths closely matching the annual incidence of the disease [reviewed in (Farrow B et al., 2008)]. About 95% of pancreatic cancers are pancreatic ductal adenocarcinomas (PDAC), which are highly invasive with aggressive local growth and rapid metastases to surrounding tissues [reviewed in (Stathis A and Moore, 2010)]. Our recent studies demonstrated that VSV is very effective against the majority of human PDAC cell lines, both in vitro and in vivo, but that some cell lines are resistant to VSV replication and oncolysis (Moerdyk-Schauwecker et al., 2013; Murphy et al., 2012). All cell lines resistant to VSV retained functional type I IFN responses (Moerdyk-Schauwecker et al., 2013; Murphy et al., 2012) and displayed constitutive high-level expression of the IFN-stimulated antiviral genes MxA and OAS (Moerdyk-Schauwecker et al., 2013; Murphy et al., 2012)). Inhibition of JAK/STAT signaling by Jak inhibitor I (Jak Inh. I) decreased levels of MxA and OAS and increased VSV replication (Moerdyk-Schauwecker et al., 2013).
Effective oncolytic virus (OV) therapy depends not only on the ability of OVs to infect and replicate in cancer cells, but also to kill them. VSV kills infected cells primarily via induction of apoptosis (Balachandran et al., 2001; Balachandran et al., 2000; Cary et al., 2011; Gadaleta et al., 2005; Gaddy DF and and Lyles, 2005; Gaddy DF, 2007; Kopecky and Lyles, 2003; Kopecky et al., 2001). The specific mechanism of apoptosis in response to VSV infection depends on both virus and cell type, and apoptosis induction has never been studied in any pancreatic cancer cells in response to VSV. Thus, the goals of this study were (1) to investigate the mechanism of apoptosis induction in PDAC cell lines by three different viruses: wt-like VSV (VSV-GFP) and VSV attenuated by M dependent and independent mechanisms (VSV-ΔM51-GFP and VSV-P1-GFP respectively; and (2) to examine whether dysregulation of apoptosis, a hallmark of PDACs as well as other cancers [reviewed in (Hamacher et al., 2008; Neesse et al., 2012; Roder et al., 2011)], contributes to the resistance of some PDACs to VSV-mediated oncolysis. For example, in chronic lymphocytic leukemia (CLL) cells overexpressing the anti-apoptotic protein Bcl-2, VSV-M51R (M51R substitution in M protein) was unable to induce apoptosis and consequently the CLL cells were resistant to VSV-induced killing (Tumilasci et al., 2008).
The use of a VSV recombinant with the M51 deletion in the M protein (unable to evade type I IFN responses), and two VSV recombinants with wt M protein revealed possible links between apoptosis and type I IFN signaling in PDAC cell lines. Moreover, the ability of PDAC cells to undergo apoptosis following non-viral stimuli was also examined. Finally, as apoptosis activation may reduce viral replication (and potentially reduce oncolytic virus efficiency), as has been reported for many viruses [reviewed in (Galluzzi et al., 2008; O’Brien, 1998)] including, in some instances, VSV (Chattopadhyay et al., 2011; Desforges et al., 2002; Sharif-Askari et al., 2007), the effect of apoptosis activation on VSV replication in PDAC cells was examined. Revealing mechanisms of VSV-mediated apoptosis in PDAC cells is important for understanding virus-host interactions in cancer cells. In addition, understanding mechanisms of resistance of cancer cells to VSV and other OVs is critical for developing effective OV approaches, identifying biomarkers and developing approaches to break resistance.
MATERIALS AND METHODS
Viruses
VSV-ΔM51-GFP and VSV-p1-GFP viruses were kindly provided by Jack Rose (Yale University), and VSV-GFP virus was kindly provided by Asit Pattnaik (University of Nebraska). All 3 VSV recombinants are based on the same full-length VSV (Indiana serotype) cDNA clone (Lawson et al., 1995), which contains the L gene and the N-terminal 49 residues of the N gene from the Mudd-Summers strain, the remainder of the genome from the San Juan strain (both belonging to the Indiana serotype). VSV-ΔM51-GFP has a deletion of methionine at amino acid position 51 of the M protein and the green fluorescent protein (GFP) ORF inserted at position 5 of the viral genome (Wollmann G et al., 2010). VSV-GFP is similar to VSV-ΔM51-GFP, but has wt M (Das et al., 2006). VSV-p1-GFP also has wt M but GFP is inserted in position 1 (Wollmann G et al., 2010). Viruses were grown in BHK-21. Viral titers were determined by standard plaque assay on BHK-21 cells and expressed as plaque forming units (PFU) per ml.
Cell lines
The human PDAC cell lines used in this study were kindly provided by the following individuals in Fall 2010: David McConkey (M. D. Anderson Cancer Center): CFPAC-1 and Hs766T cells; Randall Kimple (UNC-Chapel Hill): Capan-2 and T3M4 cells; Timothy Wang (Columbia University): AsPC-1 cells; Andrei Ivanov (University of Rochester Medical School): HPAF-II cells; Michael Hollingsworth (University of Nebraska Medical Center): Suit2 cells; Emmanuel Zervos (Tampa General Hospital): HPAC cells; Pinku Mukherjee (University of North Carolina at Charlotte): Capan-1, MIA PaCa-2 and Panc-1 cells. Cells were maintained as previously described (Moerdyk-Schauwecker et al., 2013). After receipt, the human origin of all cell lines was confirmed by partial sequencing of KRAS and actin. As expected, all PDAC cell lines had a mutation in KRAS, as is typical for PDACs (data not shown).
Drugs
The following drugs were used in this study: recombinant career-free (CF) human TNF-α (R&D Systems); recombinant human TNF-related apoptosis-inducing ligand (TRAIL) (Millipore); Fas activating antibody (Millipore, clone CH11); caspase-8 inhibitor Z-IETD-FMK (R&D Systems); pan-caspase inhibitor Z-VAD-FMK (R&D Systems) and Jak Inh. I (“InSolution”, Calbiochem).
Western Blot
Cellular lysates and Western blots were prepared as previously described (Moerdyk-Schauwecker et al., 2013). Cell lysates were collected at 17 hours (h) post infection (p.i.) following infection at MOI 15 (based on BHK-21 titer). Due to a limited amount of total protein isolated from T3M4 and HPAC cells, the following exceptions were made: for PKR T3M4 sample, no protein was loaded for uninfected T3M4 cells; for caspase 3 and FADD in HPAC cells 15 μg (half the amount) was loaded. The following primary antibodies were used in TBS-T with 5% BSA or milk and 0.02% sodium azide: 1:10,000 rabbit polyclonal anti-VSV antibodies (raised against VSV virion proteins), 1:1000 anti-MxA (clone 631–645) antibodies from Sigma, and the following antibodies from Cell Signaling Technology (1:1000 or 1:500): caspase 3, caspase 8 (clone 1C12), caspase 9, Bak, Bax (clone D2E11), BID, Bcl-2 (clone 50E3), Bcl-xL (clone 54H6), Daxx (clone 25C12), FADD, Fas (clone 4C3), Mcl-1 (clone D35A5). The following horseradish peroxidase-conjugated secondary antibodies were used: 1:2000 goat anti-mouse and 1:2000 goat anti-rabbit (Jackson-ImmunoResearch). The Amersham ECL Western Blotting Detection Kit (GE Healthcare) or Pierce SuperSignal West Pico Detection Kit (Thermo Scientific) was used for detection. Membranes were (Moyer et al., 1986) Coomassie blue stained to verify sample loading. When Jak Inh. I treatment was used, 6-well plates were seeded such that they were approximately 80% confluent at the time of inhibitor treatment. Cells were treated with 2.5 μM Jak Inh. I or vehicle (DMSO) only in cell culture media with 5% FBS for 48 h prior to infection (media was removed and replaced with fresh drug/vehicle containing media after the first 24 h). Cells were then mock infected or infected with VSV-ΔM51-GFP in DMEM without FBS at an MOI of 15 (based on BHK-21 titer). Following a 1 h absorption period, the virus containing media was aspirated and replaced with growth media with 5% FBS containing either 2.5 μM Jak Inh. I or vehicle. At 17 h p.i., cells were collected and used to prepare cellular lysates for Western blotting as described above.
Cell based apoptosis detection
For detection of apoptosis induction following virus infection, cells were seeded in 96-well plates such that they were approximately 80% confluent at the time of treatment. Cells were then mock- or virus-infected at an MOI of 15 (based on BHK-21 titer). Following a 1 h absorption period, the virus containing media was aspirated and replaced with growth media containing 5% FBS. At 24 h p.i., apoptosis activation was assessed using the Caspase Glow 3/7 assay (Promega) in accordance with manufacturer instructions. When inhibitors of apoptosis were used, cells were pretreated with 100 μM caspase-8 inhibitor Z-IETD-FMK, 100 μM pan-caspase inhibitor Z-VAD-FMK or vehicle only (DMSO diluted in PBS) in growth media for 1 h prior to infection. Cells were then mock infected or infected with VSV-ΔM51-GFP at an MOI of 15 (based on BHK-21 titer), or treated with 1μg/ml Fas activating antibody, 1μg/ml TRAIL, or 25ng/ml TNF-α in the continued presence of either inhibitor or vehicle. Following a 1 h absorption period, the virus containing media was aspirated and replaced with growth media with 5% FBS (experiments using only virus) or no FBS (experiments also utilizing other drugs) containing either inhibitor or vehicle. At 17 h p.i., apoptosis activation was assessed using the Cleaved Caspase-3 In-Cell ELISA (Thermo Scientific), in accordance with manufacturer instructions. Alternatively, cells were infected at a cell line specific MOI of 2 to ensure one-step growth kinetics, and then treated with inhibitors of apoptosis as above. At the indicated time points, virus replication was monitored by measuring virus-directed GFP fluorescence in live cells [CytoFluor Series 4000 (Perseptive Biosystems), with excitation filter of 485/20 nm, emission filter of 530/25 nm and gain=50] and by collecting virus containing media for titration on BHK-21 cells.
Statistical analysis
All statistical analyses were performed using GraphPad Prism, version 5.03 for Windows (GraphPad Software, San Diego, California). Caspase 3/7 activity and caspase 3 cleavage within a cell line were analyzed by one-way ANOVA with Bonferroni post-test for comparison to the control. Virus driven GFP expression and virus replication following caspase inhibitor treatment was analyzed by repeated measures two-way ANOVA with Bonferroni post-test, following log transformation of replication values to increase normality.
RESULTS
Human PDAC cells are highly heterogeneous in their abilities to activate apoptosis following VSV infection
Effective OV therapy is dependent on the ability of a replication-competent virus to kill infected cancer cells. Although VSV mediated apoptosis was studied in other systems (Balachandran et al., 2001; Balachandran et al., 2000; Cary et al., 2011; Gadaleta et al., 2005; Gaddy DF and and Lyles, 2005; Gaddy DF, 2007; Sharif-Askari et al., 2007), the mechanism of apoptosis induction by VSV in pancreatic cancer cells has never been examined.
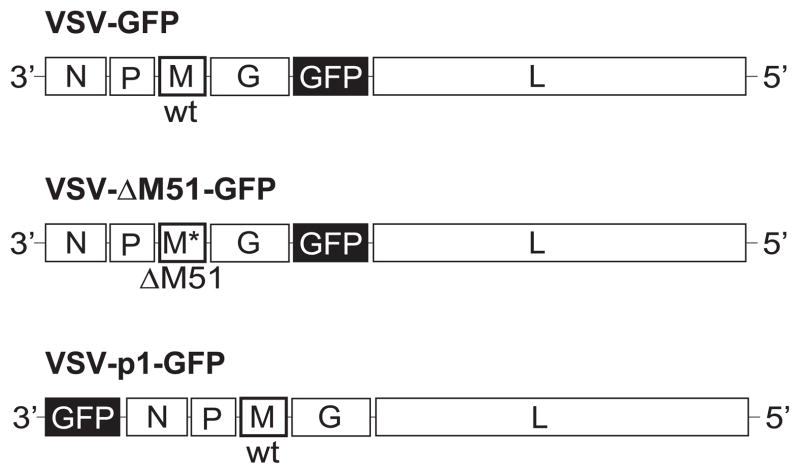
Our major focus is the commonly used VSV recombinant with the ΔM51 mutation in the M gene (Brown et al., 2009; Ebert O et al., 2005; Stojdl DF et al., 2003; Wollmann G et al., 2010). The recombinant VSV-ΔM51-GFP used in this study also contains the GFP ORF at position 5 of the viral genome (between G and L) (Fig. 1), which only marginally affects VSV replication, but allows for monitoring of VSV infection, replication, and spread, based on virus-driven GFP expression (Wollmann G et al., 2010). Several experiments also utilized two VSV recombinants encoding the wt M gene: VSV-GFP and VSV-p1-GFP (Fig. 1). VSV-GFP has the same GFP insertion as VSV-ΔM51-GFP but wt M gene, allowing study of the role of M protein in apoptosis induction. VSV-p1-GFP, also has the wt M gene, but insertion of the GFP ORF at position 1 of the VSV genome results in slower viral replication kinetics (Ramsburg et al., 2005; Wollmann G et al., 2010), allowing for examination of the role of M protein independent virus attenuation in apoptosis induction(Ramsburg et al., 2005; Wollmann G et al., 2010).
Figure 1. Viruses used in this study.
The viruses used in this study were VSV-ΔM51-GFP, VSV-p1-GFP and VSV-GFP. VSV-ΔM51-GFP has a deletion of methionine at amino acid position 51 of the M protein and the green fluorescent protein (GFP) ORF inserted at position 5 of the viral genome. VSV-GFP is similar to VSV-ΔM51-GFP, but has wt M. VSV-p1-GFP also has wt M but GFP is inserted in position 1.
To examine the ability of VSV recombinants to induce apoptosis in PDAC cells, a panel of 10 clinically relevant human PDAC cell lines was used (Table 1). These cells have been characterized in our previous studies for their permissiveness to VSV as well as their type I IFN signaling status (Moerdyk-Schauwecker et al., 2013; Murphy et al., 2012) (summarized in Table 1). Importantly, two of these PDAC cell lines (MIA PaCa-1 and Capan-1, indicated in all figures using green font) are highly permissive to VSV and defective in antiviral signaling in response to VSV infection; four cell lines (Capan-2, AsPC-1, Suit2, and T3M4, black font) are permissive to VSV but have VSV-inducible IFN signaling; two cell lines (CFPAC-1 and HPAC, blue font) are moderately permissive to VSV (resistant only at low MOIs) and have VSV-inducible IFN signaling and constitutive expression of MxA and OAS; and two cell lines (HPAF-II and Hs766T, red font) are highly resistant to VSV (at all tested MOIs) and have VSV-inducible IFN signaling and constitutive expression of MxA and OAS.
Table 1.
Relationship between apoptosis induction and IFN status of PDA cells.
| Human cell line | Origin | VSV-induced caspase 3 cleavage | VSV-induced caspase 8 cleavage* | VSV-induced caspase 9 cleavage | ** VSV-induced IFN-β mRNA | ** VSV-induced or constit. IFN-α mRNA | ** Constit. MxA and OAS | ** Resist to VSV-ΔM51 at low MOI | ** Resist to VSV-ΔM51 at high MOI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M51 | P1 | WT | M51 | P1 | WT | M51 | P1 | WT | |||||||
| Capan-1 | liver metastasis | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | N | N | N | N | N |
| MIA PaCa-2 | primary PDA | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | N | N | N | N | N |
| Capan-2 | primary PDA | ++ | + | + | ++ | + | + | ++ | ++ | ++ | Y | Y | N | N | N |
| AsPC-1 | ascites | ++ | − | + | ++ | − | − | ++ | + | ++ | Y | Y | N | N | N |
| Suit2 | liver metastasis | ++ | − | − | ++ | − | − | ++ | − | − | Y | Y | N | N | N |
| T3M4 | lymph node metastasis | ++ | + | + | + | + | + | ++ | ++ | ++ | Y | Y | N | N | N |
| CFPAC-1 | primary PDA | ++ | − | + | ++ | − | ++ | ++ | + | ++ | Y | Y | Y | Y | N |
| HPAC | primary PDA | − | − | − | − | − | − | − | − | − | Y | Y | Y | Y | N |
| HPAF-II | primary PDA | + | − | + | − | − | − | + | − | + | Y | Y | Y | Y | Y |
| Hs766T | lymph node metastasis | − | − | − | − | − | − | − | − | − | Y | Y | Y | Y | Y |
“M51” - VSV-ΔM51-GFP; “P1” - VSV-p1-GFP; “WT” - VSV-GFP; “Y” – yes; “N” – no.
− Low/not detectable levels
+ Intermediate levels
++ High levels
++ Cleaved Caspase 8 p43/41 and p18 are detected, + Cleaved Caspase 8 p 43/41 only is detected
Summarized from our previous studies (Moerdyk-Schauwecker et al., 2013; Murphy et al., 2012)
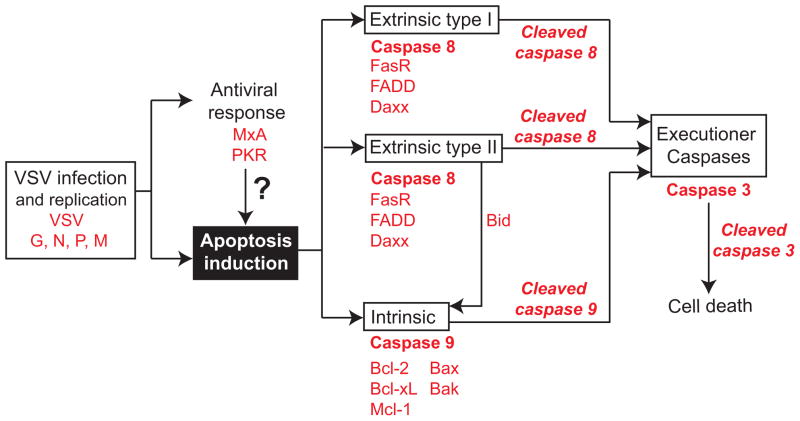
Cells were mock-treated or infected at an MOI of 15 (based on virus titration on BHK-21 cells) with VSV-ΔM51-GFP, VSV-p1-GFP or VSV-GFP. Protein was isolated at 17 h p.i. and Western blotting performed to examine VSV replication, ISG expression and the presence or absence of cleaved caspases and other apoptosis related factors. Figure 2 outlines the different apoptotic pathways PDA cell lines can undergo once infected and important proteins that regulate them.
Figure 2. Pathways and proteins studied.
Relationship between the apoptotic pathways. All proteins of interest for this study are in red.
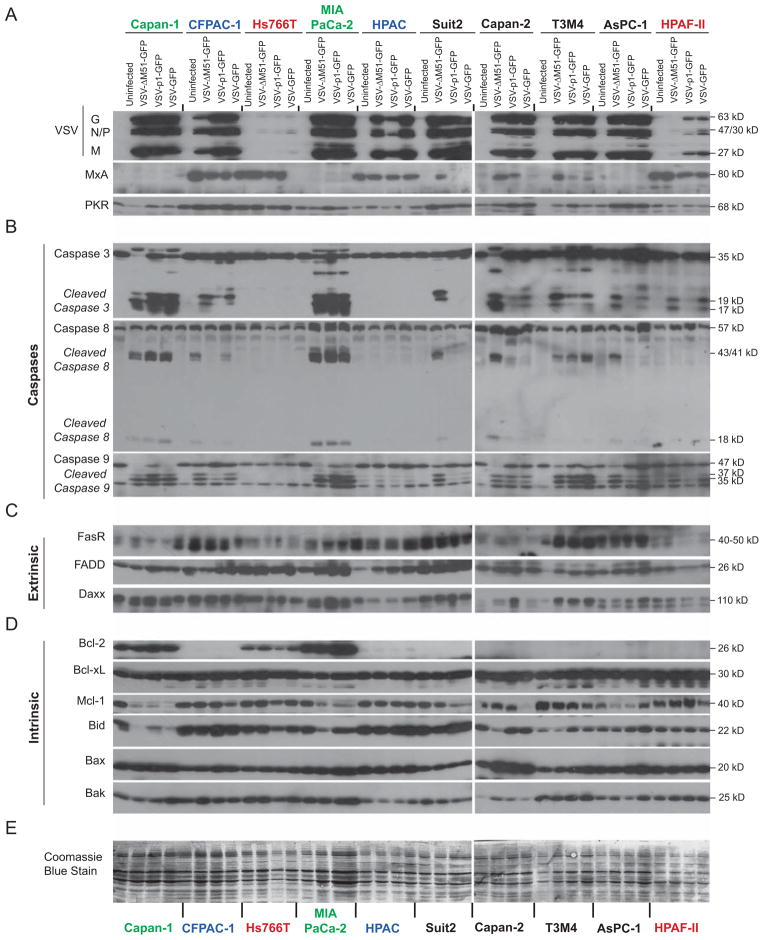
In agreement with our previous studies (Moerdyk-Schauwecker et al., 2013; Murphy et al., 2012), most cell lines supported good VSV replication (Fig. 3A), with the exception of Hs766T and HPAF-II which are resistant to VSV even at high MOI infection. Also, in agreement with our previous study (Moerdyk-Schauwecker et al., 2013), CFPAC-1, HPAC, HPAF-II and Hs766T constitutively expressed MxA, while VSV-induced expression of this ISG was observed in most other PDACs. Although basal levels of another ISG, PKR, did not differ in most cell lines [as previously shown (Moerdyk-Schauwecker et al., 2013)], PKR protein levels were increased following VSV infection in most cell lines with VSV-inducible type IFN signaling.
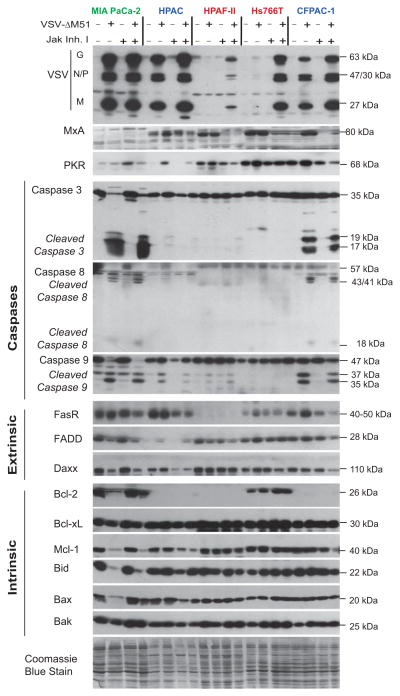
Figure 3. Protein levels of apoptosis related genes in PDAC cells following VSV-ΔM51-GFP infection.
Cells were mock-infected or infected with VSV-ΔM51-GFP, VSV-p1-GFP or VSV-GFP at an MOI of 15 (based on BHK-21 titer). At 17 h p.i, cell lysates were prepared and analyzed by Western blot for the indicated proteins associated with (A) virus replication and antiviral response, (B) caspase cleavage, (C) the extrinsic apoptosis pathway, (D) the intrinsic apoptosis pathway and (E) total protein staining. Protein (kDa) product sizes are indicated on the right. Due to a limited amount of total protein isolated from T3M4 and HPAC cells, the following exceptions were made: for PKR T3M4 sample, no protein was loaded for uninfected T3M4 cells; for caspase 3 and FADD in HPAC cells 15 μg (half the amount) was loaded.
In most PDAC cell lines, all 3 VSV recombinants induced caspase 3 cleavage following infection (Fig. 3B; cleaved products appear as a double band at p17/p19). However, all 3 viruses induced similar (and the highest) levels of caspase 3 cleavage only in Capan1 and MIA PaCa-2. Both cell lines are unable to induce Type I IFN responses to VSV (Moerdyk-Schauwecker et al., 2013; Murphy et al., 2012). In all cell lines with VSV-inducible Type I IFN responses, despite similar replication levels for the 3 tested VSVs, VSV-ΔM51-GFP induced more caspase 3 cleavage. This indicates a positive role for the ΔM51 mutation, and therefore host responses, in apoptosis induction, and is unlikely to be simply a result of virus attenuation as VSV-p1-GFP induced caspase cleavage similarly to VSV-GFP. In agreement with their general resistance to VSV infection and replication (Moerdyk-Schauwecker et al., 2013; Murphy et al., 2012), Hs766T and HPAF-II showed the lowest levels of VSV protein accumulation in infected cells (Fig. 3A). However, while Hs766T had no detectable cleaved caspase 3, HPAF-II showed low but easily detectable caspase 3 cleavage in cells infected with VSV-ΔM51-GFP and VSV-GFP (Fig. 3B.). Surprisingly, HPAC cells, while supporting good levels of replication for all 3 tested VSVs, had no detectable cleaved caspase 3. This result shows that VSV replication is likely an important determinant of apoptosis, but it is not sufficient for apoptosis induction in PDAC cells.
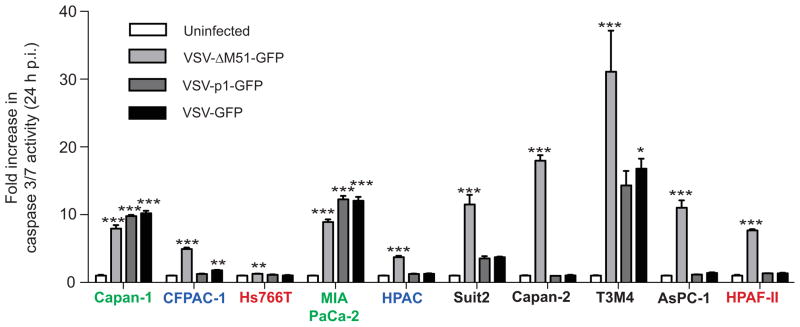
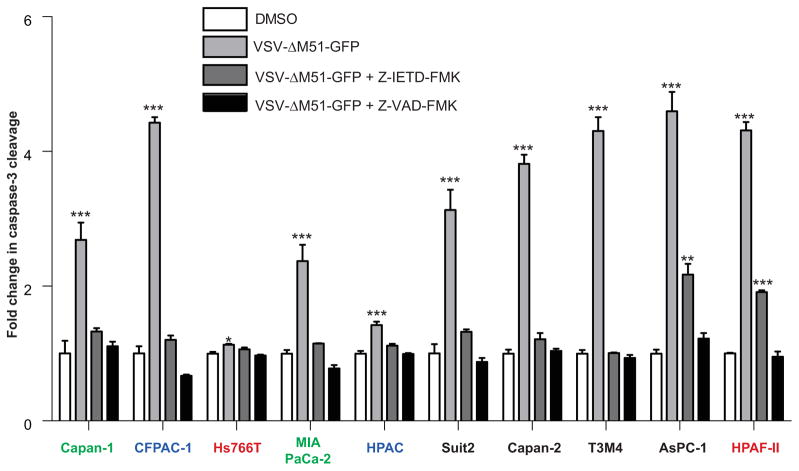
The ability of VSV recombinants to induce apoptosis in PDAC cells was also analyzed by measuring the activity of effector caspases 3 and 7 (Fig. 4). Hs766T and HPAC, which showed no detectable cleaved caspase 3 (Fig. 3B), also showed the lowest caspase 3/7 activity (Fig. 4), again indicating a possible block(s) in apoptosis upstream of caspase 3/7. Also consistent with Figure 3B, only 2 PDAC cell lines (Capan-1 and MIA PaCa-2) showed similar caspase 3/7 activity when infected with any of the three VSV recombinants, while all other cell lines showed greater induction of apoptosis by VSV-ΔM51-GFP (Fig. 4).
Figure 4. Caspase 3/7 activation in PDAC cells following VSV-ΔM51-GFP infection.
Cells were mock- or virus-infected at an MOI of 15 (based on BHK-21 titer). At 24 h p.i., apoptosis activation was assessed using the Caspase Glow 3/7 assay. Caspase 3/7 activation is expressed as fold increase over mock treated, with the mock treated activity indicated as 1. Assay was done in triplicate and data represent the mean ± standard error of mean. Treatments were compared using a 1-way ANOVA followed by the Bonferroni posttest for comparison to the control. *, p<0.05; **, p<0.01; ***, p<0.001.
Apoptosis activation mechanism depends on both VSV M protein and PDAC cell line
Previous studies in other cell types demonstrated that VSV M51 mutants typically activate apoptosis via the caspase 8 dependent extrinsic pathway (Cary et al., 2011; Gaddy DF and and Lyles, 2005; Gaddy DF, 2007), with the Fas receptor appearing to play a key role (Gaddy DF, 2007). The extrinsic apoptotic pathway can be either type I, where caspase-8 cleavage is sufficient to activate effector caspases, or type II where the signal must be amplified through the intrinsic pathway via caspase-8 cleavage of Bid (Barnhart et al., 2003; Scaffidi et al., 1998). In contrast to VSV M51 mutants, VSV encoding wt M protein typically activates apoptosis through the caspase 9 dependent intrinsic (mitochondrial) pathway (Balachandran et al., 2001; Balachandran et al., 2000; Gaddy DF and and Lyles, 2005). VSV wt M protein likely plays an important role in apoptosis induction by inhibiting host gene expression (Kopecky and Lyles, 2003; Kopecky et al., 2001). However, the specific mechanism of apoptosis in response to VSV infection depends on both virus and cell type (Balachandran et al., 2001; Balachandran et al., 2000; Cary et al., 2011; Gaddy DF and and Lyles, 2005), and the mechanism of apoptosis induction by VSV has never been studied in PDAC cells.
As seen in Figure 3B for VSV-ΔM51-GFP infected cells, where cleavage of caspase 3 took place, cleavage of both caspase 8 and 9 was also detectable in all PDACs cell lines, except for HPAF-II, indicating that this virus induces both the extrinsic and intrinsic apoptosis pathways in most PDACs. Interestingly, in HPAF-II cells, VSV-ΔM51-GFP induced caspase 9 but not caspase 8 cleavage, suggesting that only the intrinsic pathway was induced.
For VSV-GFP and VSV-p1-GFP, a predominance of caspase 9 cleavage to caspase 8 cleavage was seen in Capan-2, AsPC-1 and HPAF-II compared to other PDACs, suggesting induction of apoptosis primarily through the intrinsic pathway as previously reported for wt M. However, in all other cell lines cleavage of both caspase 8 and caspase 9 was seen even upon infection with VSV expressing wt M (Fig. 3B).
Having demonstrated heterogeneity in the ability of PDAC cell lines to undergo apoptosis depending on both virus and cell, we examined whether expression of major apoptosis regulator(s) could account for these differences. Signaling through the Fas death receptor has been shown to be required for VSV-M51R induced apoptosis (Gaddy DF, 2007). Interestingly, two PDAC cell lines, Hs766T and HPAF-II, most resistant to VSV replication and displaying no or low apoptosis induction in response to VSV, both showed low levels of Fas receptor (Fig. 3C) compared to most other PDAC cell lines, suggesting that low Fas levels could play a role in resistance of these cell lines to VSV-mediated apoptosis. However, two other PDAC cell lines (Capan-1 and Capan-2), also showed low levels of Fas expression despite the lack of resistance to VSV mediated apoptosis. Another apoptosis-resistant PDAC cell line, HPAC, showed good levels of Fas receptor. FADD and Daxx have both been shown to play important roles in Fas signaling (Balachandran et al., 2000; Gaddy DF, 2007); however there were no major differences between PDAC cell lines.
Bcl-2 family members have been shown to be altered in PDACs, as well as other cancers, and to have predictive value for prognosis and treatment response to chemotherapy and radiotherapy [reviewed in (Frenzel et al., 2009; Westphal and Kalthoff, 2003)]. This family is part of the intrinsic pathway (and thus also the type II extrinsic pathway) and contains both pro- and anti-apoptotic members. As shown in Figure 3D, Mcl-1, an anti-apoptotic protein, was consistently expressed in all uninfected cell lines. However, Mcl-1 levels decreased in most VSV-sensitive cell lines in response to infection with one or more VSV recombinants, likely as part of the apoptosis initiation process (Schache et al., 2009). Production of Bcl-2, an anti-apoptotic protein, was highly variable and undetectable in a number of cell lines. Hs766T was the only apoptosis-resistant PDAC cell line where Bcl-2 was detectable, possibly explaining why it is more resistant to VSV mediated apoptosis than other cell lines. However, Capan-1 and MIA PaCa-2 cells, which efficiently activate apoptosis in response to VSV infection, expressed Bcl-2 at equal or greater levels to Hs766T. The anti-apoptotic protein Bcl-xL was expressed evenly among all cell lines, indicating that this protein is probably not a key player in resistance to VSV induced apoptosis, as were the pro-apoptotic proteins Bak, Bax and Bid. Apoptosis-sensitive PDAC cell lines showed Bid cleavage when infected with VSV-ΔM51-GFP. While a potential biomarker of apoptosis resistance was not identified, it is known that the ratio rather than the absolute quantity of these pro- and anti-apoptotic proteins is important in the regulation of cell death [reviewed in (Wong, 2011)]. Further studies are needed to determine a role of these apoptotic regulators in the resistance of some PDACs to VSV induced apoptosis.
VSV-ΔM51 induces apoptosis in PDACs via the type II extrinsic pathway
The major focus of this study was VSV-ΔM51-GFP. Therefore, the mechanism of apoptosis induction during VSV-ΔM51-GFP infection was studied in more detail. As shown in Figure 3B, where cleavage of caspase 3 took place, cleavage of both caspase 8 and 9 was also detectable in all VSV-ΔM51-GFP infected cells (except for HPAF-II). Cleavage of both caspases 8 and 9 can result from either activation of the type II extrinsic pathway (where caspase 8 is essential for the activation of caspases 9 and 3), independent activation of intrinsic and extrinsic pathways, or death receptor-independent activation of caspase 8 as part of a positive feedback loop following cleavage of caspase 3 after initial activation of the intrinsic pathway (Liu et al., 2011). As VSV with M51 mutations frequently induces apoptosis via the extrinsic pathway (Gaddy DF and and Lyles, 2005; Gaddy DF, 2007) and pancreatic cells have been shown to utilize the type II extrinsic pathway (Hinz et al., 2000), we hypothesized that VSV-ΔM51-GFP induced apoptosis in PDAC cells via this mechanism.
To test this hypothesis, infections with VSV-ΔM51-GFP at MOI 15 (based on BHK-21 titer) were conducted in the presence of Z-VAD-FMK, a pan-caspase inhibitor, or Z-IETD-FMK, a caspase 8 specific inhibitor. Following treatment, cleavage of caspase 3 was measured by ELISA at 17 h p.i. As shown in Figure 5, most PDAC cell lines showed a strong increase in caspase 3 cleavage following VSV-ΔM51-GFP infection in agreement with the Western blot analysis (Fig. 3B) and the caspase activity assay (Fig. 4). The two PDAC cell lines (HPAC and Hs766T), which showed minimal caspase 3 activity and cleavage in the previous assays again showed low levels of cleaved caspase 3. Importantly, treatment with Z-IETD-FMK returned cleaved caspase 3 levels to near baseline in all cell lines, indicating caspase 8 is critical to apoptosis induction following VSV-ΔM51-GFP infection. As caspase 9 cleavage was also seen in all these PDAC cell lines (Fig. 3B), we concluded that apoptosis occurred through the type II extrinsic pathway. This mechanism is also consistent with the detection of cleaved Bid in many PDACs cell lines infected with VSV-ΔM51-GFP (Fig. 3D).
Figure 5. Effect of caspase inhibitors on caspase 3 cleavage.
Cells were pretreated with 100 μM caspase-8 inhibitor Z-IETD-FMK, 100 μM general caspase inhibitor Z-VAD-FMK or vehicle only (DMSO) in growth media for 1 h prior to infection. Cells were then mock infected or infected with VSV-ΔM51-GFP at an MOI of 15 PFU/cell (based on BHK-21 titer) in the continued presence of either inhibitor or vehicle. At 17 h p.i., cleaved caspase 3 was analyzed by ELISA. Caspase 3 cleavage is expressed as fold increase over mock treated, with the mock treated level indicated as 1. Assay was done in triplicate and data represent the mean ± standard error of mean. Treatments were compared using a 1-way ANOVA followed by the Bonferroni posttest for comparison to the control. *, p<0.05; **, P<0.01; ***, p<0.001.
Role of virus replication levels and type I interferon in apoptosis induction in PDAC cell lines
Under the experimental conditions shown in Figure 3 (MOI 15 infection), VSV replication was severely inhibited in HPAF-II and Hs766T. No (Hs766T) or low (HPAF-II) caspase 3 cleavage (Fig. 3B) could potentially be attributed to very low levels of virus replication in these 2 cell lines. However, HPAC cells, while supporting good levels of VSV replication, showed no detectable cleaved caspase 3, indicating that good VSV replication levels are not sufficient for apoptosis induction at least in some PDAC cells. All three cell lines (HPAC, HPAF-II and Hs766T) as well as CFPAC-1 constitutively express some ISGs, including MxA [Fig. 6 and (Moerdyk-Schauwecker et al., 2013)]. Therefore, we conducted an experiment to test the hypothesis that resistance of PDAC cells to VSV-mediated apoptosis is the result of upregulated type I IFN signaling leading to low-level VSV replication and/or expression of one or more anti-apoptotic factors. As controls, we used MIA PaCa-2 cells, which are defective in type I IFN signaling, as well as CFPAC-1 cells, which are similar to HPAC cells in their constitutive expression of ISGs, but, unlike HPAC, undergo apoptosis following VSV infection.
Figure 6. Effect of type I IFN signaling inhibition and increased VSV-ΔM51-GFP replication.
Cells were mock (DMSO) treated or treated with 2.5 μM Jak Inh. I for 48 h prior to infection with VSV-ΔM51-GFP at MOI 15 PFU/cell. Cells were harvested at 17 h p.i. and cell lysates were prepared and analyzed by Western blot for the indicated proteins. Protein (kDa) product sizes are indicated on the right.
Inhibition of type I IFN signaling and subsequent increase in VSV-ΔM51-GFP replication levels were achieved by treatment of cells with Jak Inh. I as in our previous study (Moerdyk-Schauwecker et al., 2013). Cells lines were mock-infected or infected at MOI 15 (based on BHK-21 titer) with VSV-ΔM51-GFP and protein was isolated at 17 h p.i.. MxA protein was downregulated in all treated cell lines, confirming Jak Inh. I treatment was effective. Importantly, although Jak Inh. I treatment increased VSV-ΔM51-GFP replication in PDAC cell lines with constitutive ISG expression, it did not result in increased caspase 3 cleavage (Fig. 6). These results demonstrate that even when viral replication is increased and type I IFN signaling inhibited, HPAC and Hs766T cannot efficiently activate apoptosis. Therefore, there is no clear correlation between VSV-ΔM51-GFP replication levels or type I IFN signaling and apoptosis in these two cell lines. To not miss effects of Jak Inh. I treatment upstream of caspase 3, we looked also for changes in cleavage of caspases 8 and 9 and expression of extrinsic/intrinsic regulators and did not see any major changes (Fig. 6).
Activation of apoptosis by non-viral stimulators
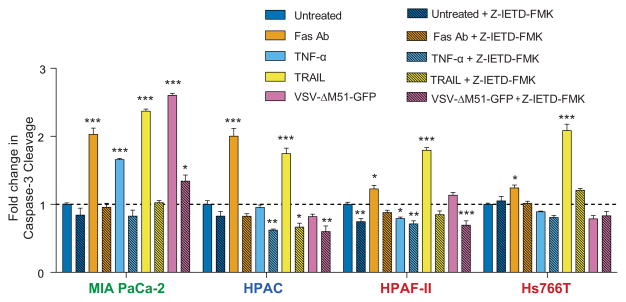
Since VSV-ΔM51-GFP was unable to effectively induce apoptosis in Hs766T and HPAC and weakly and variably induced apoptosis in HPAF-II, we wanted to know whether these cell lines resist only virus mediated apoptosis or if they have a more general defect in apoptosis. Therefore, we tested if the extrinsic apoptosis pathway could be induced using a virus independent method. Hs766T, HPAC, HPAF-II and MIA PaCa-2 were treated with Fas activating antibody CH11, TNF-α, TRAIL or VSV-ΔM51-GFP and tested for caspase-3 cleavage at 17 h p.i. All stimulators induced caspase 3 cleavage in MIA PaCa-2 but VSV-ΔM51-GFP appeared to do so most effectively (Fig. 7). TNF-α did not induce apoptosis in any of the PDAC cell lines resistant to VSV mediated apoptosis. Fas activating antibody CH11 more strongly induced caspase 3 cleavage in HPAC than in Hs766T and HPAF-II, consistent with Fas receptor levels (Fig. 3C and 6). Finally, consistent with previous data, VSV-ΔM51-GFP did not induce caspase 3 cleavage in HPAC, Hs766T and HPAF-II cells. Together, the data suggest that at least two of the three PDAC cell lines resistant to VSV mediated apoptosis have a more general defect in apoptosis.
Figure 7. Effect of Fas antibody/TNF-alfa/TRAIL on caspase 3 cleavage.
Cells were pretreated with 100 μM caspase-8 inhibitor Z-IETD-FMK or vehicle only (DMSO) in growth media for 1 h prior to treatment. Cells were then mock treated, treated with 1μg/ml Fas activating antibody, 1μg/ml TRAIL, or 25ng/ml TNF-3 or infected with VSV-ΔM51-GFP at an MOI of 15 PFU/cell (based on BHK-21 titer) in the continued presence of either inhibitor or vehicle. At 17 h p.i., cleaved caspase 3 was analyzed by ELISA. Caspase 3 cleavage is expressed as fold increase over mock treated, with the mock treated level indicated as 1. Assays were done in triplicate and data represent the mean ± standard error of mean. Treatments were compared using a 1-way ANOVA followed by the Bonferroni posttest for comparison to the control. *, p<0.05; **, p<0.01; ***, p<0.001.
Impact of apoptosis on VSV-ΔM51-GFP replication
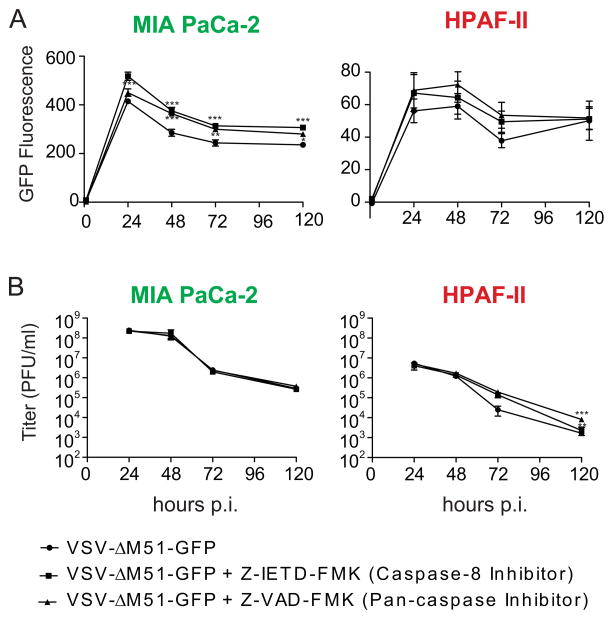
While strong inhibition of apoptosis in response to VSV-ΔM51-GFP infection can prevent oncolysis, rapid induction of apoptosis can potentially have an antiviral effect by destroying the infected cell before the virus reaches its maximum replication potential. As this could limit the effectiveness of VSV as an oncolytic virus, we examined the possible effects of apoptosis induction on VSV-ΔM51-GFP replication in PDAC cells. Two cell lines (VSV-permissive MIA PaCa-2 and VSV-resistant HPAF-II) were infected with VSV-ΔM51-GFP in the presence or absence of Z-VAD-FMK (a pan-caspase inhibitor) or Z-IETD-FMK (a caspase 8 specific inhibitor). Cells were infected at a cell line specific MOI of 2 to ensure one-step growth kinetics, and virus replication was monitored by measuring virus-directed GFP fluorescence in live cells (Fig. 8A) and measuring new particle production by titration of media on BHK-21 cells (Fig. 8B). GFP expression only modestly (although statistically significant at most time points) increased in inhibitor-treated cells (Fig. 8A). However, no statistically significant increase in new particle production was seen in MIA PaCa-2 cells at any time point, and an increase was only seen in HPAF-II at 120 h p.i. (Fig. 8B). The tolerance of VSV replication to apoptosis in MIA PaCa-2 and HPAF-II is most likely due to VSV’s relatively fast replication cycle, allowing it to outpace the apoptotic response (Koyama, 1995; Timm and Yin, 2012).
Figure 8. Effect of apoptosis inhibition on VSV-ΔM51-GFP replication.
Cells were pretreated with 100 μM caspase-8 inhibitor Z-IETD-FMK, 100 μM general caspase inhibitor Z-VAD-FMK or vehicle only (DMSO diluted in PBS) in growth media for 1 h prior to infection. Cells were then mock infected or infected with VSV-ΔM51-GFP at an MOI of 2 PFU/cell (cell line specific titer) for the study of 1-step replication kinetics based on (A) virus driven GFP expression or (B) infectious particle production (limit of detection=2×102 PFU/ml) as determined at the indicated time points. Assays were performed in triplicate and values represent mean ± standard error of mean. Treatments were compared by two-way ANOVA with Bonferroni posttest for comparison of treatments to control. *, p<0.05; **, p<0.01; ***, p<0.001.
DISCUSSION
In this study, we investigated apoptosis activation in human PDAC cells in response to three different VSV recombinants expressing ΔM51 or wild type M protein. We demonstrated that VSV-ΔM51 is a more potent inducer of apoptosis in the majority of PDAC cells than VSV with wt M, and that it activates apoptosis mainly via the type II extrinsic pathway. We also showed that resistance of some PDAC cell lines to VSV-mediated oncolysis is not due only to type I IFN responses that limit virus replication (Moerdyk-Schauwecker et al., 2013; Murphy et al., 2012), but also to cellular defects in apoptosis.
Our study investigated VSV-mediated apoptosis in pancreatic cancer cells for the first time. Previous studies in other systems showed VSV-M51R mutation activated apoptosis mainly via the caspase 8 dependent extrinsic pathway (Cary et al., 2011; Gaddy DF and and Lyles, 2005; Gaddy DF, 2007), while VSV encoding wt M protein typically activated apoptosis through the caspase 9 dependent intrinsic pathway (Balachandran et al., 2001; Balachandran et al., 2000; Gaddy DF and and Lyles, 2005). However, this distinction is not absolute as either or both pathways can be activated during infection with either variant (Balachandran et al., 2001; Cary et al., 2011). Our data show a similar situation in PDAC cells. Despite similar levels of replication in most cell lines (except for Hs766T and HPAF-II which are highly resistant to VSV), VSV-GFP virus induced both intrinsic and extrinsic pathways in most PDAC cell lines, but only the intrinsic pathway in Capan-2 and AsPC-1 cell lines. This suggests that cellular factors can determine the mechanism of apoptosis mediated by the same virus. However, viral factors can also determine the mechanism of apoptosis. For example, compared to VSV-GFP, VSV-ΔM51-GFP caused significantly stronger apoptosis in Suit2, Capan-2, T3M4 and AsPC-1 cells, even though both viruses replicated to similar levels in these 4 cell lines.
The role of type I IFN signaling and ISGs in apoptosis induction in PDAC cells is not well understood. Consistent with the anti-proliferative and pro-apoptotic effects generally associated with type I IFN responses, treatment with exogenous type I IFN has been shown to induce apoptosis in at least some PDAC cell lines, although induction efficiency varied considerably across cell lines (Booy et al., 2014). Many ISGs including Fas ligand, TRAIL and several IRFs, have known pro-apoptotic effects [reviewed in (Chawla-Sarkar et al., 2003; Cheon et al., 2014)]. Some antiviral ISGs, including OAS-RNase L and PKR, have also been shown to have pro-apoptotic roles [reviewed in (Clemens, 2003)]. However, other ISGs (for example, IFI6, also known as G1P3 or ISG6-16) are known to be anti-apoptotic (Cheriyath et al., 2007; Cheriyath et al., 2011).
The role of ISGs in VSV-mediated apoptosis is also complex. PKR in particular has been shown to be important for efficient induction of apoptosis by VSV-M51R mutation in mouse fibrosarcoma cells (Gaddy DF, 2007). In contrast, PKR does not appear to be important for induction of apoptosis by VSV with wt M protein (Balachandran et al., 2000). Moreover, cellular PKR expression was associated with resistance to wt VSV infection, apoptosis induction and killing (Balachandran et al., 2000). Together, these studies suggest that ISG expression is beneficial for apoptosis induction by VSV-M51 mutants (due to PKR and other factors), but negatively impacts apoptosis induction by VSV expressing wt M due to inhibitory effects on viral replication.
Our results are consistent with induction/enhancement of apoptosis by ISGs in PDAC cell lines permissive to VSV and showing VSV inducible type I IFN signaling (Figure 3 and Table 1) (Moerdyk-Schauwecker et al., 2013). In these cell lines, VSV-ΔM51-GFP was a more effective apoptosis inducer than VSV recombinants expressing wt M. Unlike wt M protein, M protein with an M51 mutation is unable to block type I IFN mediated antiviral responses in infected cells (Stojdl DF et al., 2003) and allows for expression of ISGs, including pro-apoptotic ISGs, in response to infection.
In contrast to PDACs with VSV inducible ISG expression, it is likely that constitutive high-level expression of ISGs in VSV-resistant PDAC cell lines negatively impacts VSV-mediated apoptosis by dramatically inhibiting virus replication (regardless of M protein variant). Furthermore, the pattern of ISG expression differs in response to acute virus infection or IFN treatment as compared to chronic virus infection (Li et al., 2014; Wilson et al., 2013) or diseases involving chronic type I IFN production, including some cancers (Cheon et al., 2014). Thus, the role of ISGs in apoptosis induction in response to VSV infection may be different in cancers with constitutive versus inducible expression of ISGs.
Importantly, our data also show that type I IFN responses are not required for robust VSV induced apoptosis in PDAC cells. Two PDAC cell lines defective in type I IFN signaling in response to VSV, MIA PaCa-2 and Capan-1, supported the highest levels of caspase cleavage in response to VSV infection regardless of M protein status. In this instance, the absence of the pro-apoptotic effect of type I IFN responses is likely compensated for by the uninhibited replication of virus.
Overall, VSV-ΔM51-GFP is a potent inducer of apoptosis in the majority of PDAC cell lines. This is significant, as PDACs are known to be highly resistant to a variety of chemotherapies in part because of defects in apoptosis (Hamacher et al., 2008; Neesse et al., 2012; Roder et al., 2011). This result not only speaks to the potential of VSV-ΔM51-GFP as an oncolytic agent but also suggests it may also be useful as a sensitizer to chemotherapy. In several cancers, VSV-M51R infection improved doxorubicin treatment by facilitating degradation of the anti-apoptotic protein Mcl-1 (Schache et al., 2009), while another study showed that wt VSV in combination with gemcitabine increased apoptosis and increased killing of lung cancer cell lines in vitro and in vivo (Li et al., 2004). If successful, such combination therapies could allow for equal or greater efficacy at lower doses of chemotherapeutic drug, reducing side effects in patients.
Furthermore, while VSV-ΔM51-GFP was a potent inducer of apoptosis in most PDAC cells, apoptosis had little or no antiviral effect on VSV-ΔM51-GFP replication in PDAC cells. This was also previously demonstrated for VSV in several other systems (Balachandran et al., 2001; Hobbs et al., 2003; Hobbs et al., 2001; Sharif-Askari et al., 2007) and is most likely due to VSV’s relatively fast replication cycle in most cells, allowing the virus to complete its replication cycle prior to cell death. Interestingly, other studies have shown that inhibition of apoptosis can increase VSV replication (Chattopadhyay et al., 2011; Desforges et al., 2002; Sharif-Askari et al., 2007), indicating that small variations in viral replication or apoptosis kinetics likely determine whether or not an apoptotic response negatively impacts VSV replication. Given this, while cancer related apoptosis defects can be a therapeutic barrier, they may actually benefit VSV oncolytic therapy in many cases by allowing VSV to “win” the race against this antiviral mechanism. Apoptosis defects may also be another mechanism increasing VSV specificity for cancerous versus normal tissue as has been shown for other oncolytic viruses (Liu and Kirn, 2005). In general, induction of apoptosis does not seriously impact VSVs ability to replicate in cancer cells, making it a promising oncolytic virus and suggesting that cotherapy with agents that induce and/or enhance apoptosis should be feasible.
While VSV-ΔM51-GFP was a potent inducer of apoptosis and subsequent cell death in most PDAC cells, this was not the case in some PDAC cell lines. Three PDAC cell lines, Hs766T, HPAC and HPAF-II, with the strongest resistance to VSV-ΔM51-GFP mediated apoptosis were known from our previous studies to have constitutive expression of some antiviral IFN-stimulated genes (Moerdyk-Schauwecker et al., 2013; Murphy et al., 2012). At least in case of HPAC cells, failure to undergo apoptosis was not due to low VSV replication levels. The inability of Hs766T and HPAF-II cells to undergo apoptosis could be due to limited VSV-ΔM51-GFP replication. However, increasing viral replication via inhibition of constitutive type I IFN responses did not improve induction of apoptosis. It should be noted that we cannot rule out that stimulation of VSV replication using Jak Inh. I was counteracted by simultaneous inhibition of the potentially pro-apoptotic effects of type I IFN signaling. In particular, this case is distinct from that of MIA PaCa-2 and Capan-1, as while Jak Inh. I facilitates virus replication and spread, initiation of infection in resistant cell lines remains relatively inefficient (Moerdyk-Schauwecker et al., 2013).
In addition to resisting apoptosis induction by VSV, Hs766T and HPAF-II were also resistant to apoptosis when treated with Fas activating antibody. Therefore, our data indicate two separate mechanisms behind resistance of PDAC cells to VSV-ΔM51-GFP mediated apoptosis: (1) virus-specific defects in apoptosis, as seen with HPAC, where Fas activating antibody can induce apoptosis but virus-induced apoptosis is significantly inhibited, or (2) general defects in Fas receptor mediated apoptosis, as seen with the Hs766T and HPAF-II cells, where apoptosis cannot be induced effectively by virus or Fas activating antibody.
As PDAC cell lines that evaded VSV-ΔM51-GFP mediated apoptosis activation showed no or low levels of cleaved caspase 8 and 9, it is likely that cellular factors upstream of these initiator caspases are involved in this resistance (Hanahan and Weinberg, 2011; Kelly and Strasser, 2011). As TRAIL was relatively effective in inducing apoptosis in all these cell lines, the defect(s) may primarily impact the Fas receptor mediated pathway. Fas receptor was expressed at lower levels in Hs766T cells than in many other cell lines, which may contribute to its apoptosis resistance. Evasion of apoptosis by PDACs is commonly impacted by overexpression of Fas decoy receptors (DcR3), Fas associated phosphatase-1 (FAP-1), and/or FLICE-inhibitory protein c-FLIP (Hamacher et al., 2008; Neesse et al., 2012; Roder et al., 2011), all of which target events upstream of caspase 8. Our future studies will examine these factors.
Although we did not find a relationship between expression of Bcl-2 proteins and resistance of PDAC cell lines to VSV-ΔM51-mediated oncolysis, further studies are also needed to determine the influence of these or other regulators downstream of caspase 8 and 9 such as inhibitors of apoptosis (IAPs) (Hamacher et al., 2008). (Samuel et al., 2010, Samuel, 2013 #8684)Our future studies will also examine the role of other types of cell death in VSV infected PDACs, particularly autophagy as VSV has already been reported to induce autophagy in different types of cancer cells (Schache et al., 2009).
Research Highlight.
We tested 3 VSVs for induction of apoptosis in 10 pancreatic cancer cell lines
In most cell lines VSVs activated both extrinsic and intrinsic apoptosis pathways
VSV-ΔM51 primarily activated the type II extrinsic pathway
Interferon status of cancer cells played a role in apoptosis induction
3 cell lines were resistant to apoptosis under most experimental conditions
Acknowledgments
We thank Eric Hastie and Marcela Cataldi for critical comments on the manuscript. This work was supported by NIH grant 1R15CA167517-01 (to V.Z.G.) from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed M, McKenzie MO, Puckett S, Hojnacki M, Poliquin L, Lyles DS. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J Virol. 2003;77:4646–4657. doi: 10.1128/JVI.77.8.4646-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S, Porosnicu M, Barber GN. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J Virol. 2001;75:3474–3479. doi: 10.1128/JVI.75.7.3474-3479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S, Roberts PC, Brown LE, Truong H, Pattnaik AK, Archer DR, Barber GN. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13:129–141. doi: 10.1016/s1074-7613(00)00014-5. [DOI] [PubMed] [Google Scholar]
- Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24:7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- Barnhart BC, Alappat EC, Peter ME. The CD95 type I/type II model. Semin Immunol. 2003;15:185–193. doi: 10.1016/s1044-5323(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Booy S, van Eijck CH, Dogan F, van Koetsveld PM, Hofland LJ. Influence of type-I Interferon receptor expression level on the response to type-I Interferons in human pancreatic cancer cells. J Cell Mol Med. 2014;18:492–502. doi: 10.1111/jcmm.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CW, Stephenson KB, Hanson S, Kucharczyk M, Duncan R, Bell JC, Lichty BD. The p14 FAST protein of reptilian reovirus increases vesicular stomatitis virus neuropathogenesis. J Virol. 2009;83:552–561. doi: 10.1128/JVI.01921-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary ZD, Willingham MC, Lyles DS. Oncolytic Vesicular Stomatitis Virus Induces Apoptosis in U87 Glioblastoma Cells by a Type II Death Receptor Mechanism and Induces Cell Death and Tumor Clearance In Vivo. J Virol. 2011;85:5708–5717. doi: 10.1128/JVI.02393-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Yamashita M, Zhang Y, Sen GC. The IRF-3/Bax-mediated apoptotic pathway, activated by viral cytoplasmic RNA and DNA, inhibits virus replication. J Virol. 2011;85:3708–3716. doi: 10.1128/JVI.02133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- Cheon H, Borden EC, Stark GR. Interferons and their stimulated genes in the tumor microenvironment. Semin Oncol. 2014;41:156–173. doi: 10.1053/j.seminoncol.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyath V, Glaser KB, Waring JF, Baz R, Hussein MA, Borden EC. G1P3, an IFN-induced survival factor, antagonizes TRAIL-induced apoptosis in human myeloma cells. J Clin Inv. 2007;117:3107–3117. doi: 10.1172/JCI31122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyath V, Leaman DW, Borden EC. Emerging roles of FAM14 family members (G1P3/ISG 6-16 and ISG12/IFI27) in innate immunity and cancer. J Interferon Cytokine Res. 2011;31:173–181. doi: 10.1089/jir.2010.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens MJ. Interferons and apoptosis. J Interferon Cytokine Res. 2003;23:277–292. doi: 10.1089/107999003766628124. [DOI] [PubMed] [Google Scholar]
- Das SC, Nayak D, Zhou Y, Pattnaik AK. Visualization of intracellular transport of vesicular stomatitis virus nucleocapsids in living cells. J Virol. 2006;80:6368–6377. doi: 10.1128/JVI.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M, Despars G, Berard S, Gosselin M, McKenzie MO, Lyles DS, Talbot PJ, Poliquin L. Matrix protein mutations contribute to inefficient induction of apoptosis leading to persistent infection of human neural cells by vesicular stomatitis virus. Virology. 2002;295:63–73. doi: 10.1006/viro.2001.1329. [DOI] [PubMed] [Google Scholar]
- Ebert O, Harbaran S, Shinozaki K, Woo SL. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 2005;12:350–358. doi: 10.1038/sj.cgt.7700794. [DOI] [PubMed] [Google Scholar]
- Farrow B, Albo D, Berger DH. The role of the tumor microenvironment in the progression of pancreatic cancer. J Surg Res. 2008;149:319–328. doi: 10.1016/j.jss.2007.12.757. [DOI] [PubMed] [Google Scholar]
- Frenzel A, Grespi F, Chmelewskij W, Villunger A. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis. 2009;14:584–596. doi: 10.1007/s10495-008-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadaleta P, Perfetti X, Mersich S, Coulombie F. Early activation of the mitochondrial apoptotic pathway in Vesicular Stomatitis virus-infected cells. Virus Res. 2005;109:65–69. doi: 10.1016/j.virusres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Gaddy DF, Lyles DS. Vesicular stomatitis viruses expressing wild-type or mutant M proteins activate apoptosis through distinct pathways. J Virol. 2005;79:4170–4179. doi: 10.1128/JVI.79.7.4170-4179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddy DF, Lyles DS. Oncolytic vesicular stomatitis virus induces apoptosis via signaling through PKR, Fas, and Daxx. J Virol. 2007;81:2792–2804. doi: 10.1128/JVI.01760-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathogens. 2008;4:e1000018. doi: 10.1371/journal.ppat.1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamacher R, Schmid RM, Saur D, Schneider G. Apoptotic pathways in pancreatic ductal adenocarcinoma. Mol Cancer. 2008;7:64. doi: 10.1186/1476-4598-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hastie E, Cataldi M, Marriott I, Grdzelishvili VZ. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16–32. doi: 10.1016/j.virusres.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie E, Grdzelishvili VZ. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J Gen Virol. 2012;93:2529–2545. doi: 10.1099/vir.0.046672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz S, Trauzold A, Boenicke L, Sandberg C, Beckmann S, Bayer E, Walczak H, Kalthoff H, Ungefroren H. Bcl-XL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosis. Oncogene. 2000;19:5477–5486. doi: 10.1038/sj.onc.1203936. [DOI] [PubMed] [Google Scholar]
- Hobbs JA, Hommel-Berrey G, Brahmi Z. Requirement of caspase-3 for efficient apoptosis induction and caspase-7 activation but not viral replication or cell rounding in cells infected with vesicular stomatitis virus. Hum Immunol. 2003;64:82–92. doi: 10.1016/s0198-8859(02)00702-4. [DOI] [PubMed] [Google Scholar]
- Hobbs JA, Schloemer RH, Hommel-Berrey G, Brahmi Z. Caspase-3-like proteases are activated by infection but are not required for replication of vesicular stomatitis virus. Virus Res. 2001;80:53–65. doi: 10.1016/s0168-1702(01)00350-1. [DOI] [PubMed] [Google Scholar]
- Kelly GL, Strasser A. The essential role of evasion from cell death in cancer. Adv Cancer Res. 2011;111:39–96. doi: 10.1016/B978-0-12-385524-4.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky SA, Lyles DS. Contrasting effects of matrix protein on apoptosis in HeLa and BHK cells infected with vesicular stomatitis virus are due to inhibition of host gene expression. J Virol. 2003;77:4658–4669. doi: 10.1128/JVI.77.8.4658-4669.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky SA, Willingham MC, Lyles DS. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J Virol. 2001;75:12169–12181. doi: 10.1128/JVI.75.24.12169-12181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama AH. Induction of apoptotic DNA fragmentation by the infection of vesicular stomatitis virus. Virus Res. 1995;37:285–290. doi: 10.1016/0168-1702(95)00026-m. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wei YQ, Wen YJ, Zhao X, Tian L, Yang L, Mao YQ, Kan B, Wu Y, Ding ZY, Deng HX, Li J, Luo Y, Li HL, He QM, Su JM, Xiao F, Zou CH, Fu CH, Xie XJ, Yi T, Tan GH, Wang L, Chen J, Liu J, Gao ZN. Induction of apoptosis and tumor regression by vesicular stomatitis virus in the presence of gemcitabine in lung cancer. Int J Cancer. 2004;112:143–149. doi: 10.1002/ijc.20276. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Duan X, Liu B, Yang C, Zeng P, McGilvray I, Chen L. Activation of endogenous type I IFN signaling contributes to persistent HCV infection. Rev Med Virol. 2014;24:332–342. doi: 10.1002/rmv.1795. [DOI] [PubMed] [Google Scholar]
- Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: reinventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Liu J, Uematsu H, Tsuchida N, Ikeda MA. Essential role of caspase-8 in p53/p73-dependent apoptosis induced by etoposide in head and neck carcinoma cells. Mol Cancer. 2011;10:95. doi: 10.1186/1476-4598-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TC, Kirn D. Viruses with deletions in antiapoptotic genes as potential oncolytic agents. Oncogene. 2005;24:6069–6079. doi: 10.1038/sj.onc.1208734. [DOI] [PubMed] [Google Scholar]
- Moerdyk-Schauwecker M, Shah NR, Murphy AM, Hastie E, Mukherjee P, Grdzelishvili VZ. Resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus: role of type I interferon signaling. Virology. 2013;436:221–234. doi: 10.1016/j.virol.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer SA, Baker SC, Lessard JL. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci USA. 1986;83:5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AM, Besmer DM, Moerdyk-Schauwecker M, Moestl N, Ornelles DA, Mukherjee P, Grdzelishvili VZ. Vesicular stomatitis virus as an oncolytic agent against pancreatic ductal adenocarcinoma. J Virol. 2012;86:3073–3087. doi: 10.1128/JVI.05640-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neesse A, Gress TM, Michl P. Therapeutic targeting of apoptotic pathways: novel aspects in pancreatic cancer. Curr Pharm Biotechnol. 2012;13:2273–2282. doi: 10.2174/138920112802501953. [DOI] [PubMed] [Google Scholar]
- O’Brien V. Viruses and apoptosis. J Gen Virolo. 1998;79:1833–1845. doi: 10.1099/0022-1317-79-8-1833. [DOI] [PubMed] [Google Scholar]
- Ramsburg E, Publicover J, Buonocore L, Poholek A, Robek M, Palin A, Rose JK. A vesicular stomatitis virus recombinant expressing granulocyte-macrophage colony-stimulating factor induces enhanced T-cell responses and is highly attenuated for replication in animals. J Virol. 2005;79:15043–15053. doi: 10.1128/JVI.79.24.15043-15053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder C, Trauzold A, Kalthoff H. Impact of death receptor signaling on the malignancy of pancreatic ductal adenocarcinoma. Eur J Cell Biol. 2011;90:450–455. doi: 10.1016/j.ejcb.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nature Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S, Tumilasci VF, Oliere S, Nguyen TL, Shamy A, Bell J, Hiscott J. VSV oncolysis in combination with the BCL-2 inhibitor obatoclax overcomes apoptosis resistance in chronic lymphocytic leukemia. Mol Ther. 2010;18:2094–2103. doi: 10.1038/mt.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schache P, Gurlevik E, Struver N, Woller N, Malek N, Zender L, Manns M, Wirth T, Kuhnel F, Kubicka S. VSV virotherapy improves chemotherapy by triggering apoptosis due to proteasomal degradation of Mcl-1. Gene Ther. 2009;16:849–861. doi: 10.1038/gt.2009.39. [DOI] [PubMed] [Google Scholar]
- Sharif-Askari E, Nakhaei P, Oliere S, Tumilasci V, Hernandez E, Wilkinson P, Lin R, Bell J, Hiscott J. Bax-dependent mitochondrial membrane permeabilization enhances IRF3-mediated innate immune response during VSV infection. Virology. 2007;365:20–33. doi: 10.1016/j.virol.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nature reviews Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin JE, Hiscott J, Bell JC. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Timm A, Yin J. Kinetics of virus production from single cells. Virology. 2012;424:11–17. doi: 10.1016/j.virol.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier MD, Lyles DS, Reiss CS. Peripheral, but not central nervous system, type I interferon expression in mice in response to intranasal vesicular stomatitis virus infection. J Neurovirol. 2007;13:433–445. doi: 10.1080/13550280701460565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumilasci VF, Oliere S, Nguyen TL, Shamy A, Bell J, Hiscott J. Targeting the apoptotic pathway with BCL-2 inhibitors sensitizes primary chronic lymphocytic leukemia cells to vesicular stomatitis virus-induced oncolysis. J Virol. 2008;82:8487–8499. doi: 10.1128/JVI.00851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal S, Kalthoff H. Apoptosis: targets in pancreatic cancer. Mol Cancer. 2003;2:6. doi: 10.1186/1476-4598-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G, Rogulin V, Simon I, Rose JK, van den Pol AN. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J Virol. 2010;84:1563–1573. doi: 10.1128/JVI.02040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]