Abstract
Neuropeptide S (NPS) is a neurotransmitter that activates the NPS receptor to modulate biological functions including anxiety-like behaviors, feeding, and drug reinforcement. RTI-118 is a novel NPS receptor antagonist that decreased cocaine self-administration in rats at doses that had little or no effect on food-maintained responding. To build on these previous findings, this study examined effects of RTI-118 on cocaine-induced facilitation of intracranial self-stimulation (ICSS) in rats. To provide a context for data interpretation, effects of RTI-118 were compared to effects of the kappa opioid receptor agonist U69,593, because the kappa opioid receptor is another peptide neurotransmitter receptor reported to modulate abuse-related cocaine effects. RTI-118 effects were also examined on ICSS facilitation produced by methylenedioxypyrovalerone (MDPV), a novel designer drug of abuse with some cocaine-like effects. Male Sprague-Dawley rats (n=12) with electrodes targeting the medial forebrain bundle responded under a fixed-ratio 1 schedule for range of brain stimulation frequencies. Under control conditions, brain stimulation maintained a frequency-dependent increase in ICSS rates. Cocaine (1.0–10 mg/kg) and MDPV (3.2 mg/kg) facilitated ICSS. RTI-118 (3.2––32 mg/kg) alone produced little effect on ICSS but dose dependently blocked cocaine-induced ICSS facilitation. U69,593 (0.25–0.5 mg/kg) also attenuated cocaine effects, but blockade of cocaine effects was incomplete even at a U69,593 dose that alone depressed ICSS. RTI-118 (32 mg/kg) failed to block MDPV-induced ICSS facilitation. These results support further consideration of NPS receptor antagonists as candidate treatments for cocaine abuse and provide evidence for differential effects of a candidate treatment on abuse-related effects of cocaine and MDPV.
Keywords: Neuropeptide S, RTI-118, cocaine, methylenedioxypyrovalerone, intracranial self-stimulation
1. INTRODUCTION
Neuropeptide S (NPS) is a 20 amino-acid peptide co-expressed with glutamate in a small population of neurons that originate in discrete brainstem regions and project to multiple brain targets (Guerrini et al., 2010; Xu et al., 2004). NPS derives its name from its conserved N-terminal serine residue, and it binds to the Gq/Gs-coupled NPS receptor to mobilize calcium, stimulate adenylate cyclase, and increase cellular excitability. NPS receptors are distributed in multiple brain regions, including ventral tegmental area, amygdala, hippocampus and hypothalamus (Leonard and Ring, 2011; Xu et al., 2007). NPS receptor agonists produce an unusual behavioral profile that includes increased wakefulness, locomotor stimulation and anxiolytic-like effects (Dal Ben et al., 2011), and both NPS and its receptor have been implicated in effects produced by abused drugs like cocaine (Guerrini et al., 2010; Kallupi et al., 2010; Paneda et al., 2009; Schmoutz et al., 2012). One outcome of these studies has been the hypothesis that NPS receptor antagonists may have utility as candidate medications for treatment of addiction to cocaine and other abused drugs (Guerrini et al., 2010).
RTI-118 (3-oxo-1,1-diphenyl-N-(2-(piperidin-1-yl)ethyl)tetrahydro-1H-oxazolo[3,4-a]pyrazine-7(3H)-carboxamide) is a novel NPS receptor antagonist that has been tested in studies of cocaine self-administration and reinstatement of extinguished cocaine self-administration in rats (Schmoutz et al., 2012; Zhang et al., 2008). Specifically, RTI-118 (5–30 mg/kg i.p.) produced a dose-dependent and nearly complete elimination of cocaine self-administration, and was more potent and efficacious to reduce cocaine self-administration than food-maintained responding (Schmoutz et al., 2012). RTI-118 (1–20 mg/kg i.p.) also blocked cocaine, cue-, and stress-induced reinstatement of extinguished cocaine self-administration (Schmoutz et al., 2012). The potency of RTI-118 to reduce abuse-related cocaine effects in rats was similar to its potency to block locomotor activation elicited by intracerebroventricular NPS in mice (Hassler et al., 2014). These results are consistent with the hypothesis that RTI-118 functions in vivo as an NPS receptor antagonist that warrants further study as a candidate medication for treatment of cocaine dependence.
This study evaluated effects of RTI-118 on cocaine-induced facilitation of intracranial self-stimulation (ICSS) in rats. ICSS is an experimental procedure that has been used to evaluate abuse-related drug effects and potential efficacy of candidate anti-addiction treatments (Bauer et al., 2014; Carlezon and Chartoff, 2007; Negus and Miller, 2014; Vlachou and Markou, 2011). In ICSS, operant responding is maintained by pulses of electrical stimulation delivered via an intracranial electrode. The frequency of stimulation can be manipulated within each session to engender a wide range of baseline behavioral rates. Cocaine and many other abused drugs increase (“facilitate”) low ICSS rates maintained by low brain-stimulation frequencies, whereas drugs that produce anhedonic effects or motor impairment decrease high ICSS rates maintained by high brain-stimulation frequencies (Bauer et al., 2013; Carlezon and Chartoff, 2007; Negus and Miller, 2014). We hypothesized that RTI-118 would block cocaine-induced facilitation of ICSS. Effects of RTI-118 were compared to effects of the kappa opioid receptor agonist U69,593 as a positive control. Kappa opioid receptors mediate effects of an endogenous peptide ligand (i.e. dynorphin) that modulates cocaine effects, and agonists like U69,593 have been examined for their impact on abuse-related cocaine effects in ICSS (Tomasiewicz et al., 2008) and other procedures (Mello and Negus, 2000; Shippenberg et al., 2001; Walsh et al., 2001). Lastly, RTI-118 effects on ICSS facilitation by methylenedioxypyrovalerone (MDPV) were also examined. MDPV is an emerging designer drug of abuse that produces cocaine-like blockade of dopamine transport (Baumann et al., 2012) and cocaine-like facilitation of ICSS (Bonano et al., 2013). To complement behavioral studies, in vitro functional studies were conducted to further evaluate pharmacological selectivity of RTI-118 for NPS receptors in comparison to a range of other representative G-protein-coupled receptors, ligand-gated ion channels and voltage-gated ion channels (5-HT1A, 5-HT2A and 5-HT2B serotonin receptors; CB1 and CB2 cannabinoid receptors; apelin receptors; mu, kappa and delta opioid receptors; TAAR1 trace amine-associated receptors; TRPV1 transient receptor potential cation channels; CaV 3.1 and CaV 3.2 voltage-gated calcium channels).
2. MATERIALS AND METHODS
2.1 In vitro studies
2.1.1 General Calcium Mobilization Assays
Studies were conducted with procedures similar to those used previously to assess antagonist effects of RTI-118 at NPS receptors (Zhang et al., 2008). Stable cell lines that over-expressed the desired human receptors were used. The day before the assay, cells were plated into 96-well black-walled assay plates in growth medium and incubated at 37°C, 5% CO2 overnight. Prior to the assay, Calcium 5 dye (Molecular Devices, Sunnyvale, CA) was reconstituted according to the manufacturer instructions. The reconstituted dye was diluted 1:40 in pre-warmed (37°C) assay buffer (1X HBSS, 20 mM HEPES, 2.5 mM probenecid, pH 7.4 at 37°C). Growth medium was removed, and the cells were gently washed with 100 μL of pre-warmed (37°C) assay buffer. The cells were incubated for 45 minutes at 37°C, 5% CO2 in 200 μL of the diluted Calcium 5 dye. For agonist assays, serial dilutions of RTI-118 were prepared at 10x the desired final concentration in 1% DMSO/assay buffer, aliquoted into 96-well polypropylene plates, and warmed to 37°C. After the dye-loading incubation period, the cells were pre-treated with 25 μL of 9% DMSO/assay buffer and incubated for 15 min at 37°C. After the pre-treatment incubation period, the plate was read in a FLIPR Tetra or FlexStation II (Molecular Devices). Calcium-mediated changes in fluorescence were monitored every 1 second over a 90 second time period, with the Tetra adding 25 μL of the test compound dilutions at the 10 second time point (excitation at 470–495 nm, detection at 515–575 nm). Maximum kinetic reduction (ScreenWorks, Molecular Devices) relative fluorescence units (RFU) were plotted against compound concentration. On the FlexStation II, calcium-mediated changes in fluorescence were monitored every 1.52 seconds over a 60 second time period, with the FlexStation II adding 25 μL of test compound dilutions at the 19 second time point (excitation at 485 nm, detection at 525 nm). Peak kinetic reduction (SoftMax, Molecular Devices) relative fluorescence units (RFU) were plotted against compound concentration. For antagonist (IC50) assays, the above procedure was followed except that cells were pre-treated with serial dilutions of RTI-118 (prepared at 10x the desired final concentration in 9% DMSO/assay buffer), and the instrument added the control agonist EC50–EC80 concentration (prepared at 10x the desired final concentration in 1% DMSO/assay buffer). Data were fit to the appropriate three-parameter logistic curve to generate either EC50 or IC50 values (GraphPad Prism 6.0, GraphPad Software, Inc., San Diego, CA).
2.1.2 Serotonin
5-HT1A,5-HT2A and 5-HT2B HEK293 cells were plated at 40,000 and 35,000 cells/well, respectively, in plates pre-coated with PEI in DMEM-HG supplemented with 10% fetal bovine serum, 100 units of penicillin and streptomycin, 15 mM HEPES, and 100 μg/ml normocin™. CHO-Gα16-5-HT1A cells were plated at 25,000 cells/well in Ham’s F12 supplemented with 10% fetal bovine serum, 100 units of penicillin and streptomycin, and 100 μg/ml normocin™. The general procedure was followed using the control agonist 5-HT.
2.1.3 Cannabinoid
CHO-Gα16-CB1 and -CB2 cells were plated at 25,000 and 30,000 cells/well, respectively, in Ham’s F12 supplemented with 10% fetal bovine serum, 100 units of penicillin and streptomycin, and 100 μg/ml normocin™. The general procedure was followed using the control agonist CP55,940 except that the pre-treatment dilutions were prepared in 2.25% BSA/4.5% DMSO/4.5% EtOH/assay buffer and treatment dilutions were prepared in 0.25% BSA/0.5% DMSO/0.5% EtOH/assay buffer.
2.1.4 APJ (Apelin)
CHO-Gα16-APJ-AGTRL1 cells were plated at 30,000 cells/well in DMEM/F12 supplemented with 10% fetal bovine serum, 100 units of penicillin and streptomycin, and 100 μg/ml normocin™. The general procedure was followed using the control agonist apelin except that the pre-treatment dilutions were prepared in 2.25% BSA/9% DMSO/assay buffer and treatment dilutions were prepared in 0.25% BSA/1% DMSO/assay buffer.
2.1.5 Opioids
CHO-Gα16-mu opioid receptor, -delta opioid receptor, and – kappa opioid receptor cells were plated at 30,000 cells/well in Ham’s F12 supplemented with 10% fetal bovine serum, 100 units of penicillin and streptomycin, and 100 μg/ml normocin™. The general procedure was followed using the control agonists Damgo, DPDPE, and U69,593, respectively.
2.1.6 TRPV1
HEK293 cells were plated at 30,000 cells/well in plates pre-coated with PEI in DMEM-HG supplemented with 10% fetal bovine serum, 100 units of penicillin and streptomycin, 15 mM HEPES, and 100 μg/ml normocin™. The general procedure was followed using the control agonist capsaicin.
2.1.7 Calcium Voltage Channel 3.1 and 3.2 Calcium Mobilization Assay
Two individual stable cell lines were created by over-expressing human CaV 3.1 or CaV 3.2 receptors in HEK293 cells. The day before the assay, cells were plated into 384-well black-walled assay plates coated with poly- -lysine in DMEM supplemented with 1% fetal bovine serum, 100 units penicillin/streptomycin, 500 μM sodium pyruvate, 4 mM -glutamine, 0.5 mM calcium chloride, and 0.225 mM magnesium sulfate and incubated at 37°C, 5% CO2 overnight. Prior to the assay, Calcium 5 dye (Molecular Devices) was reconstituted according to the manufacturer instructions. The reconstituted dye was diluted 1:10 in pre-warmed (37°C) assay buffer (1X HBSS, 20 mM HEPES, 500 μM calcium chloride, pH 7.4 at 37°C). The cells were incubated for 45 minutes at 37°C, 5% CO2 in 30μL of the diluted Calcium 5 dye. For antagonist assays, serial dilutions of RTI-118 were prepared at 10x the desired final concentration in 1% DMSO/assay buffer, aliquoted into 384-well polypropylene plates, and warmed to 37°C. Calcium chloride (5 mM final) was prepared at 10x in assay buffer. After the dye-loading incubation period, 7.5 μL of the test compound dilutions was added to the cells by the FLIPR Tetra (Molecular Devices). The plate was then incubated for 15 min at 37°C. Calcium-mediated changes in fluorescence were monitored every 1 second over a 60 second time period, with the Tetra adding 7.5 μL of calcium chloride at the 10 second time point (excitation at 470–495 nm, detection at 515–575 nm). Area under the curve kinetic reductions (ScreenWorks, Molecular Devices) relative fluorescence units (RFU) were plotted against compound concentration. Data were fit to the appropriate three-parameter logistic curve to generate IC50 values (GraphPad Prism 6.0, GraphPad Software, Inc., San Diego, CA).
2.1.8 hTAAR1 cAMP Assay
CHO cells stably expressing the human TAAR1 receptor were used. The day before the assay, cells were plated into 96-well white-walled assay plates in DMEM/F12 supplemented with 1% fetal bovine serum and 100 units of penicillin/streptomycin and incubated at 37°C, 5% CO2 overnight. The next day, medium was removed and the cells were gently washed with 100 μL of pre-warmed DMEM/F12 medium. For agonist assays, serial dilutions of R06039-229 were prepared at 3x the desired final concentration in 1% DMSO/DMEM/F12 supplemented with 1 mM IBMX final. At this point, the manufacturer instructions were followed for the cAMP HitHunter™ kit (DiscoveRx). Luminescence was measured at 3 hr post detection reagent addition on the FlexStation III (1000 ms integration). Relative luminescence units (RLU) were plotted against compound concentration. For antagonist (IC50) assays, the above procedure was followed except that serial dilutions of RTI-118 and the EC60 concentration of PEA were prepared at 6x the desired final concentration in 1% DMSO/DMEM/F12 supplemented with 1 mM IBMX final.
2.2 Intracranial self-stimulation (ICSS)
2.2.1 Subjects
Twelve adult male Sprague-Dawley rats (Harlan, Frederick, MD, USA) weighing between 327–385 g at the time of surgery were individually housed and maintained on a 12 h light/dark cycle (lights on 6:00 AM to 6:00 PM). All rats were kept in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Rats had free access to food and water except during testing. Animal maintenance and research were in compliance with the National Institutes of Health guidelines on care and use of animal subjects in research (National Research Council, 2011). All animal use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
2.2.2 Surgery
All rats were maintained under isoflurane (2.5–3% in oxygen; Webster Veterinary, Phoenix, AZ, USA) anesthesia during implantation of stainless steel electrodes (Plastics One, Roanoke, VA, USA). The cathode of each electrode was 0.25 mm in diameter and covered with polyamide insulation except at the flattened tip, while the anode was 0.125 mm in diameter and uninsulated. The cathode was implanted into the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral to the midsagittal suture, 8.8 mm ventral to the skull) using a stereotaxic device. Three screws were placed in the skull, and the anode was wrapped around one of these screws to serve as a ground. The skull screws and electrode were then secured to the skull with orthodontic resin. Ketoprofen (5 mg/kg) was used for post-operative analgesia immediately and 24 h after surgery. Animals were allowed to recover for at least 7 days before beginning ICSS training.
2.2.3 Apparatus
Experiments were conducted in sound-attenuating boxes that contained modular acrylic test chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever (4.5 cm wide, 2.0 cm deep, 3 cm off the floor), three stimulation lights (red, yellow, and green, positioned 7.6 cm directly above the response lever), a 2 W house light, and an ICSS stimulator (Med Associates, St. Albans, VT, USA). Electrodes were connected to the stimulator by a swivel commutator (Model SL2C, Plastics One). Control of experimental events and acquisition of data were accomplished with a computer operated by Med-PC IV software and connected to test chambers by an interface system (Med Associates).
2.2.4 Training
Rats were trained under a fixed-ratio 1 (FR 1) schedule of electrical brain stimulation using a behavioral procedure identical to that previously described (Bonano et al., 2013). During behavioral sessions, each lever press resulted in the delivery of a 0.5 s train of square wave cathodal pulses with each pulse lasting 0.1 ms. Brain stimulation was accompanied by 0.5 s illumination of the stimulus lights over the lever, and further lever press responding within the 0.5 s stimulation period did not earn additional stimulation. During initial 60 min training sessions, stimulation frequency was maintained at 126 Hz, and stimulation intensity was adjusted for each individual rat to the lowest intensity that sustained a high rate of reinforcement (> 30 stimulations/min). This intensity (100–240 μA across rats) was then held constant for the remainder of the study, and frequency manipulations were introduced. Sessions involving frequency manipulations consisted of three consecutive 10 min components. During each component, a descending series of 10 frequencies ranging from 158 to 56 Hz in 0.05 log increments was presented with each frequency available for a 1 min trial. Each frequency trial began with a 10 s time-out period during which responding had no scheduled consequences. Five non-contingent “priming” stimulations were delivered in conjunction with illumination of the stimulus lights during the last 5 s of the time-out to signal the frequency of stimulation that would be available during that trial. This non-contingent stimulation was followed by a 50 s “response” phase, during which responding produced electrical stimulation under a FR 1 schedule as described above. Training continued until rats reliably responded at high rates for only the first three to five frequency trials of each component over a period of at least three consecutive training days.
2.2.5 Testing
Test sessions lasted 90 min and consisted of three 10 min “baseline” components followed first by a 30 min time-out period during which test compounds were administered and then by three sequential 10 min “test” components. One group of six rats was used to examine effects of cocaine, RTI-118 or U69,593 alone, or of cocaine administered after pretreatment with RTI-118 or U69,593. In studies with cocaine alone, cocaine (0.32, 1.0, 3.2, 10 mg/kg) or saline vehicle was administered 10 min before initiation of test components. In pretreatment studies with RTI-118, rats were treated first with RTI-118 (vehicle, 3.2, 10, 32 mg/kg), then 20 min later with either 10 mg/kg cocaine or saline vehicle, and test components began 10 min after the second injection. In pretreatment studies with U69,593, rats were treated first with U69,593 (vehicle, 0.25, 0.5 mg/kg), then 10 min later with either 10 mg/kg cocaine or saline vehicle, and test components began 10 min after the second injection. Studies with cocaine alone were conducted first, followed by studies with RTI-118 and then by studies with U69,593. Within each test phase, the order of dosing was varied across subjects using a Latin-square design. A second group of six rats was used to evaluate effects of MDPV alone or after pretreatment with RTI-118. For these studies, rats were treated first with 32 mg/kg or its vehicle, then 20 min later with 3.2 mg/kg MDPV or its vehicle, and test components began 10 min after the second injection. The order of testing was varied across subjects using a Latin-square design. Doses and pretreatment times for cocaine, MDPV, RTI-118 and U69,593 were based on previous studies (Bonano et al., 2013; Rosenberg et al., 2013; Schmoutz et al., 2012; Tomasiewicz et al., 2008). Test sessions were completed on Tuesdays and Fridays, and three-component training sessions were conducted on all other weekdays.
2.2.6 Data Analysis
The primary dependent variable was reinforcement rate in stimulations per minute during each frequency trial. To normalize these data, raw reinforcement rates from each trial in each rat were converted to percent maximum control rate (%MCR), with MCR defined as the mean of the maximal rates observed during the second and third baseline components for any given rat in any given session. Thus, %MCR values for each trial were calculated as %MCR= (reinforcement rate during a frequency trial ÷ maximum control rate) × 100. For each experimental manipulation, data from all three test components were averaged first within each rat and then across rats to yield mean test curves for each manipulation. Results from test sessions were compared by repeated measures two-way ANOVA, with ICSS frequency as one factor and drug treatment as the second factor. A significant ANOVA was followed by Holm-Sidak post hoc test with the criterion for significance set at P<0.05.
2.2.7 Drugs
(−)-Cocaine HCl (National Institutes on Drug Abuse Drug Supply Program; Bethesda, MD), RTI-118 HCl (Dr. Scott Runyon; Research Triangle Institute), and (±)-3,4-methylenedioxypyrovalerone HCl (Dr. Richard Glennon; Virginia Commonwealth University) were dissolved in sterile saline. U69,593 was purchased from Sigma Chemical (St. Louis, MO) and was dissolved in sterile saline with a drop of lactic acid. All drugs were administered i.p.
3. RESULTS
3.1 In vitro functional studies
Table 1 shows results of in vitro studies that examined effects of RTI-118 on activity mediated by 13 different receptors and channels at which RTI-118 might produce off-target effects. At concentrations up to 10 μM, RTI-118 did not produce agonist or antagonist effects at any of these targets.
Table 1.
Lack of RTI-118 effects on in vitro activity mediated by a panel of 13 receptors and ion channels. ND=not determined.
| Receptor/Channel Target | Agonist EC50 | Antagonist IC50 |
|---|---|---|
| 5HT1A | > 10 μM | > 10 μM |
| 5HT2A | > 10 μM | > 10 μM |
| 5HT2B | > 10 μM | > 10 μM |
| hTAAR1 | > 10 μM | > 10 μM |
| CB1 | > 10 μM | > 10 μM |
| CB2 | > 10 μM | > 10 μM |
| Mu | > 10 μM | > 10 μM |
| Delta | > 10 μM | > 10 μM |
| Kappa | > 10 μM | > 10 μM |
| APJ (Apelin) | > 10 μM | > 10 μM |
| CaV 3.1 | ND | > 10 μM |
| CaV 3.2 | ND | > 10 μM |
| TRPV1 | > 10 μM | > 10 μM |
3.2 Intracranial self-stimulation
Across the 12 rats used in these studies, the mean ± S.E.M. maximal control rate (MCR) was 60.6 ± 4.10 stimulations per trial. Electrical brain stimulation maintained a frequency-dependent increase in ICSS rates under baseline conditions (e.g. vehicle data in Fig. 1). Generally, low brain-stimulation frequencies (1.75–1.90 log Hz) maintained low ICSS rates, intermediate frequencies (1.90–2.05 log Hz) supported an increase in ICSS rates, and high frequencies (2.05–2.20 log Hz) maintained asymptotic ICSS rates.
Fig. 1. Effects of cocaine (A), RTI-118 (B) and U69,593 (C) on ICSS.
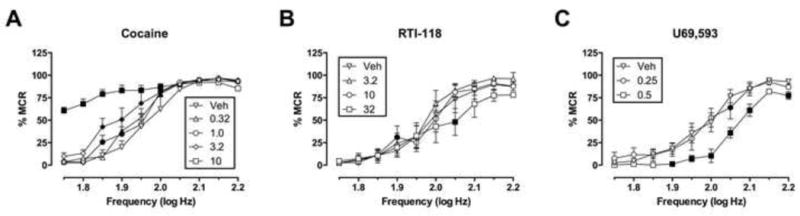
Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: percent maximum control reinforcement rate (%MCR). Drug doses are indicated in legends in units of mg/kg. Filled points represent frequencies at which reinforcement rates were statistically different from vehicle rates as determined by two-way ANOVA followed by Holm-Sidak post hoc test, P<0.05. All data show mean ± S.E.M. for five (RTI-118) or six (cocaine, U69,593) rats.
Figure 1 shows effects of cocaine alone (0.32–10 mg/kg), RTI-118 alone (3.2–32 mg/kg), and U69,593 alone (0.25, 0.5 mg/kg). Two-way ANOVA indicated significant main effects of frequency and dose and significant frequency × dose interactions for all drugs, and only frequency × dose interaction effects are reported below for each drug. Cocaine [F(36,180)=11.95, P<0.0001] dose-dependently facilitated low ICSS rates maintained by low brain stimulation frequencies, and the highest dose of 10 mg/kg cocaine facilitated ICSS across a broad range of six frequencies (1.75–2.0 log Hz). RTI-118 also significantly altered ICSS [F(27,108)=2.269, P=0.0016], although effects were modest across the dose range examined compared to the other drugs evaluated. Post hoc analysis indicated that 10 mg/kg RTI-118 significantly increased ICSS at one frequency (1.9 log Hz), and 32 mg/kg RTI-118 significantly decreased ICSS at only one frequency (2.05 log Hz). U69,593 [F(18,90)=3.225, P=0.0001] dose-dependently depressed ICSS, with the highest dose reducing ICSS across a broad range of six intermediate to high frequencies (1.9–2.2 log Hz, excluding 2.15 log Hz).
Figure 2 shows effects of 10 mg/kg cocaine after pretreatment with RTI-118 (3.2–32 mg/kg) or U69,593 (0.25, 0.5 mg/kg). For RTI-118 + cocaine, two-way ANOVA revealed significant main effects of frequency [F(9,36)=8.127, P<0.0001] and treatment [F(3,12)=4.481, P=0.0249], but the interaction was not significant [F(27,108)=0.9666, P=0.5199]. Furthermore, attenuation of cocaine effects was statistically significant only at the highest RTI-118 dose (32 mg/kg), which also significantly depressed ICSS when administered alone (see Fig. 1B). For U69,593 + cocaine, two-way ANOVA revealed significant main effects of frequency [F(9,45)=22.51, P<0.0001] and treatment [F(2,10)=5.863, P=0.0207], but the interaction was not significant [F(18,90)=1.237, P=0.2499]. U69,593 significantly and dose-dependently attenuated cocaine-induced facilitation of ICSS at doses that depressed ICSS when administered alone (see Fig. 1C).
Fig. 2. Effects of pre-treatment with RTI-118 (A) or U69,593 (B) on cocaine-facilitated ICSS.
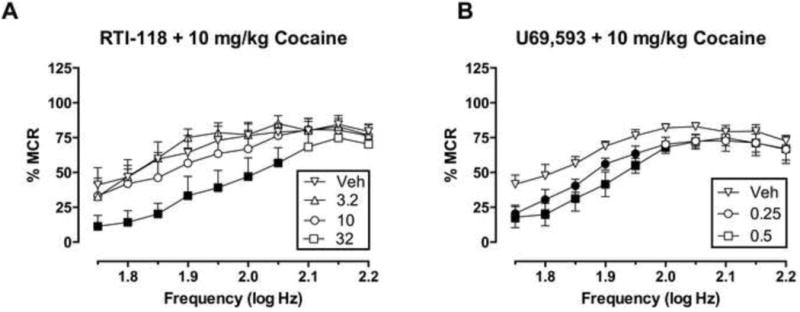
Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: percent maximum control reinforcement rate (%MCR). Pre-treatment doses of RTI-118 or U69,593 are indicated in legends in units of mg/kg. Filled points represent frequencies at which reinforcement rates were statistically different from vehicle + 10 mg/kg cocaine as determined by two-way ANOVA followed by Holm-Sidak post hoc test, P<0.05. All data show mean ± S.E.M. for five (RTI-118 + cocaine) or six (U69,593 + cocaine) rats.
Figure 3 compares effects of treatment with vehicle + vehicle to effects of the highest dose of either RTI-118 or U69,593 + 10 mg/kg cocaine. For RTI-118 + cocaine, two-way ANOVA revealed a significant main effect of frequency [F(9,36)=20.05, P<0.0001], but not of treatment [F(1,4)=0.1671, P=0.7037], and the interaction was also not significant [F(9,36)=1.929, P=0.0789]. For U69,593 + cocaine, two-way ANOVA indicated a significant main effect for frequency [F(9,45)=66.55, P<0.0001], but not treatment [F(1,5)=0.8709, P=0.3935], and a significant interaction [F(9,45)=5.058, P=0.0001]. Relative to vehicle + vehicle treatment, treatment with 0.5 mg/kg U69,593 + 10 mg/kg cocaine significantly facilitated low ICSS rates maintained by low stimulation frequencies (1.75–2.0 log Hz) and significantly depressed high ICSS rates maintained by high stimulation frequencies (2.15–2.2 log Hz). Thus, by this comparison, RTI-118 completely blocked cocaine-induced facilitation of ICSS, whereas U69,593 failed to completely block cocaine-induced ICSS facilitation even at a U69,593 dose that recruited depression of high ICSS rates.
Fig. 3. Comparison of ICSS after treatment with vehicle + vehicle and either RTI-118 + cocaine (A) or U69,593 + cocaine (B).
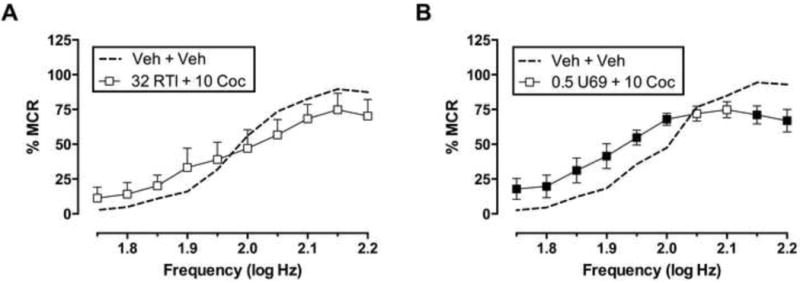
Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: percent maximum control reinforcement rate (%MCR). Treatments are indicated in legends, and drug doses are presented in units of mg/kg. Filled points represent frequencies at which ICSS rates after drug treatments were different from vehicle treatment as determined by two-way ANOVA followed by Holm-Sidak post hoc test, P<0.05. All data show mean ± S.E.M. for five (RTI-118 + cocaine) or six (U69,593 + cocaine) rats.
Figure 4 shows effects of RTI-118 (32 mg/kg or vehicle) + MDPV (3.2 mg/kg or vehicle). There were significant main effects of frequency [F(9,36)=35.46, P<0.0001] and treatment [F(3,12)=4.545, P=0.0238] and a significant interaction [F(27,108)=2.619, P=0.0002]. Relative to the vehicle + vehicle treatment, MDPV alone facilitated ICSS across a broad range of frequencies (1.75–2.05 log Hz), whereas in this group of rats, 32 mg/kg RTI-118 alone did not significantly alter ICSS at any frequency. RTI-118 pretreatment did not alter MDPV-induced facilitation of ICSS.
Fig. 4. Effects of RTI-118 on MDPV-facilitated ICSS.
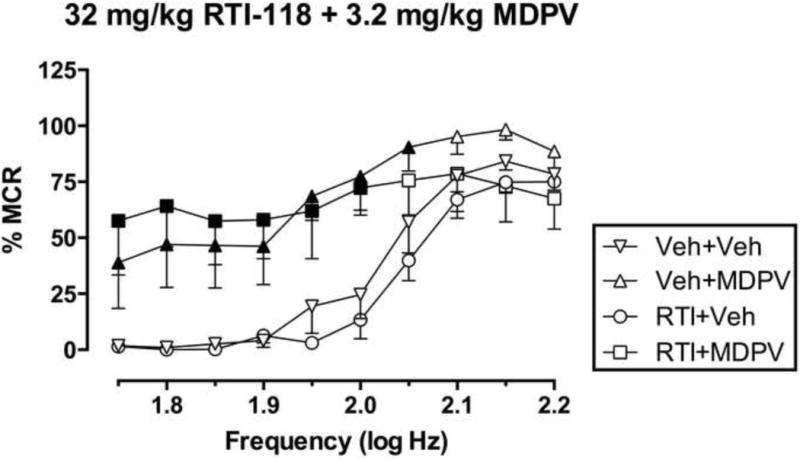
Abscissa: frequency of electrical brain stimulation in log Hz. Ordinate: percent maximum control reinforcement rate (%MCR). Filled points represent frequencies at which reinforcement rates were statistically different from vehicle rates as determined by two-way ANOVA followed by Holm-Sidak post hoc test, P<0.05. All data show mean ± S.E.M. for five rats.
4. DISCUSSION
4.1 In vitro functional selectivity of RTI-118
Previously published data with an in vitro calcium mobilization assay reported potency of RTI-118 as an antagonist at NPS receptors with a Ke value of 109±23 nM (Zhang et al., 2008). In contrast, the present study showed that RTI-118 concentrations up to 10 μM had no agonist or antagonist effects on representative Gs-, Gq- and Gi-protein-coupled receptors or ligand- and voltage-gated ion channels. These results suggest that RTI-118 is at least 100-fold selective for NPS receptors relative to these other targets.
4.2 Effects of RTI-118 and U69,593 alone
When administered alone, low doses of 3.2–10 mg/kg RTI-118 had little effect on ICSS, whereas a higher dose of 32 mg/kg RTI-118 produced a significant but modest depression of ICSS in one group of rats but not in a second group of rats. This agrees with a previous study showing that RTI-118 doses up to 20 mg/kg IP had no effect on rates of food-maintained responding, and a higher dose of 30 mg/kg RTI-118 produced a significant but small (≤20%) reduction in rates of food-maintained responding (Schmoutz et al., 2012). One implication of these findings is that endogenous signaling in NPS/NPS receptor systems is low, because blockade of NPS receptors has little overt effect on behavior.
Depressant effects of RTI-118 are also small in comparison to effects of the kappa opioid receptor agonist U69,593. In contrast to NPS receptors, which couple to Gq/Gs to increase cellular excitability, kappa opioid receptors are coupled to Gi and inhibit cellular excitability (Tejeda et al., 2012). In particular, kappa opioid receptors are located anatomically on mesolimbic dopamine neurons that project from ventral tegmental area to forebrain targets including nucleus accumbens (Chavkin, 2011; Shippenberg et al., 2001; Wee and Koob, 2010). Accordingly, activation of kappa opioid receptors by agonists like U69,593 reduces activity of and dopamine release by these neurons, and opposes rewarding effects of stimuli such as electrical stimulation of the medial forebrain bundle or of abused drugs like cocaine that depend on mesolimbic dopamine release. The depression of ICSS produced by U69,593 in this study agrees with many previous studies showing depression of ICSS by kappa opioid receptor agonists (Chartoff et al., 2008; Negus et al., 2012; Rosenberg et al., 2013; Todtenkopf et al., 2004).
4.3 Effects of RTI-118 and U69,593 on cocaine-induced facilitation of ICSS
In the present study, RTI-118 produced a dose-dependent and complete blockade of cocaine-induced facilitation of ICSS at RTI-118 doses that produced little or no effect on ICSS when administered alone. This finding agrees with a previous study reporting that RTI-118 also produced a dose-dependent and nearly complete elimination of cocaine self-administration in rats while producing little or no effect on food-maintained responding (Schmoutz et al., 2012). RTI-118 and other NPS receptor antagonists have also been found to block cue-, cocaine-, and stress-induced reinstatement of cocaine self-administration in rats and mice (Kallupi et al., 2010; Kallupi et al., 2013; Paneda et al., 2009; Schmoutz et al., 2012). Taken together, these results support further consideration of NPS receptor antagonists as treatments for cocaine abuse and dependence.
In agreement with a previous study, the kappa opioid receptor agonist U69593 also produced a dose-dependent attenuation of cocaine-induced facilitation of ICSS (Tomasiewicz et al., 2008). However, even the high dose of 0.5 mg/kg U69,593 failed to completely block cocaine-induced facilitation of lCSS despite producing a robust depression of ICSS when administered alone. Overall, U69,593 produced weaker antagonism than RTI-118 of cocaine-induced ICSS facilitation despite being tested up to doses that produced greater depression of ICSS when administered alone. This agrees with previous drug self-administration studies, which found that selective kappa opioid receptor agonists including U69,593 reduce cocaine self-administration only at doses that also reduce food-maintained responding (Mello and Negus, 1998; 2000; Negus et al., 1997).
The comparison of effects produced by RTI-118 and U69,593 also highlights one other facet of drug testing that will be important for future studies with RTI-118 as a candidate treatment for cocaine dependence. Although cocaine self-administration can be reduced by acute treatment with kappa opioid receptor agonists, drugs used for addiction treatment are typically administered chronically. During chronic administration, tolerance develops to kappa opioid receptor agonist effects on cocaine self-administration (Mello and Negus, 1998; 2000; Negus et al., 1997), and chronic kappa opioid receptor agonist treatment also failed to alter cocaine self-administration in human laboratory studies (Walsh et al., 2001). The effects of chronic RTI-118 treatment on cocaine self-administration or cocaine-induced facilitation of ICSS remain to be determined, but this will be an important step in further evaluation.
4.4 Effects of RTI-118 on MDPV-induced facilitation of ICSS
MDPV is a derivative of methcathinone that has recently emerged as designer drug of abuse and a constituent in drug mixtures denoted by street names that include “bath salts” (Baumann et al., 2013; De Felice et al., 2014; Gregg and Rawls, 2014). Like cocaine, MDPV functions as an inhibitor of dopamine transporters, and it produces various cocaine-like effects that include increased dopamine concentrations in nucleus accumbens, locomotor activation, reinforcing effects in drug self-administration procedures, and facilitation of ICSS with a potency approximately 3-fold higher than that of cocaine (Baumann et al., 2012; Bonano et al., 2013; Cameron et al., 2013; Watterson et al., 2012). In agreement with these previous studies, the present study found that 3.2 mg/kg MDPV produced a facilitation of ICSS similar to that produced by 10 mg/kg cocaine. However, a dose of RTI-118 that completely blocked effects of cocaine failed to alter effects of MDPV. The mechanisms for this difference require further study. MDPV differs from cocaine primarily in its higher affinity for dopamine transporters, its higher selectivity for dopamine vs. norepinephrine and serotonin transporters, and its longer duration of action (Baumann et al., 2012; Bonano et al., 2013; Watterson et al., 2012). Regardless of the underlying mechanism, these studies provide evidence to suggest that treatments effective in blocking cocaine may be less effective in blocking effects of other abused stimulants like MDPV.
4.5 Conclusions
These results support further consideration of NPS receptor antagonists in general, and RTI-118 in particular, as candidate medications for treatment of cocaine abuse. However, the failure of RTI-118 to block MDPV effects suggests that NPS receptor antagonists may not be useful to treat MDPV abuse despite the similar mechanism of action of cocaine and MDPV as dopamine uptake inhibitors.
Acknowledgments
This project was supported by grants R01 DA033930 (SSN, RAG), R01 DA026946 (SSN), F30 DA037649 (JSB), and R01 MH081247 (SPR). The authors thank Dr. Ann Decker for in vitro evaluation of RTI-118.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors declare no conflict of interest.
References
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol. 2013;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Negus SS. The effect of chronic amphetamine treatment on cocaine-induced facilitation of intracranial self-stimulation in rats. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-013-3405-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR. Psychoactive “bath salts”: not so soothing. Eur J Pharmacol. 2013;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-Methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2012;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano J, Glennon RA, De Felice L, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2013;231:199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Verkariya R, De Felice L, Glennon RA. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology (Berl) 2013;227:493–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Exposure to the selective kappa-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C. The therapeutic potential of kappa-opioids for treatment of pain and addiction. Neuropsychopharmacology. 2011;36:369–370. doi: 10.1038/npp.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Ben D, Antonini I, Buccioni M, Lambertucci C, Marucci G, Thomas A, Volpini R, Cristalli G. Neuropeptide S receptor: recent updates on nonpeptide antagonist discovery. Chem Med Chem. 2011;6:1163–1171. doi: 10.1002/cmdc.201100038. [DOI] [PubMed] [Google Scholar]
- De Felice LJ, Glennon RA, Negus SS. Synthetic cathinones: Chemical phylogeny, physiology, and neuropharmacology. Life Sci. 2014;97:20–26. doi: 10.1016/j.lfs.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Rawls SM. Behavioral pharmacology of designer cathinones: A review of the preclinical literature. Life Sci. 2014;97:27–30. doi: 10.1016/j.lfs.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini R, Salvadori S, Rizzi A, Regoli D, Calo G. Neurobiology, pharmacology, and medicinal chemistry of neuropeptide S and its receptor. Med Res Rev. 2010;30:751–777. doi: 10.1002/med.20180. [DOI] [PubMed] [Google Scholar]
- Hassler C, Zhang Y, Gilmour B, Graf T, Fennell T, Snyder R, Deschamps JR, Reinscheid RK, Garau C, Runyon SP. Identification of neuropeptide S antagonists: structure-activity relationship studies, X-ray crystallography, and in vivo evaluation. ACS Chem Neurosci. 2014 doi: 10.1021/cn500113c. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Cannella N, Economidou D, Ubaldi M, Ruggeri B, Weiss F, Massi M, Marugan J, Heilig M, Bonnavion P, de Lecea L, Ciccocioppo R. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc Natl Acad Sci U S A. 2010;107:19567–19572. doi: 10.1073/pnas.1004100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, de Guglielmo G, Cannella N, Li HW, Calo G, Guerrini R, Ubaldi M, Renger JJ, Uebele VN, Ciccocioppo R. Hypothalamic neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2013;226:347–355. doi: 10.1007/s00213-012-2910-y. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Ring RH. Immunohistochemical localization of the neuropeptide S receptor in the rat central nervous system. Neuroscience. 2011;172:153–163. doi: 10.1016/j.neuroscience.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J Pharmacol Exp Ther. 1998;286:812–824. [PubMed] [Google Scholar]
- Mello NK, Negus SS. Interactions between kappa opioid agonists and cocaine. Preclinical studies. Ann N Y Acad Sci. 2000;909:104–132. doi: 10.1111/j.1749-6632.2000.tb06678.x. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. National Academy Press; Washington DC: 2011. [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation (ICSS) to evaluate abuse potential of drugs. Pharmacol Rev. 2014 doi: 10.1124/pr.112.007419. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC. Effects of peripherally restricted kappa opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther. 2012;340:501–509. doi: 10.1124/jpet.111.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. J Pharmacol Exp Ther. 2008;325:893–901. doi: 10.1124/jpet.107.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneda C, Huitron-Resendiz S, Frago LM, Chowen JA, Picetti R, de Lecea L, Roberts AJ. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. J Neurosci. 2009;29:4155–4161. doi: 10.1523/JNEUROSCI.5256-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M, Carroll FI, Negus SS. Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain. 2013;14:246–259. doi: 10.1016/j.jpain.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoutz CD, Zhang Y, Runyon SP, Goeders NE. Antagonism of the neuropeptide S receptor with RTI-118 decreases cocaine self-administration and cocaine-seeking behavior in rats. Pharmacol Biochem Behav. 2012;103:332–337. doi: 10.1016/j.pbb.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Chefer VI, Zapata A, Heidbreder CA. Modulation of the behavioral and neurochemical effects of psychostimulants by kappa-opioid receptor systems. Ann N Y Acad Sci. 2001;937:50–73. doi: 10.1111/j.1749-6632.2001.tb03558.x. [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Shippenberg TS, Henriksson R. The dynorphin/kappa-opioid receptor system and its role in psychiatric disorders. Cell Mol Life Sci. 2012;69:857–896. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Markou A. Intracranial self-stimulation. In: Olmstead MC, editor. Animal Models of Drug Addiction. Humana Press; New York: 2011. pp. 3–56. [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–158. [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gilmour BP, Navarro HA, Runyon SP. Identifying structural features on 1,1-diphenyl-hexahydro-oxazolo[3,4-a]pyrazin-3-ones critical for Neuropeptide S antagonist activity. Bioorg Med Chem Lett. 2008;18:4064–4067. doi: 10.1016/j.bmcl.2008.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]


