Abstract
Overexpression of c-met and suppression of matrix metalloproteinase-10 (MMP-10) and cathepsin F genes was previously shown to normalize wound healing, epithelial and stem cell marker patterns in organ-cultured human diabetic corneas. We now examined if gene therapy of limbal cells only would produce similar effects.
Eight pairs of organ-cultured autopsy human diabetic corneas were used. One cornea of each pair was treated for 48 hours with adenoviruses (Ad) harboring full-length c-met mRNA or a mixture (combo) of Ad with c-met and shRNA to MMP-10 and cathepsin F genes. Medium was kept at the limbal level to avoid transduction of central corneal epithelium. Fellow corneas received control Ad with EGFP gene. After additional 5 (cmet) or 10 days (combo) incubation, central corneal epithelial debridement with nheptanol was performed, and wound healing times were determined microscopically. Corneal cryostat sections were immunostained for diabetic and putative limbal stem cell markers, α3β1 integrin, nidogen-1, fibronectin, laminin γ3 chain, ΔNp63α, keratins 14, 15, and 17, as well as for activated signaling intermediates, phosphorylated EGFR, Akt, and p38.
Limbal c-met overexpression significantly accelerated healing of 8.5-mm epithelial wounds over EGFP controls (6.3 days vs. 9.5 days, p<0.02). Combo treatment produced a similar result (6.75 days vs. 13.5 days, p<0.03). Increased immunostaining vs. EGFP controls for most markers and signaling intermediates accompanied c-met gene or combo transduction.
Gene therapy of limbal epithelial stem cell compartment has a beneficial effect on the diabetic corneal wound healing and on diabetic and stem cell marker expression, and shows potential for alleviating symptoms of diabetic keratopathy.
Keywords: Cornea, Diabetes, Limbal gene therapy, C-met, Cathepsin F, MMP-10, Stem cell marker, Signaling pathways
1. INTRODUCTION
Diabetic retinopathy (DR) is the major complication of diabetes mellitus in the eye. It occurs both in type I (insulin-dependent) and type II (non-insulin-dependent) diabetes (Antonetti et al. 2012). Vision loss from diabetes is mainly due to the retinal changes but other parts of the eye are also affected. In the iris, it is neovascularization and neovascular glaucoma; in the lens, diabetic cataract; in the optic nerve, glaucomatous optic neuropathy (Nakamura et al. 2005; Blum et al. 2007; Helbig, 2007; Murta and Cavallerano, 2007). Depending on disease duration and retinopathy stage, these symptoms are encountered in less than 30% of diabetic patients. The diabetic corneal disease includes recurrent erosions, delayed and incomplete wound healing, ulcers and edema, complications after vitrectomy and corneal surgery, and neuropathy / loss of corneal sensation; up to 70% diabetics have corneal problems (Schultz et al. 1981; Herse, 1988; Didenko et al. 1999; Wylegała et al. 2006; Chen et al. 2009; Bikbova et al. 2012; Calvo-Maroto et al. 2014). The most severe proliferative stage of DR cannot be recapitulated in animal models. We have previously validated human diabetic corneal organ culture system that reproduces wound healing dynamics and diabetic marker distribution (Kabosova et al. 2003), most probably due to the existence of epigenetic metabolic memory (Roy et al. 1990; Grant et al. 1998; Kowluru et al. 2013). We have further developed specific gene therapy that effectively reversed slow diabetic corneal epithelial wound healing and restored similar to normal expression of several diabetic markers and putative stem cell-associated proteins. This therapy was based on overexpression in diabetic corneas of matrix metalloproteinase-10 (MMP-10) and cathepsin F that were knocked-down using adenovirus (Ad)-driven shRNA transduction (Saghizadeh et al. 2010a, 2013). Additionally, c-met proto-oncogene, receptor of hepatocyte growth factor (HGF), was reduced in diabetic corneas and its levels were successfully restored using its Ad-driven overexpression (Saghizadeh et al. 2005, 2010b). The best normalizing effect on diabetic corneas was obtained using combination therapy approach, upon changing the levels of all three targets (Saghizadeh et al. 2013). As stem cells are necessary for epithelial wound healing, and diabetes-altered stem cell marker patterns became much closer to normal after gene therapy, we hypothesized that gene therapy could be also efficient if applied only to the limbus that harbors corneal epithelial stem cells (Lehrer et al. 1998; Lu et al. 2001; Rama et al. 2010; Amitai-Lange et al. 2014). It was, therefore, examined whether gene therapy of the limbus, to increase expression of c-met and knock-down MMP-10 and cathepsin F only in limbal cells, would restore putative stem cell marker expression and normalize wound healing in diabetic corneas. To ensure limbal involvement, healing of large 8.5 mm wounds was studied in organ-cultured corneas.
2. METHODS
2.1. Human Tissue and Ethics Statement
Postmortem diabetic human donor eyes were purchased from the National Disease Research Interchange (NDRI, Philadelphia, PA). NDRI has a human tissue collection protocol approved by a managerial committee and subject to National Institutes of Health oversight. This work was covered by an approved Cedars-Sinai Medical Center exempt IRB protocol EX-1055.
2.2. Organ Culture, Viral Transduction and Wound Healing
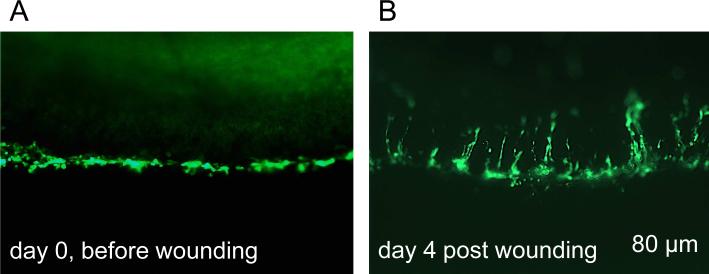
Eight pairs of age-matched diabetic corneas (4 per group; Table 1) were organ-cultured in serum-free DMEM with insulin-transferrin-selenite (Kabosova et al. 2003). Ad harboring shRNA to MMP-10 and cathepsin F genes (Saghizadeh et al. 2013) were obtained from Capital Biosciences (Gaithersburg, MD), and Ad overexpressing full-length c-met cDNA was engineered in the laboratory (Saghizadeh et al. 2010b). Control Ad (Capital Biosciences) drove enhanced green fluorescent protein (EGFP) expression. Virus incubation with corneas was performed for 48 hours with medium level at the limbus to ensure that central corneas were not transduced (Saghizadeh et al. 2010a, 2010b, 2013). This was verified by EGFP expression in live corneas showing EGFP signal only in the limbal compartment (Fig. 1A). Details of the procedure have been published. After 5 days of additional incubation with Ad-c-met (Saghizadeh et al. 2010b) or 10 days with Ad-c-met and Ad-shRNAs (combo treatment; Saghizadeh et al. 2013), central corneal epithelial wounds were made for 1 min with round paper disks soaked in n-heptanol. Fellow corneas of each pair received Ad-EGFP and were also wounded.
TABLE 1.
Donor Characteristics
| Case number | Age, years | Sex | DM type | Duration, years | Cause of death | Culture treatment |
|---|---|---|---|---|---|---|
| DR 11-20 | 62 | M | NIDDM | 10 | Congestive heart failure | c-Met |
| DM 11-21 | 73 | M | NIDDM | 10 | Pulmonary edema | c-Met |
| DM 11-28 | 71 | F | NIDDM | 8 | Myocardial infarction | c-Met |
| DM 11-29 | 84 | M | NIDDM | 30 | Congestive heart failure | c-Met |
| DM 12-19 | 79 | M | NIDDM | Over 10 | Hyperkalemic cardiac arrest | Combo |
| DM 12-21 | 84 | F | NIDDM | 20 | Alzheimer's disease | Combo |
| DM 12-22 | 60 | M | NIDDM | 10 | Cardiovascular disease | Combo |
| DM 12-23 | 71 | F | NIDDM → IDDM | 14 → 1 | Anoxic encephalopathy | Combo |
Combo, sh-cathepsin F + sh-MMP-10 + c-met. Arrows denote the number of years with a particular type of diabetes.
Figure 1.
Transduction of organ-cultured diabetic cornea with Ad-EGFP (live images). A, only limbal region is transduced (EGFP+ cells, green). B, during healing of a large 8.5 mm wound, limbal cell migrate centripetally. Days are relative to wound healing study. e, epithelium, s, stroma. Bar = 80 μm.
Wound closure was monitored microscopically as described (Saghizadeh et al. 2013), and expressed as the number of days to complete healing. In a pilot experiment, Ad-cmet transduced cornea and fellow Ad-EGFP control had a 5-mm wound made as described before (Saghizadeh et al. 2010a, 2010b, 2013). All other corneas had 8.5-mm wounds made to ensure limbal cell involvement in the healing process.
2.3. Immunostaining
Upon completion of the healing process, corneas were cut in half and embedded in O.C.T. compound (Sakura Finetek USA, Torrance, CA). Cryosections were immunostained using specific antibodies for a variety of markers (Table 2; Saghizadeh et al. 2013). These included proteins used for transduction (c-met, MMP-10, and cathepsin F), proteins with reduced expression in diabetic corneas (α3β1 integrin, nidogen-1, laminin γ3 chain, and fibronectin), putative limbal epithelial stem cell (LESC) markers (ΔNp63α, keratins 14, 15, and 17), as well as phosphorylated (p-) activated signaling intermediates (p-p38, p-EGFR, and p-Akt). Controls without primary antibodies were routinely included and were negative. Pictures were taken in an Olympus BX40 microscope (Olympus USA, Melville, NY), equipped with a high-sensitivity MicroFire 2-megapixel color digital camera (Imaging Planet, Goleta, CA). In each experiment, the same exposure times were used across all cases for each marker. The results were assessed by two independent observers upon immunostaining in three independent experiments. Although there was some typical staining variability among individual cases, possibly due to differences in donor ages and/or disease duration and severity, the presented pictures are representative of most studied cases.
TABLE 2.
Antibodies Used in the Study
| Antigen | Antibody | Source | Dilution |
|---|---|---|---|
| MMP-10 | Goat pAb sc-9941 | Santa Cruz Biotechnology (Dallas, TX) | 1:10 |
| Cathepsin F | Goat pAb AF2075 | R&D Systems (Minneapolis, MN) | 1:25 of 0.5 mg/ml stock |
| c-Met | Goat pAb sc-161 | Santa Cruz Biotechnology | 1:10 |
| Keratin 14 | Mouse mAb NB100-65109 | Novus Biologicals (Littleton, CO) | Undiluted |
| Keratin 15 | Mouse mAb sc-47697 | Santa Cruz Biotechnology | 1:10 |
| Keratin 17 | Mouse mAb sc-58726 | Santa Cruz Biotechnology | Undiluted |
| ANp63 | Goat pAb sc-8609 | Santa Cruz Biotechnology | 1:200 |
| Fibronectin | Mouse mAb 618 | Ugarova et al. 1996 | Undiluted |
| Laminin γ3 chain | Rabbit pAb R96 | Saghizadeh et al. 2011 | 1:500 |
| Nidogen-1 | Mouse mAb MAB2570 | R&D Systems | 1:50 |
| Integrin α3β1 | Mouse mAb MAB1992 | EMD Millipore (Billerica, MA) | 1:50 |
| p-EGFR (Tyr845) | Rabbit pAb 44-784G | Thermo Fisher Scientific (Waltham, MA) | 1:50 |
| p-Akt (Ser473) | Rabbit pAb 9271 | Cell Signaling Technology (Danvers, MA) | 1:50 |
| p-p38 (Thr180/Tyr182) | Rabbit pAb 05-1059 | EMD Millipore | Undiluted |
mAb, monoclonal antibody; pAb, polyclonal antibody. Undiluted antibodies refer to straight hybridoma supernatants.
2.4. Statistical Analysis
Wound healing times in transduced corneas vs. controls were statistically compared with two-tailed Student t test using Prism5 program (GraphPad Software, San Diego, CA), with p<0.05 considered significant. Data are presented as the mean ± standard error of mean (SEM).
3. RESULTS
3.1. Epithelial wound healing acceleration by gene therapy
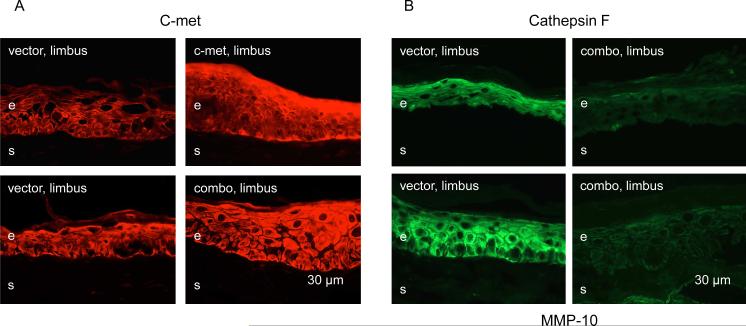
Limbal transduction of organ-cultured diabetic corneas with Ad-c-met caused increased immunostaining for the transgene in the limbal epithelial cells in comparison with Ad-EGFP (Fig. 2A). Such an increase was also noted when corneas were transduced with a mixture of three Ad harboring c-met gene and shRNA for MMP-10 and cathepsin F genes (Fig. 2B), suggesting that the additional viruses did not negatively impact the expression of respective transgenes. In the latter case (combo treatment), immunostaining for MMP-10 and cathepsin F in the limbal epithelium was reproducibly and markedly reduced. These results were in complete accordance with previous results obtained using quantitative RT-PCR after transduction of whole corneas (Saghizadeh et al. 2010b, 2013).
Figure 2.
Verification of viral transduction by immunostaining. A, limbal c-met expression is increased upon limbal transduction with Ad-c-met or combined Ads with MMP-10 and cathepsin F shRNA and full-length c-met (combo). B, both MMP-10 and cathepsin F expression in the limbus is markedly decreased upon combo treatment. Here and below, representative pictures were taken with the same exposure times for each row and each marker. Here and below, the transduced components are indicated in white on the panels, and the markers revealed by immunostaining of corneal sections are indicated in black above and below the respective panels. e, epithelium, s, stroma. Bar = 30 μm.
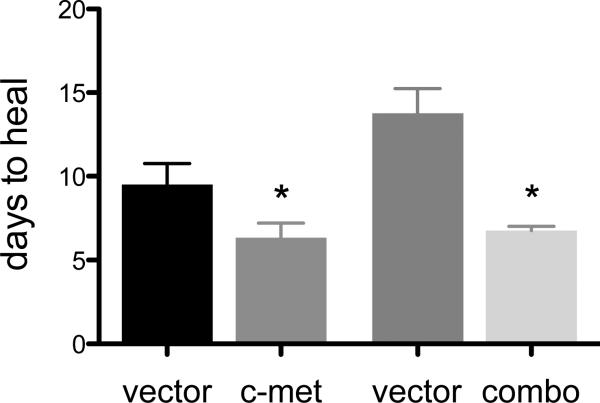
When healing of large epithelial wounds was examined, it was noted that at least in some corneas, centripetally oriented streaks of EGFP-positive cells were seen extending to the central cornea (Fig. 1B), in accordance with published evidence (Amitai-Lange et al. 2014; Di Girolamo et al. 2014). Statistical analysis of epithelial wound healing showed significant decrease in healing times both for c-met and combo treatments vs. vector controls (Fig. 3). Limbal transduction of c-met gene reduced healing time by 33% (6.3 days vs. 9.5 days, p<0.02), whereas combo treatment caused a reduction of 51% (6.75 days vs. 13.5 days, p<0.03), which was very similar to the 55% decrease obtained using transduction of the whole corneal epithelium (Saghizadeh et al. 2013). Therefore, limbal gene therapy was able to normalize healing of large epithelial wounds in diabetic corneas.
Figure 3.
Epithelial wound healing after various treatments. C-met overexpression significantly reduced healing time (by 33%). Combo treatment resulted in complete normalization of epithelial wound healing time (51% healing time reduction). *p<0.05.
3.2. Normalization of diabetic and stem cell markers by limbal gene therapy
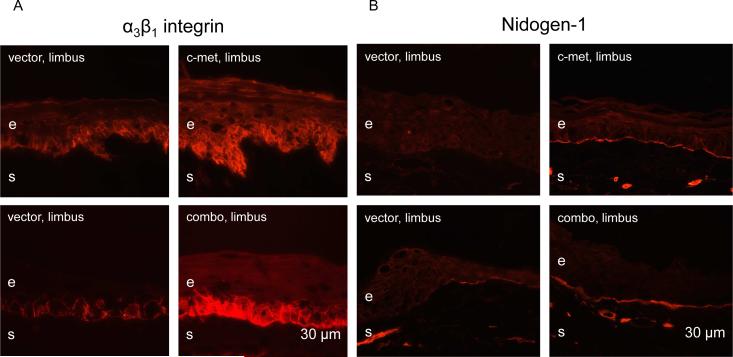
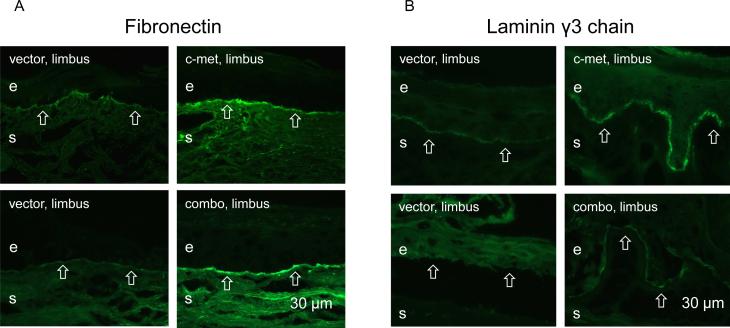
The effects of limbal gene therapy were next examined on the patterns of diabetic markers, α3β1 integrin and nidogen-1/entactin, that were previously shown to have a markedly decreased expression in diabetic corneas (Ljubimov et al. 1998; Kabosova et al. 2003). α3β1 integrin staining was consistently irregular in the limbal epithelium of vector-transduced corneas, whereas the staining became strong and more uniform upon both treatments (Fig. 4A). Nidogen-1 staining was weak and discontinuous in limbal basement membrane of vector-transduced corneas. Upon both treatments, strong and nearly continuous staining was consistently observed across cases (Fig. 4B). Staining for fibronectin and laminin γ3 chain was discontinuous or absent over large areas in the limbal basement membrane of vector-transduced corneas. It became nearly continuous upon c-met and combo treatments (Fig. 5A, B).
Figure 4.
Normalization of patterns of diabetic markers upon limbal gene therapy. A, epithelial α3β1 integrin immunostaining becomes markedly stronger and more regular in the limbus following c-met overexpression and combo treatment. B, staining for nidogen-1 in the limbal basement membrane after vector transduction is weak and discontinuous over large areas (arrows). It becomes bright and nearly continuous following both treatments. e, epithelium, s, stroma. Bar = 30 μm.
Figure 5.
Normalization of basement membrane protein patterns after gene therapy. Staining of the limbal basement membrane (open arrows) for fibronectin (A) and laminin γ3 chain becomes strong and nearly continuous following both treatments. e, epithelium, s, stroma. Bar = 30 μm.
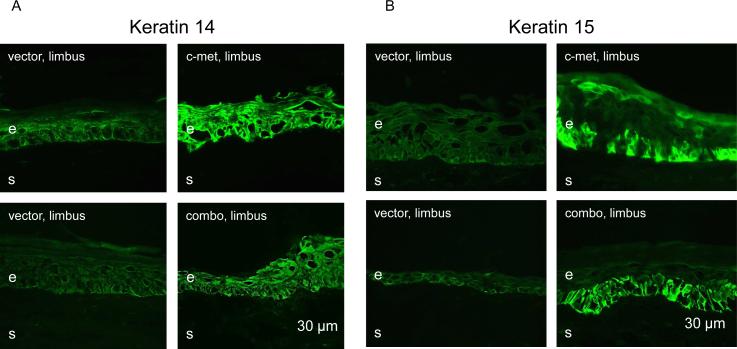
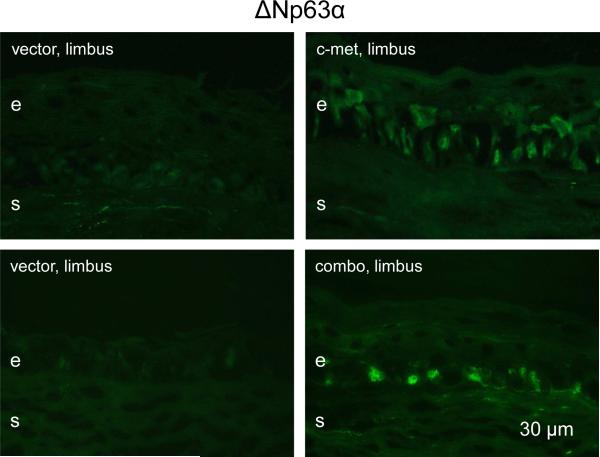
In our previous study we observed marked reduction of immunostaining for several putative LESC markers in diabetic ex vivo and organ-cultured corneas (Saghizadeh et al. 2011). C-met and combo transduction of whole corneal epithelium was accompanied by restoration of normal patterns of these markers (Saghizadeh et al. 2011, 2013). Therefore, it was further examined whether limbal gene therapy would also normalize the expression patterns of such markers. As expected, both c-met and combo gene therapies were able to markedly increase limbal immunostaining for keratin 14 (Fig. 6A) and keratin 15 (Fig. 6B). Additionally, ΔNp63, a putative limbal stem cell marker enriched in limbal epithelial holoclones (Pellegrini et al. 2001, 2014; Rama et al. 2010; Joe and Yeung, 2014) was also increased both in staining intensity and the number of positive/bright cells upon both treatments (Fig. 7). Because of the much higher abundance of the α isoform of ΔNp63 in the cornea (Robertson et al. 2008), it may be assumed that this result pertains mostly to the ΔNp63α. Thus, the treatments apparently provided functional normalization of limbal stem cell compartment. Of the tested markers, only keratin 17 did not show appreciable changes in staining intensity upon treatments (data not shown here).
Figure 6.
Increased expression of putative limbal stem cell markers upon limbal gene therapy. Both treatments dramatically increase limbal staining for keratin 14 (A) and keratin 15 (B). e, epithelium, s, stroma. Bar = 30 μm.
Figure 7.
Increased expression of putative stem cell marker ΔNp63α following limbal gene therapy. The number of positive cells in the limbus and staining intensity are markedly increased upon both treatments. e, epithelium, s, stroma. Bar = 30 μm.
3.3. Increased activation of signaling intermediates in limbal cells by gene therapy
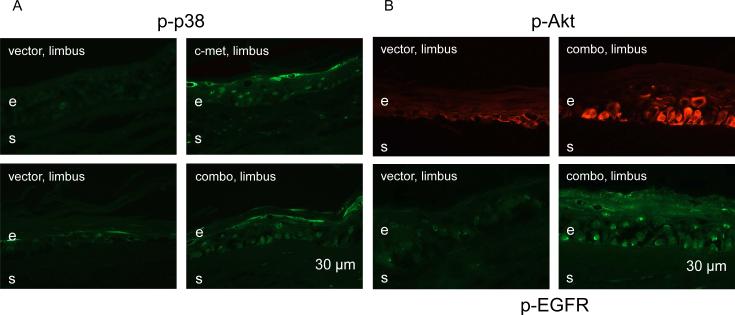
In previous experiments it was shown that c-met normalization of epithelial wound healing in diabetic corneas was apparently due to activation of p38 mitogen-activated protein kinase (MAPK) (Saghizadeh et al. 2010b). At the same time, suppression of proteinase expression in the whole corneal epithelium was accompanied by the activation of wound healing-promoting EGFR-Akt axis (Saghizadeh et al. 2010a, 2013). Therefore, we verified whether limbal gene therapy also caused activation of respective signaling pathways. C-met transduction of the diabetic limbus was associated with limbal upregulation of p-p38, and the same effect was observed after combo treatment (Fig. 8A). Additionally, in accordance with previous results, limbal p-EGFR and p-Akt were also upregulated following combo treatment, as evidenced by consistently increase in staining intensity and in the numbers of positive cells (Fig. 8B). Therefore, both pathways important for corneal wound healing, through EGFR-Akt (Xu et al. 2009; Xu and Yu, 2011; Saghizadeh et al. 2010a, 2013) and p38 (Sharma et al. 2003; Saika et al. 2004; Saghizadeh et al. 2010b) apparently mediated the observed effects of limbal gene therapy.
Figure 8.
Increased limbal expression of signaling intermediates after gene therapy. A, limbal p-p38 staining (intensity and number of positive cells) is markedly enhanced upon both treatments. B, limbal staining for p-EGFR and p-Akt is dramatically increased following combo treatment. e, epithelium, s, stroma. Bar = 30 μm.
4. DISCUSSION
Compared to many types of therapies, gene therapy is unique because it affects primarily one disease-associated target with high specificity and can result either in a gain or a loss of function of the targeted gene. This property largely determined huge interest in this approach and led to the development of various genetic drugs, delivery vehicles and clinical trials. The cornea is a very attractive tissue for gene therapy because of superficial location that facilitates gene delivery by various means and follow-up of the effects and possible adverse reactions. Major advances in corneal gene therapy have been recently reviewed in detail (Mohan et al. 2013). Despite successful use in different experimental models, clinical development of corneal gene therapy has been hampered by insufficient information about genetic causes of most common diseases and targets for intervention.
Our previous work has identified several markers reproducibly altered in human corneas from long-term diabetic patients (Saghizadeh et al. 2001, 2005, 2011) and, hence, amenable to gene therapy. Because these markers were related to epithelial basement membrane structure, as well as growth factors, proteinases and stem cell-associated proteins, it was suggested that their alterations could contribute to the poor cell migration and slow wound healing clinically observed in diabetic patients. Therefore, wound healing and marker expression were chosen as functional tests to monitor gene therapy efficacy applied to diabetic organ-cultured corneas. Organ-cultured diabetic corneas reproduce changes in wound healing seen in diabetic patients and marker expression alterations typical for ex vivo diabetic corneas (Kabosova et al. 2003; Saghizadeh et al. 2011). The feasibility of using corneal organ cultures was corroborated by the existence of metabolic memory, most probably related to stable epigenetic changes elicited by long-term hyperglycemic insult (Kowluru et al. 2013). For this reason, there was no need to keep organ-cultured corneas in high glucose. Using this model system and adenoviral gene delivery we showed that overexpression of cmet proto-oncogene and/or downregulation of specific proteinase genes (MMP-10 and cathepsin F) in the whole corneal epithelium normalized epithelial wound healing and the expression of several markers in diabetic corneas (Saghizadeh et al. 2010b, 2013). It was also shown that many putative LESC markers had significantly reduced immunostaining in diabetic corneas, which could be corrected by the same gene therapy (Saghizadeh et al. 2011).
It is well established that LESC concentrated at the corneal periphery are important for epithelial maintenance, renewal and wound healing (Notara et al. 2010; Mort et al. 2012; Joe and Yeong, 2014; Ksander et al. 2014). As they become altered with diabetes, judged by significantly reduced expression of a number of their putative markers (Saghizadeh et al. 2011), this dysfunction could cause epithelial abnormalities typical for diabetic keratopathy. Therefore, targeting gene therapy to limbal cells could alleviate critical problems in diabetic corneal epithelium. This hypothesis was tested here using limbal gene therapy with subsequent healing of large corneal wounds, in which there is known participation of limbal stem cell compartment (Amitai-Lange et al. 2014).
As expected, limbal gene therapy produced similar effects on the diabetic corneas as gene therapy of whole corneal epithelium (Saghizadeh et al., 2010b, 2013). Wound healing times were reduced in half and the expression patterns of several diabetic (α3β1 integrin, nidogen-1, fibronectin, and laminin γ3 chain) and LESC markers (keratins 14 and 15, and ΔNp63α) became similar to normal. Combination therapy with overexpression of c-met gene and shRNA-mediated suppression of MMP-10 and cathepsin F genes produced the strongest effect. Therefore, targeted gene therapy of corneal stem cell compartment is sufficient to reverse and normalize important alterations of the diabetic cornea.
The effects of specific gene therapy involved activation of major signaling pathways in the limbal cells that could constitute a molecular mechanism of wound healing and marker normalization. Key signaling intermediates previously associated with corneal wound healing (Sharma et al. 2003; Saika et al. 2004; Xu et al. 2009; Saghizadeh et al. 2010a, 2010b, 2013; Xu and Yu, 2011; 2013), including phosphorylated/activated EGFR, Akt, and p38 MAPK, had higher expression in treated corneas. It should be noted that EGFR-Akt axis is important for expansion of undifferentiated LESC (He et al. 2006). Additionally, p38 can influence LESC marker expression including ΔNp63α, and their growth factor-induced self-renewal (Cheng et al. 2009; Ho et al. 2013). In case of combined gene therapy these two pathways (EGFR-Akt and ERK-p38) could act in concert because of existing cross-talk between HGF/c-met system and EGFR (Spix et al. 2007; Xu and Yu, 2007). The data suggest that activation of these pathways following gene therapy could have dual effect on LESC maintenance/proliferation and LESC-driven epithelial wound healing.
The presented data attest to the feasibility of limbal gene therapy for alleviating important abnormalities of diabetic keratopathy including slow wound healing and LESC dysfunction. For potential future clinical use, gene therapy of the limbus only could be easier performed and would require smaller amounts of virus than the therapy of the whole cornea. In severe diabetic keratopathy with persistent epithelial defects and recurrent erosions or abnormal healing after vitrectomy, there may be a need for replacing ailing limbal epithelium. In these cases, expansion of these cells in culture and normalization with gene therapy could pave the way for future transplantation purposes to diabetic patients with advanced diabetic keratopathy. Additionally, our approach could be expanded using silencing of other promising diabetes-altered corneal targets, such as microRNAs (Funari et al. 2013) and opioid growth factor receptor (McLaughlin et al. 2012).
CONCLUSIONS
Specific adenoviral gene therapy of limbal compartment of organ-cultured diabetic corneas was sufficient to normalize defective epithelial wound healing.
Combined therapy with overexpression of c-met gene (downregulated in diabetic cornea) and shRNA silencing of MMP-10 and cathepsin F genes (upregulated in diabetic cornea) was more efficient than c-met therapy in restoring normal limbal expression patterns of diabetic and putative limbal epithelial stem cell markers.
The effects of gene therapy are apparently mediated by activation of EGFR-Akt and p38 signaling pathways.
Our data provide an experimental proof of principle on the importance and feasibility of limbal stem cell gene therapy for normalization of diabetic corneas.
HIGHLIGHTS.
– adenovirus-driven gene therapy of limbal cells in human organ-cultured diabetic corneas is sufficient to restore normal epithelial wound healing time
– overexpression of c-met gene and silencing of MMP-10 and cathepsin F genes led to normalization of the expression of diabetic and putative stem cell markers in treated corneas
– effects of gene therapy appear to be mediated by wound-healing related signaling pathways involving EGFR-Akt and p38 axes
ACKNOWLEDGMENTS
The study was supported by NIH R01 EY13431 (AVL), CTSI grant UL 1RR033176 (AVL), Cedars-Sinai Regenerative Medicine Institute (MS), and Department of Surgery (AVL); RPB Challenge Grant to Department of Ophthalmology and NIH R01 EY12676 (WJB). Results have been presented in part at the Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO), Orlando, FL, May 2014.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: M. Saghizadeh, None; C.M. Dib, None; W.J. Brunken, None; A.V. Ljubimov, None.
REFERENCES
- Amitai-Lange A, Altshuler A, Bubley J, Dbayat N, Tiosano B, Shalom-Feuerstein R. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells. 2014 doi: 10.1002/stem.1840. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N. Engl. J. Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- Bikbova G, Oshitari T, Tawada A, Yamamoto S. Corneal changes in diabetes mellitus. Curr. Diabetes Rev. 2012;8:294–302. doi: 10.2174/157339912800840479. [DOI] [PubMed] [Google Scholar]
- Blum M, Kloos C, Müller N, Mandecka A, Berner R, Bertram B, Müller UA. [Prevalence of diabetic retinopathy. Check-up program of a public health insurance company in Germany 2002-2004]. [in German] Ophthalmologe. 2007;104:499–500. 502–504. doi: 10.1007/s00347-007-1522-0. [DOI] [PubMed] [Google Scholar]
- Calvo-Maroto AM, Perez-Cambrodí RJ, Albarán-Diego C, Pons A, Cerviño A. Optical quality of the diabetic eye: a review. Eye (Lond) 2014 doi: 10.1038/eye.2014.176. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WL, Lin CT, Ko PS, Yeh PT, Kuan YH, Hu FR, Yang CM. In vivo confocal microscopic findings of corneal wound healing after corneal epithelial debridement in diabetic vitrectomy. Ophthalmology. 2009;116:1038–1047. doi: 10.1016/j.ophtha.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Cheng CC, Wang DY, Kao MH, Chen JK. The growth-promoting effect of KGF on limbal epithelial cells is mediated by upregulation of ΔNp63α through the p38 pathway. J. Cell Sci. 2009;122:4473–4480. doi: 10.1242/jcs.054791. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, Bobba S, Raviraj V, Delic NC, Slapetova I, Nicovich PR, Halliday GM, Wakefield D, Whan R, Lyons GJ. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells. 2014 doi: 10.1002/stem.1769. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Didenko TN, Smoliakova GP, Sorokin EL, Egorov VV. [Clinical and pathogenetic features of neurotrophic corneal disorders in diabetes]. [in Russian] Vestn. Oftalmol. 1999;115:7–11. [PubMed] [Google Scholar]
- Funari VA, Winkler M, Brown J, Dimitrijevich SD, Ljubimov AV, Saghizadeh M. Differentially expressed wound healing-related microRNAs in the human diabetic cornea. PLoS One. 2013;8:e84425. doi: 10.1371/journal.pone.0084425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Cho HT, Li W, Kawakita T, Jong L, Tseng SC. Signaling-transduction pathways required for ex vivo expansion of human limbal explants on intact amniotic membrane. Invest. Ophthalmol. Vis. Sci. 2006;47:151–157. doi: 10.1167/iovs.05-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig H. Surgery for diabetic retinopathy. Ophthalmologica. 2007;221:103–111. doi: 10.1159/000098255. [DOI] [PubMed] [Google Scholar]
- Herse PR. A review of manifestations of diabetes mellitus in the anterior eye and cornea. Am. J. Optom. Physiol. Opt. 1988;65:224–230. doi: 10.1097/00006324-198803000-00013. [DOI] [PubMed] [Google Scholar]
- Ho TC, Chen SL, Wu JY, Ho MY, Chen LJ, Hsieh JW, Cheng HC, Tsao YP. PEDF promotes self-renewal of limbal stem cell and accelerates corneal epithelial wound healing. Stem Cells. 2013;31:1775–1784. doi: 10.1002/stem.1393. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yeung SN. Concise review: identifying limbal stem cells: classical concepts and new challenges. Stem Cells Transl. Med. 2014;3:318–322. doi: 10.5966/sctm.2013-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabosova A, Kramerov AA, Aoki AM, Murphy G, Zieske JD, Ljubimov AV. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp. Eye Res. 2003;77:211–217. doi: 10.1016/s0014-4835(03)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, McGuire SP, Gregory MS, Vincent WJ, Perez VL, Cruz-Guilloty F, Kao WW, Call MK, Tucker BA, Zhan Q, Murphy GF, Lathrop KL, Alt C, Mortensen LJ, Lin CP, Zieske JD, Frank MH, Frank NY. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511:353–357. doi: 10.1038/nature13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer MS, Sun TT, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J. Cell Sci. 1998;111:2867–2875. doi: 10.1242/jcs.111.19.2867. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Huang ZS, Huang GH, Burgeson RE, Gullberg D, Miner JH, Ninomiya Y, Sado Y, Kenney MC. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J. Histochem. Cytochem. 1998;46:1033–1041. doi: 10.1177/002215549804600907. [DOI] [PubMed] [Google Scholar]
- Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp. Biol. Med. 2001;226:653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Zagon IS. The opioid growth factor-opioid growth factor receptor axis: homeostatic regulator of cell proliferation and its implications for health and disease. Biochem. Pharmacol. 2012;84:746–755. doi: 10.1016/j.bcp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Rodier JT, Sharma A. Corneal gene therapy: basic science and translational perspective. Ocul. Surf. 2013;11:150–164. doi: 10.1016/j.jtos.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort RL, Douvaras P, Morley SD, Dorà N, Hill RE, Collinson JM, West JD. Stem cells and corneal epithelial maintenance: insights from the mouse and other animal models. Results Probl. Cell Differ. 2012;55:357–394. doi: 10.1007/978-3-642-30406-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtha T, Cavallerano J. The management of diabetic eye disease in the setting of cataract surgery. Curr. Opin. Ophthalmol. 2007;18:13–18. doi: 10.1097/ICU.0b013e32801129fc. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kanamori A, Negi A. Diabetes mellitus as a risk factor for glaucomatous optic neuropathy. Ophthalmologica. 2005;219:1–10. doi: 10.1159/000081775. [DOI] [PubMed] [Google Scholar]
- Notara M, Alatza A, Gilfillan J, Harris AR, Levis HJ, Schrader S, Vernon A, Daniels JT. In sickness and in health: Corneal epithelial stem cell biology, pathology and therapy. Exp. Eye Res. 2010;90:188–195. doi: 10.1016/j.exer.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Rama P, Di Rocco A, Panaras A, De Luca M. Concise review: Hurdles in a successful example of limbal stem cell-based regenerative medicine. Stem Cells. 2014;32:26–34. doi: 10.1002/stem.1517. [DOI] [PubMed] [Google Scholar]
- Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- Robertson DM, Ho SI, Cavanagh HD. Characterization of ΔNp63 isoforms in normal cornea and telomerase-immortalized human corneal epithelial cells. Exp. Eye Res. 2008;86:576–585. doi: 10.1016/j.exer.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Brown DJ, Castellon R, Chwa M, Huang GH, Ljubimova JY, Rosenberg S, Spirin KS, Stolitenko RB, Adachi W, Kinoshita S, Murphy G, Windsor LJ, Kenney MC, Ljubimov AV. Overexpression of matrix metalloproteinase-10 and matrix metalloproteinase-3 in human diabetic corneas: a possible mechanism of basement membrane and integrin alterations. Am. J Pathol. 2001;158:723–734. doi: 10.1016/S0002-9440(10)64015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Epifantseva I, Hemmati DM, Ghiam CA, Brunken WJ, Ljubimov AV. Enhanced wound healing, kinase and stem cell marker expression in diabetic organ-cultured human corneas upon MMP-10 and cathepsin F gene silencing. Invest. Ophthalmol. Vis. Sci. 2013;54:8172–8180. doi: 10.1167/iovs.13-13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Tajbakhsh J, Aoki AM, Wang C, Chai NN, Ljubimova JY, Sasaki T, Sosne. G, Carlson MR, Nelson SF, Ljubimov AV. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Invest. Ophthalmol. Vis. Sci. 2005;46:3604–3615. doi: 10.1167/iovs.04-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Yaghoobzadeh Y, Hu J, Ljubimova JY, Black KL, Castro MG, Ljubimov AV. Adenovirus-driven overexpression of proteinases in organ-cultured normal human corneas leads to diabetic-like changes. Brain Res. Bull. 2010a;81:262–272. doi: 10.1016/j.brainresbull.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Yu FS, Castro MG, Ljubimov AV. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Invest. Ophthalmol. Vis. Sci. 2010b;51:1970–1980. doi: 10.1167/iovs.09-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Soleymani S, Harounian A, Bhakta B, Troyanovsky SM, Brunken WJ, Pellegrini G, Ljubimov AV. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Mol. Vis. 2011;17:2177–2190. [PMC free article] [PubMed] [Google Scholar]
- Saika S, Okada Y, Miyamoto T, Yamanaka O, Ohnishi Y, Ooshima A, Liu CY, Weng D, Kao WW. Role of p38 MAP kinase in regulation of cell migration and proliferation in healing corneal epithelium. Invest. Ophthalmol. Vis. Sci. 2004;45:100–109. doi: 10.1167/iovs.03-0700. [DOI] [PubMed] [Google Scholar]
- Sareen D, Saghizadeh M, Ornelas L, Winkler MA, Narwani K, Sahabian A, Funari VA, Tang J, Spurka L, Punj V, Maguen E, Rabinowitz YS, Svendsen CN, Ljubimov AV. Differentiation of human limbal-derived induced pluripotent stem cells into limbal-like epithelium. Stem Cells Transl. Med. 2014;3:1002–1012. doi: 10.5966/sctm.2014-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Trans. Am. Ophthalmol Soc. 1981;79:180–199. [PMC free article] [PubMed] [Google Scholar]
- Sharma GD, He J, Bazan HE. P38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J. Biol Chem. 2003;278:21989–21997. doi: 10.1074/jbc.M302650200. [DOI] [PubMed] [Google Scholar]
- Spix JK, Chay EY, Block ER, Klarlund JK. Hepatocyte growth factor induces epithelial cell motility through transactivation of the epidermal growth factor receptor. Exp. Cell Res. 2007;313:3319–3325. doi: 10.1016/j.yexcr.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarova TP, Ljubimov AV, Deng L, Plow EF. Proteolysis regulates exposure of the IIICS-1 adhesive sequence in plasma fibronectin. Biochemistry. 1996;35:10913–10921. doi: 10.1021/bi960717s. [DOI] [PubMed] [Google Scholar]
- Wylegała E, Moćko L, Woyna-Orlewicz A, Teper S, Orzhechowska-Wylegała B. [Diabetic complications within ocular surface] [in Polish]. Pol. Merkur Lekarski. 2006;21:495–497. [PubMed] [Google Scholar]
- Xu KP, Yu FS. Cross talk between c-Met and epidermal growth factor receptor during retinal pigment epithelial wound healing. Invest. Ophthalmol. Vis. Sci. 2007;48:2242–2248. doi: 10.1167/iovs.06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Yu FS. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Invest. Ophthalmol. Vis. Sci. 2011;52:3301–3308. doi: 10.1167/iovs.10-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KP, Li Y, Ljubimov AV, Yu FS. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes. 2009;58:1077–1085. doi: 10.2337/db08-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]