Abstract
Natural products are characterized by high chemical diversity and biochemical specificity; therefore, they are appealing as lead compounds for drug discovery. Given the importance of angiogenesis to many pathologies, numerous natural products have been explored as potential anti-angiogenic drugs. Ocular angiogenesis underlies blinding eye diseases such as retinopathy of prematurity (ROP) in children, proliferative diabetic retinopathy (DR) in adults of working age, and age-related macular degeneration (AMD) in the elderly. Despite the presence of effective therapy in many cases, these diseases are still a significant health burden. Anti-VEGF biologics are the standard of care, but may cause ocular or systemic side effects after intraocular administration and patients may be refractory. Many anti-angiogenic compounds inhibit tumor growth and metastasis alone or in combination therapy, but a more select subset of them has been tested in the context of ocular neovascular diseases. Here, we review the promise of natural products as anti-angiogenic agents, with a specific focus on retinal and choroidal neovascularization. The multifunctional curcumin and the chalcone isoliquiritigenin have demonstrated promising anti-angiogenic effects in mouse models of DR and choroidal neovascularization (CNV) respectively. The homoisoflavanone cremastranone and the flavonoid deguelin have been shown to inhibit ocular neovascularization in more than one disease model. The isoflavone genistein and the flavone apigenin on the other hand are showing potential in the prevention of retinal and choroidal angiogenesis with long-term administration. Many other products with antiangiogenic potential in vitro such as the lactone withaferin A, the flavonol quercetin, and the stilbenoid combretastatin A4 are awaiting investigation in different ocular disease relevant animal models. These natural products may serve as lead compounds for the design of more specific, efficacious, and affordable drugs with minimal side effects.
Keywords: angiogenesis, natural compounds, blinding diseases, small molecules, choroidal neovascularization, retinal neovascularization, polyphenols
1. Introduction
The medicinal use of natural compounds derived from plants, animals and microorganisms was introduced in ancient medicine thousands of years ago (Ji et al., 2009). Natural products served as the sole pharmacological source for the treatment of diseases for most of human history, where some herbs were chewed to relieve pain, and others were applied topically on wounds to improve healing. Screening natural products to provide novel human therapeutics was maximized by the Western pharmaceutical industry resulting in a broad spectrum of pharmaceuticals from non-synthetic molecules (Newman et al., 2003). In the 19th century, the development of analytical and structural chemistry provided the tools to purify different compounds from natural sources and to determine their structures, which in turn, provided information about their possible molecular targets in the human body (Ji et al., 2009). In the 20th century, most approved drugs were natural products or analogues derived from them. Natural source-derived antibiotics such as the penicillins, immunosuppressants for organ transplants such as cyclosporine, and anticancer drugs such as taxols revolutionized medicine and improved quality of life (Li and Vederas, 2009). The functions and structures of natural products and their molecular targets are believed to have evolved to interact with one another (Schmidt et al., 2007), suggesting that natural products might serve as optimal small molecule ligands for some human targets.
Despite these advantages, a decline in natural products-based drug discovery has been experienced in the past decades with the advent of molecular biology and rational, structure-based design that made it possible to design synthetic chemicals to target specific proteins. However, the past few years have witnessed a resurgence of interest in the use of natural compounds as a basis for drug development, with several promising compounds having undergone clinical evaluation for the treatment of varied conditions such as neoplastic, immunological, and inflammatory diseases (Mishra and Tiwari, 2011).
Interest in natural products will continue for many reasons: they are a matchless source of novel drug leads and inspiration for the synthesis of synthetic or semi-synthetic molecules (Mishra and Tiwari, 2011), they can work synergistically to potentiate the activity of other drugs and overcome drug resistance (Schmidt et al., 2007), and they can offer powerful leads with favorable absorption, distribution, metabolism, excretion, and toxicity (ADMET) characteristics (Corson and Crews, 2007). Advances in separation and structure determination technologies, along with the ability to modulate biological activity through structural modifications, have made a wide variety of natural products and derivatives readily available (Koehn and Carter, 2005).
Numerous natural compounds have been tested as inhibitors of uncontrolled angiogenesis in various pathological conditions, such as cancer and inflammation, and also in blinding eye diseases. The concept of angiogenesis as an important therapeutic target was initially introduced due to its involvement in tumor growth and metastasis (Folkman, 1995). Pathological ocular angiogenesis (neovascularization) however, underlies several non-neoplastic ocular disorders that can cause blindness. Ocular neovascularization has a significant contribution to ROP, DR, AMD, neovascular glaucoma, retinal vein occlusion, as well as other ocular diseases (Zhang and Ma, 2007). In this review, we will discuss promising natural compounds that demonstrate inhibition of ocular angiogenesis. We will explore their potential in the prevention/treatment of ocular neovascular diseases either alone or in combination with the standard therapies.
2. Angiogenesis in ocular health and disease
Angiogenesis is a highly regulated process that involves the formation of new blood vessels from existing ones, which is kept under the control of positive (angiogenic) and negative (anti-angiogenic) endogenous factors (Carmeliet and Jain, 2011). This process is clearly different from vasculogenesis, the de novo synthesis of blood vessels from endothelial progenitor cells. Angiogenesis not only requires endothelial cell migration and proliferation, but also vessel maturation, vessel remodeling, and degradation of extracellular matrix. Angiogenesis is the major mechanism of vascular growth during embryonic development and wound healing. Under normal conditions, endothelial cells are quiescent without significant proliferation, due to a balance in the expression level and function of angiogenic factors such as vascular endothelial growth factor (VEGF) and angiostatic factors such as pigment epithelium derived factor (PEDF) (Folkman and Ingber, 1992).
During rapid uncontrolled ocular angiogenesis, fragile and leaky vasculature is formed. This leads to hemorrhage and accumulation of fluids and protein exudates in ocular cavities, causing reduction in the transparency of the cornea and impairment of the structure and function of retinal neurons resulting in vision loss. These vessels may induce the formation of fibrous scarring, causing irreversible damage to retinal function that can eventually result in blindness if left untreated (Zhang and Ma, 2007).
3. Existing anti-angiogenic drug therapies
Therapeutic approaches currently available for ocular neovascular diseases aim to seal off the leaky vasculature using laser photocoagulation and/or photodynamic therapy as well as inhibit new vessel formation (Dorrell et al., 2007). The major angiogenic factor in neovascularization is VEGF. Therefore, several anti-VEGF drugs have been recently used such as pegaptanib (Macugen®, Valeant), bevacizumab (Avastin®, Genentech), ranibizumab (Lucentis®, Genentech) and aflibercept (Eylea®, Regeneron). Pegaptanib is an aptamer engineered to bind specifically to VEGF165, the isoform primarily responsible for pathological ocular angiogenesis (Ng et al., 2006). Bevacizumab is a humanized monoclonal antibody to VEGF inhibiting VEGF-receptor interaction (Gunther and Altaweel, 2009). Ranibizumab is a recombinant humanized fraction of anti-VEGF antibody that binds to all VEGF isoforms (Rosenfeld et al., 2006). Aflibercept, known as VEGF Trap, is a fusion protein that consists of VEGF receptor-binding sequences fused to a segment of a human antibody backbone (Stewart, 2012a).
These drugs all act by targeting the VEGF signaling pathway at the level of ligand-receptor interaction. They have been shown to be successful in many AMD patients. Meanwhile, they are still under investigation for their potential therapeutic effect on ROP and DR (Andreoli and Miller, 2007). However, a significant number of AMD patients remain unresponsive (Lux et al., 2007). As biologics, these drugs have an unfavorable cost to benefit ratio (Mitchell et al., 2011). Moreover, since VEGF signaling is also required for the survival of quiescent endothelial cells and glial cells that nourish endothelial cells in almost all the tissues of the body, these drugs can cause significant systemic side effects such as myocardial infarction, stroke, delayed wound healing and non-ocular hemorrhage even when the drugs are administered intravitreously (Stewart, 2012b). Additionally, several ocular side effects can be associated with intravitreous injections of anti-VEGF drugs, such as intraocular inflammation, ocular hemorrhage, and retinal detachment (Falavarjani and Nguyen, 2013). Therefore, there is a strong need to develop new, affordable drugs specifically targeted for ocular angiogenesis with minimal side effects to complement and perhaps combine with existing therapies.
4. Anti-angiogenic natural products and ocular neovascular diseases
A select subset of natural compounds, spanning a variety of compound classes, have been tested for their effects in ocular neovascular diseases specifically, and some have very promising activity (Table 1). Polyphenols are the most abundant secondary metabolites, constituting the active substances in many medicinal plants. They have long been recognized for their antioxidant properties (Manach et al., 2004). Therefore, they have been tested for their potential therapeutic effects in many diseases such as cancer and inflammatory and cardiovascular diseases. Polyphenols are loosely defined as having several hydroxyl groups on aromatic rings. They are divided into classes such as phenolic acids, flavonoids, stilbenoids and lignans, according to the number of phenolic groups and the structures that connect these rings to one another (Manach et al., 2004). The flavonoids are the most common class of polyphenolic compounds that are found ubiquitously in plants. They share a common structure of two aromatic rings that are connected together by three carbon atoms that form an oxygenated heterocycle (Manach et al., 2004). They are divided into subclasses according to the substitutions on the heterocycle and the position and length of the linker between the cyclic moieties, and include flavonols (e.g., quercetin), flavones (e.g., luteolin and apigenin), isoflavones (e.g., genistein), flavanones (e.g., hesperetin) and homoisoflavanones (e.g., cremastranone) (Figure 1). Many flavonoids have been studied for their beneficial roles in ocular diseases (Majumdar and Srirangam, 2010).
Table 1.
Summary of the potential effects of promising natural compounds in inhibiting ocular angiogenesis in vitro and in vivo.
| Compound | Source | In vitro system and effects | In vivo models tested | |
|---|---|---|---|---|
| Luteolin (1) | Fruits and vegetables (Miean and Mohamed, 2001) | HUVECs, 5 µM, inhibited VEGF-induced VEGFR2 autophosphorylation and activation of PI3K/Akt but not ERK1/2 (Bagli et al., 2004) HRECs, 1 µM, decreased VEGF-induced migration and tube formation (Park et al., 2012) |
|
|
| Apigenin (2) | Fruits and vegetables (Horinaka et al., 2006) | HUVECs and choroidal endothelial cells (CECs), 3 & 10 µg/mL: inhibited proliferation, migration and tube formation (Zou and Chiou, 2006) | Daily i.p. injection for 4 weeks, 15 and 30 mg/kg, reduced CNV after laser photocoagulation in rats (Zou and Chiou, 2006) | |
| Genistein (3) | Soybeans (Fotsis et al., 1993) | BAECs, 20 µM, inhibited migration and proliferation due to non-specific inhibition of tyrosine kinases (Koroma and de Juan, 1994) |
|
|
| Hesperetin (4) | Citrus fruits (Kawaii et al., 1999) | HRECs, 16 µM, inhibited proliferation (Basavarajappa et al., 2013) | Long-term oral dosing, 100 mg/kg, in STZ rats:
|
|
| Cremastranone (5) | Cremastra appendiculata (Shim et al., 2004) | HUVECs, 5 µM:
|
|
|
| SH-11052 | Synthetic isomer of cremastranone | HUVECs, GI50 18 µM, inhibited proliferation (Basavarajappa et al., 2014) HRECs, GI50 43 µM,
|
||
| SH-11037 | Synthetic derivative of cremastranone | HRECs, GI50 150 nM, very potent in inhibiting proliferation with 10-fold selectivity over HUVECs (Basavarajappa et al., 2014) | ||
| Quercetin (6) | Abundant in human food – e.g. apples and onions (Formica and Regelson, 1995) |
|
|
|
| Isoliquiritigenin (7) | Licorice root (Vaya et al., 1997) | HUVECs:
|
|
|
| Deguelin (8) | Mundulea sericea (Gerhauser et al., 1997) | HUVECs, 0.1 µM, inhibited tube formation without affecting cell viability and dramatically reduced VEGF expression (Kim et al., 2008b) |
|
|
| Curcumin (9) | Indian spice turmeric (Kuttan et al., 1987) |
|
|
|
| Resveratrol (10) | Red wine and grape skin (Soleas et al., 1997) |
|
|
|
| Honokiol (11) | Magnolia species (Lee et al., 2011) |
|
|
|
| Combretastatin A4 (12) | Combretum caffrum (Young and Chaplin, 2004) | HUVECs, 80 ng/ml, inhibited proliferation, migration and tube formation, with induction of apoptosis after 48 hours (Ahmed et al., 2003) |
|
|
| Decursin (13) | Angelica gigas (Konoshima et al., 1968) |
|
|
|
| Withaferin A (14) | Withania somnifera (Mohan et al., 2004) |
|
|
|
Fig. 1.
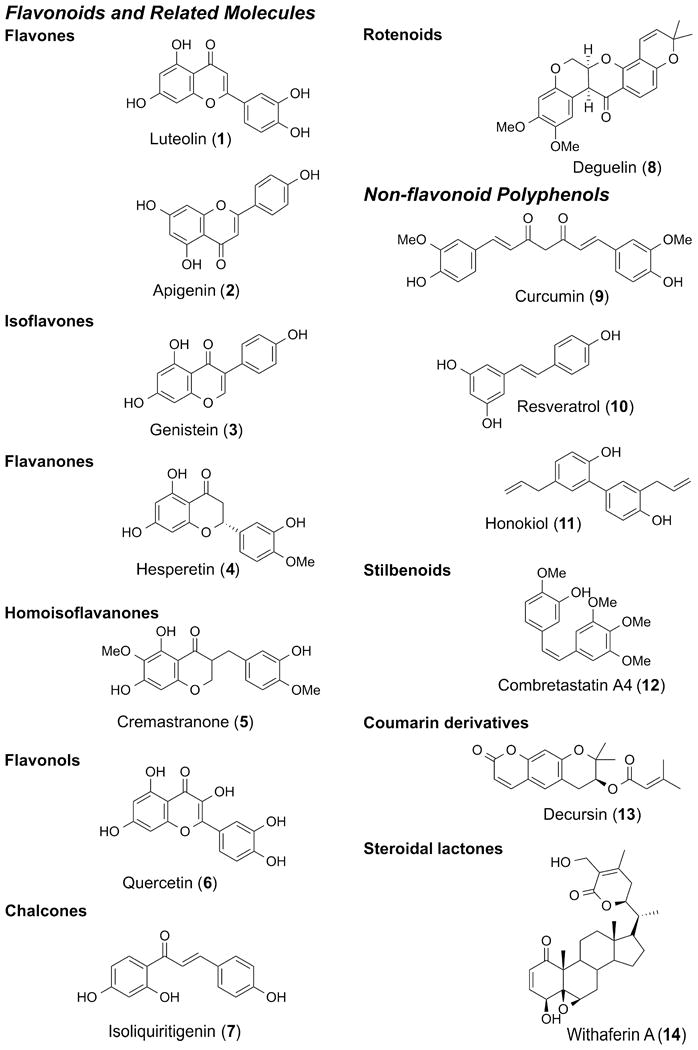
Chemical structures of anti-angiogenic natural products tested in the context of ocular neovascular diseases.
5. Natural products in ocular neovascularization models
5.1. In vitro models
In vitro models for angiogenesis utilize cultured endothelial cells to test the effects of the compounds on cell proliferation, migration and tube formation. Endothelial cell proliferation can be investigated by multiple assays, such as MTT (Denizot and Lang, 1986) or EdU incorporation assays (Buck et al., 2008). Migration of endothelial cells can be evaluated in vitro by techniques such as the scratch wound migration assay (Liang et al., 2007). Tube formation is one of the most common tests for angiogenesis, which measures the ability of endothelial cells to form three-dimensional structures (tubes) (Madri et al., 1988).
Human umbilical vein endothelial cells (HUVECs) and bovine aortic endothelial cells (BAECs) are established cell systems. These in vitro systems provide a rapid, practical and reproducible way for screening of large numbers of compounds. However, for screening of compounds specifically for ocular angiogenesis, it is important to use more relevant, tissue specific endothelial cells; human retinal microvascular endothelial cells (HRECs) are a popular choice in this case, and are commercially available. Nearly all the natural compounds discussed below were effective in angiogenesis inhibition in vitro. The majority of them were tested in HUVECs, a model endothelial cell line, but not from the same vascular bed as those endothelial cells implicated in ocular diseases. Withaferin A (14) was the most potent in inhibiting proliferation with GI50 12 nM (Mohan et al., 2004). Genistein (3) and quercetin (6) showed equal potencies in inhibiting BAEC proliferation at 20 µM concentration (Jackson and Venema, 2006; Koroma and de Juan, 1994). Luteolin (1), hesperetin (4), cremastranone (5) and synthetic derivatives, curcumin (9), and decursin (13) were tested in the most disease-relevant cells, HRECs. SH-11037, a synthetic derivative of cremastranone (5) that we developed, showed the highest potency in these cells, compared to other compounds, with GI50 150 nM, and with 10-fold selectivity over HUVECs (Basavarajappa et al., 2014).
5.2. In vivo models
5.2.1. Proliferative DR
In healthy adults, the ocular vasculature is mainly quiescent under the control of endogenous anti-angiogenic factors such as PEDF and angiostatin (Qazi et al., 2009). The abnormal growth of new blood vessels, such as retinal neovascularization, interferes with normal functions in regulating light transmission. Numerous clinical and experimental studies have identified ischemia as one of the major causes of retinal neovascularization (Ashton et al., 1954). One of the most common forms of retinal neovascularization is proliferative DR, a common cause of blindness in patients between 25 and 65 years old. Persistent hyperglycemia, high blood pressure and hypoxia in diabetic patients contribute to retinopathy and damage to retinal capillaries. This is followed by the proliferative stage, where new, aberrant blood vessels grow along the retina and into the vitreous; this is sometimes exacerbated by the formation of fibrovascular scarring and retinal detachment (Grossniklaus et al., 2010). With an increasing diabetic population in the United States, approximately 700,000 Americans have diabetic retinopathy, with an annual incidence of 65,000 new cases (Zhang et al., 2010).
Currently, there is no perfect model for proliferative DR; the most commonly used animal model is induced by intraperitoneal (i.p.) administration of streptozotocin (STZ) in mice and rats (Jo et al., 2013). The hyperglycemic action of STZ induces diabetes and the development of retinopathy later with the disease progression. Many natural compounds including genistein (3), hesperetin (4), curcumin (9), resveratrol (10), and decursin (13) demonstrated potential in ameliorating retinopathy in the STZ model after oral administration. Due to the lack of proliferative DR in this model (Lai and Lo, 2013), retinopathy was defined as increased vascular leakage that was ameliorated by genistein (3) and hesperetin (4). In the case of other compounds, retinopathy was evaluated by the extent of diabetes-induced metabolic abnormalities that are known to be important in development of DR. Effects seen were due to inhibiting retinal oxidative stress and/or increasing antioxidant defense systems as seen with hesperetin (4), and curcumin (9) (Kowluru and Kanwar, 2007; Kumar et al., 2013), or due to suppressing the production of pro-inflammatory mediators and growth factors as in the case of resveratrol (10), and decursin (13) (Soufi et al., 2012; Yang et al., 2013). Meanwhile, combretastatin A4 (12) failed to show an effect in long-term galactose fed dogs, a preclinical model for DR where neovascularization slowly develops over a period of months to years as in clinical cases (Kador et al., 2007). To our knowledge, no other negative ocular angiogenesis studies on any compound discussed here have been presented.
5.2.2. ROP
ROP is a form of retinal neovascularization that is developmental in origin (Smith, 2002). Retinal vascularization starts at approximately the 16th week of gestation and completes by full-term pregnancy (i.e. 40th week of gestation). Hence, premature babies have incomplete retinal vasculature and upon exposure to oxygen therapy (hyperoxia) during neonatal intensive care, this incomplete retinal vasculature decays. This condition is more pronounced in premature babies with birth weight less than 1250 g (Sapieha et al., 2010). During this hyperoxia, the expression of hypoxia-driven angiogenic factors is downregulated, resulting in a retardation of existing retinal blood growth, which increases the metabolic requirement of the retina on return to normoxia, stimulating abnormal blood vessel formation. ROP severity ranges from mild with no visual defects to aggressive neovascularization causing blindness and retinal detachment that is responsible for 6–18% of total childhood blindness cases (Coats, 2005). Vision loss in about 1300 children every year has been estimated in the United States alone, with many more cases worldwide, especially as survival of premature infants increases in developing countries (Javitt et al., 1993).
Oxygen-induced retinopathy (OIR) is the major model in use for study and evaluation of pathological angiogenesis resulting from ischemia such as retinal neovascularization (Smith et al., 1994). In this model, early postnatal animals are exposed to hyperoxia during early retinal development and remain in the oxygen chamber for 5 days (P7). After their return to room air, neovascularization is noticed within about 5 days (P12). The OIR model mimics the pathological characteristics of ROP, with a consistent and reproducible angiogenic response; therefore, it has become important for studying the disease mechanisms and investigating potential treatments for ROP (Smith et al., 1994). Several natural compounds demonstrated anti-angiogenic effects in the OIR model with different potencies suggesting their potential in the treatment of ROP. Luteolin (1) and deguelin (8) showed equal potencies in suppressing retinal neovascularization after intravitreal injection of 0.1 µM compound concentration on P14 (Kim et al., 2008c; Park et al., 2012). Meanwhile, genistein (3), resveratrol (10), honokiol (11), and combretastatin A4 (12) were effective after multiple systemic intraperitoneal injections (Griggs et al., 2002; Kim and Suh, 2010; Vavilala et al., 2013; Wang et al., 2005).
5.2.3. AMD
The choroid, a highly vascularized compartment of the eye responsible for delivering nutrients and oxygen to the photoreceptors, is also susceptible to neovascularization. In this case, aberrant vasculature can originate in the choroid and grow through a break in Bruch's membrane to the subretinal space, precipitating vision loss. This break may be secondary to trauma, a degenerative process, and/or tissue inflammation (Grossniklaus and Green, 2004). Choroidal neovascularization (CNV) is seen in “wet” AMD, one of the major causes of vision loss among people over 55 years old (Jager et al., 2008). Wet AMD accounts for about 90% of AMD-related blindness with about 200,000 new cases diagnosed every year in the United States (Congdon et al., 2004). CNV can lead to edema and damage of the macula, causing vision loss in the center of the retina.
Laser-induced CNV, in mouse or rat, is the most popular model that has been used to study the role of tissue factors in CNV formation, as well as the evaluation of potential pharmacologic therapies for CNV (Dobi et al., 1989). In this model, laser photocoagulation spots are introduced into the otherwise normal choroid through the dilated mouse/rat pupil. These burns create breaks in Bruch's membrane, which lead to the growth of new blood vessels from the choroid into the subretinal space, recapitulating the main pathological features in wet AMD (Tobe et al., 1998). Despite the artificial nature of the laser-induced CNV model, and the fact that mice and rats do not have a macula, it is currently a standard animal model in AMD research (Lambert et al., 2013). Several natural compounds such as apigenin (2), genistein (3), cremastranone (5), quercetin (6), isoliquiritigenin (7), deguelin (8), and combretastatin A4 (12) showed a significant reduction in neovascularization after laser photocoagulation. Deguelin (8) was the most potent in inhibiting neovascularization after intravitreal injection at 0.1 µM concentration (Kim et al., 2008b). Cremastranone (5) was of moderate potency at 1 µM concentration (Kim et al., 2008a), compared to isoliquiritigenin (7), which was effective in the 10-200 µM range (Jhanji et al., 2011). Apigenin (2) and combretastatin A4 (12) suppressed CNV formation when administered systemically (Nambu et al., 2003; Zou and Chiou, 2006). Genistein (3) showed partial inhibition of CNV formation, compared to specific inhibitors of VEGF/PEDF receptor kinases, when given systemically (Kwak et al., 2000). While systemic drug administration is favored over the intravitreal route, it is influenced by different challenges, such as the blood-retinal barrier, that will control the effectiveness of the drug reaching the eye (Macha and Mitra, 2003). On the other hand, the intravitreal route is more effective in delivering maximum concentration of the drug to the eye with minimal systemic side effects; therefore, it is the most commonly used for treatments of ocular neovascular diseases.
6. Mechanisms of angiogenesis inhibition by natural products
Identification of the mechanisms of action of anti-angiogenic compounds is a crucial step for them to proceed to clinical trials. Most of the natural compounds under investigation have been tested for their effect on known angiogenic pathways (Figure 2). However, elucidating novel pathways for angiogenesis inhibition is vital to overcome the resistance that might emerge with long-term administration of angiogenic inhibitors (Kerbel and Folkman, 2002).
Fig. 2.
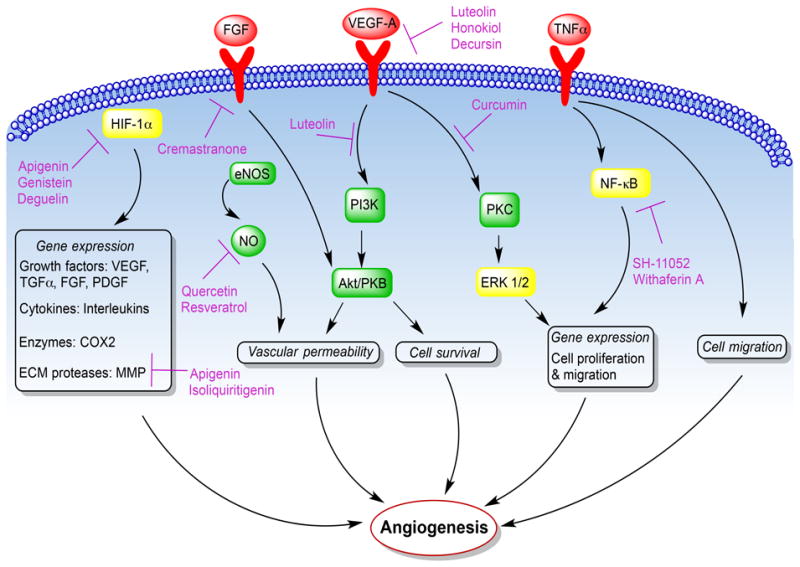
The known locations of natural products' effects on selected angiogenic pathways. Angiogenic factors act on their receptors (red) on endothelial cells to activate various downstream signaling molecules (green) and transcription factors (yellow) to mediate angiogenesis. Several natural products interfere with these angiogenic pathways as indicated in purple.
Apart from VEGF, the most dominant angiogenic factor in neovascularization, other growth factors such as fibroblast growth factor (FGF) and angiogenin have also been shown to promote angiogenesis (Qazi et al., 2009). On the other hand, there are several endogenous anti-angiogenic factors such as PEDF, thrombospondin-1 (TSO-1), and angiostatin that are required to maintain homeostasis of angiogenesis (Nyberg et al., 2005). The balance between endogenous pro- and anti-angiogenic factors tightly regulates homeostasis of ocular vasculature. When this balance is disturbed, formation of new blood vessels occurs, which is implicated in the diseases discussed above. Under certain conditions, the production of angiogenic stimuli causes endothelial cells to secrete proteases such as matrix metalloproteases (MMP) and collagenases, which leads to degradation and remodeling of the extracellular matrix (ECM). Apigenin (2) and isoliquiritigenin (7) were shown to inhibit HUVEC migration and tube formation by downregulating MMPs (Kang et al., 2010; Kim, 2003).
Proangiogenic stimuli such as VEGF and FGF bind to cell surface receptors and activate downstream signaling cascades to promote migration and proliferation of endothelial cells through the newly formed gaps generating new capillaries through which blood begins to flow. Interference with angiogenesis at the receptor level has been demonstrated by natural compounds such as luteolin (1) and honokiol (11), which inhibited VEGFR2 autophosphorylation and activation in HUVECs (Bagli et al., 2004; Bai et al., 2003), and decursin (13) in HRECs (Yang et al., 2013). Other compounds inhibited the activation of downstream signaling pathways of growth factors such as luteolin (1) which inhibited VEGF-induced PI3K/Akt activation in HUVECs (Bagli et al., 2004), and curcumin (9) which blocked VEGF-induced PKCβII translocation in HRECs (Figure 2) (Premanand et al., 2006). Cremastranone (5) inhibited FGF signaling in HUVECs (Shim et al., 2004). Growth factors also increase vascular permeability through stimulation of nitric oxide (NO) synthesis and release from endothelial cells, a step that was inhibited by quercetin (6) and resveratrol (10) (Jackson and Venema, 2006; Kim and Suh, 2010).
Tumor necrosis factor-α (TNF-κ) is a very potent angiogenic stimulator that binds to TNF receptors and activates nuclear factor-kappa B (NF-kB), a transcription factor that is central to the regulation of many genes, such as those encoding adhesion molecules and angiogenic mediators (Grilli et al., 1993). Some natural compounds interfered with NF-κB activation and expression of downstream genes in vitro either by blocking NF-κB nuclear translocation as we showed with SH-11052, a synthetic isomer of cremastranone (5) (Basavarajappa et al., 2014), or as seen with withaferin A (14) by targeting the ubiquitin proteasome pathway (UPP) which regulates NF-κB activation (Figure 2) (Palombella et al., 1994).
Endothelial cells respond to oxygen, too. Under normal conditions, the oxygen sensor prolyl hydroxylase domain protein (PHD) hydroxylates hypoxia-inducible factor 1α (HIF-1α), which is then targeted for proteasomal degradation. During hypoxia, PHD is inactivated and HIF-1α activates the transcription of multiple genes including growth factors such as VEGF and TNF-α, cytokines such as interleukins (IL-6 and IL-8), proinflammatory enzymes such as cyclooxygenase 2 (COX2), and proteases such as MMPs. This broad transcriptional response increases blood flow and oxygen supply by angiogenesis (Fraisl et al., 2009). However, HIF-1α can be activated under non-hypoxic conditions by growth factors such as VEGF, causing a vicious cycle of uncontrolled neovascularization. Apigenin (2) interfered with HIF-1α signaling in vitro, by initiating HIF-1α degradation and inhibiting the expression of downstream VEGF in HUVECs (Zou and Chiou, 2006). Meanwhile, genistein (3) inhibited hypoxia-induced expression of HIF-1α, and deguelin (8) reduced HIF-1α expression and mediated its degradation in vivo in the OIR model (Figure 2) (Kim et al., 2008c; Wang et al., 2005).
A clear characterization of the molecular targets by which the above natural products interfere with angiogenesis is still needed. However, unlike most natural products discussed here, a direct target of withaferin A (14) is known. It binds to the intermediate filament (IF) protein vimentin, which plays a critical role in angiogenesis and cancer growth, causing IF degradation and down-regulation of their expression levels. This leads to attenuation of retinal gliosis associated with several degenerative conditions in the eye including AMD (Bargagna-Mohan et al., 2007; Bargagna-Mohan et al., 2010). Further elucidation of the direct targets of other natural compounds is important to develop more specific and potent analogs with minimal side effects and to test the combination of different compounds targeting distinct angiogenic mechanisms.
7. What does the future hold?
There is considerable promise amongst the natural compounds that are currently under investigation specifically for ocular neovascular diseases (Table 1). Almost all compounds discussed here were effective in inhibiting in vitro angiogenesis. Withaferin A (14) showed high potency in inhibiting angiogenesis in HUVECs. Interestingly, SH-11037, a synthetic derivative of cremastranone (5), demonstrated the highest potency among the compounds discussed in inhibiting proliferation, migration and tube formation of HRECs, a tissue specific and disease relevant cell type. We saw this effect at nanomolar concentrations, suggesting a strong potential of SH-11037 in angiogenesis inhibition that is awaiting a clear understanding of its mechanism of action and demonstration of its therapeutic potential in animal models of ocular neovascular diseases. Intravitreally injected deguelin (8) was very potent in suppressing retinal and choroidal neovascularization in vivo in the OIR and laser-induced CNV models, respectively. However, some undesirable cell toxicity, over a certain dose, could be an obstacle for its clinical use (Kim et al., 2008d). Synthetic derivatives of deguelin have been studied as an alternative to maintain its therapeutic potential with fewer side effects (Kim et al., 2008d). A novel candidate, SH-14, demonstrated high apoptotic activity and less cytotoxicity than deguelin on several cancer cell lines, suggesting a better potential for cancer prevention and therapy (Kim et al., 2008d). Further studies are needed to demonstrate the anti-angiogenic effects of this or other derivatives on ocular neovascular diseases.
The pleiotropic effects of curcumin (9) make it an interesting natural product in the ocular context. It caused the induction of apoptosis and G0/G1 cell cycle arrest in HUVECs (Singh et al., 1996), interfered with VEGF-induced signaling in HRECs (Premanand et al., 2006) and blocked FGF-2- signaling in the corneal pocket assay (Mohan et al., 2000). Synthesis of curcumin derivatives to target ocular angiogenesis specifically, as well as improve bioavailability while minimizing effects on other tissues/pathways might be a reasonable approach to develop an effective drug for ocular neovascularization.
Withaferin A (14) interfered with the UPP in HUVECs and choroidal endothelial cells (CECs), suggesting a conserved mechanism of action among different endothelial cell types (Bargagna-Mohan et al., 2006). Testing withaferin A activity in ocular disease-relevant animal models is required to demonstrate its therapeutic potential in ocular neovascular diseases such as ROP, AMD and DR. Importantly, with a direct target of withaferin A known (the IF protein vimentin), development of more specific or potent analogs is a possibility. Meanwhile, a clear and comprehensive understanding of the mechanism of action of other natural compounds in vitro is still needed. This should be done using tissue specific endothelial cells and disease-relevant animal models as discussed, which will assist in the synthesis of novel analogues to specifically target ocular neovascularization with minimal systemic effects.
Other natural compounds, not discussed here, have been tested in a more limited fashion on ocular neovascularization, such as astaxanthin (Izumi-Nagai et al., 2008), baicalin (Yang et al., 2014), and the fumagillin derivative lodamin (Benny et al., 2010), but still may prove promising. Any of these compounds could potentially be effective on their own or after medicinal chemistry optimization to increase specificity and potency and improve pharmacokinetics. Topical, intravitreal, and systemic administration could achieve drug delivery to the ocular tissues. However, drug delivery remains a challenge that depends on the physicochemical properties of the compound, physiological barriers and ocular permeability. The topical route is the most favored, but the drug formulation and ocular permeability may limit the drug bioavailability following this route. Physiological barriers such as the blood-retinal barrier and potential undesirable systemic exposure to the drug challenge systemic administration as an option for drug delivery to ocular tissues. An intravitreal route is invasive by nature but is very effective, therefore the most commonly used for experimental studies (Matsumoto et al., 2006; Srirangam et al., 2012; Zhang et al., 2009), and is of course the delivery route used for existing biologic therapies in the clinic.
The therapeutic use of these natural compounds as inhibitors of ocular angiogenesis is awaiting further studies to clearly elucidate their molecular targets and to thoroughly test them in disease-relevant animal models before they can proceed towards clinical trials. Better understanding of known angiogenic mechanisms and identification of novel pathways to interfere with angiogenesis is important for future therapeutic development of more effective angiogenesis inhibitors. This strategy could be achieved by further synthesizing derivatives that selectively target a specific molecular pathway and/or use of combination therapies to target multiple pathways in this complicated pathological process, which could offer better therapeutic outcomes for patients afflicted by blinding neovascular eye diseases.
8. Conclusion
Medicinal plants continue to provide new sources of compounds for the treatment of disease. Natural products that have been shown to reduce angiogenesis provide an appealing alternative to the available biologic pharmacotherapies for ocular neovascular diseases, and hopefully more of these compounds will be tested in the ocular context, drawing on the wealth of compounds that show antiangiogenic activity in cancer models (Sagar et al., 2006a, b). Successful compounds could be used alone or in combination treatment with standard therapies to reduce the effective dosage and thereby significantly decrease side effects. In order to develop specific anti-angiogenic natural compounds for ocular disease, extensive testing still needs to be done.
Highlights.
Small molecule inhibitors of pathological ocular angiogenesis would be an appealing addition to the available biologic therapies
A growing subset of natural-source compounds are promising suppressors of ocular neovascularization in vitro and in vivo
With further mechanistic studies, some of these compounds would be ready to progress toward clinical trials
Acknowledgments
We thank Drs. Michael Boulton and Dulcie Mulholland for critical comments on the manuscript. Related work in the authors' laboratory is supported by the International Retinal Research Foundation, the Showalter Research Trust, the Retina Research Foundation, the Carl Marshall and Mildred Almen Reeves Foundation, and an IUPUI FORCES grant. This publication was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc. HDB is a Kemin Health Ausich Graduate Scholar and TWC is an Indiana CTSI KL2 Scholar (NIH/NCATS KL2TR001106).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed B, Van Eijk LI, Bouma-Ter Steege JC, Van Der Schaft DW, Van Esch AM, Joosten-Achjanie SR, Lambin P, Landuyt W, Griffioen AW. Vascular targeting effect of combretastatin A-4 phosphate dominates the inherent angiogenesis inhibitory activity. Int J Cancer. 2003;105:20–25. doi: 10.1002/ijc.11010. [DOI] [PubMed] [Google Scholar]
- Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol. 2007;18:502–508. doi: 10.1097/ICU.0b013e3282f0ca54. [DOI] [PubMed] [Google Scholar]
- Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38:397–432. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagli E, Stefaniotou M, Morbidelli L, Ziche M, Psillas K, Murphy C, Fotsis T. Luteolin inhibits vascular endothelial growth factor-induced angiogenesis; inhibition of endothelial cell survival and proliferation by targeting phosphatidylinositol 3′-kinase activity. Cancer Res. 2004;64:7936–7946. doi: 10.1158/0008-5472.CAN-03-3104. [DOI] [PubMed] [Google Scholar]
- Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, Murad E, Dubiel W, Soff G, Arbiser JL. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- Bargagna-Mohan P, Hamza A, Kim YE, Ho YKA, Mor-Vaknin N, Wendschlag N, Liu J, Evans RM, Markovitz DM, Zhan CG, Kim KB, Mohan R. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem Biol. 2007;14:623–634. doi: 10.1016/j.chembiol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargagna-Mohan P, Paranthan RR, Hamza A, Dimova N, Trucchi B, Srinivasan C, Elliott GI, Zhan CG, Lau DL, Zhu H, Kasahara K, Inagaki M, Cambi F, Mohan R. Withaferin A targets intermediate filaments glial fibrillary acidic protein and vimentin in a model of retinal gliosis. J Biol Chem. 2010;285:7657–7669. doi: 10.1074/jbc.M109.093765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargagna-Mohan P, Ravindranath PP, Mohan R. Small molecule anti-angiogenic probes of the ubiquitin proteasome pathway: potential application to choroidal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:4138–4145. doi: 10.1167/iovs.05-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa HD, Lee B, Fei X, Magaña C, Waller C, Crouch NR, Mulholland DA, Seo SY, Corson TW. Structure-activity relationship studies of a natural product inhibitor of choroidal angiogenesis. Invest Ophthalmol Vis Sci. 2013;54:3282. E-abstract. [Google Scholar]
- Basavarajappa HD, Lee B, Quigley J, Sulaiman RA, Rajashekhar G, Seo SY, Corson TW. Identification and characterization of a novel synthetic homoisoflavonoid as an inhibitor of retinal angiogenesis. Invest Ophthalmol Vis Sci. 2014;55:1266. E-abstract. [Google Scholar]
- Basavarajappa HD, Lee B, Fei X, Lim D, Callaghan B, Mund JA, Case J, Rajashekhar G, Seo SY, Corson TW. Synthesis and mechanistic studies of a novel homoisoflavanone inhibitor of endothelial cell growth. PLoS One. 2014;9:e95694. doi: 10.1371/journal.pone.0095694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benny O, Nakai K, Yoshimura T, Bazinet L, Akula JD, Nakao S, Hafezi-Moghadam A, Panigrahy D, Pakneshan P, D'Amato RJ. Broad spectrum antiangiogenic treatment for ocular neovascular diseases. PLoS One. 2010;5:e12515. doi: 10.1371/journal.pone.0012515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakenhielm E, Cao R, Cao Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 2001;15:1798–1800. doi: 10.1096/fj.01-0028fje. [DOI] [PubMed] [Google Scholar]
- Buck SB, Bradford J, Gee KR, Agnew BJ, Clarke ST, Salic A. Detection of S-phase cell cycle progression using 5-ethynyl-2′-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2′-deoxyuridine antibodies. Biotechniques. 2008;44:927–929. doi: 10.2144/000112812. [DOI] [PubMed] [Google Scholar]
- Cao L, Liu H, Lam DS, Yam GH, Pang CP. In vitro screening for angiostatic potential of herbal chemicals. Invest Ophthalmol Vis Sci. 2010;51:6658–6664. doi: 10.1167/iovs.10-5524. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li XX, Xing NZ, Cao XG. Quercetin inhibits choroidal and retinal angiogenesis in vitro. Graefes Arch Clin Exp Ophthalmol. 2008;246:373–378. doi: 10.1007/s00417-007-0728-9. [DOI] [PubMed] [Google Scholar]
- Coats DK. Retinopathy of prematurity: involution, factors predisposing to retinal detachment, and expected utility of preemptive surgical reintervention. Trans Am Ophthalmol Soc. 2005;103:281–312. [PMC free article] [PubMed] [Google Scholar]
- Congdon N, O'Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P Eye Diseases Prevalence Research G. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769–774. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Dobi ET, Puliafito CA, Destro M. A new model of experimental choroidal neovascularization in the rat. Arch Ophthalmol. 1989;107:264–269. doi: 10.1001/archopht.1989.01070010270035. [DOI] [PubMed] [Google Scholar]
- Dorrell M, Uusitalo-Jarvinen H, Aguilar E, Friedlander M. Ocular neovascularization: basic mechanisms and therapeutic advances. Surv Ophthalmol. 2007;521(Suppl 1):S3–19. doi: 10.1016/j.survophthal.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 2013;27:787–794. doi: 10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J, Ingber D. Inhibition of angiogenesis. Semin Cancer Biol. 1992;3:89–96. [PubMed] [Google Scholar]
- Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, Schweigerer L. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci U S A. 1993;90:2690–2694. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev Cell. 2009;16:167–179. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Fugner A. Inhibition of immunologically induced inflammation by the plant steroid withaferin A. Arzneimittelforschung. 1973;23:932–935. [PubMed] [Google Scholar]
- Gerhauser C, Lee SK, Kosmeder JW, Moriarty RM, Hamel E, Mehta RG, Moon RC, Pezzuto JM. Regulation of ornithine decarboxylase induction by deguelin, a natural product cancer chemopreventive agent. Cancer Res. 1997;57:3429–3435. [PubMed] [Google Scholar]
- Griggs J, Skepper JN, Smith GA, Brindle KM, Metcalfe JC, Hesketh R. Inhibition of proliferative retinopathy by the anti-vascular agent combretastatin-A4. Am J Pathol. 2002;160:1097–1103. doi: 10.1016/S0002-9440(10)64930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M, Chiu JJ, Lenardo MJ. NF-κB and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137:496–503. doi: 10.1016/j.ajo.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 2010;29:500–519. doi: 10.1016/j.preteyeres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther JB, Altaweel MM. Bevacizumab (Avastin) for the treatment of ocular disease. Surv Ophthalmol. 2009;54:372–400. doi: 10.1016/j.survophthal.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Sakai T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor-related apoptosis-inducing ligand. Mol Cancer Ther. 2006;5:945–951. doi: 10.1158/1535-7163.MCT-05-0431. [DOI] [PubMed] [Google Scholar]
- Izumi-Nagai K, Nagai N, Ohgami K, Satofuka S, Ozawa Y, Tsubota K, Ohno S, Oike Y, Ishida S. Inhibition of choroidal neovascularization with an anti-inflammatory carotenoid astaxanthin. Invest Ophthalmol Vis Sci. 2008;49:1679–1685. doi: 10.1167/iovs.07-1426. [DOI] [PubMed] [Google Scholar]
- Jackson SJ, Venema RC. Quercetin inhibits eNOS, microtubule polymerization, and mitotic progression in bovine aortic endothelial cells. J Nutr. 2006;136:1178–1184. doi: 10.1093/jn/136.5.1178. [DOI] [PubMed] [Google Scholar]
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- Javitt J, Dei Cas R, Chiang YP. Cost-effectiveness of screening and cryotherapy for threshold retinopathy of prematurity. Pediatrics. 1993;91:859–866. [PubMed] [Google Scholar]
- Jhanji V, Liu H, Law K, Lee VY, Huang SF, Pang CP, Yam GH. Isoliquiritigenin from licorice root suppressed neovascularisation in experimental ocular angiogenesis models. Br J Ophthalmol. 2011;95:1309–1315. doi: 10.1136/bjophthalmol-2011-300110. [DOI] [PubMed] [Google Scholar]
- Ji HF, Li XJ, Zhang HY. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009;10:194–200. doi: 10.1038/embor.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo DH, Cho CS, Kim JH, Jun HO, Kim JH. Animal models of diabetic retinopathy: doors to investigate pathogenesis and potential therapeutics. J Biomed Sci. 2013;20:38. doi: 10.1186/1423-0127-20-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MH, Lee SH, Ahn EM, Lee YM. Decursin and decursinol angelate inhibit VEGF-induced angiogenesis via suppression of the VEGFR-2-signaling pathway. Carcinogenesis. 2009;30:655–661. doi: 10.1093/carcin/bgp039. [DOI] [PubMed] [Google Scholar]
- Kador PF, Blessing K, Randazzo J, Makita J, Wyman M. Evaluation of the vascular targeting agent combretastatin a-4 prodrug on retinal neovascularization in the galactose-fed dog. J Ocul Pharmacol Ther. 2007;23:132–142. doi: 10.1089/jop.2006.0103. [DOI] [PubMed] [Google Scholar]
- Kang SW, Choi JS, Choi YJ, Bae JY, Li J, Kim DS, Kim JL, Shin SY, Lee YJ, Kwun IS, Kang YH. Licorice isoliquiritigenin dampens angiogenic activity via inhibition of MAPK-responsive signaling pathways leading to induction of matrix metalloproteinases. J Nutr Biochem. 2010;21:55–65. doi: 10.1016/j.jnutbio.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M. Quantitation of flavonoid constituents in citrus fruits. J Agric Food Chem. 1999;47:3565–3571. doi: 10.1021/jf990153+. [DOI] [PubMed] [Google Scholar]
- Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim JH, Lee YM, Ahn EM, Kim KW, Yu YS. Decursin inhibits retinal neovascularization via suppression of VEGFR-2 activation. Mol Vis. 2009;15:1868–1875. [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim JH, Yu YS, Jun HO, Kwon HJ, Park KH, Kim KW. Inhibition of choroidal neovascularization by homoisoflavanone, a new angiogenesis inhibitor. Mol Vis. 2008a;14:556–561. [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim JH, Yu YS, Park KH, Kang HJ, Lee HY, Kim KW. Antiangiogenic effect of deguelin on choroidal neovascularization. J Pharmacol Exp Ther. 2008b;324:643–647. doi: 10.1124/jpet.107.132720. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim JH, Yu YS, Shin JY, Lee HY, Kim KW. Deguelin inhibits retinal neovascularization by down-regulation of HIF-1α in oxygen-induced retinopathy. J Cell Mol Med. 2008c;12:2407–2415. doi: 10.1111/j.1582-4934.2008.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim KH, Kim JH, Yu YS, Kim YM, Kim KW, Kwon HJ. Homoisoflavanone inhibits retinal neovascularization through cell cycle arrest with decrease of cdc2 expression. Biochem Biophys Res Commun. 2007;362:848–852. doi: 10.1016/j.bbrc.2007.08.100. [DOI] [PubMed] [Google Scholar]
- Kim MH. Flavonoids inhibit VEGF/bFGF-induced angiogenesis in vitro by inhibiting the matrix-degrading proteases. J Cell Biochem. 2003;89:529–538. doi: 10.1002/jcb.10543. [DOI] [PubMed] [Google Scholar]
- Kim WT, Suh ES. Retinal protective effects of resveratrol via modulation of nitric oxide synthase on oxygen-induced retinopathy. Korean J Ophthalmol. 2010;24:108–118. doi: 10.3341/kjo.2010.24.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Chang DJ, Hennessy B, Kang HJ, Yoo J, Han SH, Kim YS, Park HJ, Seo SY, Mills G, Kim KW, Hong WK, Suh YG, Lee HY. A novel derivative of the natural agent deguelin for cancer chemoprevention and therapy. Cancer Prev Res. 2008d;1:577–587. doi: 10.1158/1940-6207.CAPR-08-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- Konoshima M, Chi HJ, Hata K. Coumarins from the root of Angelica gigas Nakai. Chem Pharm Bull (Tokyo) 1968;16:1139–1140. doi: 10.1248/cpb.16.1139. [DOI] [PubMed] [Google Scholar]
- Koroma BM, de Juan E., Jr Phosphotyrosine inhibition and control of vascular endothelial cell proliferation by genistein. Biochem Pharmacol. 1994;48:809–818. doi: 10.1016/0006-2952(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab. 2007;4:8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B, Gupta SK, Srinivasan BP, Nag TC, Srivastava S, Saxena R, Jha KA. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc Res. 2013;87:65–74. doi: 10.1016/j.mvr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73:29–31. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is major stimulator in model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:3158–3164. [PubMed] [Google Scholar]
- Lai AK, Lo AC. Animal models of diabetic retinopathy: summary and comparison. J Diabetes Res. 2013;2013:106594. doi: 10.1155/2013/106594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert V, Lecomte J, Hansen S, Blacher S, Gonzalez ML, Struman I, Sounni NE, Rozet E, de Tullio P, Foidart JM, Rakic JM, Noel A. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat Protoc. 2013;8:2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- Lee B, Basavarajappa HD, Sulaiman RS, Fei X, Seo SY, Corson TW. The first synthesis of the antiangiogenic homoisoflavanone, cremastranone. Org Biomol Chem. 2014;12:7673–7677. doi: 10.1039/c4ob01604a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Lee YM, Lee CK, Jung JK, Han SB, Hong JT. Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther. 2011;130:157–176. doi: 10.1016/j.pharmthera.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Li JW, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Lin MT, Yen ML, Lin CY, Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol. 2003;64:1029–1036. doi: 10.1124/mol.64.5.1029. [DOI] [PubMed] [Google Scholar]
- Lux A, Llacer H, Heussen FM, Joussen AM. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol. 2007;91:1318–1322. doi: 10.1136/bjo.2006.113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macha S, Mitra AK. Ophthalmic Drug Delivery Systems. Vol. 2. Marcel Dekker, Inc.; New York: 2003. [Google Scholar]
- Madri JA, Pratt BM, Tucker AM. Phenotypic modulation of endothelial cells by transforming growth factor-β depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988;106:1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Srirangam R. Potential of the bioflavonoids in the prevention/treatment of ocular disorders. J Pharm Pharmacol. 2010;62:951–965. doi: 10.1211/jpp.62.08.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Nakamura Y, Iida H, Ito K, Ohguro H. Comparative assessment of distribution of blackcurrant anthocyanins in rabbit and rat ocular tissues. Exp Eye Res. 2006;83:348–356. doi: 10.1016/j.exer.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- Mishra BB, Tiwari VK. Natural products: an evolving role in future drug discovery. Eur J Med Chem. 2011;46:4769–4807. doi: 10.1016/j.ejmech.2011.07.057. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Annemans L, White R, Gallagher M, Thomas S. Cost effectiveness of treatments for wet age-related macular degeneration. Pharmacoeconomics. 2011;29:107–131. doi: 10.2165/11585520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Mohan R, Hammers HJ, Bargagna-Mohan P, Zhan XH, Herbstritt CJ, Ruiz A, Zhang L, Hanson AD, Conner BP, Rougas J, Pribluda VS. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7:115–122. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- Mohan R, Sivak J, Ashton P, Russo LA, Pham BQ, Kasahara N, Raizman MB, Fini ME. Curcuminoids inhibit the angiogenic response stimulated by fibroblast growth factor-2, including expression of matrix metalloproteinase gelatinase B. J Biol Chem. 2000;275:10405–10412. doi: 10.1074/jbc.275.14.10405. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Cooney MJ, Tu AH, Chang KY, Cao J, Ando A, An GJ, Melia M, de Juan E., Jr Normalization of retinal vascular permeability in experimental diabetes with genistein. Invest Ophthalmol Vis Sci. 2001;42:2110–2114. [PubMed] [Google Scholar]
- Nambu H, Nambu R, Melia M, Campochiaro PA. Combretastatin A-4 phosphate suppresses development and induces regression of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3650–3655. doi: 10.1167/iovs.02-0985. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Ng EW, Shima DT, Calias P, Cunningham ET, Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Paranthan RR, Bargagna-Mohan P, Lau DL, Mohan R. A robust model for simultaneously inducing corneal neovascularization and retinal gliosis in the mouse eye. Mol Vis. 2011;17:1901–1908. [PMC free article] [PubMed] [Google Scholar]
- Park SW, Cho CS, Jun HO, Ryu NH, Kim JH, Yu YS, Kim JS, Kim JH. Anti-angiogenic effect of luteolin on retinal neovascularization via blockade of reactive oxygen species production. Invest Ophthalmol Vis Sci. 2012;53:7718–7726. doi: 10.1167/iovs.11-8790. [DOI] [PubMed] [Google Scholar]
- Premanand C, Rema M, Sameer MZ, Sujatha M, Balasubramanyam M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Invest Ophthalmol Vis Sci. 2006;47:2179–2184. doi: 10.1167/iovs.05-0580. [DOI] [PubMed] [Google Scholar]
- Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. J Genet. 2009;88:495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 1. Curr Oncol. 2006a;13:14–26. [PMC free article] [PubMed] [Google Scholar]
- Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 2. Curr Oncol. 2006b;13:99–107. [PMC free article] [PubMed] [Google Scholar]
- Sapieha P, Joyal JS, Rivera JC, Kermorvant-Duchemin E, Sennlaub F, Hardy P, Lachapelle P, Chemtob S. Retinopathy of prematurity: understanding ischemic retinal vasculopathies at an extreme of life. J Clin Invest. 2010;120:3022–3032. doi: 10.1172/JCI42142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BM, Ribnicky DM, Lipsky PE, Raskin I. Revisiting the ancient concept of botanical therapeutics. Nat Chem Biol. 2007;3:360–366. doi: 10.1038/nchembio0707-360. [DOI] [PubMed] [Google Scholar]
- Sheu ML, Chiang CK, Tsai KS, Ho FM, Weng TI, Wu HY, Liu SH. Inhibition of NADPH oxidase-related oxidative stress-triggered signaling by honokiol suppresses high glucose-induced human endothelial cell apoptosis. Free Radic Biol Med. 2008;44:2043–2050. doi: 10.1016/j.freeradbiomed.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Shim JS, Kim JH, Lee J, Kim SN, Kwon HJ. Anti-angiogenic activity of a homoisoflavanone from Cremastra appendiculata. Planta Med. 2004;70:171–173. doi: 10.1055/s-2004-815496. [DOI] [PubMed] [Google Scholar]
- Singh AK, Sidhu GS, Deepa T, Maheshwari RK. Curcumin inhibits the proliferation and cell cycle progression of human umbilical vein endothelial cell. Cancer Lett. 1996;107:109–115. doi: 10.1016/0304-3835(96)04357-1. [DOI] [PubMed] [Google Scholar]
- Smith LE. Pathogenesis of retinopathy of prematurity. Acta Paediatr. 2002;(Suppl 91):26–28. doi: 10.1111/j.1651-2227.2002.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: a molecule whose time has come? And gone? Clin Biochem. 1997;30:91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- Soufi FG, Mohammad-Nejad D, Ahmadieh H. Resveratrol improves diabetic retinopathy possibly through oxidative stress - nuclear factor κB - apoptosis pathway. Pharmacol Rep. 2012;64:1505–1514. doi: 10.1016/s1734-1140(12)70948-9. [DOI] [PubMed] [Google Scholar]
- Srirangam R, Hippalgaonkar K, Majumdar S. Intravitreal kinetics of hesperidin, hesperetin, and hesperidin G: effect of dose and physicochemical properties. J Pharm Sci. 2012;101:1631–1638. doi: 10.1002/jps.23047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012a;96:1157–1158. doi: 10.1136/bjophthalmol-2011-300654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MW. The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clin Proc. 2012b;87:77–88. doi: 10.1016/j.mayocp.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavilala DT, O'Bryhim BE, Ponnaluri VK, White RS, Radel J, Symons RC, Mukherji M. Honokiol inhibits pathological retinal neovascularization in oxygen-induced retinopathy mouse model. Biochem Biophys Res Commun. 2013;438:697–702. doi: 10.1016/j.bbrc.2013.07.118. [DOI] [PubMed] [Google Scholar]
- Vaya J, Belinky PA, Aviram M. Antioxidant constituents from licorice roots: isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Radic Biol Med. 1997;23:302–313. doi: 10.1016/s0891-5849(97)00089-0. [DOI] [PubMed] [Google Scholar]
- Wang B, Zou Y, Li H, Yan H, Pan JS, Yuan ZL. Genistein inhibited retinal neovascularization and expression of vascular endothelial growth factor and hypoxia inducible factor 1α in a mouse model of oxygen-induced retinopathy. J Ocul Pharmacol Ther. 2005;21:107–113. doi: 10.1089/jop.2005.21.107. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Jo H, Kim JG, Jung SH. Baicalin attenuates laser-induced choroidal neovascularization. Curr Eye Res. 2014;39:745–51. doi: 10.3109/02713683.2013.868908. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yang K, Li Y, Li X, Sun Q, Meng H, Zeng Y, Hu Y, Zhang Y. Decursin inhibited proliferation and angiogenesis of endothelial cells to suppress diabetic retinopathy via VEGFR2. Mol Cell Endocrinol. 2013;378:46–52. doi: 10.1016/j.mce.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Young SL, Chaplin DJ. Combretastatin A4 phosphate: background and current clinical status. Expert Opin Investig Drugs. 2004;13:1171–1182. doi: 10.1517/13543784.13.9.1171. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang J, Wang L, Xia H. Ocular pharmacokinetics and availability of topically applied baicalein in rabbits. Curr Eye Res. 2009;34:257–263. doi: 10.1080/02713680902725962. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Ma JX. Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy. Prog Retin Eye Res. 2007;26:1–37. doi: 10.1016/j.preteyeres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, Gregg EW, Albright AL, Klein BE, Klein R. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang P, Shen Y, Lin BQ, Zhang WY, Chiou GC. Effect of quercetin on formation of choroidal neovascularization (CNV) in age-related macular degeneration (AMD) Eye Sci. 2011;26:23–29. doi: 10.3969/j.issn.1000-4432.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Zou Y, Chiou GC. Apigenin inhibits laser-induced choroidal neovascularization and regulates endothelial cell function. J Ocul Pharmacol Ther. 2006;22:425–430. doi: 10.1089/jop.2006.22.425. [DOI] [PubMed] [Google Scholar]


