Abstract
Striatal-enriched protein tyrosine phosphatase (STEP) is a brain-specific tyrosine phosphatase that plays a major role in the development of synaptic plasticity. Recent findings have implicated STEP in several psychiatric and neurological disorders, including Alzheimer’s disease, schizophrenia, fragile X syndrome, Huntington’s disease, stroke/ischemia, and stress-related psychiatric disorders. In these disorders, STEP protein expression levels and activity are dysregulated, contributing to the cognitive deficits that are present. In this review, we focus on the most recent findings on STEP, discuss how STEP expression and activity are maintained during normal cognitive function, and how disruptions in STEP activity contribute to a number of illnesses.
Keywords: Alzheimer’s disease, fragile X syndrome, Huntington’s disease, ischemia, schizophrenia, STEP, stroke
Introduction
Synaptic networks are the basis of normal brain function, in which billions of neurons make contact with one another through synaptic connections. Synaptic strengthening is required for normal cognition, while disruption to synaptic function contributes to a growing number of neuropsychiatric and neurodegenerative disorders (Blanpied and Ehlers, 2004; Fiala et al., 2002; Garner and Wetmore, 2012; Huttenlocher, 1991; Van den Oever et al., 2012).
Many proteins functioning in synaptic networks are regulated by phosphorylation. Functional disruptions of some of these proteins contribute to the development of neuropsychiatric disorders and key players in this process are the kinases and phosphatases that add or remove phosphate groups from target substrates. While several serine/threonine kinases are critical for brain function, the tyrosine kinases also phosphorylate target proteins to regulate intracellular mechanisms required for memory consolidation. Regulating these intracellular events requires modulation of both tyrosine kinases and the family of protein tyrosine phosphatases (PTPs) that dephosphorylate target proteins involved in synaptic strengthening.
Striatal-enriched protein tyrosine phosphatase (STEP) is a brain-specific tyrosine phosphatase that is expressed in the striatum, cortex, hippocampus, and other related brain regions with the exception of the cerebellum (Boulanger et al., 1995; Lombroso et al., 1991). The current model of STEP function is that it opposes the development of synaptic strengthening. Recent findings from multiple laboratories indicate that STEP is involved in the pathophysiology of several neuropsychiatric disorders. One of the more important findings over the last several years is that both high and low levels of STEP activity are involved in these illnesses (Table 1). For example, increased STEP activity is associated with Alzheimer’s disease, schizophrenia, Parkinson’s disease, and fragile X syndrome (Carty et al., 2012; Goebel-Goody et al., 2012b; Kurup et al., 2012; Zhang et al., 2010). In contrast, decreased STEP activity is associated with Huntington’s disease, stroke, brain ischemia, and stress-related anxiety disorders (Deb et al., 2013; Saavedra et al., 2011; Yang et al., 2012). It therefore appears that both high and low levels of STEP can disrupt synaptic functioning, with several mechanisms maintaining STEP expression at optimal levels. This review examines mechanisms that regulate STEP activity in relation to these neuropsychiatric disorders.
Table 1.
STEP and neuropsychiatric disorders. Due to space limitations, only some of these disorders are discussed in this review.
| Disorder | STEP | Brain Region | Disruption of STEP homeostasis |
Model | Citations |
|---|---|---|---|---|---|
| Alzheimer’s Disease | ↑ | Dorsolateral prefrontal cortex | Aβ inhibition of proteasome and PP1 dephosphorylation of STEP | Human Mouse |
-Snyder et al., 2005 -Kurup et al., 2010 -Zhang et al., 2010, 2011 |
| Cognitive deficits of aging | ↑ | Hippocampus | Disruption of STEP ubiquitination | Human Monkey Rat Mouse |
-Brouillette et al., under review |
| Fragile X syndrome | ↑ | Hippocampus | FMRP normally inhibits STEP mRNA | Mouse | -Darnell et al., 2011 -Goebel-Goody et al., 2012 |
| Parkinson’s disease | ↑ | Striatum, but not cortex | Disruption of STEP ubiquitination | Human Rat Mouse |
-Kurup et al., under review |
| Schizophrenia | ↑ | Dorsolateral prefrontal cortex, cingulate | Disruption of STEP ubiquitination | Human Mouse |
-Carty et al., 2012 |
| Cocaine | ↑ | Striatum | Adenosine A2A receptors | Mouse | -Chiodi et al., 2014 |
| Epilepsy | ↓ | Hippocampus | STEP KO mice protective | Mouse | -Briggs et al., 2011 |
| Alcoholism | ↑ ↓ |
Hippocampus Dorsomedial striatum but not DLS or Nuc acc. |
Unknown Phosphorylation in DMS inactivates STEP |
Mouse | -Hicklin et al., 2011 -Darcq et al., 2014 |
| Huntington’s disease | ↓ | Striatum | Unknown | Mouse | -Saavedra et al., 2011 -Gladding et al., 2012 |
| Ischemia/stroke | ↓ | Cortex | Unknown | Mouse | -Deb et al., 2013 |
| Stress Anxiety related disorders | ↓ | Cortex Bed nucleus of stria terminalis | Unknown Unknown |
Mouse | -Yang et al., 2012 -Dabrowska et al., 2013 |
Molecular biology
STEP (PTPN5) is an intracellular PTP consisting of two major isoforms: a cytosolic STEP46 and a membrane-associated STEP61 (Fig. 1) (Boulanger et al., 1995; Bult et al., 1996). Both STEP61 and STEP46 contain the conserved PTP catalytic domain ([I/V]HCxAGxxR[S/T]G) (Bult et al., 1996). Alternative splicing of a single STEP gene produces an additional 172 amino acids at the N-terminus of STEP61, which targets STEP61 to the endoplasmic reticulum and, in part, to postsynaptic terminals (Boulanger et al., 1995; Bult et al., 1996; Oyama et al., 1995).
Figure 1.

Structure of STEP. Two splice variants are shown, and their names are based on their mobility in SDS-gel electrophoresis. Both STEP61 (541 amino acids) and STEP46 (369 amino acids) have conserved PTP catalytic domain at the C-terminal and contain a KIM (kinase-interacting motif) domain. STEP61 has an additional N-terminal 172 amino acids that contain polyproline-rich domains (PP) and transmembrane domains (TM). Each of PPs is required for binding to Fyn and Pyk2, an interaction that also requires the KIM domain. The phosphorylation site within the KIM domain (S221 or S49) is phosphorylated by PKA. The S160 in STEP61 is also phosphorylated by PKA but its role remains unknown. The cysteine residues (C65 and C76) mediate STEP dimerization.
STEP61 and STEP46 contain a kinase interacting motif (KIM), which is the domain in STEP isoforms necessary for interacting with all substrates identified to date. A small families of PTPs containing KIM domains are highly homologous among themselves and consist of STEP, PTP-STEP-like (PTP-SL), and hematopoietic tyrosine phosphatase (He-PTP), each of which dephosphorylates members of the mitogen-activated protein kinase (MAPK) family (Adachi et al., 1992; Bult et al., 1996; Hendriks et al., 2009; Pulido et al., 1998).
The N-terminal sequence of STEP61 contains two hydrophobic domains and two polyproline-rich (PP) and PEST (peptide sequences that are rich in proline [P], glutamic acid [E], serine [S], and threonine [T]) domains (Bult et al., 1996) (Figure 1). The PP domains are required for the interactions of STEP61 with Fyn and Pyk2, indicating that these domains (in addition to the KIM domain) contribute to the substrate specificity of STEP61 (Nguyen et al., 2002; Xu et al., 2012). The PEST sequences mediate proteolytic processes in several proteins, although their function in STEP61 remains less clear (Shumway et al., 1999; Spencer et al., 2004). Calpain-mediated proteolysis of STEP61 results after extrasynaptic stimulation of N-methyl-D-aspartate receptors (NMDAR) (Xu et al., 2009); but proteolysis occurs within the KIM domain and not within the PEST sequence. The resulting cleaved fragment (STEP33) no longer contains an intact KIM domain and does not bind to or dephosphorylate STEP substrates (Nguyen et al., 1999; Xu et al., 2009).
The current model of STEP is that it opposes the development of synaptic strengthening (Goebel-Goody et al., 2012a). As is discussed further below, this is due to the targets of STEP. STEP dephosphorylation of regulatory subunits of NMDARs and α-amino-3-hydroxy-5-methyl 4-isoxazolepropionic acid receptors (AMPARs) leads to their internalization, while dephosphorylation of a regulatory Tyr within the activation loop of several kinase results in their inactivation. The rapid removal or inactivation of STEP at synaptic terminals is a theme that has emerged and that permits the development of synaptic strengthening.
Each STEP isoform displays a distinct distribution within the central nervous system. STEP61 is expressed in the hippocampus, neocortex, lateral amygdala, and spinal cord, while both STEP61 and STEP46 are found in the striatum, central nucleus of the amygdala, and optic nerve (Boulanger et al., 1995; Bult et al., 1996; Lorber et al., 2004). STEP is not expressed in the cerebellum, where the related PTP-SL is localized (Watanabe et al., 1998) and whether PTP-SL has similar functions to STEP in the cerebellum remains to be determined. STEP isoforms are also differentially expressed during development. In rodents, STEP61 is expressed prenatally, although strong expression occurs postnatally and throughout life. In contrast, STEP46 expression emerges at postnatally and progressively increases to adult levels over the first month (Okamura et al., 1997; Raghunathan et al., 1996). Interestingly, STEP expression has recently been found to increase with normal aging, and significant increases in STEP expression occurs in aged rodents and non-human primates with cognitive deficits compared to aged animals with no cognitive deficits, as well as in humans with mild cognitive deficits (Brouillette and Lombroso, unpublished data).
Synaptic activity can also result in the rapid translation of specific STEP isoforms that are not present at base line. For example, STEP46 is not normally detected in neurons of the lateral amygdala; however, within minutes of fear conditioning, STEP46 is rapidly translated and increased levels of STEP46 correlate with the subsequent dephosphorylation and inactivation of extracellular signal-regulated kinase 1/2 (ERK1/2), a member of the MAPK family (Olausson et al., 2012).
In addition, although STEP61 and STEP46 can dephosphorylate the same substrates in vitro, there appears to be some substrate specificity in vivo. Thus, STEP46 interacts much more strongly to phosphorylated ERK1/2 than does STEP61, while STEP61 binds with higher affinity to Pyk2 and Fyn (Paul et al., 2003; Xu et al., 2012).
Neuropsychiatric disorders associated with increased STEP levels
Alzheimer’s disease
Alzheimer’s disease (AD) is a common, progressive neurodegenerative disorder characterized by increasingly severe memory impairments. The number of affected individuals with AD is projected to double over the next several decades (Forlenza et al., 2010). The risk of AD increases with age and represents a serious problem among the elderly as quality of life diminishes with progression of the disease. Pathological features of AD at the cellular level include beta amyloid (Aβ) accumulation, tau protein hyperphosphorylation and accumulation, and amyloid plaque and neurofibrillary tangles formation (Hardy and Selkoe, 2002; Lacor et al., 2004). Aβ accumulation leads to oligomerization of the peptide, and Aβ oligomers reduce long-term potentiation (LTP) by disrupting signaling through NMDAR and AMPAR (Kim et al., 2001; Selkoe, 2002; Snyder et al., 2005).
Recent studies indicate that Aβ accumulation is associated with increased STEP expression and phosphatase activity. Two independent signaling mechanisms initiate the up-regulation of STEP activity by Aβ. The first mechanism involves dephosphorylation of STEP itself. Aβ binding to the α7-nicotinic receptor promotes Ca2+ influx and activation of protein phosphatase 2B (PP2B, also known as calcineurin) and protein phosphatase 1 (PP1). PP1 dephosphorylates STEP directly, which increases the affinity of STEP to bind to its substrates. Phosphorylation of STEP occurs at Ser221 within the KIM domain and sterically prevents association of STEP with target proteins (Snyder et al., 2005). The second mechanism is through Aβ inhibition of the proteasome. This causes accumulation of STEP protein that is normally degraded by the proteasomal pathway, and results in increased STEP activity (Kurup et al., 2010).
Elevated levels of active STEP result in dephosphorylation of the NMDAR subunit GluN2B (Tyr1472) and promote internalization of GluN1/GluN2B NMDARs, as well as GluA1/GluA2 AMPARs (Kurup et al., 2010; Zhang et al., 2011; Zhang et al., 2010; Zhang et al., 2008). Excessive glutamate receptor internalization disrupts synaptic function and is thought to contribute to the cognitive deficits observed in AD.
Compelling evidence in support of a direct role for STEP in the pathophysiology of AD comes from a series of studies in AD mouse models. The triple transgenic model (3xTg-AD or LaFerla mouse) contains three mutated genes that were found in cases of familial AD, and consist of the mutated versions of the amyloid precursor protein, presenilin, and tau proteins (Oddo et al., 2003). The mutated genes were introduced into the mouse to produce the triple transgenic strain. These mice have both cognitive and biochemical deficits (elevated beta amyloid, plaques and tau deposits) and are used extensively to investigate the pathophysiology of AD in mice (Oddo et al., 2003).
Deletion of the STEP gene in the LaFerla mouse model reversed the cognitive deficits observed in these mice, restored NMDARs and AMPARs to synaptosomal membranes, and significantly enhanced LTP in hippocampal slices (Zhang et al., 2010 and 2011). Elevated levels of STEP have also been reported in three other mouse models, including transgenic mice containing a mutation in the amyloid precursor protein gene (Tg2576) (Kurup et al., 2010), transgenic mice containing two mutations in the amyloid precursor protein gene (J20) (Chin et al., 2005), and transgenic mice containing mutations to both the amyloid precursor protein and presenilin genes (APP/PS1) (Zhang et al., 2013).
More recently, increased STEP expression and activity was observed in animal models of normal aging as well as aged humans (Brouillette and Lombroso, unpublished data). Significant increases of STEP61 were observed in the hippocampus of aged animals with cognitive decline compared to aged animals without cognitive deficits, including aged rodents, non-human primates, and humans diagnosed with mild cognitive impairments. The increased expression of active STEP again promotes GluN2B dephosphorylation, GluN1/GluN2B internalization, and the dephosphorylation and inactivation of another STEP substrate, ERK1/2. Furthermore, aged STEP knockout (KO) mice performed as well as young mice in several cognitive tasks. This observation is in contrast to aged wild-type mice that had elevated STEP levels and significant cognitive deficits, supporting the hypothesis that increases in STEP activity induces signaling mechanisms that oppose synaptic strengthening. The mechanism for the increase in STEP expression in aged animals with cognitive deficits compared to aged animals that do not show cognitive deficits remains unclear, although preliminary data suggest a disruption in the normal ubiquitination and degradation of STEP over time, leading to increases in its expression.
Schizophrenia
Schizophrenia (SZ) is a chronic and disabling neuropsychiatric disorder that affects nearly 1% of the world population. Symptoms of SZ typically manifest during late adolescence, and include hallucinations, delusions, motivational loss, social withdrawal, and cognitive deficits. The glutamate hypothesis of SZ posits a reduction in NMDAR signaling (Goff and Coyle, 2001). This hypothesis is supported by anatomical studies conducted on postmortem brains of SZ patients, which showed a reduction in the expression of ionotropic glutamate receptors in the prefrontal cortex, thalamus, and temporal lobe compared to controls (Gao et al., 2000; Ibrahim et al., 2000). Moreover, postnatal elimination of NMDAR subunits in cortical and hippocampal interneurons induces several SZ-like behaviors in mice (Belforte et al., 2010). Additionally, NMDAR antagonists such as phenylcyclohexylpiperidine (PCP) and ketamine are psychotomimetic and induce SZ-like symptoms in animal models and humans (Javitt and Zukin, 1991; Krystal et al., 1994; Rosenbaum et al., 1959). Taken together, these findings have suggested that glutamate signaling may be disrupted in SZ.
A recent study of postmortem brains of SZ patients from two clinical cohorts showed elevated levels of STEP expression compared to age, sex, and postmortem-interval matched controls (Carty et al., 2012). Increased STEP expression was observed in the anterior cingulate cortex and dorsolateral prefrontal cortex of patients with SZ. Mice treated with PCP or MK-801, another NMDAR noncompetitive inhibitor, also showed greater STEP expression compared to saline-treated control mice. STEP KO mice were more resistant to the effects of both acute and chronic PCP-administration, such as increased locomotion and cognitive deficits (Carty et al., 2012). The mechanism for the increase in STEP expression appeared to involve a disruption in the normal ubiquitination of STEP isoforms. As a result, STEP was not normally degraded by the proteasome and expression levels and activity were significantly increased (Carty et al., 2012).
These results are consistent with the glutamate hypothesis of SZ. Thus, high levels of STEP result in an increase in the internalization of glutamate receptors and a loss of these receptors from synaptic membranes. In addition, an important finding by Carty et al. (2012) was that drugs used for the treatment of SZ result in the protein kinase A (PKA)-mediated phosphorylation and inactivation of STEP. Many neuroleptics used in the treatment of SZ block dopamine D2 receptors, which results in increased PKA activity and phosphorylation of STEP by PKA at its regulatory Ser221 within the KIM domain, blocking the association of STEP with its substrates (Carty et al., 2012; Turalba et al., 2004). STEP substrates therefore have increased phosphorylation at regulatory Tyr residues, including GluN2B, resulting in enhanced surface expression of NMDARs. Finally, although not a genome-wide association study, an initial linkage analysis showed that four STEP single nucleotide polymorphisms (SNPs) were associated with an Israeli population of patients with SZ (Pelov et al., 2012).
Fragile X Syndrome
Fragile X syndrome (FXS) is the most common hereditary form of mental retardation. Patients with FXS show behavior and intellectual abnormalities, including cognitive deficits, anxiety, hyperactivity, and seizures and approximately 30% of patients meet the criteria for autism spectrum disorder (Cornish et al., 2008). FXS results from decreased transcription of the Fragile X Mental Retardation 1 (Fmr1) gene, which is caused by a CGG-expansion in the first exon of Fmr1 immediately downstream of the Fmr1 promoter (Oberle et al., 1991). Large repeats of the trinucleotide lead to hypermethylation and subsequent silencing of gene expression (Chen et al., 1995). Fragile X mental retardation protein (FMRP), the protein encoded by Fmr1, binds to and suppresses translation of a group of mRNAs, some of which are downstream of metabotropic glutamate receptor (mGluR) signaling (Antar and Bassell, 2003; Bear et al., 2004; Li et al., 2001). In the absence of FMRP, affected mRNAs are released from inhibition and translation increases for several proteins (Gross et al., 2010; Hou et al., 2006; Lu et al., 2004; Zalfa et al., 2003).
Fmr1 KO mice were found to have exaggerated mGluR-dependent long-term depression (LTD) (Huber et al., 2002). Both NMDARs and AMPARs are internalized after mGluR stimulation with the pharmacological mGluR agonist (RS)-3,5-dihydroxyphenylglycine (DHPG) (Snyder et al., 2001). As STEP regulates the internalization of both NMDARs and AMPARs (Zhang et al., 2008), it is a candidate protein that was proposed to regulate aspects of the pathogenesis of FXS, and it was found that a tyrosine phosphatase was involved in the expression of mGluR-dependent LTD and that blocking tyrosine phosphatase activity prevented the expression of DHPG-induced LTD (Moult et al., 2006).
It has now been shown that STEP regulates AMPAR internalization after mGluR stimulation (Zhang et al., 2008). STEP levels are increased following DHPG stimulation and the increase in STEP expression and activity is followed by internalization of GluA1/GluA2 receptors (Zhang et al., 2008). In addition, DHPG-induced AMPAR internalization does not occur in STEP KO hippocampal cultures, but is restored in these cultures by reintroduction of active STEP protein. As a result of these findings, STEP has been termed an “LTD protein” due to its role in AMPAR endocytosis after mGluR activation and is one of a handful of proteins that regulate LTD expression (Luscher and Huber, 2010).
The mechanism by which STEP protein levels are increased in FXS involves the regulation of STEP mRNA by FMRP. STEP mRNA is one of the messages that associates with and is inhibited by FMRP (Darnell et al., 2011) and as a result, STEP expression is aberrantly high in Fmr1 KO mice (Goebel-Goody et al., 2012b). As discussed above, excessive STEP levels lead to internalization of glutamate receptors, which contributes to synaptic dysfunction. To test the hypothesis that the excess expression of STEP was contributing to the FXS phenotype, STEP levels were reduced in Fmr1 KO mice (Goebel-Goody et al., 2012b). Audiogenic seizures were significantly reduced in the Fmr1 KO mice that were also null for STEP. In addition, there was a significant reversal of some of the characteristic social abnormalities that have been observed in these mice. As one example of several tasks used to test for socialization, reduction of STEP corrected non-social anxiety-related behaviors, such as open arm exploration in the elevated plus maze. The findings demonstrated that genetically reducing STEP significantly diminished seizures and restored social and non-social anxiety-related behaviors in Fmr1 KO mice, suggesting that strategies to inhibit STEP activity may be effective for treating patients with FXS.
In a possible molecular mechanism of FXS, STEP expression aberrantly elevates in the absence of FMRP by two separate pathways. First, STEP mRNA translation is not suppressed by FMRP and STEP translation rate increases. Translation of amyloid precursor protein (APP) is also increased in the absence of FMRP, which results in more Aβ production. As discussed above, Aβ in turn inhibits the ubiquitin proteasome system, leading to reduction in STEP protein degradation. Increased STEP activity is suggested to maintain the persistent internalization of AMPARs and exaggerated mGluR-dependent LTD, causing synaptic dysfunction. Although STEP involvement in FXS pathology has been suggested in mouse models, there are no human data yet published on FXS postmortem samples.
Neuropsychiatric disorders associated with decreased STEP levels
Huntington’s disease
Huntington’s disease (HD) is a neurodegenerative disorder that affects motor coordination, and leads to cognitive decline and psychiatric difficulties. The onset of HD is typically in mid-adult life. As with FXS, HD results from a triplet repeat expansion, in this case within the coding sequence of the gene huntingtin. The gene normally has a CAG repeat of 10 to 28, and patients with HD have abnormally high number of these triplets within the coding sequence, resulting in a long sequence of glutamines. Exactly how the abnormal stretch of glutamines affects the function of huntingtin remains unclear. The role of STEP in this disease was investigated because striatal projection neurons are affected in HD.
In contrast to the increase in STEP expression observed in AD, STEP expression levels are significantly decreased in the striatum of an HD mouse model (R6/1) (Saavedra et al., 2011). The R6/1 transgenic mouse was one of the first transgenic mouse models to investigate HD and express exon 1 of the human HD gene with approximately 115 CAG repeats and is a widely used model to study the pathogenesis and therapeutic interventions in the disorder (Mangiarini et al., 1996). In addition to the decrease in STEP expression, its phosphorylation levels are also increased, further reducing STEP activity. Increased phosphorylated STEP may be due to decreased calcineurin activity and/or increased PKA activity. Due to low levels of STEP activity, phosphorylated ERK and p38 MAPK accumulate in the striatum of the mice, causing cell death and inducing HD pathology. R6/1 mice show increased resistance to quinolinic acid (QUIN)-induced excitotoxicity (Saavedra et al., 2011).
Another HD mouse model, YAC128, is excitotoxin-sensitive. These mice express full-length huntingtin with 128 CAG repeats, and are considered a model of early stage HD, because they are excitotoxin-resistant and are thought to represent later stages of the disease. In these mice, NMDARs are increased at extrasynaptic sites in the striatum, and intracellular Ca2+ levels are abnormally high (Gladding et al., 2012). Elevated Ca2+ levels activate the calcineurin/PP1 pathway, which dephosphorylates and increases STEP activity. Increased STEP activity in turn reduces expression of the NMDARs on synaptic membranes, and was proposed to contribute to HD pathogenesis (Gladding et al., 2012). Therefore, it is plausible that, in striatum of HD patients, STEP expression temporarily increases by extrasynaptic NMDARs at early stages, enhancing NMDAR internalization at synaptic sites. Then, at late stages, due to elevated activation of dopamine receptors by mutant huntingtin, calcineurin activity decreases and/or PKA activity increases, reducing STEP activity to cause cell death. The molecular mechanisms of the dysfunctional huntingtin proteins affecting NMDARs and dopamine receptors still remain unknown. Future studies will likely recognize STEP as a protein that contributes to the pathophysiology of HD.
Stroke/Ischemia
Stroke is the leading cause of death and long-term disability worldwide. On average, someone in the USA suffers from a stroke every 40 seconds. Ischemic stroke is by far the most common kind of stroke, accounting for about 88% of all forms of stroke. Despite advances in understanding the pathophysiology of ischemic stroke, successful treatment remains a major challenge in clinical medicine. Intravenous thrombolysis with tissue plasminogen activator to restore blood supply to the brain (reperfusion) remains the only pharmacologic therapy (Green, 2008; Kwiatkowski et al., 1999). Rapid reperfusion, although necessary for restoration of brain metabolic activity, is also associated with additional risks (Aronowski et al., 1997; Fujimura et al., 1999; Kuroda and Siesjo, 1997). The development of strategies to protect neurons from both ischemia and reperfusion injury is therefore an important goal.
Emerging evidence indicate that STEP is regulated at both transcriptional and post-transcriptional levels following transient focal cerebral ischemia. Using a rat model of transient ischemic stroke, an earlier study showed that occlusion of the middle cerebral artery for 2 hours followed by reperfusion leads to a time-dependent decrease in STEP mRNA level in the ischemic hemisphere. At the protein level, decrease in STEP is also observed in the ischemic hemisphere with a concomitant increase in the appearance of STEP33, the cleaved form that no longer interacts with STEP substrates (Braithwaite et al., 2008). A more extensive study evaluating the role of STEP following cerebral ischemia in rats showed that initial activation of STEP during the insult disrupts signaling through p38 MAPK, a substrate of STEP that is involved in excitotoxicity and ischemic brain damage (Deb et al., 2013). Degradation of active STEP during reperfusion precedes ischemic brain damage and is associated with secondary activation of p38 MAPK. These findings suggest that rapid activation of STEP during an ischemic insult may provide initial neuroprotection, while degradation of active STEP over time limits the efficacy of STEP, resulting in secondary activation of cytotoxic pathways that eventually leads to brain damage.
To further evaluate whether calpain-dependent cleavage of STEP61 is neurotoxic, a recent study used a 16 amino acid peptide that spans the cleavage site in STEP and showed that it inhibits calpain-mediated proteolysis of STEP61, possibly by competitive inhibition of the binding of calpain to STEP61 (Xu et al., 2009). Using oxygen glucose deprivation (OGD) as an in vitro model of ischemia, the study further showed that the tissue diffusible form of this peptide inhibitor significantly reduces OGD-induced STEP33 production. By inhibiting the degradation of endogenous STEP61, the peptide inhibitor reduced neuronal death caused by OGD in cortical slices (Xu et al., 2009), indicating a neuroprotective role of endogenous STEP. Consistent with this interpretation, a more recent study demonstrated that a mild ischemic insult by middle cerebral artery occlusion (MCAO) for 30 minutes (reperfusion 24 hours) in mice with a targeted deletion of the STEP gene results in a dramatic increase in brain injury that encompasses the striatum, cortex and hippocampus (Deb et al., 2013). To confirm the neuroprotective role of STEP, they tested the efficacy of a different STEP-derived peptide from the peptide that blocked calpain-mediated proteolysis and tested its ability to limit stroke injury. The peptide lacked the N-terminal hydrophobic region and the C-terminal phosphatase domain, bound irreversibly to p38 MAPK, was resistant to ubiquitin-mediated proteasomal degradation, and crossed the blood-brain barrier. Restoration of the STEP signaling pathway with this novel STEP mimetic in STEP KO mice was effective in reducing p38 MAPK activation and attenuating ischemic brain damage when administered at the onset of reperfusion. Using a rat model of focal cerebral ischemia (MCAO 90 minutes, reperfusion 24 hours), the study further showed that delayed administration of the STEP-derived peptide, 6 hours after the onset of stroke, following the degradation of endogenous STEP was effective in reducing ischemic brain damage. Taken together, these studies demonstrated the neuroprotective role of endogenous STEP and established the blood-brain barrier permeable STEP-mimetic as a promising new tool for stroke therapy.
Stress-related psychiatric disorders
Psychiatric disorders triggered by stress often exhibit cognitive deficits. The role of STEP in resisting the effects of stress in the brain is the subject of a recent study in rats by Yang et al., 2012, although these studies still need to be expanded to include human populations. The study by Yang and colleagues used a restraint paradigm to analyze impairments in object location memory (OLM), a cognitive task disrupted by stress. The authors show a correlation between STEP expression in the hippocampus and the development of stress-induced cognitive changes. The number of dendritic spines in the hippocampus was also correlated with STEP expression levels, suggesting that STEP is a determinant of susceptibility to stress-induced cognitive deficits. STEP knockdown in the hippocampus made experimental rats more susceptible to stress and associated OLM impairments. At the cellular level, STEP knockdown in the hippocampus resulted in increased LTP, decreased LTD, and increased ERK1/2 phosphorylation. Conversely, STEP over-expression resulted in decreased LTP, increased LTD, and reduced ERK1/2 phosphorylation compared to controls. More importantly, these rats showed more resistance to stress-induced cognitive and morphological changes. Prolonged ERK1/2 activation by STEP knockdown led to elevated Cav1.2 expression, which induced excitatory overload, a mediator of glutamate toxicity. Therefore, increasing STEP expression or reducing Cav1.2 channel activity may provide resistance to stress-related psychiatric disorders.
Molecular mechanisms regulating STEP levels and phosphorylation
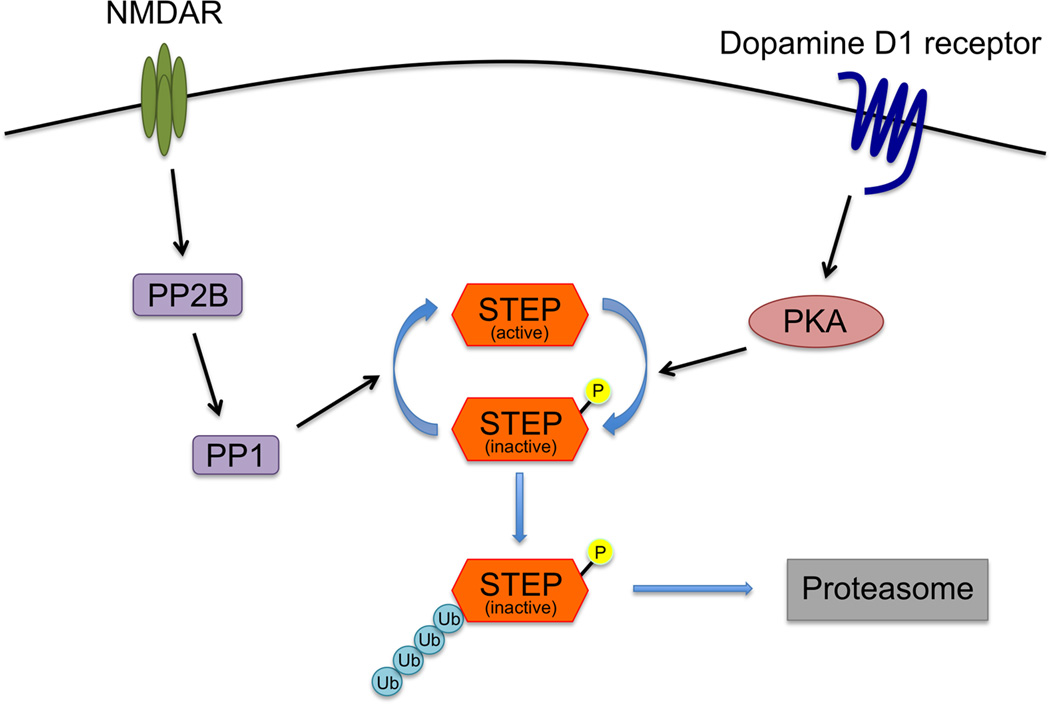
As mentioned above, the phosphorylation of a serine residue within the STEP KIM domain regulates substrate binding due to steric hindrance (Paul et al., 2003; Paul et al., 2000). Dopamine D1 receptor activation leads to PKA activation, which phosphorylates STEP at this regulatory serine. PKA also phosphorylates and activates dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32), which in turn inhibits PP1, a phosphatase that dephosphorylates the KIM domain serine residue (Paul et al., 2000; Valjent et al., 2005). PP1 and PP2B activation resulting from NMDAR and α7 nicotinic acetylcholine receptor stimulation increases dephosphorylated STEP and increases STEP activity (Paul et al., 2003; Snyder et al., 2005; Valjent et al., 2005). Therefore, a balance between dopamine receptor signaling and glutamate/acetylcholine receptor signaling maintains STEP activity by regulating STEP phosphorylation status.
STEP expression levels are also regulated by ubiquitination and degradation. Synaptic NMDAR activation induces rapid STEP polyubiquitination, which results in proteasomal degradation (Xu et al., 2009). Therefore, where synaptic activity is high, STEP levels are kept low by NMDAR activation and proteasomal degradation. STEP translation is also regulated by FMRP in mGluR-dependent LTD. However, the mechanisms that regulate STEP transcription are not well understood, and analysis of the promoter on the STEP gene will be useful for this purpose as well as the involvement of microRNAs in the regulation of STEP mRNA levels.
Although not yet been implicated in neuropsychiatric disorders, a few other mechanisms modify STEP activity. For example, calpain-mediated cleavage of STEP61 induced by extrasynaptic NMDAR stimulation generates STEP33, which has no phosphatase activity (Xu et al., 2009). Extrasynaptic NMDAR stimulation activates p38 MAPK, which maintains the active, phosphorylated state due to reduction in STEP61 levels, triggering the cell death cascade. Another example to modify STEP activity is the dimerization of STEP61. This dimerization requires the cysteine residues, Cys65 and Cys76, which reside within the hydrophobic region of STEP61 (Deb et al., 2011). Some STEP61 dimers are present at basal levels, and dimerization increases by hydrogen peroxide-induced oxidative stress, which decreases STEP’s phosphatase activity. STEP46, on the other hand, is not dimerized at basal conditions as it lacks the cysteine residues. However, oxidative stress can generate STEP46 oligomers as well as dimers and oligomers of STEP61 (Deb et al., 2011).
STEP translation by synaptic stimulation also modifies STEP activity. mGluR activation with DHPG induces STEP translation at dendrites through MAPK and phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K) signaling pathways. This kind of local translation occurs for a number of proteins at dendrites and is an essential process in the development of synaptic strengthening. Certain situations also modify STEP activity. For example, fear conditioning in rodents results in the de novo synthesis of STEP46 in the basal lateral amygdala (Paul et al., 2007). Prior to fear conditioning, only STEP61 is expressed in this region, and was proposed to be a mechanism to down-regulate ERK1/2 activity (Paul et al., 2007).
STEP-interacting kinases in neuropsychiatric disorders
Thus far, we have described STEP involvements in neuropsychiatric disorders. Since STEP is phosphorylated by PKA and dephosphorylates other kinases, these target kinases may play roles in these disorders. Although the STEP substrates ERK1/2 and p38 MAPK have not directly been described in neuropsychiatric disorders, these are critical regulators in cell survival or death. At synaptic sites, NMDAR activation leads to ERK1/2 phosphorylation and activation, inducing cell survival signaling (Xu et al., 2009). At extrasynaptic sites, NMDAR activation leads to p38 MAPK phosphorylation and activation, leading to apoptotic signaling (Xu et al., 2009). By dephosphorylating each of them, STEP can regulate cell survival or death mechanisms.
The kinase that regulates STEP activity, PKA, has been implicated in mood disorders, HD, and age-associated neurodegeneration, and PKA activity is high in these disorders (Carlyle et al., 2014; Dwivedi and Pandey, 2008; Giralt et al., 2011). A Src family kinase, Fyn, has been associated with AD, and Fyn is up-regulated in the disease (Lee et al., 2004; Roberson et al., 2011). Importantly, STEP dephosphorylates Fyn and suppresses its kinase activity, and also dephosphorylates NMDARs at the site phosphorylated by Fyn. Molecular mechanisms that involve PKA and Fyn to cause these psychiatric disorders are fairly complex, because there is tau, a microtubule-associated protein, which is a key player in these mechanisms (Boehm, 2013). Although the connection between tau and STEP is still unclear, future studies will uncover whether mechanisms exist that involve both proteins.
Lastly, a newly identified substrate of STEP, Pyk2, is a tyrosine kinase related to focal adhesion kinase (Xu et al., 2012). It up-regulates NMDARs via activation of Src and promotes ERK1/2 activation (Dikic et al., 1996; Huang et al., 2001). While Pyk2 involvements in neuropsychiatric disorders are unknown, it likely functions in the mechanisms that contribute to these disorders.
Conclusion
This review presents recent findings establishing a role of STEP in the pathogenesis of several neuropsychiatric disorders. There are some differences in underlying mechanisms for each illness. For example, Aβ accumulation in AD leads to multiple different signaling pathways, including ones that lead to increased STEP expression. In SZ, the ubiquitin proteasome system is disrupted, leading to the accumulation of several proteins, including STEP. In FXS, loss of FMRP protein leads to aberrant expression of several proteins including STEP. Although STEP-independent pathways likely contribute to these illnesses, cognitive deficits are caused by dysregulated STEP function and its signaling.
In all diseases in which STEP is increased, we have examined the levels of STEP substrates and these have decreased Tyr phosphorylation levels. Our working hypothesis is that STEP regulates the activity of ERK1/2, Fyn, Pyk2, and the glutamate receptors in parallel and that dephosphorylation of these targets work together to oppose the development of synaptic strengthening. This hypothesis is supported by the reports of Fyn, NR2B, GluR2, and ERK1/2 KO mice, in which each was shown to have cognitive deficits (Jia et al., 1996; Kojima et al., 1997; Kutsuwada et al., 1996; Mazzucchelli et al., 2002). Although NMDARs are clearly central in cognitive functions, we do not have evidence at the present time that the regulation by STEP of Tyr1472 on the GluN2B subunit of NMDARs by itself is sufficient to reverse cognitive deficits. This is likely a critical event, but the regulation of local protein translation and gene transcription by the STEP substrate ERK1/2 is also necessary for long-term cognitive processes. These results suggest that although disruption of any of these STEP substrates can contribute to cognitive deficits, STEP functions to regulate these proteins in a coordinated fashion.
It is now clear that appropriate STEP levels must be maintained within an optimal range in the brain for normal cognitive function. An interesting question arising from these recent findings is why does one develop AD and not PD or FXS if STEP levels are elevated in all of these disorders? Although the answer to this question has not yet been fully investigated, it is likely that there is a differential regulation of STEP posttranslational modifications, depending on different brain regions. For example, phosphorylation of STEP61 after alcohol consumption was described in the dorsomedial striatum but not in the nearby dorsolateral striatum or the nucleus accumbens (Darcq et al., 2014), while dephosphorylation of STEP61 was reported within the hippocampus after alcohol consumption (Hicklin et al., 2011). The differential regulation of STEP is an open area of research in the biology of STEP and should provide additional insights into mechanisms by which disruption of STEP activity may give rise to one distinct disorder compared to another.
For those disorders in which STEP is hyperactive, a potential clinical strategy is the use of STEP inhibitors. A study using a substrate activity screening method identified a platform of STEP inhibitors, although much work is needed to establish a viable STEP inhibitor (Baguley et al., 2013). In addition, a more recent study using a classical high-throughput screen identified a novel STEP inhibitor (TC-2153, a pentathiepin with an IC50 of 25 nM). Administration of TC-2153 in vivo was sufficient to reverse the cognitive deficits in an AD mouse model (Xu et al., 2014). Neuropsychiatric disorders associated with low levels of STEP could benefit from peptide delivery such as one used to study ischemia as described above, or from viral-based STEP gene delivery. Future studies will hopefully identify pharmacological agents to modulate STEP activity and potentially treat neuropsychiatric disorders in which STEP activity is disrupted.
Figure 2.
Regulation of STEP expression and activity. NMDAR activation leads to dephosphorylation of STEP through the activation of a PP2B (calcineurin) / PP1 pathway, and dephosphorylation of STEP results its activation. D1 receptor stimulation induces phosphorylation and inactivation of STEP via PKA signaling. Phosphorylated STEP undergoes ubiquitination, which results in proteasomal degradation of STEP.
Highlights.
The tyrosine phosphatase STEP is plays a major role in synaptic function.
STEP is involved in pathophysiology of several neuropsychiatric disorders.
Increased STEP levels are associated with diseases like Alzheimer’s disease.
Decreased STEP levels are associated with diseases like Huntington’s disease.
STEP expression must be maintained at optimal levels for normal cognitive function.
Acknowledgements
NIH grants MH091037 and MH52711 (PJL) supported the research reported in this review. We thank Drs. Surojit Paul, Hisayuki Yokokura and Zachery Oestreicher for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi M, Sekiya M, Arimura Y, Takekawa M, Itoh F, Hinoda Y, Imai K, Yachi A. Protein-tyrosine phosphatase expression in pre-B cell NALM-6. Cancer Res. 1992;52:737–740. [PubMed] [Google Scholar]
- Antar LN, Bassell GJ. Sunrise at the synapse: the FMRP mRNP shaping the synaptic interface. Neuron. 2003;37:555–558. doi: 10.1016/s0896-6273(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Baguley TD, Xu HC, Chatterjee M, Nairn AC, Lombroso PJ, Ellman JA. Substrate-based fragment identification for the development of selective, nonpeptidic inhibitors of striatal-enriched protein tyrosine phosphatase. J Med Chem. 2013;56:7636–7650. doi: 10.1021/jm401037h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55:1121–1127. doi: 10.1016/j.biopsych.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Boehm J. A ‘danse macabre’: tau and Fyn in STEP with amyloid beta to facilitate induction of synaptic depression and excitotoxicity. Eur J Neurosci. 2013;37:1925–1930. doi: 10.1111/ejn.12251. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite SP, Xu J, Leung J, Urfer R, Nikolich K, Oksenberg D, Lombroso PJ, Shamloo M. Expression and function of striatal enriched protein tyrosine phosphatase is profoundly altered in cerebral ischemia. Eur J Neurosci. 2008;27:2444–2452. doi: 10.1111/j.1460-9568.2008.06209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs SW, Walker J, Asik K, Lombroso P, Naegele J, Aaron G. STEP regulation of seizure thresholds in the hippocampus. Epilepsia. 2011;52:497–506. doi: 10.1111/j.1528-1167.2010.02912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillette J, Ménard C, Quirion R, Bontempi B, Schneider JS, Norris CM, Ferland G, Bézard E, Gaudreau P, Lombroso PJ. The tyrosine phosphatase STEP is implicated in age-related memory decline across different species. (under review) [Google Scholar]
- Bult A, Zhao F, Dirkx R, Jr, Sharma E, Lukacsi E, Solimena M, Naegele JR, Lombroso PJ. STEP61: a member of a family of brain-enriched PTPs is localized to the endoplasmic reticulum. J Neurosci. 1996;16:7821–7831. doi: 10.1523/JNEUROSCI.16-24-07821.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyle BC, Nairn AC, Wang M, Yang Y, Jin LE, Simen AA, Ramos BP, Bordner KA, Craft GE, Davies P, Pletikos M, Sestan N, Arnsten AF, Paspalas CD. cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proc Natl Acad Sci U S A. 2014;111:5036–5041. doi: 10.1073/pnas.1322360111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty NC, Xu J, Kurup P, Brouillette J, Goebel-Goody SM, Austin DR, Yuan P, Chen G, Correa PR, Haroutunian V, Pittenger C, Lombroso PJ. The tyrosine phosphatase STEP: implications in schizophrenia and the molecular mechanism underlying antipsychotic medications. Transl Psychiatry. 2012;2:e137. doi: 10.1038/tp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Mariappan SV, Catasti P, Ratliff R, Moyzis RK, Laayoun A, Smith SS, Bradbury EM, Gupta G. Hairpins are formed by the single DNA strands of the fragile X triplet repeats: structure and biological implications. Proc Natl Acad Sci U S A. 1995;92:5199–5203. doi: 10.1073/pnas.92.11.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Palop JJ, Puolivali J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodi V, Mallozzi C, Ferrante A, Chen JF, Lombroso PJ, Di Stasi AM, Popoli P. Cocaine-induced changes of synaptic transmission in the striatum are modulated by adenosine A2A receptors and involve the tyrosine phosphatase STEP. Neuropsychopharmacology. 2014;39:569–578. doi: 10.1038/npp.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Turk J, Hagerman R. The fragile X continuum: new advances and perspectives. J Intellect Disabil Res. 2008;52:469–482. doi: 10.1111/j.1365-2788.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo JD, Li C, Dewitt S, Xu J, Lombroso PJ, Rainnie DG. Striatal-enriched protein tyrosine phosphatase-STEPs toward understanding chronic stress-induced activation of corticotrophin releasing factor neurons in the rat bed nucleus of the stria terminalis. Biol. Psychiatry. 2013;74:817–826. doi: 10.1016/j.biopsych.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Hamida SB, Wu S, Phamluong K, Kharazia V, Xu J, Lombroso P, Ron D. Inhibition of striatal-enriched tyrosine phosphatase 61 in the dorsomedial striatum is sufficient to increased ethanol consumption. J Neurochem. 2014;129:1024–1034. doi: 10.1111/jnc.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb I, Manhas N, Poddar R, Rajagopal S, Allan AM, Lombroso PJ, Rosenberg GA, Candelario-Jalil E, Paul S. Neuroprotective role of a brain-enriched tyrosine phosphatase, STEP, in focal cerebral ischemia. J Neurosci. 2013;33:17814–17826. doi: 10.1523/JNEUROSCI.2346-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb I, Poddar R, Paul S. Oxidative stress-induced oligomerization inhibits the activity of the non-receptor tyrosine phosphatase STEP61. J Neurochem. 2011;116:1097–1111. doi: 10.1111/j.1471-4159.2010.07165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Pandey GN. Adenylyl cyclase-cyclicAMP signaling in mood disorders: role of the crucial phosphorylating enzyme protein kinase A. Neuropsychiatr Dis Treat. 2008;4:161–176. doi: 10.2147/ndt.s2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Forlenza OV, Diniz BS, Gattaz WF. Diagnosis and biomarkers of predementia in Alzheimer’s disease. BMC Med. 2010;8:89. doi: 10.1186/1741-7015-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842:92–100. doi: 10.1016/s0006-8993(99)01843-0. [DOI] [PubMed] [Google Scholar]
- Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- Garner CC, Wetmore DZ. Synaptic pathology of Down syndrome. Adv Exp Med Biol. 2012;970:451–468. doi: 10.1007/978-3-7091-0932-8_20. [DOI] [PubMed] [Google Scholar]
- Giralt A, Saavedra A, Carreton O, Xifro X, Alberch J, Perez-Navarro E. Increased PKA signaling disrupts recognition memory and spatial memory: role in Huntington’s disease. Hum Mol Genet. 2011;20:4232–4247. doi: 10.1093/hmg/ddr351. [DOI] [PubMed] [Google Scholar]
- Gladding CM, Sepers MD, Xu J, Zhang LY, Milnerwood AJ, Lombroso PJ, Raymond LA. Calpain and STriatal-Enriched protein tyrosine phosphatase (STEP) activation contribute to extrasynaptic NMDA receptor localization in a Huntington’s disease mouse model. Hum Mol Genet. 2012;21:3739–3752. doi: 10.1093/hmg/dds154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, Lombroso PJ. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev. 2012a;64:65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody SM, Wilson-Wallis ED, Royston S, Tagliatela SM, Naegele JR, Lombroso PJ. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav. 2012b;11:586–600. doi: 10.1111/j.1601-183X.2012.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Green AR. Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. Br J Pharmacol. 2008;1(153 Suppl):S325–S338. doi: 10.1038/sj.bjp.0707594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, Warren ST, Bassell GJ. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci. 2010;30:10624–10638. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hendriks WJ, Dilaver G, Noordman YE, Kremer B, Fransen JA. PTPRR protein tyrosine phosphatase isoforms and locomotion of vesicles and mice. Cerebellum. 2009;8:80–88. doi: 10.1007/s12311-008-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicklin TR, Wu PH, Radcliffe RA, Freund RK, Goebel-Goody SM, Correa PR, Proctor WR, Lombroso PJ, Browning MD. Alcohol inhibition of the NMDA receptor function, long-term potentiation, and fear learning requires striatal-enriched protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 2011;108:6650–6655. doi: 10.1073/pnas.1017856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, MacDonald JF. CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron. 2001;29:485–496. doi: 10.1016/s0896-6273(01)00220-3. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Dendritic and synaptic pathology in mental retardation. Pediatr Neurol. 1991;7:79–85. doi: 10.1016/0887-8994(91)90001-2. [DOI] [PubMed] [Google Scholar]
- Ibrahim HM, Hogg AJ, Jr, Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH. Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am J Psychiatry. 2000;157:1811–1823. doi: 10.1176/appi.ajp.157.11.1811. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna FA, Velumian A, MacDonald J, Carlen P, Abramow-Newerly W, Roder J. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron. 1996;17:945–956. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- Kim JH, Anwyl R, Suh YH, Djamgoz MB, Rowan MJ. Use-dependent effects of amyloidogenic fragments of (beta)-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J Neurosci. 2001;21:1327–1333. doi: 10.1523/JNEUROSCI.21-04-01327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N, Wang J, Mansuy IM, Grant SG, Mayford M, Kandel ER. Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene. Proc Natl Acad Sci U S A. 1997;94:4761–4765. doi: 10.1073/pnas.94.9.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Siesjo BK. Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clin Neurosci. 1997;4:199–212. [PubMed] [Google Scholar]
- Kurup P, Xu J, Ononenyi C, Nairn AC, Lombroso C. Role of Striatal-Enriched protein tyrosine Phosphatase (STEP) in Parkinsons’s disease. Poster, SFN, Washington, 44.10/C65: 2012. [Google Scholar]
- Kurup P, Xu J, Videira RA, Ononenyi C, Baltazar G, Nairn AC, Lombroso PJ. Role of striatal-enriched protein tyrosine phosphatase (STEP) in Parkinson’s disease. (under review) [Google Scholar]
- Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, Nairn AC, Lombroso PJ. Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TG, Libman RB, Frankel M, Tilley BC, Morgenstern LB, Lu M, Broderick JP, Lewandowski CA, Marler JR, Levine SR, Brott T. Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. N Engl J Med. 1999;340:1781–1787. doi: 10.1056/NEJM199906103402302. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, Do LH, Andreadis A, Van Hoesen G, Ksiezak-Reding H. Phosphorylation of tau by fyn: implications for Alzheimer’s disease. J Neurosci. 2004;24:2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001;29:2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso PJ, Murdoch G, Lerner M. Molecular characterization of a protein-tyrosine-phosphatase enriched in striatum. Proc Natl Acad Sci U S A. 1991;88:7242–7246. doi: 10.1073/pnas.88.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber B, Berry M, Hendriks W, den Hertog J, Pulido R, Logan A. Stimulated regeneration of the crushed adult rat optic nerve correlates with attenuated expression of the protein tyrosine phosphatases RPTPalpha, STEP, and LAR. Mol Cell Neurosci. 2004;27:404–416. doi: 10.1016/j.mcn.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O’Donnell W T, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci U S A. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pages G, Valverde O, Marowsky A, Porrazzo A, Orban PC, Maldonado R, Ehrengruber MU, Cestari V, Lipp HP, Chapman PF, Pouyssegur J, Brambilla R. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–2554. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Paul S, Xu Y, Gurd JW, Lombroso PJ. Calcium-dependent cleavage of striatal enriched tyrosine phosphatase (STEP) J Neurochem. 1999;73:1995–2001. [PubMed] [Google Scholar]
- Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas MF, Mandel JL. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Okamura A, Goto S, Nishi T, Yamada K, Yoshikawa M, Ushio Y. Postnatal ontogeny of striatal-enriched protein tyrosine phosphatase (STEP) in rat striatum. Exp Neurol. 1997;145:228–234. doi: 10.1006/exnr.1997.6435. [DOI] [PubMed] [Google Scholar]
- Olausson P, Venkitaramani DV, Moran TD, Salter MW, Taylor JR, Lombroso PJ. The tyrosine phosphatase STEP constrains amygdala-dependent memory formation and neuroplasticity. Neuroscience. 2012;225:1–8. doi: 10.1016/j.neuroscience.2012.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T, Goto S, Nishi T, Sato K, Yamada K, Yoshikawa M, Ushio Y. Immunocytochemical localization of the striatal enriched protein tyrosine phosphatase in the rat striatum: a light and electron microscopic study with a complementary DNA-generated polyclonal antibody. Neuroscience. 1995;69:869–880. doi: 10.1016/0306-4522(95)00278-q. [DOI] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Moran TD, Tronson N, Mills E, Hakim S, Salter MW, Taylor JR, Lombroso PJ. The striatal-enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry. 2007;61:1049–1061. doi: 10.1016/j.biopsych.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Snyder GL, Yokakura H, Picciotto MR, Nairn AC, Lombroso PJ. The Dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci. 2000;20:5630–5638. doi: 10.1523/JNEUROSCI.20-15-05630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelov I, Teltsh O, Greenbaum L, Rigbi A, Kanyas-Sarner K, Lerer B, Lombroso P, Kohn Y. Involvement of PTPN5, the gene encoding the striatal-enriched protein tyrosine phosphatase, in schizophrenia and cognition. Psychiatr Genet. 2012;22:168–176. doi: 10.1097/YPG.0b013e3283518586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido R, Zuniga A, Ullrich A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J. 1998;17:7337–7350. doi: 10.1093/emboj/17.24.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan A, Matthews GA, Lombroso PJ, Naegele JR. Transient compartmental expression of a family of protein tyrosine phosphatases in the developing striatum. Brain Res Dev Brain Res. 1996;91:190–199. doi: 10.1016/0165-3806(95)00176-x. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu GQ, Palop JJ, Noebels JL, Mucke L. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum G, Cohen BD, Luby ED, Gottlieb JS, Yelen D. Comparison of sernyl with other drugs: simulation of schizophrenic performance with sernyl, LSD-25, and amobarbital (amytal) sodium; I. Attention, motor function, and proprioception. AMA Arch Gen Psychiatry. 1959;1:651–656. doi: 10.1001/archpsyc.1959.03590060113013. [DOI] [PubMed] [Google Scholar]
- Saavedra A, Giralt A, Rue L, Xifro X, Xu J, Ortega Z, Lucas JJ, Lombroso PJ, Alberch J, Perez-Navarro E. Striatal-enriched protein tyrosine phosphatase expression and activity in Huntington’s disease: a STEP in the resistance to excitotoxicity. J Neurosci. 2011;31:8150–8162. doi: 10.1523/JNEUROSCI.3446-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shumway SD, Maki M, Miyamoto S. The PEST domain of IkappaBalpha is necessary and sufficient for in vitro degradation by mu-calpain. J Biol Chem. 1999;274:30874–30881. doi: 10.1074/jbc.274.43.30874. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Spencer ML, Theodosiou M, Noonan DJ. NPDC-1, a novel regulator of neuronal proliferation, is degraded by the ubiquitin/proteasome system through a PEST degradation motif. J Biol Chem. 2004;279:37069–37078. doi: 10.1074/jbc.M402507200. [DOI] [PubMed] [Google Scholar]
- Turalba AV, Leite-Morris KA, Kaplan GB. Antipsychotics regulate cyclic AMP-dependent protein kinase and phosphorylated cyclic AMP response element-binding protein in striatal and cortical brain regions in mice. Neurosci Lett. 2004;357:53–57. doi: 10.1016/j.neulet.2003.11.059. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB. The synaptic pathology of drug addiction. Adv Exp Med Biol. 2012;970:469–491. doi: 10.1007/978-3-7091-0932-8_21. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Shiozuka K, Ikeda T, Hoshi N, Hiraki H, Suzuki T, Hashimoto S, Kawashima H. Cloning of PCPTP1-Ce encoding protein tyrosine phosphatase from the rat cerebellum and its restricted expression in Purkinje cells. Brain Res Mol Brain Res. 1998;58:83–94. doi: 10.1016/s0169-328x(98)00100-4. [DOI] [PubMed] [Google Scholar]
- Xu J, Chatterjee M, Baguley TD, Brouillette J, Kurup P, Ghosh D, Kanyo J, Zhang Y, Seyb K, Onomenyi C, Foscue E, Anderson GM, Gressack J, Cuny GD, Glicksman MA, Greengard P, Lam T, Nairn AC, Tautz L, Ellman JA, Lombroso PJ. Inhibitors of the tyrosine phosphatase STEP reverse cognitive deficits in a mouse model of Alzheimer’s disease. PLoS Biol. 2014;12:e1001923. doi: 10.1371/journal.pbio.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kurup P, Bartos JA, Patriarchi T, Hell JW, Lombroso PJ. Striatal-enriched protein-tyrosine phosphatase (STEP) regulates Pyk2 kinase activity. J Biol Chem. 2012;287:20942–20956. doi: 10.1074/jbc.M112.368654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. A critical role for protein tyrosine phosphatase nonreceptor type 5 in determining individual susceptibility to develop stress-related cognitive and morphological changes. J Neurosci. 2012;32:7550–7562. doi: 10.1523/JNEUROSCI.5902-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xie JW, Yang J, Cao YP. Tyrosine phosphatase STEP61 negatively regulates amyloid beta-mediated ERK/CREB signaling pathways via alpha7 nicotinic acetylcholine receptors. J Neurosci Res. 2013;91:1581–1590. doi: 10.1002/jnr.23263. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Anderson GM, Greengard P, Nairn AC, Lombroso PJ. Reduced levels of the tyrosine phosphatase STEP block beta amyloid-mediated GluA1/GluA2 receptor internalization. J Neurochem. 2011;119:664–672. doi: 10.1111/j.1471-4159.2011.07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB, Pittenger C, Greengard P, Strittmatter SM, Nairn AC, Lombroso PJ. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer’s disease mouse model. Proc Natl Acad Sci U S A. 2010;107:19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]