Abstract
The therapeutic management of chronic pain associated with many cancers is problematic due to development of tolerance and other adverse effects during the disease progression. Recently we reported on a bivalent ligand (MMG22) containing both mu agonist and mGluR5 antagonist pharmacophores that produced potent antinociception in mice with LPS-induced acute inflammatory pain via a putative MOR-mGluR5 heteromer. In the present study we have investigated the antinociception of MMG22 in a mouse model of bone cancer pain to determine its effectiveness in reducing this type of chronic nociception. There was a 572-fold increase in the potency of MMG22 over a period of 3–21 days that correlated with the progressive increase in hyperalgesia induced by bone tumor growth following implantation of fibrosarcoma cells in mice. The enhancement of antinociception with the progression of the cancer is possibly due to inhibition of NMDA receptor-mediated hyperalgesia via antagonism of mGluR5 and concomitant activation of MOR by the MMG22-occupied heteromer. Notably, MMG22 was 3.6-million-fold more potent than morphine at PID 21. Since MMG22 exhibited a 250,000-times greater potency than that of a mixture of the mu opioid (M19) agonist and mGluR5 antagonist (MG20) monovalent ligands, the data suggest that targeting the putative MOR-mGluR5 heteromer is far superior to univalent interaction with receptors in reducing tumor-induced nociception. In view of the high potency, long duration (>24 h) of action and minimal side effects, MMG22 has the potential to be a superior pharmacological agent than morphine and other opiates in the treatment of chronic cancer pain and to serve as a novel pharmacologic tool.
Keywords: hyperalgesia, fibrosarcoma, MPEP, inflammatory pain
1. Introduction
In spite of the prevalence of chronic pain associated with cancer, the number of systematic studies to develop new analgesics to reduce cancer pain is substantially less than efforts to treat the disease. Consequently, there have been few if any new drugs approved for treatment of chronic cancer pain in recent years (Cleeland et al., 2011). While analgesics such as morphine remain the mainstay treatment option for severe cancer pain, they provide inadequate analgesia in more than half of the patients (Te et al., 2013) and display dose-limiting adverse effects (Muralidharan and Smith, 2013).
Reports have revealed that coadministration of metabotropic glutamate receptor-5 (mGluR5) antagonists suppress morphine tolerance and dependence and enhance antinociception when coadministered (Kozela et al., 2003; Sotgiu et al., 2003; Walker et al., 2000; 2001a; Walker et al., 2001b). Based on these reports and evidence for a MOR-mGluR5 heteromer in cultured cells (Schröder et al., 2009), a bivalent ligand (MMG22) that contains a mu opioid agonist and mGluR5 antagonist pharmacophores was developed (Akgün et al., 2013). MMG22 displayed exceptionally potent antinociception upon intrathecal (i.t.) administration in an acute inflammatory pain mouse model. Based on structure-activity relationship studies, it was concluded that the target of MMG22 is a putative MOR-mGluR5 heteromer in the spinal cord. The involvement of MOR-mGluR5 heteromer is consistent with the finding that in the inflammatory state, colocalized MOR and mGluR5 are upregulated in glia and neurons within the dorsal horn where they play a role in neuromodulation of pain (Neugebauer, 2001; Ren et al., 2012).
In view of the exceptional i.t. potency (ED50 ~9 fmol/mouse) and high therapeutic ratio (~106) of MMG22, here we present a study relevant to the effectiveness of MMG22 in reducing chronic tumor-induced hyperalgesia (Cavalheiro and Olney, 2001; Choi et al., 2011; Park et al., 2007; Prickett and Samuels, 2012; Sontheimer, 2003), using an established chronic bone cancer model in mice. Significantly, our results reveal that i.t. administration of MMG22 produces profound, long duration antinociception that becomes more potent during cancer progression.
2. Methods and Materials
2.1 Cell Culture
National Collection of Type Cultures (NCTC) clone 2472 fibrosarcoma cells, originally derived from a connective tissue tumor in a C3H mouse, were obtained from the American Type Cell Culture Collection (Rockville, MD). Cells were grown and maintained in accordance with standard cell culturing techniques. NCTC cells were grown to 80–90% confluence in 75 cm2 flasks (Corning, Lowell, MA) in Dulbecco’s Modification of Eagles Medium (Invitrogen, Carlsbad, CA) fortified with 10% Horse Serum and sodium bicarbonate. Cell cultures were housed in a water-jacketed incubator with 5% carbon dioxide at 37°C.
2.2 Animals
Male C3H mice (National Cancer Institute, Bethesda, MA) were used for all experiments. The inbred C3H/He line is synergistic to the fibrosarcoma cells used in these experiments and allows these cells to grow tumors without rejection. Animals were maintained on a 12 h light/dark cycle with food and water ad libitum. Each compound had a separate group of mice that were used for 21 days.
2.3 Implantation
Tumor cells were manually injected by boring into the calcaneus bone using a 29 ½ gauge needle connected to a sterile 0.3 ml insulin syringe in the left hind paw. Following injection, mice were allowed to recover in cages on a heating pad (Smeester et al., 2012; Smeester et al., 2013; Wacnik et al., 2001.
2.4 Intrathecal Injection
All compounds (Fig 1) were synthesized according to previously published protocols (Akgün et al., 2013). For in vivo studies, all compounds were dissolved in 10% DMSO and then diluted to less than 1% DMSO in the test solutions. DMSO when given i.t. in a 1% or less concentrated solution did not show any antinociception. Morphine (Mallinckrodt) was dissolved in sterile saline. Compounds were administered i.t. in a 5-μl volume in conscious mice to determine peak time and ED50/80 (Hylden and Wilcox, 1980).
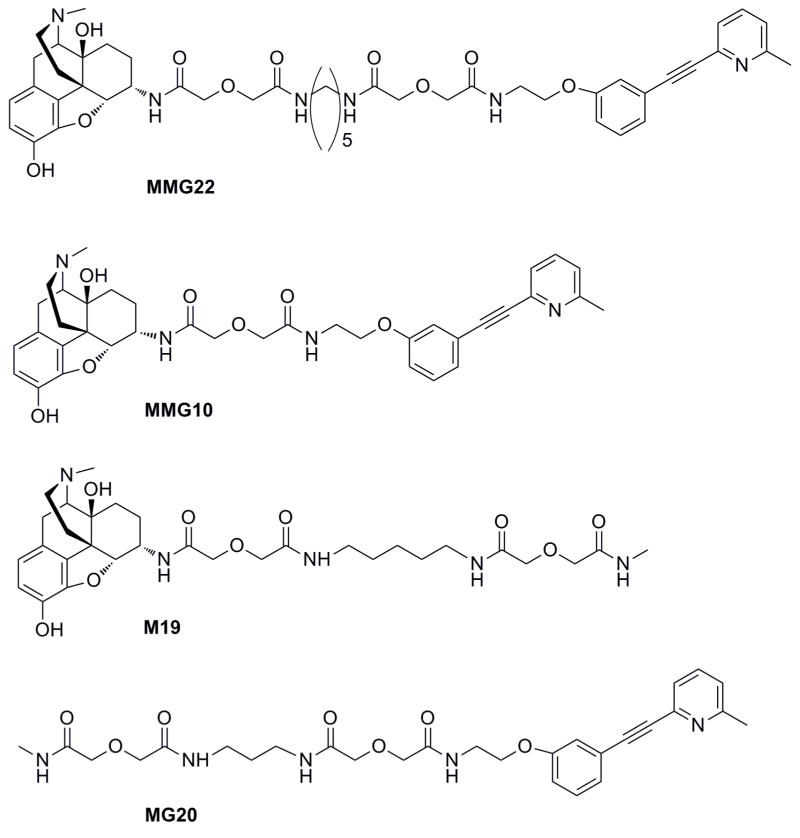
Fig. 1.
Chemical structures of bivalent (MMG22; MMG10) and monovalent (M19, MG20) ligands evaluated in C3H mouse bone cancer studies.
2.5 Mechanical Hyperalgesia
Animals were placed under clear glass cups on a wire grid and allowed to acclimate for 30 min (Smeester et al., 2012; Smeester et al., 2013). Mechanical hypersensitivity was tested using a von Frey filament #3.61, which produces a force of 70 mg, was applied to the plantar surface of both hind paws with enough force to cause it to bow slightly, the numbers of positive responses out of a total of 10 applications were recorded (Raghavendra et al., 2003; Smeester et al., 2012; Wacnik et al., 2001). Baseline von Frey measurements were obtained prior to tumor-implantation or saline injection into the calcaneus. Subsequent von Frey measurements were taken prior to compound injection as well as 5, 10 and 20 minutes post-injection on days (PID) 0, 3, 7, 10, 14, 17 and 21. Animals with saline injection served as controls for tumor cell implantation and tumor-induced nociception. The %MPE was calculated [(Time-point value − Day# baseline)/(Day 0 baseline − Day# baseline)] × 100 (Akgün et al., 2013) Behavioral assessments were conducted during the light cycle at approximately the same time each day. The investigator performing the von Frey testing was blinded to the animal and compound injected.
2.6 Dose Optimization
The individual monovalents, M19 (mu agonist) and MG20 (mGluR5 antagonist) were evaluated for efficacy prior to combined i.t. administration. M19 showed increasing potency from PID 3 to PID 21 (ED80 250,000 fmol and 50 fmol, respectively). Latent and increasing %MPE was observed with a MG20 dose of 250,000 fmol on PID 3 was 25%, but was 75% on PID 21 with identical dose. To test for synergism based on the individual doses, a final concentration of 50 fmol M19 and 250,000 of MG20 was tested over 17 days. On PID 21, the dose was decreased to 1/4th of the original since this was when M19 and MG20 had the highest %MPE (Figure 3). The dose for MMG22 is the highest point in the dose response curves from Fig 2.
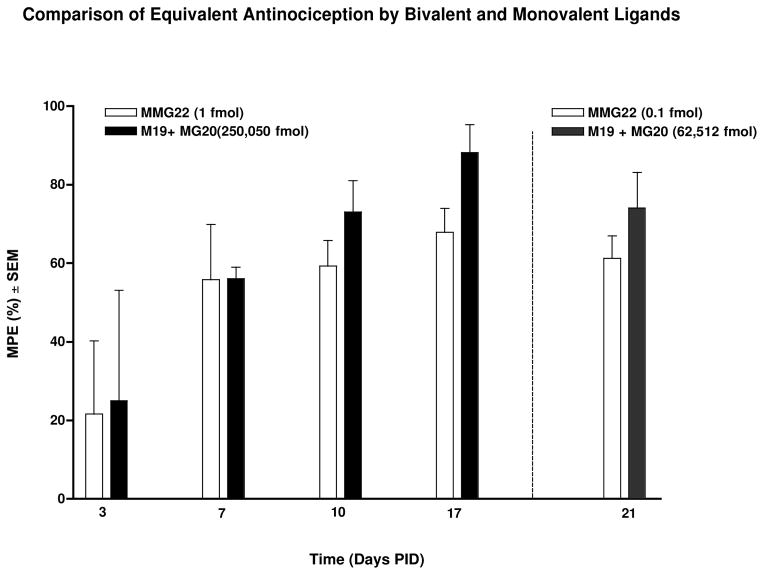
Fig. 3.
Comparison of equivalent antinociception by MMG22 and monovalent ligands (M19 + MG20). Beginning on PID 3 and continuing through the course of the experiment, intrathecal (i.t.) administration of either MMG22 or a mixture of monovalent ligands, M19 (mu agonist) + MG20 (mGluR5 antagonist), produced antinociception in tumor-bearing animals. MMG22 was exceptionally more potent than the combination of monovalent ligands M19 and MG22 at all time points evaluated. MMG22 or its co-administered monovalents (M19 + MG20) were tested at lower doses to show the increase in potency PID 21 (n = 4–11/group).
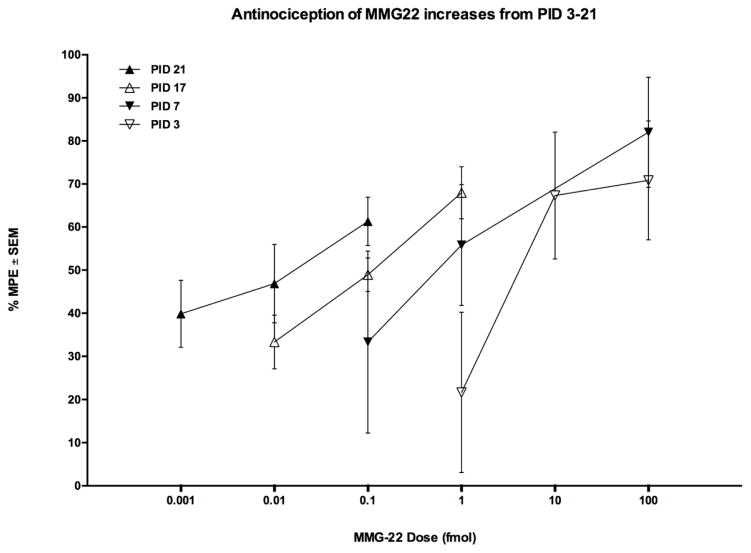
Fig. 2.
Antinociception of i.t. MMG22 increases from PID 3–21. The increasing potency occurred at all subsequent time points tested, The shift in dose-response over a 18-day period (PID 3–21) resulted in a ~570-fold increase in potency.
2.7 Statistics
Data shown as mean ± S.E.M. Comparisons between groups were performed using two-way ANOVA with post hoc comparisons using Bonferroni’s method (Bonferroni, 1936). The level of significance was set at P ≤ 0.05.
2.8 Study Approval
Procedures were performed in accordance with the guidelines recommended by the International Association for the Study of Pain and all experimental protocols were approved by the Animal Care and Use Committee at the University of Minnesota.
3. Results
3.1 The Potency of MMG22 Increases with Growth of Fibrosarcoma
Administration of MMG22 (i.t., 5 minute peak time) was evaluated for its anti-hyperalgesic effect in fibrosarcoma-bearing mice. Beginning from PID 3, increased potency was observed at all-time points tested (Fig 2). The ED50’s were: PID 3, 5.72 fmol/mouse (0.99 – 32.98); PID 7, 0.60 (0.04 – 9.0); PID 17, 0.10 (.003 – 0.266); PID 21, 0.01 fmol/mouse (0.001 – 0.08). The potency increase was 572-times greater on PID 21 than on PID 3. No tolerance was evident for MMG22 over the 21 days of repeated i.t. injections. Moreover, we did not observe any evidence of chronic drug toxicity over the 21-day experimental period even though in some experiments the dose of MMG22 was a million-fold greater than its ED50. In this regard, following drug administration animals maintained normal body weight and exhibited normal eating and drinking behavior, normal respiratory rate and normal urination over the entire course of the study. We did not examine MMG22 beyond the 21-day endpoint for humane reasons, since the tumor in some of the animals reaches a diameter of 2 cubic centimeters beyond this time point.
3.2 Single Dose of MMG22 Promotes Long Duration Antinociception
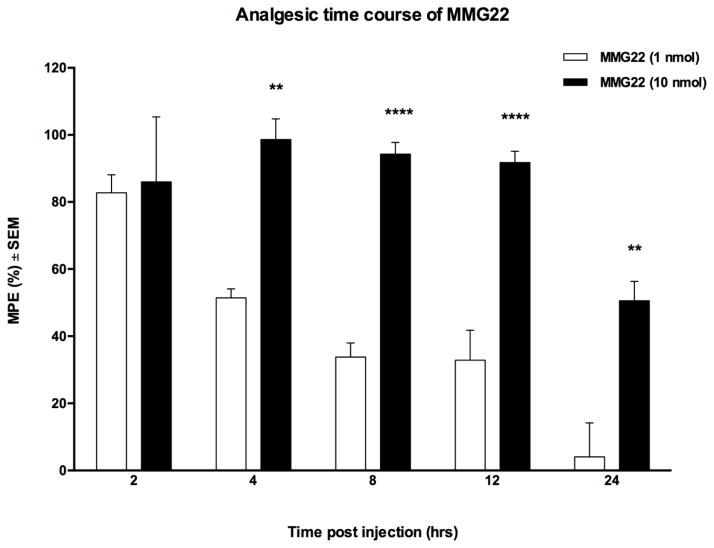
With peak hyperalgesia evident between PID 17–21, MMG22 was evaluated for effectiveness over a period of 24 h using 1 and 10 nmol/mouse doses in tumor-bearing animals between PID 17 and 21. These doses were based on previous work in LPS pretreated mice that produced long duration of antinociception (Akgün et al., 2013). The results of this study (Fig 5) revealed that potent antinociception was maintained over 24 h in mice receiving a single 10 nmol dose/mouse i.t. of MMG22. Mice that received a 1 nmol dose exhibited a significantly shorter duration of antinociception. (Fig 4, **P < 0.01, **P < 0.0001).
Fig. 4.
The time-course of antinociception produced after i.t. administration of MMG22 (1 or 10 nmol) in tumor-bearing mice on PID 21. The half-maximal dose effect for 10 nmol was 24 h, whereas mice receiving 1 nmol exhibited half-maximal effect at 4 h. (n = 5/group; **P < 0.01 and ****P < 0.0001
3.3 MMG22 Produces Optimal Potency
Monovalents M19 and MG20 were evaluated for synergism in comparison with the bivalent MMG22 (see compound optimization). Beginning on PID 3, administration of either the bivalent ligand MMG22 or the mixture of the monovalents produced antinociception in tumor bearing animals. However, MMG22 was exceptionally more potent (~250,000-fold) than the mixture of monovalent ligands at all-time points (PID 3–17) and 625,000 times more potent on PID 21. To investigate the effect of bivalent ligand linker length on potency, a member of this series with a 10-atom linker, MMG10, was tested over 21 days on tumor-bearing mice for its anti-hyperalgesic effectiveness. Unlike MMG22, the dose of MMG10 to maintain its ED80 potency increased from 6.25 pmol to 25.0 pmol/mouse between PID 3–17, and to 100 pmol/mouse on PID 21, which represents a million-fold lower potency relative to MMG22.
3.4 Evaluation of MMG22 Against the ‘Gold Standard’ Opioid Analgesic, Morphine
To compare the anti-hyperalgesic effectiveness of MMG22 against a standard opioid agonist, we evaluated morphine’s antinociceptive effect and compared it to that of MMG22 using the same paradigm as MMG22 over the course of 21 days in our murine fibrosarcoma tumor model. Beginning on PID 10 and lasting the entirety of the experiment, MMG22 was significantly more potent than morphine (PID 10, MMG22 was 23,000 times more potent than morphine (ED50: 13.7 pmol/mouse (3.38 – 55.50)); PID 17 MMG22 was 495,600 times more potent than morphine (ED50: 49.56 pmol/mouse (15.20 – 161.6)); and on PID 21, MMG22 was 3.6 million times more potent than morphine (ED50: 36.26 pmol/mouse (14.79 – 88.89)). Unlike MMG22, i.t. administration of morphine did not result in increased potency over time. No changes in the ED50 values of morphine were observed at any time point tested.
4. Discussion
Recently (Neugebauer, 2001; Ren et al., 2012) reported that mGluR5 becomes progressively upregulated in tumor-bearing mice over 21 days, with the largest increase occurring between days 14 and 21. Similarly, in our present study, implantation of mice with fibrosarcoma cells produced a robust mechanical hyperalgesia following a similar time course mimicking what we found previously with either fibrosarcoma cells or osteosarcoma cells implanted into the mouse hindpaw (Smeester et al., 2012; Smeester et al., 2013). Importantly, in the present study the potency of MMG22 was found to progressively increase over time following implantation. This is in contrast to morphine whose potency decreased between PID 10 and 17. This is reflected by the 3.6 million-fold greater potency of MMG22 over morphine on PID 21. Moreover, MMG22 did not show evidence of chronic toxicity over the 3-week period of this study nor was there any evidence of the development of tolerance. Thus, MMG22 has the potential to be superior to current opioid drugs because of its high potency, long duration of action and lack of tolerance.
Both mGluR5 and NMDA receptor (NMDAR) are localized as signaling partners on postsynaptic terminals of spinal neurons, and they are physically linked through a Homer-Shank postsynaptic density protein complex (Niswender and Conn, 2010; Piers et al., 2012a). The reported physical association of mGluR5 and MOR with NMDAR subunits NR2 (Perroy et al., 2008; Piers et al., 2012b; Sanchez-Blazquez et al., 2013) and NR1 (Rodriguez-Munoz et al., 2012), respectively, raises the possibility that during inflammation, activation putative MOR-mGluR5 heteromers rather than individual homomers associate with these subunits, given the structure-activity evidence for MMG22 and the reported upregulation of MOR and mGluR5 as a consequence of inflammation. From this perspective, MMG22 may mediate potent antinociception by blockage of NMDAR-hyperalgesia via antagonism of the mGluR5 protomer and activation of the MOR protomer of MOR-mGluR5.
That the bivalency of MMG22 is essential for its high potency in bone cancer, is consistent with the 250,000-times lower potency that was observed for a mixture of monovalent ligands (M19 + MG20). This is because the length of the linker that tethers the pharmacophores in the bivalent MMG series is critical for targeting MOR-mGluR5 heteromer. In this regard, the 22-atom linker in MMG22 appears to be optimal for simultaneous interaction with both protomers in the heteromer, while the short linker (10 atoms) in MMG10 does not permit this to occur. In contrast to the increased potency of MMG22 as the cancer progresses, the potency of MMG10 decreased by a million-fold on post-implantation day 21 (PID 21). The greater potency of MMG22 over MMG10 reflects the importance of linker length for bridging of the pharmacophores to the protomers in MOR-mGluR5. The large potency decrease as a function of cancer progression is likely due to development of tolerance, given that MMG10 in LPS-pretreated mice was reported to exhibit this adverse effect.
In summary, the orders of magnitude greater intrathecal antinociception of MMG22 relative to a mixture of monovalent ligands that contain identical mu agonist and mGluR5 pharmacophores suggests that its exceptional potency is related to selective activation of the MOR protomer and antagonism of the mGluR5 protomer in a putative MOR-mGluR5 heteromer. Based on the similar profiles for both hyperalgesia and the potency of MMG22 over a period of 21 days after implantation of fibrosarcoma cells in mice, we propose that the tumor-induced hyperalgesia is mediated via antagonism of the mGluR5 protomer which allosterically inhibits the NMDA receptor. The prominent features observed for MMG22 in this chronic bone cancer pain model are: a) progressive increase of antinociception over a period of 21 days following implantation of fibrosarcoma cells in mice that is similar to the time-course profile of hyperalgesia, and b) a single 10 nmol intrathecal dose of MMG22 produces a long duration of antinociception (>24 h). It is noteworthy that the potency of MMG22 is 3.6 million-times greater than that of morphine after implantation of fibrosarcoma cells. These properties suggest that clinical development of MMG22 for spinal delivery is a viable option for the efficacious pharmacotherapy of chronic cancer pain, since pain management in patients with cancer still remains suboptimal (Simon and Schwartzberg, 2014).
Acknowledgments
This project was supported by NIH research grant R01DA030316 from the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akgün E, Javed MI, Lunzer MM, Smeester BA, Beitz AJ, Portoghese PS. Ligands that interact with putative MOR-mGluR5 heteromer in mice with inflammatory pain produce potent antinociception. Proceedings of the National Academy of Sciences. 2013;110:11595–11599. doi: 10.1073/pnas.1305461110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonferroni CE. Teoria statistica delle classi e calcolo delle probabilit\’{a} Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali di Firenze. 1936;8:3–62. [Google Scholar]
- Cavalheiro EA, Olney JW. Glutamate antagonists: deadly liaisons with cancer. Proc Natl Acad Sci U S A. 2001;98:5947–5948. doi: 10.1073/pnas.121179198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KY, Chang K, Pickel JM, Badger JD, II, Roche KW. Expression of the metabotropic glutamate receptor 5 (mGluR5) induces melanoma in transgenic mice. Proc Natl Acad Sci U S A. 2011;108:15219–15224. S15219/15211–S15219/15213. doi: 10.1073/pnas.1107304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS, O’Mara A, Zagari M, Baas C. Integrating pain metrics into oncology clinical trials. Clin Cancer Res. 2011;17:6646–6650. doi: 10.1158/1078-0432.CCR-11-1109. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Kozela E, Pilc A, Popik P. Inhibitory effects of MPEP, an mGluR5 antagonist, and memantine, an N-methyl-D-aspartate receptor antagonist, on morphine antinociceptive tolerance in mice. Psychopharmacology (Berlin, Ger) 2003;165:245–251. doi: 10.1007/s00213-002-1287-8. [DOI] [PubMed] [Google Scholar]
- Muralidharan A, Smith MT. Pathobiology and management of prostate cancer-induced bone pain: recent insights and future treatments. Inflammopharmacology. 2013;21:339–363. doi: 10.1007/s10787-013-0183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V. Peripheral metabotropic glutamate receptors: fight the pain where it hurts. Trends Neurosci. 2001;24:550–552. doi: 10.1016/s0166-2236(00)02007-5. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Lee S-A, Han I-H, Yoo B-C, Lee S-H, Park J-Y, Cha I-H, Kim J, Choi S-W. Clinical significance of metabotropic glutamate receptor 5 expression in oral squamous cell carcinoma. Oncol Rep. 2007;17:81–87. [PubMed] [Google Scholar]
- Perroy J, Raynaud F, Homburger V, Rousset M-C, Telley L, Bockaert J, Fagni L. Direct Interaction Enables Cross-talk between Ionotropic and Group I Metabotropic Glutamate Receptors. J Biol Chem. 2008;283:6799–6805. doi: 10.1074/jbc.M705661200. [DOI] [PubMed] [Google Scholar]
- Piers TM, Kim DH, Kim BC, Regan P, Whitcomb DJ, Cho K. Translational concepts of mGluR5 in synaptic diseases of the brain. Front Neuropharmacol. 2012a;6:199. doi: 10.3389/fphar.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piers TM, Kim DH, Kim BC, Regan P, Whitcomb DJ, Cho K. Translational Concepts of mGluR5 in Synaptic Diseases of the Brain. Front Pharmacol. 2012b;3:199. doi: 10.3389/fphar.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickett TD, Samuels Y. Molecular Pathways: Dysregulated Glutamatergic Signaling Pathways in Cancer. Clin Cancer Res. 2012;18:4240–4246. doi: 10.1158/1078-0432.CCR-11-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Attenuation of Morphine Tolerance, Withdrawal-Induced Hyperalgesia, and Associated Spinal Inflammatory Immune Responses by Propentofylline in Rats. Neuropsychopharmacology. 2003;29:327–334. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- Ren BX, Gu XP, Zheng YG, Liu CL, Wang D, Sun YE, Ma ZL. Intrathecal injection of metabotropic glutamate receptor subtype 3 and 5 agonist/antagonist attenuates bone cancer pain by inhibition of spinal astrocyte activation in a mouse model. Anesthesiology. 2012;116:122–132. doi: 10.1097/ALN.0b013e31823de68d. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Munoz M, Sanchez-Blazquez P, Vicente-Sanchez A, Berrocoso E, Garzon J. The Mu-Opioid Receptor and the NMDA Receptor Associate in PAG Neurons: Implications in Pain Control. Neuropsychopharmacology. 2012;37:338–349. doi: 10.1038/npp.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Rodriguez-Munoz M, Berrocoso E, Garzon J. The plasticity of the association between mu-opioid receptor and glutamate ionotropic receptor N in opioid analgesic tolerance and neuropathic pain. Eur J Pharmacol. 2013;716:94–105. doi: 10.1016/j.ejphar.2013.01.066. [DOI] [PubMed] [Google Scholar]
- Schröder H, Wu DF, Seifert A, Rankovic M, Schulz S, Hölt V, Koch T. Allosteric modulation of metabotropic glutamate receptor 5 affects phosphorylation, internalization, and desensitization of the [mu]-opioid receptor. Neuropharmacology. 2009;56:768–778. doi: 10.1016/j.neuropharm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Smeester BA, Al-Gizawiy M, Beitz AJ. Effects of different electroacupuncture scheduling regimens on murine bone tumor-induced hyperalgesia: sex differences and role of inflammation. Evidence-based complementary and alternative medicine : eCAM. 2012;2012:671386. doi: 10.1155/2012/671386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeester BA, Al-Gizawiy M, O’Brien EE, Ericson ME, Triemstra JL, Beitz AJ. The effect of electroacupuncture on osteosarcoma tumor growth and metastasis: analysis of different treatment regimens. Evidence-based complementary and alternative medicine : eCAM. 2013;2013:387169. doi: 10.1155/2013/387169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H. Malignant gliomas: perverting glutamate and ion homeostasis for selective advantage. Trends Neurosci. 2003;26:543–549. doi: 10.1016/j.tins.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Sotgiu ML, Bellomi P, Biella GEM. The mGluR5 selective antagonist 6-methyl-2-(phenylethynyl)-pyridine reduces the spinal neuron pain-related activity in mononeuropathic rats. Neurosci Lett. 2003;342:85–88. doi: 10.1016/s0304-3940(03)00259-3. [DOI] [PubMed] [Google Scholar]
- Te BN, Vernooij-Dassen M, Burger N, Ijsseldijk M, Vissers K, Engels Y. Pain and its interference with daily activities in medical oncology outpatients. Pain Physician. 2013;16:379–389. [PubMed] [Google Scholar]
- Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, Wilcox GL. Functional interactions between tumor and peripheral nerve: Morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci. 2001;21:9355–9366. doi: 10.1523/JNEUROSCI.21-23-09355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Bowes M, Panesar M, Davis A, Gentry C, Kesingland A, Gasparini F, Spooren W, Stoehr N, Pagano A, Flor PJ, Vranesic I, Lingenhoehl K, Johnson EC, Varney M, Urban L, Kuhn R. Metabotropic glutamate receptor subtype 5 (mGlu5) and nociceptive function. I. Selective blockade of mGlu5 receptors in models of acute, persistent and chronic pain. Neuropharmacology. 2000;40:1–9. doi: 10.1016/s0028-3908(00)00113-1. [DOI] [PubMed] [Google Scholar]
- Walker K, Bowes M, Panesar M, Davis A, Gentry C, Kesingland A, Gasparini F, Spooren W, Stoehr N, Pagano A, Flor PJ, Vranesic I, Lingenhoehl K, Johnson EC, Varney M, Urban L, Kuhn R. Metabotropic glutamate receptor subtype 5 (mGlu5) and nociceptive function. I. Selective blockade of mGlu5 receptors in models of acute, persistent and chronic pain. Neuropharmacology. 2001a;40:1–9. doi: 10.1016/s0028-3908(00)00113-1. [DOI] [PubMed] [Google Scholar]
- Walker K, Reeve A, Bowes M, Winter J, Wotherspoon G, Davis A, Schmid P, Gasparini F, Kuhn R, Urban L. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology. 2001b;40:10–19. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]