Abstract
Natural languages contain countless regularities. Extraction of these patterns is an essential component of language acquisition. Here we examined the hypothesis that memory processing during sleep contributes to this learning. We exposed participants to a hidden linguistic rule by presenting a large number of two-word phrases, each including a noun preceded by one of four novel words that functioned as an article (e.g., gi rhino). These novel words (ul, gi, ro and ne) were presented as obeying an explicit rule: two words signified that the noun referent was relatively near, and two that it was relatively far. Undisclosed to participants was the fact that the novel articles also predicted noun animacy, with two of the articles preceding animate referents and the other two preceding inanimate referents. Rule acquisition was tested implicitly using a task in which participants responded to each phrase according to whether the noun was animate or inanimate. Learning of the hidden rule was evident in slower responses to phrases that violated the rule. Responses were delayed regardless of whether rule-knowledge was consciously accessible. Brain potentials provided additional confirmation of implicit and explicit rule-knowledge. An afternoon nap was interposed between two 20-min learning sessions. Participants who obtained greater amounts of both slow-wave and rapid-eye-movement sleep showed increased sensitivity to the hidden linguistic rule in the second session. We conclude that during sleep, reactivation of linguistic information linked with the rule was instrumental for stabilizing learning. The combination of slow-wave and rapid-eye-movement sleep may synergistically facilitate the abstraction of complex patterns in linguistic input.
Keywords: language acquisition, implicit learning, slow-wave sleep, REM, event-related potentials
Introduction
The extraction of patterns from linguistic input lies at the core of language learning. Natural languages are governed by complex regularities at virtually every level. For example, within a given language, certain sound combinations commonly co-occur while others are illegal (e.g., pl versus tl onsets in English). Words can be combined into phrases and sentences only in limited ways, specified by syntactic rules (e.g., articles such as the or my are not followed by verbs). Subtle regularities can even predict the lexical category of a word (Farmer, Christiansen & Monaghan, 2006). Most native speakers have little insight into these regularities, even though this knowledge is essential for comprehension and production (Paradis, 2004). Acquisition of these regularities typically occurs implicitly in children, in the absence of intention to learn or awareness of what has been learned (Paradis, 2004; Ullman, 2004). Pattern extraction for learning linguistic regularities certainly occurs online during training, but here we consider whether offline processes during sleep may also play a role.
The general importance of sleep for memory consolidation, as well as for the extraction of rules, has been repeatedly demonstrated (Stickgold & Walker, 2013). For example, sleep can lead to insight in a rote mathematical task (Wagner, Gais, Haider, Verleger, & Born, 2004), gains in transitive inference (Ellenbogen, Hu, Payne, Titone & Walker, 2007), improvements in statistical sequence learning (Durrant, Taylor, Cairney & Lewis, 2011, Durrant, Cairney & Lewis, 2013), and enhanced category learning (Djonlagic et al., 2009). Memories that share common elements may be reactivated during sleep in a way that promotes shared connections (Lewis & Durrant, 2011). If idiosyncratic aspects of each memory are also lost over time, a general schema may result. In the context of language acquisition, this schema could represent overarching linguistic rules abstracted over multiple exemplars and learning episodes (e.g., knowledge that the –s morpheme indicates plurality).
Our aim was to test whether sleep mechanisms promote rule generalization in a language-learning context. We built upon a paradigm developed by Leung and Williams (2012, in press), in which participants were presented with phrases containing four novel articles (gi, ul, ro and ne). Participants were explicitly instructed that these novel articles encode distance, with two of the articles used when the accompanying noun refers to a nearby object, and the other two used when the accompanying noun refers to objects that are far away. However, unbeknownst to participants, the use of these articles was also governed by a second semantic feature involving noun animacy: two of the articles (gi and ul) were used for animate nouns and the other two (ro and ne) for inanimate nouns. Participants responded to each phrase by indicating whether it contained an animate or inanimate object, such that processing of noun animacy was assured. A final violation block, consisting of phrases in which the mapping between articles and animacy values was reversed, was presented at the end of the experiment. Using this paradigm, Leung and Williams found that participants’ responses to trials in the violation block were delayed, even when they reported no awareness of this regularity. This finding provides evidence that adults can implicitly learn mappings between grammatical form and meaning. This ability is a key component of language acquisition, as associations between form and meaning underlie virtually all aspects of language.
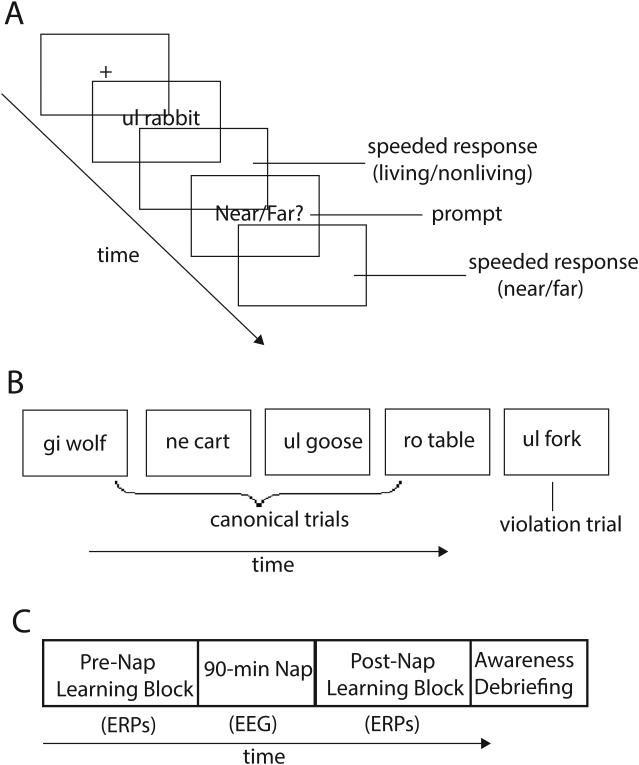
In the present study, as in Leung and Williams, participants responded to phrases composed of a novel article and noun (e.g., ul spider) that either conformed to or violated a hidden linguistic animacy rule. However, we adapted Leung and Williams’ original paradigm by presenting violation trials interspersed throughout the learning block, rather than in a separate block at the end of learning, in order to track the time course of learning effects (Figure 1). We also recorded event-related brain potentials (ERPs) to provide additional measures of learning and rule awareness. We hypothesized that learning of the hidden rule should be evident in slower responses to phrases that violated the rule, similar to previous findings (Leung & Williams, 2012, in press). We additionally hypothesized that ERP differences would emerge between canonical and violation phrases as participants implicitly learned the hidden rule, representing a neural index of learning. In addition, we hypothesized that participants who became aware of the rule would show a P600 effect to violation phrases, a positive-going deflection with a typical latency between 600-1000 ms (Friederici, 2002). This component has been previously linked to the conscious detection of a syntactic violation (Batterink & Neville, 2013). In contrast, participants who remained unaware of the rule should not show this effect. To examine whether sleep influences the implicit learning of associations between form and meaning, participants were exposed to phrases containing the four novel articles, subsequently napped, and were then tested on new phrases upon awakening.
Figure 1.
Summary of experimental task and overall paradigm.
A) Sequence of events in a typical trial.
B) Representation of the trial structure in the experimental task. One out of every seven trials was a violation trial (~14%). Violation trials were interspersed unpredictably throughout the experimental task.
C) Each learning block was comprised of 308 unique trials. A 90-min nap separated the two learning blocks.
The critical experimental question was whether measures of learning changed as a function of sleep mechanisms. We examined SWS and REM (slow-wave sleep and REM sleep), as well as their interactions, guided by theories about the roles of these sleep stages (Diekelmann & Born, 2010; Walker & Stickgold, 2010; Stickgold, Whidbee, Schirmer, Patel & Hobson, 2000). We adopted a correlational approach, which previously implicated a synergism between SWS and REM sleep (e.g., Stickgold et al., 2000; Cairney, Durrant, Power & Lewis, in press). This approach avoids a shortcoming of conventional sleep/wake comparisons, wherein improvements in behavioral performance can be attributed either to memory enhancement over a retention interval with sleep compared to one without sleep, or to memory reduction secondary to interference (i.e., greater interference during waking than during sleep) and/or arousal effects (i.e., higher alertness after sleep than an equivalent period of wake). We thus focused on the degree to which the learning changed after sleep, in order to determine whether sleep processing contributes to the abstraction of linguistic rules. In particular, we predicted that duration of SWS, REM, and/or interactions between SWS and REM would correlate with an increase in implicit knowledge of the hidden rule, as reflected by larger reaction time (RT) differences to violation versus canonical phrases after sleep.
Materials and Methods
Participants
Twenty-nine right-handed, neurologically normal native English speakers (17 female; age range, 18.3 – 25.4 years) participated in this study.
Experimental Task
Building on the methodology used by Leung and Williams (2012; in press), as described above, participants were trained on an artificial article system composed of four novel articles: gi, ro, ul, and ne. They were instructed that these articles functioned like the English word “the” but that they also designated relative distance, with two of them (gi and ro) meaning “near” and the other two (ul and ne) meaning “far.” However, participants were not told that the four novel articles also predicted the animacy of the subsequent noun (Table 1). Before beginning the main experimental task, participants were pre-trained for approximately 15 min on the overt meanings (near or far) of the articles. Pre-training consisted of studying flashcards and completing computerized tasks that required forward and backward translation between the novel articles and their English meanings (near/far).
Table 1.
Miniature Article System1
| Participants were not told… |
||
|---|---|---|
| Animate | Inanimate | |
| Participants were told… | ||
| Near | gi | ro |
| Far | u1 | ne |
Williams (2005) showed that the precise assignment of articles to animacy values (i.e., whether ul and gi are assigned to animate nouns and ne and ro to inanimate nouns, or vice versa) had no significant effect on learning. Therefore, in the present study, animacy assignment for the four articles was kept consistent across participants.
The main experimental task involved presenting participants with two-word phrases consisting of a novel article (gi, ul, ro, or ne) and an accompanying noun (Figure 1). Half of the nouns were animate (e.g., horse, puppy), and the other half inanimate (e.g., table, kettle). Unbeknownst to participants, the four novel articles predicted the animacy of the subsequent noun, with gi and ul usually preceding animate objects and ro and ne inanimate objects. This correlation was probabilistic, mirroring regularities found in natural languages. Six out of every seven of trials conformed to this rule, in which gi and ul were paired with animate nouns and ro and ne with inanimate nouns. On a random basis, one out of every seven trials were violation trials, in which ro and ne were paired with animate nouns and gi and ul with inanimate nouns. Participants’ task was to make two speeded responses to each trial, indicating (1) whether the phrase referred to a living or nonliving object and (2) whether the phrase referred to an object that was near or far. The critical behavioral measure was the delay in reaction times (RTs) for the animacy response to phrases that violated the hidden rule. This difference, termed the Rule Learning Index (RLI), provides a measure of the influence of the learned hidden rule. This effect has been previously shown to be sensitive to learning (Leung & Williams, 2012; in press), and can be interpreted as an interference effect, similar to the Stroop effect (MacLeod, 1991). We presumed that due to the automatic nature of reading, both the article and noun should be processed concurrently, prior to the animacy response. As participants learn the associations between the articles and noun animacy, the articles should begin to serve as an additional animacy cue. This additional cue should then facilitate responses on canonical trials, but would conflict with the animacy of the noun on violation trials, leading to delayed RTs and potentially decreased accuracy. Thus, the paradigm functioned both as a learning task and an online test, as it included phrases that usually conformed to the hidden animacy rule and provided measures of differential processing of canonical versus violation trials. Because participants were not informed of the underlying regularity governing the novel articles and because violation trials were interspersed throughout the task, there was no obvious difference between violation and canonical trials from the point of view of the (naïve) participant. Each learning block contained a total of 308 test trials (264 canonical trials and 44 violation trials intermixed together).
Each trial began with the presentation of a fixation cross for 1000 ms, followed by the simultaneous presentation of an article-plus-noun pair. The two words remained on the screen for 500 ms or until the participant made the animacy response, and was then replaced by the cue “Near/Far?” This cue remained on the screen until the second response. For both responses, the display advanced when a correct response was made. Four response buttons were configured so that each response (living/nonliving/near/far) was associated with a unique button. Note that our method of stimulus presentation represents a departure from most ERP studies of language processing, which typically present only a single word at a time.
After pre-training, participants completed the first Pre-Nap learning block. They then reclined in a quiet, darkened room to sleep. The nap period ended after 90 min, but was extended if the participant was still in SWS. After wakening, participants were given a 10-min break before completing a second Post-Nap learning block. The second block was identical to the first except that different nouns were presented; participants were exposed to each individual noun only once across the two sessions. The two sessions were separated by 110 min on average.
All stimuli were visually presented on a computer monitor placed approximately 130 cm in front of the participant. Stimuli were counterbalanced within cycles of seven participants, such that a given noun was presented as part of a canonical trial for six out of seven participants, and as part of a violation trial for the seventh participant. Assignment of trials to the Pre-Nap or Post-Nap test block was counterbalanced across participants. Finally, the list of nouns assigned to the canonical and violation conditions for every subject were matched on a group level for overall frequency and length using the Kucera-Francis database (mean written frequency range = 9.4-11.3, mean word length range = 5.8-6.3 letters).
Procedure
The experimental session began between 12:00 and 3:00 PM with electrode application for ERP analysis and standard sleep EEG recording (see below). Electrode application was immediately followed by the Pre-Nap learning session, the 90-min nap, and the Post-Nap learning session.
After completing the second session, awareness of the hidden animacy rule was assessed via a structured interview, in which the questions became progressively more specific as the interview went on. First, participants were asked whether they had formed any impressions about when the different novel articles were used. If they did not spontaneously report animacy as a relevant factor, they were then specifically asked whether they had noticed if any of the articles had been used more often for living versus nonliving things. If they reported noticing such a relationship, they were asked to describe the pattern for each article and to recall at which point during the experiment they had become aware of the pattern (i.e., Pre-Nap learning session, Post-Nap learning session, or only when asked directly about these patterns during the interview). If they claimed not to have noticed any relationship with animacy, they were asked to guess whether each article had been used more frequently for living versus nonliving things. Participants who were unable to accurately describe the pattern even after prompting, or who reported not becoming aware of the pattern until being directly questioned about it during the interview stage were classified as Rule-Unaware. Participants who described the pattern more or less accurately and who reported becoming aware of the pattern while performing the online experimental task (during either the Pre-Nap or Post-Nap session) were classified as Rule-Aware.
Two self-report questionnaires were administered to assess the subjective quality and duration of participants’ sleep in the preceding night as well as over the 90-min nap period. Participants were asked to report the time they went to bed, the time they woke up, total time asleep, length of time required to fall asleep, and number and length of awakenings. They also responded on a 1-5 scale to several questions aimed at assessing subjective quality of sleep: how they slept overall, how refreshed they felt upon awakening, whether they slept soundly or restlessly, whether they slept throughout the time allotted for sleep, how easy it was to fall asleep, and how easy it was to wake up.
EEG Recording & Analysis
EEG was recorded from 21 tin electrodes mounted in an elastic cap, along with two electrooculogram (EOG) channels and one chin electromyogram (EMG) channel, using a 250-Hz sampling rate. EEG was recorded throughout both learning blocks and over the nap period.
For sleep analyses, data from EEG and EOG channels were filtered with a bandpass from 0.5-30 Hz, and EMG data were filtered from 10-62 Hz. Sleep staging was conducted offline using standard criteria. EEG spectral analyses were conducted following artifact removal based on visual inspection. Time-frequency decompositions were computed using fast Fourier transform with a Hamming window over 5-s epochs. In addition to duration data obtained from sleep staging, we computed delta power as a measure of SWS quality. Mean delta power (0.5–4 Hz) was computed at electrode Fz , as delta power is maximal frontally (Grigg-Damberger, 2012).
For ERP analyses, data were band-pass filtered from 0.1-30 Hz. Artifact correction and rejection was accomplished through visual inspection and Independent Component Analysis (EEGLAB; Delorme & Makeig, 2004), according to standard analysis procedures (Batterink & Neville, 2011). Epochs were extracted from −200 to 1200 ms relative to the onset of each article-noun pair.
Statistical Analyses
Analyses focused on the RLI and corresponding accuracy data for the animacy response. Data from each session (Pre-Nap and Post-Nap) were divided into four equal epochs in order to examine the time course of learning. For each epoch, the RLI was calculated by excluding incorrect trials and then subtracting the median RT to canonical trials from the median RT to violation trials. Accuracy and RT data were analyzed using a repeated-measures ANOVA with Condition (canonical, violation) and Epoch (1-8) as within-subjects factors. Follow-up analyses conducted separately for each epoch were conducted to examine when the violation effect was significant. To examine the influence of conscious awareness of the rule, additional analyses based on results from debriefing were conducted with Awareness Classification (Rule-Aware, Rule-Unaware) as a between-subjects factor and Condition and Epoch as within-subjects factors.
For ERP statistical analysis, time intervals were selected based on visual inspection of the waveform: we focused on an earlier interval from 400-800 ms (capturing the first observed effect, a negativity), and a later interval from 800-1100 ms (capturing the second observed effect, a positivity). Mean amplitudes at electrodes F7, F3, F4, F8, T3, C3, C4, T4, T5, P3, P4, T6 were computed and entered into a repeated-measures ANOVA for each interval, with Condition (canonical, violation), Hemisphere (left, right), Anterior/Posterior (3 levels), and Lateral/Medial (2 levels) as within-subjects factors and Awareness Classification (Aware, Unaware) as a between-subject factor. Separate analyses were also conducted over midline sites (Fz, Cz, Pz, and Oz), though these results are not reported as they did not contribute additional information beyond what was yielded by the main factorial ANOVA. Incorrect trials were excluded from analysis.
The chief analysis involved measuring behavioral and electrophysiological changes across the two sessions. Learning effects would normally be expected to decline over this delay via forgetting, unless mechanisms operative during this interval actively promoted the retention (consolidation) of newly acquired information. We computed the pre- to post-nap change in behavioral sensitivity to violations (denoted as f).RLI) by subtracting the RLI over the last half of the Pre-Nap Session (i.e., after RTs had stabilized and the RLI effect emerged; Figure 2) from the RLI over the first half of the Post-Nap Session. A positive value indicates an increase in sensitivity to the rule after the delay. This f).RLI represents our main dependent measure for sleep analyses. Multiple regression was used to test whether duration of SWS (SWSdur), duration of REM (REMdur), and/or the product of SWS and REM durations (SWSdurxREMdur) predicted this behavioral RT change. These three predictor variables were selected based on current theories on sleep and memory consolidation (Diekelmann & Born, 2010; Walker & Stickgold, 2010; Stickgold et al., 2000). The product of SWS and REM in particular has been proposed to model sequential SWS and REM throughput (Stickgold et al., 2000), consistent with sequential hypotheses of sleep function (Ambrosini & Giuditta, 2001; Ficca & Salzarulo, 2004; Walker & Stickgold, 2010). Total sleep duration (Sleepdur) and sleep onset latency (Sleeplat) was also included in the model as a way of controlling for general effects of fatigue and for total sleep time, allowing us to assess the specificity of sleep-stage effects.
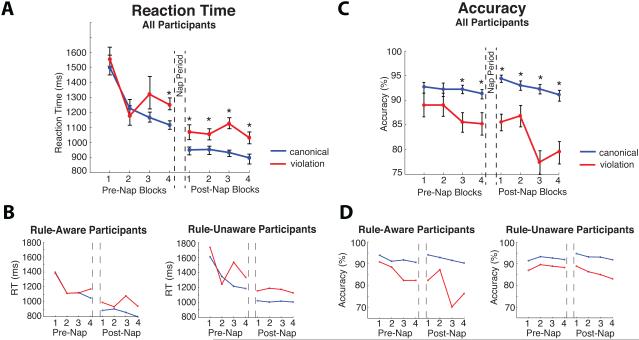
Figure 2.
A) Median RTs, pooled across participants, to canonical trials and violation trials as a function of learning over time. The RLI is the RT delay to violation trials relative to canonical trials. An asterisk indicates a significant difference between conditions (p < 0.05). Error bars represent the within-subjects SEM, computed using the procedure recommended by Morey (2008).
B) Median RTs in Rule-Aware (n = 15) and Rule-Unaware (n = 14) participants. The RLI was not significantly different between the two groups.
C) Accuracy rate to canonical trials and violation trials as a function of learning over time. An asterisk indicates a significant difference between conditions. Error bars represent the within-subjects SEM.
D) Accuracy rate in Rule-Aware and Rule-Unaware participants. Both groups showed significantly reduced accuracy to violation trials.
To be sure our results were not dependent on the decision to include only the first half of trials in the Post-Nap session when computing f).RLI, pre- to post-nap behavioral change in the RLI was also calculated by comparing the entire Post-Nap Session to the last half of the Pre-Nap Session. Because performance plateaued during the Post-Nap Session, results were very similar to those in the first analysis and are not reported.
In an additional exploratory analysis, we used ERPs to examine whether sleep measures (SWSdurxREMdur, SWSdur, or REMdur) predicted participants’ level of explicit awareness of the hidden rule during the Post-Nap block. Because our behavioral assessment of Rule Awareness consisted of a binary measure of whether or not participants became aware of the hidden rule during the experimental task, we used the P600 effect during the Post-Nap block as a graded, potentially more sensitive measure of explicit awareness. We theorized that a larger P600 should indicate a higher level of awareness (e.g., more complete explicit rule knowledge, greater confidence about the rule, and/or becoming aware of the rule earlier on in the task). We conducted a repeated-measures ANCOVA across posterior electrodes where the P600 is maximal (T5, P3, Pz, P4, T6, O1, Oz, O2), with SWSdurxREMdur, SWSdur, and REMdur as covariates. Only Rule-Aware participants (n = 14) were included in this analysis, as a significant P600 was present only in this group.
To examine whether participants who subsequently obtained different amounts of SWS and REM differed in terms of overall RT and accuracy in the Pre-Nap block, a repeated-measures ANCOVA with SWSdurxREMdur as a covariate and Condition and Epoch (1-4, Pre-Nap blocks only) was run. Pearson’s correlations were used to examine potential relationships between self-reported sleep data and SWSdurxREMdur. These measures consisted of self-reported total sleep time and total minutes awake over the sleep interval, and ratings on a 1-5 scale of overall sleep quality, degree to which the participant felt refreshed upon awakening, soundness/restlessness of sleep, the extent to which sleep occurred throughout the entire time period allocated for sleep, ease of falling asleep, and ease of waking up, for both the preceding night and the 90-min nap.
Results
Behavior
Data from the Pre-Nap and Post-Nap sessions revealed the time course of learning. Both accuracy and RTs indicated that participants became sensitive to the hidden animacy rule during the first session (Figure 2). Despite the consistency of these effects, many participants remained unaware of the hidden rule.
RTs
Across all eight epochs, RTs were significantly delayed to violation trials relative to canonical trials, indicating significant rule learning as measured by RLI (F(1,28) = 24.62, p < 0.0001, ηp2 = 0.47; Figure 2). An Epoch × Condition interaction suggested that the RLI became larger as learning progressed across the eight epochs (8 epochs: linear contrast: F(1,28) = 3.42, p = 0.074; Pre-Nap epochs alone: linear contrast: F(1,28) = 4.76, p = 0.038, ηp2 = 0.15). Follow-up analyses showed that the RLI effect accrued gradually with a reliable difference emerging during the fourth epoch (F(1,28) = 27.3, p < 0.0001; ηp2 = 0.49) after no reliable differences in the first 3 epochs (all p values > 0.17, all ηp2 values < 0.065). The RLI effect remained significant for every epoch thereafter throughout the post-nap session (all p values < 0.008, range in ηp2 values = 0.23 — 0.56). A comparison of the final two Pre-Nap epochs with the first two Post-Nap epochs indicated that overall RLI magnitude neither significantly increased nor decreased directly after the nap period (Pre/Post Nap × Condition: F(1,28) = 0.25, p = 0.62, ηp2 = 0.009).
Accuracy
Across all eight epochs, participants showed a significant reduction in accuracy for article-noun pairs that violated the hidden rule (F(1,28) = 54.5, p < 0.001, ηp2 = 0.66). An Epoch × Condition interaction indicated that the violation effect differed as a function of epoch (F(7,196) = 3.49, p = 0.004, ηp2 = 0.11), becoming larger as learning progressed (linear contrast: F(1,28) = 13.3, p = 0.001, ηp2 = 0.32). Follow-up analyses indicated that there were no significant accuracy differences between canonical and violation trials in the first two epochs (Epoch 1: F(1,28) = 3.58, p = 0.069; ηp2 =0.11; Epoch 2: F(1,28) = 2.58, p = 0.12, ηp2 =0.084), but that a significant accuracy violation effect emerged for the third epoch (F(1,28) = 14.2, p = 0.001, ηp2 = 0.34) and remained reliable for every epoch thereafter (all p values < 0.04, range in ηp2 values = 0.15-0.58). A comparison of the final two Pre-Nap epochs with the first two Post-Nap epochs indicated that the overall accuracy violation effect neither significantly increased nor decreased directly after the nap period (Pre/Post Nap × Condition: F(1,28) = 0.35, p = 0.56, ηp2 = 0.012).
Rule-aware versus Rule-unaware Participants
To examine whether accuracy violation effects and the RLI differed as a function of awareness of the hidden animacy rule, we divided participants into two groups based on verbal reports about awareness of the rule. Five participants reported becoming aware of the hidden rule during the Pre-Nap session, prior to napping, whereas ten reported becoming aware of the rule during the Post-Nap session. These participants were classified as Rule-Aware (n = 15). Participants who reported remaining unaware of the relevance of animacy during the experimental task were classified as Rule-Unaware (n = 14).
RTs
The RLI was not significantly different for the Rule-Aware versus Rule-Unaware participants (Group × Violation Condition: F(1,27) = 2.08, p = 0.16, ηp2 = 0.071; Figure 2), nor were there differences in the time course of this effect across the eight epochs between these two groups (Group × Condition × Epoch: F(7,189) = 1.70, p = 0.18). This indicates that the RLI did not emerge significantly earlier in either group. Follow-up analyses confirmed that the RLI effect was robust in both groups (Rule-Aware group: F(1,14) = 13.1, p = 0.003, ηp2 = 0.48; Rule-Unaware group: F(1,13) = 13.8, p = 0.003, ηp2 = 0.51). Rule-Aware participants responded somewhat faster overall than Rule-Unaware participants (Rule-Aware: 1095 ms; SEM = 62.7 ms; Rule-Unaware: 1202.2 ms; SEM = 63.6 ms), although this difference was not significant (F(1,27) = 2.34, p = 0.14, ηp2 = 0.080).
Accuracy
Across all eight epochs, the accuracy violation effect was marginally larger in Rule-Aware compared to Rule-Unaware participants (F(1,27) = 3.94, p = 0.057; ηp2 = 0.13). There were no significant group differences in the time course of this effect across the eight epochs (Group × Condition × Epoch: F(7,189) = 1.42, p = 0.22). Follow-up analyses showed that the accuracy violation effect was significant in each group separately (Rule-Aware: F(1,14) = 52.7, p < 0.001; ηp2 = 0.79; Rule-Unaware: F(1,13) = 14.5, p = 0.002; ηp2 = 0.53).
ERPs
Pre-Nap Session
Across all subjects, no significant ERP violation effects were found during the initial session in the two analysis intervals (400-800 ms: F(1,27) = 0.51, p = 0.48, ηp2 = 0.018; 800-1100 ms: F(1,27) = 0.34, p = 0.56, ηp2 = 0.012). This result is not surprising, as accuracy and RT did not show robust sensitivity to article-animacy violations until approximately halfway through the test (Figure 3). ERP differences may have been present in the second half of the session, but if so they were overshadowed by data from the first half. Due to insufficient number of trials, it was not possible to examine this hypothesis.
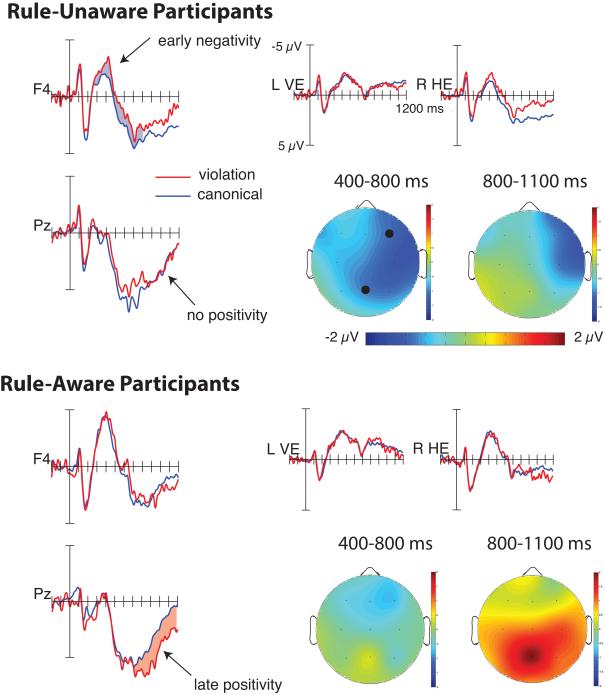
Figure 3.
Grand average ERPs to canonical and violation trials in the Post-Nap learning block, in Rule-Aware (n = 15) versus Rule-Unaware (n = 14) participants. ERPs are shown for eye channels (left vertical eye and right vertical eye) and at F4 and Pz. Approximate electrode scalp locations (F4 and Pz) are denoted with black dots on the upper left scalp map. Rule-Unaware participants showed a significantly right-lateralized negativity from 400-800 ms, whereas Rule-Aware participants showed a significant P600 effect from 800-1100 ms.
Post-Nap Session
Across all subjects, violations elicited a negativity from 400 to 800 ms that showed a right medial distribution, equally distributed over anterior and posterior sites (400-800 ms: F(1,27) = 15.56, p = 0.001, ηp2 = 0.366; Condition × Hemisphere F(1,27) = 4.14, p = 0.052; Condition × Laterality F(1,27) = 6.56, p = 0.016). This result indicates that the presence of an animacy violation modulated neural processing of article-noun pairs. Although this negative effect resembles the N400 in terms of latency and polarity, its distribution is not similar to the N400, which generally shows a posterior distribution (e.g., Kutas & Federmeier, 2011), or any other known language-related component. Thus it may partially reflect domain-general learning mechanisms that are not specific to language processing. No significant effects were observed in the 800-1100 ms interval (F(1,27) = 0.13, p = 0.72, ηp2 = 0.005).
Rule-Aware versus Rule-Unaware Participants
To investigate whether there was a correspondence between neural measures of learning and rule awareness indexed by subjective report, we examined whether ERPs in the post-nap session differed as a function of rule awareness. From 400 to 800 ms, Rule-Unaware participants showed a significantly larger negativity to violation versus canonical trials than did Rule-Aware participants (Figure 3; Awareness Group × Canonical/Violation Condition: F(1,27) = 15.56, p = 0.001; ηp2 = 0.37). Follow-up analyses revealed that Rule-Unaware participants showed a significant negativity to violation versus canonical trials (F(1,13) = 16.8, p = 0.001; ηp2 = 0.56), an effect that was maximal over the right hemisphere (Condition × Hemisphere: F(1,13) = 4.83, p = 0.047). In contrast, Rule-Aware participants did not show a significant violation effect in this time range, although there was a hint of a weak negativity over right anterior electrodes (Figure 3; F(1,14) = 1.35 , p = 0.27; ηp2 = 0.088; Condition × Lateral/Medial: F(1,14) = 5.00, p = 0.042; Follow-up analysis over medial sites ns, F(1,14) = 2.97, p = 0.11).
From 800-1100 ms, Rule-Aware participants showed a positive violation effect, while Rule-Unaware participants showed a negative violation effect in this time range (Figure 3; Awareness Group × Canonical/Violation Condition: F(1,27) = 11.39, p = 0.002; ηp2 =0.30). Follow-up analyses confirmed that the positivity in Rule-Aware participants was significant, with a medial posterior distribution similar to a P600 effect (Condition: F(1,14) = 10.2, p = 0.007; ηp2 = 0.42; Condition × Anterior/Posterior × Laterality). Note that the latency of the P600 in our participants was somewhat delayed (800-1100 ms) compared to that in most previous reports, perhaps because our paradigm required participants to process two words simultaneously, whereas in typical ERP studies there is only one word. In contrast, in Rule-Unaware participants the negative violation effect observed during the earlier interval at 400-800 ms persisted into the later interval over right electrodes, though the magnitude of the effect was somewhat weaker (Condition: F(1,13) = 3.36, p = 0.090; ηp2 = 0.21; Condition × Hemisphere: F(1,13) = 6.34, p = 0.026; Follow-up over Right Hemisphere: Condition: F(1,13) = 5.32, p = 0.038; ηp2 = 0.290). There was no hint of the posterior positivity observed in Rule-Aware participants. The finding that ERPs differed between Rule-Aware and Rule-Unaware participants supports the veracity of participants’ subjective verbal reports.
Effects of Sleep Physiology on Behavioral Measures
Table 2 shows average sleep measures. Figure 4A displays RLI across all participants as a function of learning over time. The primary measure of the effect of sleep is the change in RLI from pre to post nap, denoted as f).RLI. A linear regression model comprising SWSdur, REMdur, SWSdurxREMdur, Sleepdur, and Sleeplat significantly predicted f).RLI (F(4,26) = 3.4, p = 0.021). Of the five predictor variables, only SWSdurxREMdur significantly contributed to the model (/J = 0.87, p = 0.023). This result indicates that participants who had greater and more equally distributed durations of both SWS and REM (leading to higher SWSdurxREMdur values), showed a larger increase in sensitivity to the hidden rule after napping. These results are consistent with the idea that sequential time in both SWS and REM facilitates memory consolidation for this type of generalized information. With the exception of Sleeplat, which was a marginal predictor (/J = 0.45, p = 0.064), none of the other variables (SWSdur, REMdur, or Sleepdur) were significant predictors in the model (all p values > 0.2).
Table 2.
Sleep Measures (n = 27)
| Time Awake | Time in Stage I | Time in Stage 2 | Time in SWS | Time in REM |
|---|---|---|---|---|
| 21.9 (18.8) | 5.48 (3.18) | 36.8 (13.5) | 20.3 (12.8) | 7.72 (9.62) |
Values are min ± SD.
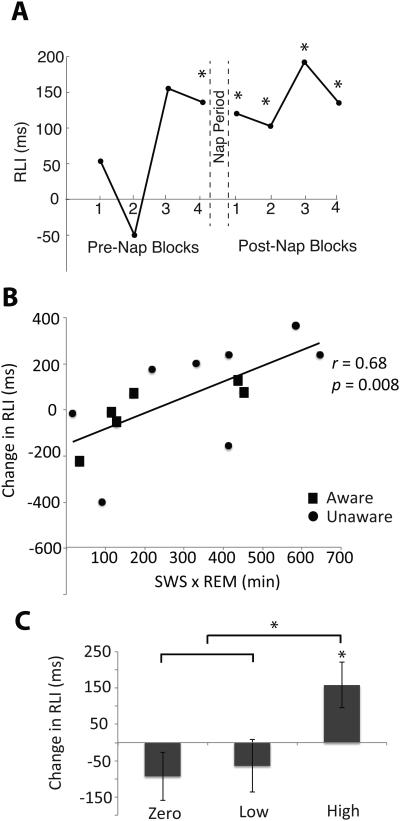
Figure 4.
A) RLI across all participants for each block. An asterisk indicates that the RLI is significant (p < 0.05).
B) Scatterplot showing relation between change in the RLI from Pre-Nap to Post-Nap sessions and product of SWS and REM duration. This correlation includes only participants who reached both SWS and REM. The mean change in RLI for participants with no REM or no SWS (zero group) was −93 ms.
C) Pre- to Post-Nap change in the RLI (f).RLI) for the three SWS × REM groups. Participants were subdivided into the zero group (n = 13), low group (n = 7), and high group (n = 7), according to SWS × REM values. The high group showed a significantly greater violation effect at Post-Nap compared to that in the other two groups. Error bars represent SEM. An asterisk indicates a significant effect (p < 0.05).
To permit further analyses of sleep/memory relationships, we computed several correlations (Table 3). As expected based on the regression results, the correlation between f).RLI and SWSdurxREMdur was significant (Figure 4B). As a point of comparison, we also computed correlations between f).RLI and the sum of time spent in SWS and REM and found only a marginal correlation (Table 3). In addition, we computed delta power over intervals of SWS as an alternative measure of the quality of SWS in place of time in SWS. Duration of REM multiplied by delta power in SWS also showed significant correlations with f).RLI, corroborating our main finding. Finally, we ran another correlation between SWSdurxREMdur and f).RLI designed to assess the effect of the “0” SWSdurxREMdur values from participants who failed to reach SWS or REM sleep during the nap; only participants who reached both SWS and REM were included. This correlation was also significant, and it was numerically stronger than the original correlation that included all participants (Table 3; Figure 4B).
Table 3.
Correlations with Change in RLI and Sleep Physiology (n = 27 unless otherwise noted)
| Change in RLI | SWS × REM Duration |
SWS × REM Duration* |
SWS + REM Duration |
Delta Power (0.5 to 4 Hz) During SWS × Duration of REM † |
|---|---|---|---|---|
| r | 0.52 | 0.68 | 0.37 | 0.45 |
| p | 0.005 | 0.008 | 0.057 | 0.021 |
n = 14, includes only those participants who obtained both SWS and REM.
n = 26, excludes one participant who did not achieve SWS.
This systematic relationship between sleep physiology and rule learning can also be readily observed via a median split on the basis of SWSdurxREMdur values. First, participants who failed to reach REM sleep during the nap (yielding SWSdurxREMdur values of 0) were designated group “Zero” (n = 13). Group “Low” was comprised of participants with the lowest SWSdurxREMdur values and group “High” those with the highest SWSdurxREMdur values (n = 7 in each group). f).RLI was significantly different among the three groups (Group × Pre/Post: F(2,24) = 3.44, p = 0.049; Figure 4C). Contrasts revealed that the Zero and Low groups did not significantly differ on this measure (t(24) = 0.29, p = 0.77), but the High group showed a significantly larger increase in f).RLI compared to that in the two other groups combined (t(24) = 2.99, p = 0.010). Additional follow-up tests showed that f).RLI was significant greater than zero for the High Group (t(6) = 2.53, p = 0.044), but not for the Low (t(6) = −0.89, p = 0.41) or Zero groups (t(12) = −0.14, p = 0.19). These results suggest that enhancement in f).RLI was specific to those participants with the highest SWS and REM throughput. Differences in the accuracy violation effect from Pre-Nap to Post-Nap did not differ significantly between the three sleep groups (Group × Pre/Post: F(2,24) = 2.37, p = 0.12).
Although the pre-post computation allowed us to assess changes in the RLI across the 110-min period that included the nap, the correlations we observed could have been influenced by individual differences at the Pre-Nap session. In this sense, all participants did not have the same baseline. Further analyses were thus conducted separately for the Pre-Nap (second half) and Post-Nap (first half) results. Whereas differences between the three groups at Pre-Nap were nonsignificant (F(2,26) = 1.28, p = 0.30), the RLI significantly differed between the groups at Post-Nap (F(2,26) = 3.47, p = 0.048). Contrasts on the Post-Nap data showed that the RLI was not significantly different between the Zero and Low groups (t(24) = 0.85, p = 0.40), but was significantly larger in the High group compared to the two other groups (t(24) = 2.33, p = 0.029; Figure 4D).
To further evaluate the possible influence of Pre-Nap learning differences among individuals, we computed another multiple regression model with the RLI at Post-Nap (first half) as the dependent measure, and SWSdurxREMdur and the RLI at baseline (second half of Pre-Nap block) as predictor variables. The model significantly predicted the RLI at Post-Nap (F(2,26) = 3.81, p = 0.037). Importantly, only SWSdurxREMdur significantly contributed to the model (/J = 0.45, p = 0.026); the RLI at baseline was not a significant predictor (p = 0.65). This result indicates that the relationship between SWSdurxREMdur and the RLI at Post-Nap cannot be attributed to baseline differences in the RLI.
It is possible that participants who subsequently obtained greater amounts of SWS and REM (those with higher SWSdurxREMdur values) were more fatigued prior to the nap. In this case, they might be expected to perform more poorly in the Pre-Nap block than participants who went on to obtain lesser amounts of SWS and REM. To address this question, we used overall RT and accuracy in the Pre-Nap block (collapsed across canonical and violation trials) as a proxy for general performance and level of alertness. There was no significant effect of subsequent SWSdurxREMdur on either RT (F(1,25) = 0.43, p = 0.52) or accuracy (F(1,25) = 2.76, p = 0.11). While the p value for accuracy approaches marginal significance, this finding reflects that participants in the High SWSdurxREMdur group were more accurate than the other two groups (t(25) = 2.16, p = 0.040). Taken together, these results are inconsistent with the idea that participants who later obtained more SWS and REM were systematically less alert during the Pre-Nap block than participants who obtained less SWS and REM.
Finally, we directly tested whether the main sleep finding—that SWSdurxREMdur predicts f).RLI—differed as a function of whether participants became explicitly aware of the hidden animacy rule. A multiple regression model with SWSdurxREMdur and rule awareness (aware, unaware) as predictor variables significantly predicted f).RLI (F(2,26) = 4.86, p = 0.017). Only SWSdurxREMdur significantly contributed to the model (/J = 0.56, p = 0.005); rule awareness was not a significant predictor (p = 0.51). These results indicate that SWSdurxREMdur predicted f).RLI similarly in Rule-Aware and Rule-Unaware participants.
Effects of Sleep Physiology on Rule-Awareness
We examined whether sleep measures (SWSdurxREMdur, SWSdur, or REMdur) predicted participants’ level of explicit awareness of the hidden rule, with the P600 during the Post-Nap session serving as a proxy for level of rule awareness. Interestingly, in Rule-Aware participants (n = 14), SWSdur significantly predicted P600 amplitude (F(1,10) = 6.63, p = 0.028), with greater SWS durations correlating with larger P600 effects. In contrast, SWSdurxREMdur (F(1,10) = 0.190, p = 0.67) and REMdur (F(1,10) = 0.34, p = 0.57) did not significantly predict P600 amplitude. While exploratory, these results provide support for the idea that sleep influences the extraction of explicit knowledge.
Self-Report Sleep Measures
We examined whether self-reported sleep duration and subjective sleep quality for the preceding night correlated with SWSdurxREMdur. No relationship was found between SWSdurxREMdur and duration of sleep, number of minutes awake, or subjective sleep quality on any measure for the previous night (all p values > 0.18). Similarly, the three SWSdurxREMdur groups (“Zero,” “Low,” and “High”) did not significantly differ on any of these measures (all p values > 0.18). These results suggest that participants who subsequently differed in SWSdurxREMdur slept similarly in the preceding night and were at comparable fatigue levels before beginning their naps, despite the fact that they went on to obtain different amounts of SWS and REM. In contrast, self-reported sleep duration and subjective sleep quality for the 90-min nap interval were found to significantly differ as a function of SWSdurxREMdur. Higher SWSdurxREMdur values were associated with a decreased number of reported awakenings (r = −0.55, p = 0.003), better self-reported sleep quality (r = 0.43, p = 0.025) and less restless/more sound sleep (r = 0.45, p = 0.018). At the group level, the three SWSdurxREMdur groups significantly differed on these same measures (number of reported awakenings: F(2,26) = 6.76, p = 0.005; sleep quality: F(2,26) = 4.23, p = 0.027; sleep soundness: F(2,26) = 4.61, p = 0.020). These results indicate that higher SWSdurxREMdur as determined physiologically is associated with better subjective sleep quality, as assessed through self-report measures.
Discussion
Effects of Sleep on Rule Generalization in Language
Our findings support the hypothesis that SWS and REM synergistically facilitate the abstraction of rules in linguistic input. Slowed responses to animacy judgments for stimuli that violated the hidden rule (measured as RLI) indicated participants learned the regularity, even if they remained entirely unaware of the rule. After the 110-min pause that included a nap, the degree to which the measure of learning increased was strongly correlated with the product of SWS and REM sleep achieved. These results provide evidence for the importance of sleep in the consolidation of newly acquired linguistic knowledge.
The influence of sleep on language acquisition has been studied in the context of lexical learning (Dumay & Gaskell, 2007), speech recognition (Fenn, Nusbaum & Margoliash, 2003), speech production (Gaskell et al., in press), and artificial grammar learning (Gomez, Bootzin & Nadel, 2006; Nieuwenhuis, Folia, Forkstam, Jensen, & Petersson, 2013). For example, lexical competition, indexing the integration of a newly acquired word into the mental lexicon, emerges only after a period of sleep, and not after an equivalent period of wakefulness (Dumay & Gaskell, 2007). It appears that sleep is especially important for pattern or rule generalization within language. Infants who slept after exposure to an artificial grammar showed greater rule abstraction, whereas infants who remained awake after learning showed enhanced veridical memory for learned items (Gomez et al., 2006). Similarly, sleep promotes rule abstraction in adults exposed to an artificial finite-state grammar, with classification performance of new strings improving after a period containing sleep compared to an equivalent period of wakefulness (Nieuwehuis et al., 2013). Sleep also facilitates the learning of phonotactic constraints in speech production. Participants who slept after repeating sequences of syllables made up of specific phoneme combinations produced speech errors consistent with the phonotactic rules acquired during training, whereas participants who remained awake during this interval did not, and furthermore, the amount of SWS predicted phonotactic learning (Gaskell et al., in press). The present study contributes to this literature, showing that sleep is also involved in the generalization of associations between form and meaning, which is arguably the most essential component of language acquisition. This type of associative learning underlies both first- and second-language acquisition across every higher-level linguistic subsystem, from morphology (e.g., learning that the English morpheme “-s” encodes plurality), to syntax (e.g., learning when to use “him” versus “he”), to semantics (e.g., learning the meanings of words such as “dog” or “cat”). Remarkably, the abstraction of linguistic rules over time is influenced not only by experiences during wake, but also by neurophysiological sleep mechanisms.
Given the delay between the Pre- and Post-nap sessions, learning effects would normally be expected to decline as memory storage gradually decays. Whereas a slight decline in learning was observed for participants who failed to achieve a high SWS/REM throughput (Figure 4C), those who did showed a significant enhancement in learning immediately after their naps. Presumably, mechanisms at work during both SWS and REM actively promoted the consolidation and abstraction of rules governing the newly acquired information.
A potential objection that may be raised against our interpretation of these results is that fatigue may contribute to the relationship between SWSdurxREMdur and f).RLI. It is conceivable that participants who subsequently obtained greater amounts of SWS and REM (i.e., those in the High SWSdurxREMdur group) were more fatigued initially, and thus showed larger gains as a function of napping. In this case, varying levels of fatigue between participants, rather than specific benefits associated with interactions between SWS and REM per se, could account for our main finding. However, several lines of evidence rule against this explanation. First, using overall RTs and accuracy as a proxy for performance, we found that participants who later obtained more SWS and REM did not perform more poorly during the Pre-Nap block. In fact, participants in the High SWSdurxREMdur group actually achieved higher accuracy levels than participants in the other two groups. This result is incompatible with the idea that participants who subsequently achieved greater durations of SWS and REM were less alert prior to napping. Secondly, self-reported sleep duration and sleep quality for the preceding night did not significantly differ as a function of subsequent SWSdurxREMdur. This suggests that high SWSdurxREMdur participants came in to the lab no more or less sleep deprived than their low SWSdurxREMdur counterparts. In contrast, several measures of sleep quality assessed over the 90-min nap period correlated with SWSdurxREMdur, supporting the validity of these self-report measures. The finding that SWSdurxREMdur, but not Sleepdur and Sleeplat, significantly predict f).RLI is also inconsistent with a general fatigue explanation. The underlying assumption here is that sleep duration and sleep latency over the nap period are effective proxies for overall fatigue, with more fatigued participants experiencing greater sleep pressure and correspondingly shorter sleep latencies and longer sleep durations over the 90-min nap interval. If recovery from fatigue attributable to the nap were primarily responsible for an increase in sensitivity to the rule, one would expect sleep duration and sleep latency to predict f).RLI more strongly than SWSdurxREMdur, These results suggest that participants who obtained more SWS and REM were not necessarily more fatigued than participants who achieved shorter durations of these stages. In other words, two equally fatigued participants may fall asleep within comparable latencies and for similar overall durations, but may nonetheless differ in the quality of sleep that they obtain. Although the present study cannot account for why such differences in sleep quality occur, it appears to be sleep quality rather than simply overall sleep quantity that contributes to rule abstraction.
Implicit Learning of Associations between Form and Meaning
Along with RTs, ERPs also showed sensitivity to animacy violations. Rule-Unaware participants showed an earlier right-lateralized negativity to violations, while Rule-Aware learners showed a P600 effect. We speculate that the early right negativity to violations indexes the development of implicit knowledge about the hidden rule. It is possible that Rule-Aware participants also acquired implicit knowledge, but that the negativity did not reach statistical significance in this group because of overlap from the early portion of the P600 effect (Figure 3). Together with subjective verbal reports, these ERPs findings indicate that sensitivity to the animacy rule occurred implicitly in many participants; conscious awareness of the rule was optional.
The RLI did not vary as a function of rule awareness. This result may be interpreted in at least two different ways. The first possibility is that the RLI represents an implicit index of learning, occurring independently of rule awareness. Because the learning task involved making responses that were speeded and orthogonal to the article-animacy correlation, it was designed to encourage automatic responding rather than deliberative or strategic processing. Thus, equal amounts of implicit learning may have occurred in both groups, with additional explicit processing occurring in the Rule-Aware group that did not directly contribute to speeded responding. The present ERP evidence neither supports nor rules out this idea. An additional piece of evidence is the finding that SWSdurxREMdur predicted f).RLI similarly in both Rule-Aware and Rule-Unaware participants (Figure 4B). If the effect were driven by different mechanisms between the two groups, effects of sleep physiology might be expected to exert different effects. Thus, results support the idea that the RLI reflects implicit learning in both groups.
An alternative interpretation to consider is that although the RLI is similar between the two groups, it reflects different underlying causes. For example, after becoming aware of the rule, Rule-Aware participants may have adopted a different strategy for processing the stimuli, perhaps forming conscious expectations of the article noun pairings. Thus, a similar RT delay may reflect implicit learning in Rule-Unaware participants, and strategic, explicit processing in Rule-Aware participants. Under this interpretation, the lack of a significant negativity and the presence of a P600 in the Rule-Aware participants may reflect greater reliance on explicit over implicit processing. However, the finding that SWSdurxREMdur predicted f).RLI in both groups supports the idea that the RLI reflects the same underlying mechanism in both groups. In sum, it is difficult to distinguish whether the RT delay reflects implicit learning in both groups, or implicit learning in the Rule-Unaware group and explicit learning in the Rule-Aware group. The strongest statement that can be made is that sleep physiology influenced subsequent sensitivity to the animacy rule, regardless of whether participants became explicitly aware of the underlying rule.
We did not directly test whether sleep facilitated explicit awareness of the underlying linguistic rule, a finding that would be consistent with previous evidence showing that sleep promotes the extraction of explicit knowledge. For example, sleep leads to sudden insight into a hidden mathematical problem (Wagner et al., 2004) and to explicit sequence knowledge in the serial reaction time task (Fischer, Drosopoulos, Tsen, & Born, 2006; Wilhelm et al., 2013). Nonetheless, it is worth noting that only 5 participants in the present study reported becoming aware of the rule during the Pre-Nap Session, while twice this number became aware of the rule after napping, during the Post-Nap Session. If there were a linear relation between exposure to learning materials and probability of becoming aware of the rule, we would expect roughly the same number of participants to reach awareness in both the Pre-Nap and Post-Nap sessions, rather than the proportions we observed. In addition, we found that SWSdur significantly predicted P600 amplitude in Rule-Aware participants, suggesting that SWS increased participants’ level of explicit awareness about the hidden rule. For example, participants who experienced SWS during their naps may have become aware of the hidden rule at an earlier point in the task, or may have gained access to a more complete set of explicit rule knowledge (e.g., becoming aware of the animacy correlations for all four articles, rather than only a subset). These results are generally consistent with the idea that sleep facilitates explicit awareness of hidden patterns or rules (e.g., Wagner et al., 2004; Fischer et al., 2006).
Although not the major focus of the study, our findings also have implications for the field of Second Language Acquisition (SLA). In this field, there is an ongoing debate about the extent to which second language learning in adults can occur in the absence of awareness of learning. A number of theoretical accounts of SLA propose that some degree of attention and awareness is necessary for the acquisition of second language forms (e.g., Robinson, 1996, 2012; VanPatten, 1996, 2004, 2007; Leow, 2001; Schmidt, 1990, 2001; Tomlin & Villa, 1994). Contrary to these views, some evidence implicates implicit second language learning—that is, learning that occurs in the absence of awareness. For example, Williams (2005) exposed participants to sentences containing the same miniature article system as in the present study (ul, gi, ro, and ne). Most participants remained unaware of the correlation between the article and noun animacy. However, when given a choice between a correct and incorrect article for a given noun (e.g., ul giraffe versus ro giraffe), rule-unaware participants selected the correct article more often than would be expected by chance. This result suggests that adults can make form-meaning connections in the absence of awareness. However, this finding has been difficult to replicate (Hama & Leow, 2010; Stutenberg & Morgan-Short, 2011), suggesting that implicit second language learning may occur at only a weak level and/or that there may be considerable variability in this ability among different participants. Nonetheless, later studies have found additional evidence for implicit second language learning in adults using reaction-time methodology, which may be more sensitive to implicit learning than a forced-choice decision task. For example, Leung and Williams (2011) found RT evidence for implicit learning of mappings between novel articles and thematic role (i.e., whether a noun is an agent or patient). Similarly, using a paradigm that provided the methodological basis for the present study, Leung and Williams (2012; in press) found RT delays to phrases that violated a hidden noun animacy rule, providing evidence for implicit learning of associations between forms and animacy.
At a basic level, the present study replicated these behavioral findings, demonstrating that responses were made more slowly to violations of the hidden animacy rule than to canonical instances of the rule. This effect was equivalently demonstrated whether or not participants evinced awareness of the underlying pattern. Moreover, our ERP data provided new evidence in support of this demonstration, converging with the behavioral findings. One limitation of prior studies of implicit second language learning (e.g, Williams 2005, Leung and Williams, 2012, in press) is that they rely solely on behavioral and self-report data. Thus, the concern can always be raised that participants categorized as “rule-unaware” may simply be underreporting their level of knowledge. ERP data from the present study countered this concern by supporting a distinction between aware and unaware participants’ online neural processing. Specifically, rule-unaware participants showed no evidence of a P600 effect, a component previously linked to the conscious processing of syntactic violations (Batterink & Neville, 2013). In addition, ERPs demonstrated that rule-unaware participants nonetheless showed a robust neural response to hidden animacy violations, providing an additional measure of learning that coincides with effects observed at the behavioral level. Taken together, these results support the idea that adults can implicitly acquire associations between form and meaning, at least for certain concepts.
Interactions Between SWS and REM
Our main finding — that maintained sensitivity to the hidden rule was predicted by sleep organization — supports sequential views of sleep-dependent memory processing. Optimal memory processing may require a cyclic succession of SWS and REM (Ambrosini & Giuditta, 2001; Ficca & Salzarulo, 2004; Walker & Stickgold, 2010), rather than merely one sleep stage. According to one hypothesis, non-adaptive memory traces are weakened or eliminated during SWS, while the remaining traces are then strengthened and integrated during REM sleep (Ambrosini & Giuditta, 2001). A more recent proposal is that SWS functions to consolidate new episodic item memories while keeping individual memory representations separate and distinct; REM then facilitates integration of these new memories with older memories, forming rich associative networks (Walker & Stickgold, 2010). Our results fit with these general ideas, suggesting that memory integration during sleep is more tightly linked to the completion of a sleep cycle than the total loading on any given sleep state.
The current study extends research linking sleep organization and behavioral learning into a novel domain—the extraction of an implicitly acquired syntactic rule. Previous evidence on SWS-REM interactions was primarily derived from visual discrimination paradigms. One study found that the product of early-night SWS and late-night REM strongly predicts visual discrimination performance, accounting for 80% of intersubject variance (Stickgold et al., 2000). Similarly, nappers who obtained both SWS and REM showed significant improvements in visual discrimination, while those with no REM showed no change in performance (Mednick, Nakayama, & Stickgold, 2003). Visual discrimination skills also improved more after an entire night of both SWS and REM, relative to either early sleep or late sleep containing relatively more SWS or REM, respectively (Gais, Plihal, Wagner, & Born, 2000). A more recent study has extended these findings, showing that SWS and REM also play complementary roles in emotional memory consolidation (Cairney et al., in press). More SWS was associated with disengagement of the hippocampus during emotional recollection, whereas more REM predicted an increase in hippocampal-neocortical connectivity. Our results suggest that reciprocal interactions between SWS and REM play a role not only in basic perceptual learning or emotional memory consolidation, but also in more abstract, higher-level learning mechanisms that are central to the acquisition of language.
To summarize, we found that experiences during both wake and sleep contribute to the extraction of a novel rule from linguistic input. Learning was operative during wake, given that exposure to the rule was sufficient for some initial acquisition of rule knowledge, whereas interactions between SWS and REM predicted the continued retention or strengthening of this newly acquired information. These findings may have important implications for language learners, suggesting that high quality sleep plays a role in retaining and perhaps enhancing linguistic knowledge acquired while awake.
Highlights.
Implicit learning of a hidden linguistic rule was observed behaviorally
Greater [SWS*REM] durations predicted increased post-sleep sensitivity to the rule
Slow-wave and REM sleep contributed to stabilization of new linguistic knowledge
Some participants became rule-aware, leading to distinct brain potentials
Sleep facilitated rule learning regardless of whether learning was explicit
Acknowledgments
We thank John Williams for input leading to the development of our experimental task. This work was supported by NIH grants T32 NS 47987 and F32 HD 078223. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrosini MV, Giuditta A. Learning and sleep: the sequential hypothesis. Sleep Medicine Reviews. 2001;5:477–490. doi: 10.1053/smrv.2001.0180. [DOI] [PubMed] [Google Scholar]
- Batterink L, Neville H. Implicit and explicit mechanisms of word learning in a narrative context: an event-related potential study. Journal of Cognitive Neuroscience. 2011;23:3181–3196. doi: 10.1162/jocn_a_00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink L, Neville H. The human brain processes syntax in the absence of conscious awareness. Journal of Neuroscience. 2013;33:8528–8533. doi: 10.1523/JNEUROSCI.0618-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney SA, Durrant SJ, Power R, Lewis PA. Complementary Roles of Slow-Wave Sleep and Rapid Eye Movement Sleep in Emotional Memory Consolidation. Cerebral Cortex. doi: 10.1093/cercor/bht349. in press. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nature Reviews Neuroscience. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dumay N, Gaskell MG. Sleep-associated changes in the mental representation of spoken words. Psychological Science. 2007;18:35–39. doi: 10.1111/j.1467-9280.2007.01845.x. [DOI] [PubMed] [Google Scholar]
- Durrant S, Taylor C, Cairney S, Lewis P. Sleep-dependent consolidation of statistical learning. Neuropsychologia. 2011;49:1322–1331. doi: 10.1016/j.neuropsychologia.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Durrant SJ, Cairney SA, Lewis PA. Overnight consolidation aids the transfer of statistical knowledge from the medial temporal lobe to the striatum. Cerebral Cortex. 2013;23:2467–2478. doi: 10.1093/cercor/bhs244. [DOI] [PubMed] [Google Scholar]
- Djonlagic I, Rosenfeld A, Shohamy D, Myers C, Gluck M, Stickgold R. Sleep enhances category learning. Learning and Memory. 2009;16:751–755. doi: 10.1101/lm.1634509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proceedings of the National Academy of Sciences, U.S.A. 2007;104:7723–7728. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer TA, Christiansen MH, Monaghan P. Phonological typicality influences on-line sentence comprehension. Proceedings of the National Academy of Sciences, U.S.A. 2006;103:12203–12208. doi: 10.1073/pnas.0602173103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn KM, Nusbaum HC, Margoliash D. Consolidation during sleepDof perceptual learning of spoken language. Nature. 2003;425:614–616. doi: 10.1038/nature01951. [DOI] [PubMed] [Google Scholar]
- Ficca G, Salzarulo P. What in sleep is for memory. Sleep Medicine. 2004;5:225–230. doi: 10.1016/j.sleep.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Fischer S, Drosopoulos S, Tsen J, Born J. Implicit learning–explicit knowing:DA role for sleep in memory system interaction. Journal of Cognitive Neuroscience. 2006;18:311–319. [PubMed] [Google Scholar]
- Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nature Neuroscience. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- Gaskell MG, Warker J, Lindsay S, Frost R, Guest G, Snowdon R, Stackhouse A. Sleep underpins the plasticity of language production. Psychological Science. doi: 10.1177/0956797614535937. in press. [DOI] [PubMed] [Google Scholar]
- Gómez RL, Bootzin RR, Nadel L. Naps promote abstraction in language-learning infants. Psychological Science. 2006;17:670–674. doi: 10.1111/j.1467-9280.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- Grigg-Damberger MM. The AASM Scoring Manual four years later. Journal of Clinical Sleep Medicine. 2012;8:323–332. doi: 10.5664/jcsm.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: Finding meaning in the N400 component of the Event-Related Brain Potential (ERP) Annual Review of Psychology. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow RP. Attention, awareness, and foreign language behavior. Language Learning. 2001;51:113–155. [Google Scholar]
- Leung JHC, Williams JN. Crosslinguistic differences in implicit language learning. Studies in Second Language Acquisition. in press. [Google Scholar]
- Leung JHC, Williams JN. Constraints on implicit learning of grammatical form-meaning connections. Language Learning. 2012;37:634–662. [Google Scholar]
- Lewis PA, Durrant SJ. Overlapping memory replay during sleep builds cognitive schemata. Trends in Cognitive Science. 2011;15:343–351. doi: 10.1016/j.tics.2011.06.004. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Morey RD. Confidence Intervals from Normalized Data: A correction to Cousineau (2005) Tutorial in Quantitative Methods for Psychology. 2008;4:61–64. [Google Scholar]
- Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nature Neuroscience. 2003;6:697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis ILC, Folia V, Forkstam C, Jensen O, Petersson KM. Sleep promotes the extraction of grammatical rules. PLOS ONE. 2013;8:e65046. doi: 10.1371/journal.pone.0065046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis M. A neurolinguistic theory of bilingualism. John Benjamins Publishing Co.; Philadelphia PA: 2004. [Google Scholar]
- Payne JD, Schacter DL, Propper PE, Huang L, Wamsley EJ, Tucker MA, Walker MP, Stickgold R. The role of sleep in false memory formation. Neurobiology of Learning and Memory. 2009;92:327–334. doi: 10.1016/j.nlm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. Consciousness, rules, and instructed second language acquisition. Peter Lang; New York: 1996. [Google Scholar]
- Robinson P, Mackey A, Gass S, Schmidt R. Attention and awareness in second language acquisition. In: Gass S, Mackey A, editors. The Routledge handbook of second language acquisition. Routledge; New York: 2012. pp. 247–267. [Google Scholar]
- Schmidt R. The role of consciousness in second language learning. Applied Linguistics. 1990;11:129–158. [Google Scholar]
- Schmidt R. Attention. In: Robinson P, editor. Cognition and second language instruction. Cambridge University Press; Cambridge, UK: 2001. pp. 3–32. [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nature Neuroscience. 2013;16:139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. Journal of Cognitive Neuroscience. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- Tomlin RS, Villa V. Attention in cognitive science and second language acquisition. Studies in Second Language Acquisition. 1994;12:287–301. doi: 10.1017/S0272263100012833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman MT. Contributions of memory circuits to language: The declarative/procedural model. Cognition. 2004;92:231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- VanPatten B. Input processing and grammar instruction: Theory and research. Ablex; Norwood, NJ: 1996. [Google Scholar]
- VanPatten B. Input processing in second language acquisition. In: VanPatten B, editor. Processing instruction: Theory, research, and commentary. Erlbaum; Mahwah, NJ: 2004. pp. 5–31. [Google Scholar]
- VanPatten B. Input procesing in adult second language acquisition. Theories in second language acquisition. In: VanPatten B, Williams J, editors. Lawrence Erlbaum Associates; Mahwah, NJ: 2007. pp. 115–135. [Google Scholar]
- Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Overnight alchemy: sleep-dependent memory evolution. Nature Reviews Neuroscience. 2010;11:218–219. doi: 10.1038/nrn2762-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Rose M, Imhof KI, Rasch B, Büchel C, Born J. The sleeping child outplays the adult’s capacity to convert implicit into explicit knowledge. Nature Neuroscience. 2013;16:391–395. doi: 10.1038/nn.3343. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends in Cognitive Sciences. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Williams JN. Learning without awareness. Studies in Second Language Acquisition. 2005;27:269–304. [Google Scholar]
- Hama M, Leow RP. Learning without awareness revisited. Studies in Second Language Acquisition. 2010;32:465–491. [Google Scholar]
- Granena Gisela, et al., editors. Selected Proceedings of the 2010 Second Language Research Forum. Cascadilla Proceedings Project; Somerville, MA: 2005. pp. 18–28. Learning without awareness revisited: A replication of Williams. [Google Scholar]
- Leung JHC, Williams JN. The implicit learning of mappings between forms and contextually derived meanings. Studies in Second Language Acquisition. 2011;33:33–55. [Google Scholar]