Abstract
Dexamethasone is a glucocorticoid that is widely used in the ophthalmic arena. The recent FDA approved dexamethasone implant can provide a three month efficacy but with high rate of drug related cataract and high intraocular pressure (IOP). It seems that higher steroid in aqueous humor and around lens may be associated with these complications based on clinical fact that higher IOP was observed with intravitreal triamcinolone acetonide (TA) than with subtenon TA. We hypothesize that placing a sustained dexamethasone release system near back of the eye through a fine needle can maximize efficacy while mitigate higher rate of IOP rise and cataract. To develop a sustained intravitreal dexamethasone delivery system, porous silicon dioxide (pSiO2) microparticles were fabricated and functionalized with amines as well as carboxyl groups. Dexamethasone was conjugated to pSiO2 through the Steglich Esterificaion Reaction between hydroxyl of dexamethasone and carboxyl groups on the pSiO2. The drug loading was confirmed by Fourier transform infrared spectroscopy (FTIR) and loading efficiency was quantitated using thermogravimetric analysis (TGA). In vitro release was conducted for three months and dexamethasone was confirmed in the released samples using liquid chromatography-tandem mass spectrometry (LC/MS/MS). A pilot ocular safety and determination of vitreous drug level was performed in rabbit eyes. The drug loading study demonstrated that loading efficiency was from 5.96% to 10.77% depending on the loading reaction time, being higher with longer loading reaction time before reaching saturation around 7 days. In vitro drug release study revealed that dexamethasone release from pSiO2 particles was sustainable for over 90 days and was 80 days longer than free dexamethasone or infiltration-loaded pSiO2 particle formulation in the same setting. Pilot in vivo study demonstrated no sign of ocular adverse reaction in rabbit eyes following a single 3 mg intravitreal injection and free drug level at 2-week was 107.23+/−10.54 ng/mL that is well above the therapeutic level but only around 20% level of dexamethasone released from OZURDEX ® (dexamethasone intravitreal implant) in a rabbit eye model. In conclusion, dexamethasone is able to covalently load to the pSiO2 particles and provide sustained drug release for at least 3 months in vitro. Intravitreal injection of these particles were well tolerated in rabbit eyes and free drug level in vitreous at 2-week was well above the therapeutic level.
Keywords: Intravitreal drug delivery, Porous silicon particle, Dexamethasone, Sustained release, Rabbit eye
1. Introduction
The delivery of therapeutics to the back of the eye has been a challenge for eye clinicians and eye researchers due to the formidable blood-eye barrier and the difficulty in accessing the posterior pole of the eye globe where the macula (which is responsible for sharp vision in mammals) is located. Unfortunately, many vision-threatening diseases, such as macular degeneration and diabetic retinopathy, attack or affect the macula. (Bressler, 2004; Williams et al., 2004) Unlike anterior segmental eye diseases, such as keratitis or even anterior uveitis, (2012; Sasaki et al., 2000) topical eye drops often do not work well on posterior segmental eye diseases, and yield very low drug levels in the vitreous. (Vemulakonda et al., 2008) (Weijtens et al., 2002) Therefore, the direct placement of therapeutics into the posterior segment of the eye globe by intravitreal injection with a fine needle has become a standard treatment modality for many chorioretinal dieases. (2011) (Heier et al., 2012) Because such diseases are chronic and refractory, they require frequent intravitreal injections, which not only increases the risk of injection-associated complications such as lens damage, vitreous hemorrhage, retinal detachment, and even endophthalmitis, but also adversely affects quality of life for patients. (Raghava et al., 2004) There is a need to develop a sustained intravitreal drug delivery system to reduce the frequency of injections.
Porous silicon (pSi) is a nanostructured material with tunable large surface area (400–800 m2 /g) and porosity, which make it an attractive material as a drug delivery platform. (Stewart and Buriak, 2000) It has good ocular biocompatibility (Cheng et al., 2008) and it is biodegradable into various protonated forms of the orthosilicate ion, which is removed from the aqueous humor and vitreous humor by normal turnover processes. (Nieto et al., 2013) Furthermore, a range of surface modifications are available for this material, such as oxidation, hydrosilylation, and silanization to increase its vitreous residence time as well as functionalize its surface for loading of various drugs. (Cheng et al., 2008) (Chhablani et al., 2013) (Hartmann et al., 2013) We have reported a porous silicon (pSi) sustained intravitreal drug delivery system in which daunorubicin was covalently bonded to the inner pore surfaces of pSi and the in vitro drug release demonstrated > 100-fold longer residence time when compared to a bolus injection of free drug. (Chhablani et al., 2013) In this current study, we aim to investigate if a similar strategy would work for a different small molecule compound, dexamethasone.
Dexamethasone is a glucocorticoid that is widely used in the medical field including ophthalmology. It has been used for anterior segment ocular inflammation, and recently for posterior segment inflammation, such as uveitis and various macular edema. (2004; Williams et al., 2009) (Lowder et al., 2011) (Haller et al., 2011) The recent FDA approved dexamethasone implant can provide a three month efficacy with a 15 to 20% complication of cataract and high intraocular pressure (IOP). (Haller et al., 2011) Cataract and high IOP are common complications for ocular use of steroids including the popular option of triamcinolone acetonide that is administered intravitreally or subtenon. (Jonas et al., 2005) (Chew et al., 2011) It seems that higher steroid in aqueous is associated with higher IOP incidence because we observed that subtenon triamcinolone acetonide (TA) had a much lower free TA in aqueous as well as a lower incidence of IOP elevation than intravitreal TA injection. (Beer et al., 2003) (Shen et al., 2010) We hypothesize that therapeutic, but lower steroid concentration in the aqueous and around the lens will mitigate steroid-associated ocular complications. For posterior eye diseases, the targeting and delivery of payload closer to disease site, while providing sustainable drug release over a long period of time, should maximize efficacy while limit lateral adverse side effects. Instead of placing a drug delivery device at pars plana, which is away from macula but closer to lens and aqueous pathways, we tried to engineer dexamethasone loaded porous silicon particles that can be placed through a fine needle injection near the macula.
2. Materials and methods
2.1. Materials
Dexamethasone (Dex), 3-aminopropyltrimethoxysilane (APS), succinic anhydride, ethanol, 4-dimethylaminopyridine, dicyclohexylcarbodiimide (DCC), N,N-dimethylformamide (DMF), and hydrochloric acid (HCl) were purchased from Sigma-Aldrich. Ethanol was purchased from Thermo Fisher Scientific. (100)-oriented boron-doped p-type silicon wafers were purchased from Siltronix Inc. (0.99 mω·cm resistivity, Archamps, France)
2.2. Methods
2.2.1 Preparation of porous silicon microparticles
Porous silicon microparticles were prepared by electrochemical etch of highly doped, (100)-oriented p-type silicon wafers in a 48% aqueous HF (hydrofluoric acid):ethanol (3:1 by volume) electrolyte solution. A silicon wafer with an exposed area of 8.04 cm2 was contacted on the backside with a strip of aluminum foil and mounted on a Teflon etching cell with a platinum counter-electrode. The wafer was etched at a constant current density of 112 mA/cm2 for 200 seconds. The resulting porous layer was then lifted off by electropolishing in a 1:29 solution of 48% aqueous hydrofluoric acid to ethanol for 100 seconds at a current density of 6.2 mA/cm2. The etching and electropolishing procedure was repeated 20 times per wafer. The microparticles were harvested every four etches, and the resulting porous layers were ultrasonicated (model FS5 dual-action ultrasonic cleaner; Thermo Fisher Scientific) in ethanol for 30 minutes to form the microparticles as we reported previously. (Chhablani et al., 2013) The porous silicon microparticles obtained had an average pore size of 20 nm as measured from the scanning electron microscopic (SEM) images, and using Adobe Photoshop software (Adobe Photoshop CS6 for Mac; Adobe Systems Inc., San Jose, CA). The mean sizes of the particles were 50 μm by 60 μm by 20 μm (Figure 1).
Figure 1.

SEM image of the pSi microparticles (left panel) and SEM image of the pore structure on a plan-view of the particle. The scale bar in the left panel represents 50 micrometers and the scale bar in the right panel represents 1 micrometer.
2.2.2 Surface modification of porous silicon particles
Surface modifications were done according to our previous reports. (Chhablani et al., 2013; Wu et al., 2011) For complete oxidation of porous silicon (pSi) to porous silica (SiO2, OPS), the particles were placed in a ceramic boat and heated from room temperature to 800°C in a furnace chamber for 2 hours (Thermo Fisher Scientific). The furnace was allowed to cool to room temperature for an additional 3 hours prior to the removal of the samples. The resulting porous silica particles (OPS) were then reacted with 2% concentrated hydrochloric acid in water for 1.5 hours, then rinsed three times with water and then rinsed once with ethanol, and dried in a vacuum oven. The particles were then rotated in a 2% 3-aminopropyltrimethoxysilane (APS; Sigma-Aldrich) in ethanol solution for 2 hours for amine functionalization, then rinsed with ethanol for three times, and dried. Finally, amine-functionalized OPS were reacted with 0.2 M succinic anhydride (99%; Sigma-Aldrich) in N,N-dimethylformamide (DMF; Sigma-Aldrich) for 16 hours and rinsed with water and ethanol (3 times) to obtain a carboxylic acid functionalized surface (OPS-CO2H). The carboxylic acid group resulted from the ring opening of succinic anhydride through a reaction with the amine group on the surface of the particles, as previously described. (Wu et al., 2011)
2.2.3. Dexamethasone (Dex) loading
For loading of dexamethasone by covalent attachment, the following was added to a centrifuge tube: OPS-CO2H (7.5 mg) particles, dicyclohexylcarbodiimide (DCC, 13 mg), 4-N,N-dimethylamminopyridine (DMAP, 3 mg) and dichloromethane (DCM, 1.2 mL). The mixture was rotated for 20 min at room temperature. Dexamethasone (4 mg) was added to the mixture system, and rotated for 7 days at room temperature. Particles were separated by centrifugation, and then carefully washed with DCM four times, and washed in ethanol for three times in order to remove unloaded drug and other chemicals before they were isolated and dried in a vacuum oven. With the three times of ethanol washes, the particles were sterilized. After drug loading, the particles were stored in a capped vial in the dark under an argon protective atmosphere. A schematic illustration of the procedure that covalently grafts Dex to oxidized porous silicon microparticles is shown in figure 2.
Figure 2.
Synthesized routes of oxidized porous silicon microparticles covalently grafted with dexamethasone.
For comparison purposes, the physical adsorption of Dex (ads-OPS-COOH:DEX) was also performed. OPS-CO2H (7.5 mg) particles, dexamethasone (4 mg), and dichloromethane (DCM, 1.2 mL) were added to a centrifuge tube, rotated for 3 hours at room temperature, then centrifuged and rinsed briefly with DCM and ethanol, then isolated and dried in vacuum oven. After drug loading, the particles were stored in a capped vial in the dark under an argon protective atmosphere.
2.2.4. Characterization of microparticles
Surface chemistry modifications and drug loading was characterized by Fourier transform infrared (FTIR) spectroscopy using an attenuated total reflectance (ATR) mode on a Nicolet 6700 Smart-iTR spectrometer (Thermo Fisher Scientific, Pittsburg, PA). Sample spectra was collected from 600 to 4000 cm−1 in absorbance mode, with a resolution of 1 cm−1. Each spectrum had an average of 128 scans.
2.2.5. In vitro Dex release and LC/MS/MS quantitation
For in vitro release, free Dex, ads-OPS-COOH:Dex, and cov-OPS-COOH:Dex microparticles (3 mg each) were weighed and placed in a 1.5 mL conical vial, and 1000 μL of cell culture grade water was added. The vial was incubated at 37°C. Every day the vial was centrifuged at 12500 rpm for 10 minutes, and 800 μL of the supernatant was taken out and stored at −80 °C, protected from light, until analysis. Fresh cell culture grade water (800 μL) was added to restore the volume in the vial in order to maintain a constant volume. It was expected that the in vitro release study would last long; therefore, cell culture grade water was used instead of saline or phosphate-buffered saline which may cause phosphate or sodium accumulation at the bottom of the vial and possibly alter pH value and salt concentration of the drug release medium over time.
An Agilent 1260 liquid chromatograph (LC) system coupled with a Thermo LCQdeca mass spectrometer was used for LC-UV-MS/MS analysis using positive ion mode electrospray ionization (ESI) as the ion source. A Phenomenex Kinetex XB-C18 column (ID 2.1 mm x length 50mm, particle size 2.6 um) with a guard column was employed for LC separation. HPLC grade water with 2.5% methanol and 0.1% formic acid was used as mobile phase A and HPLC grade methanol with 0.1% formic acid was used as the mobile phase B. The LC flow rate was set at 0.30 ml/minute. The LC gradient was increased from 30% mobile phase B to 95% mobile phase B in 10 minutes, held at 95% B for 2 minutes, returned to 30% B in 1 minute, and then held at 30% B for 7 minutes. Quantitative detection of drug released from particles containing physisorbed dexamethasone was performed by HPLC with a UV detection wavelength at 240 nm (bandwidth 6 nm) and a reference wavelength at 600 nm (bandwidth 20 nm).
2.3. Animal study
Six pigmented rabbits were used for a pilot in vivo study. The average body weight was 2.91±0.18 kg at the start of the study and 3.0±0.13 kg at sacrifice. For proof of concept, only two time points were used and the first time point was elected to be one week because our in vitro data suggested that the release will be a sustained mode. Animal use was in accordance to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research, and was approved by Animal Care and Use Committee of University of California, San Diego. Only one eye of each animal was used for intravitreal injection of cov-OPS-COOH:Dex microparticles. Intravitreal injection was performed as described previously. (Hou et al., 2014) Three milligrams of the particles were injected in one eye, and the remaining eye was untouched and used as a control. After the injection, the eyes were monitored using slit-lamp for anterior segment, indirect ophthalmoscope, and fundus camera (Canon F-A; Canon Inc., Japan) for posterior segment, tonometer (Tonopen; Medtronic, Jacksonville, FL) for intraocular pressure, and dark-adapted ERGs for retinal physiology. For ERGs, both eyes were dilated and dark-adapted for 30 min before anesthesia with 19mg/kg Ketamine and 3.4mg/kg Xylazine. The ERGs were performed with a LKC UTAS visual electrodiagnostic system (LKC Technologies, Gaithersburg, MD). The flash intensity was 0.24 cd·s/m2 at the surface of the dome. Two lower intensities with addition of neutral density filter 1.0 and 2.0 were also used. Each rabbit had three sets of ERGs in the order from lower to higher intensity. Clinical exams were conducted at day 3, 7 and 14 after injection, and three rabbits were sacrificed at day 7 and 14. After sacrifice, the eye globes were enucleated. After the cornea and lens were removed, the vitreous was sampled using a 3-mL syringe as described previously. (Cheng et al., 1999) Whole vitreous was centrifuged for 20 min at 5,000 rpm, and vitreous supernatant was subjected to HPLC/MS/MS ((An Agilent (Santa Clara, CA) 1260 HPLC system coupled with a Thermo (Waltham, MA) LCQdeca mass spectrometer)) analysis using positive ion mode electrospray ionization (ESI) as described previously. (Chhablani et al., 2013)
3 Results
3.1 FTIR of the pSi microparticles pre drug-loading and post drug-loading
As shown in figure 3, in pure dexamethasone spectra (blue), the characteristic absorption bands at 3390 was due to the stretching vibration of O-H bonds; the stretching vibration at 1706, 1662 and 1620 cm−1 was due to –C=O and double bond framework conjugated to –C=O bonds. For OPS-COOH, characteristic vibrational bands were observed at 1722, 1631, 1553; For ads-OPS-COOH:DEX, characteristic vibrational bands were observed at 1721, 1632, 1561. In covalent OPS-COO-DEX, four characteristic absorption bands at 1722, 1663, 1632 and 1548 cm−1 were detected. The vibrational bands 1663, 1632 were attributed to double bond framework conjugated to –C=O bonds vibration overlapping the amide I vibration. In contrast to the ads-OPS-COOH:DEX, the signal at 1663 for OPS-COO-DEX was obviously strengthened due to formation of new ester bonds. These characteristic vibrational bands provide evidence of successful drug loading by covalent attachment.
Figure 3.
ATR-FTIR spectra of dexamethasone (blue), physical adsorption (ads-OPS-COOH: DEX; red), functional linker on the OPS-COOH (purple) and covalent attachment (cov-OPS-COO-DEX, green)
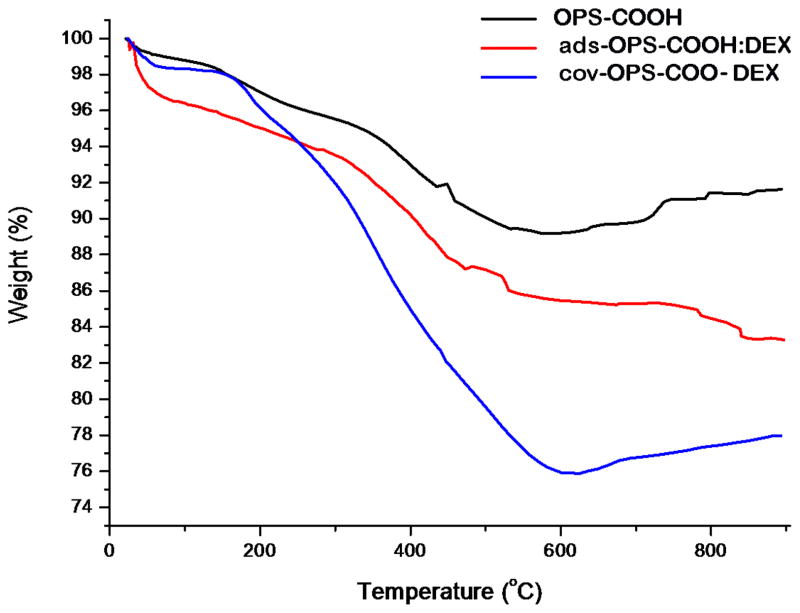
3.2 Thermogravimetric analysis (TGA) determined drug loading
The dexamethasone-loaded samples (~3 mg) were placed in a 90 μL alumina sample cup. Samples were heated at a constant rate of 10 °C/min up to 900°C in nitrogen atmosphere with a purge rate of 10 mL/min using a Q600 simultaneous TGA/DSC apparatus (TA Instruments, Newcastle, DL). As determined by thermogravimetric analysis (TGA), the mass loading of dexamethasone by covalent attachment was 134 ± 13 μg/mg particles and was 44 ± 1 μg/mg particles for physical adsorption loading (Figure 4). Weight percent is reported relative to initial weight of sample, prior to heating. The initial decrease in weight at 100°C is attributed to the loss of water from the sample. The decrease in weight is attributed to the organic functionalization, and the drug loaded into the particles. The weight loss difference obtained by subtraction between particles before (OPSCOOH) and after drug loading (ads-OPS:DEX or cov-OPS-DEX) accounts exclusively for the organic matter corresponding to the drug loaded into the particles.
Figure 4.
Thermogravimetric analysis (TGA) curves of OPS-COOH (oxidized porous silicon with carboxylic acid functional surface), ads-OPS-COOH:DEX (oxidized porous silicon particles containing physically adsorbed dexamethasone), and cov-OPS-COO-DEX (oxidized porous silicon particles containing dexamethasone covalently attached to the pore walls as described in the text).
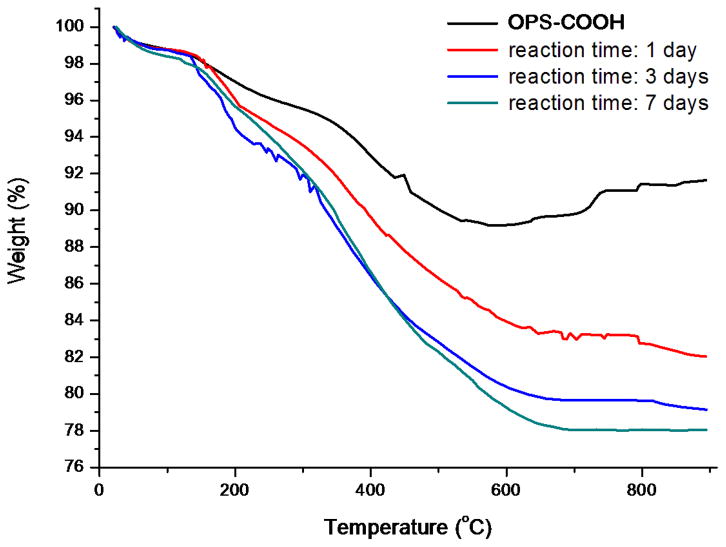
3.3. Drug loading efficiency and reaction time
In order to study the effect of reaction time on the drug loading efficiency, we carried out the drug loading using three different reaction times (1 day, 3 days and 7 days) before TGA determination of drug loading. The results were shown in Table 1 and Figure 5.
Table 1.
Drug loading capacity and loading reaction time
| Reaction time (day) | 1 | 3 | 7 |
|---|---|---|---|
| Cov-OPS-COOH:DEX (by TGA) | 15.57% | 19.10% | 20.38% |
| OPS-COOH (by TGA) | 9.61% | 9.61% | 9.61% |
| Drug loading efficiency | 5.96% | 9.49% | 10.77% |
Cov=Covalent
OPS=Oxidized porous silicon
DEX=Dexamethasone
TGA= Thermogravimetric analysis
Figure 5.
Thermogravimetric analysis (TGA) curves of OPS-COOH (oxidized porous silicon with carboxylic acid functional surface), and cov-OPS-COO-DEX (oxidized porous silicon particles containing dexamethasone covalently attached to the pore walls, drug loading reaction time for 1, 3 or 7 days).
As shown in Table 1 and Figure 5, when the loading time extended from 1 day to 3 days, the drug loading efficiency improved from 5.96% to 9.49% with a nearly 50% increase; however, further extension from 3 day to 7 days, the loading efficiency only improved slightly from 9.49% to 10.77%, indicating 3-day of loading may approach reactive equilibrium.
3.4. Drug Release Study in vitro
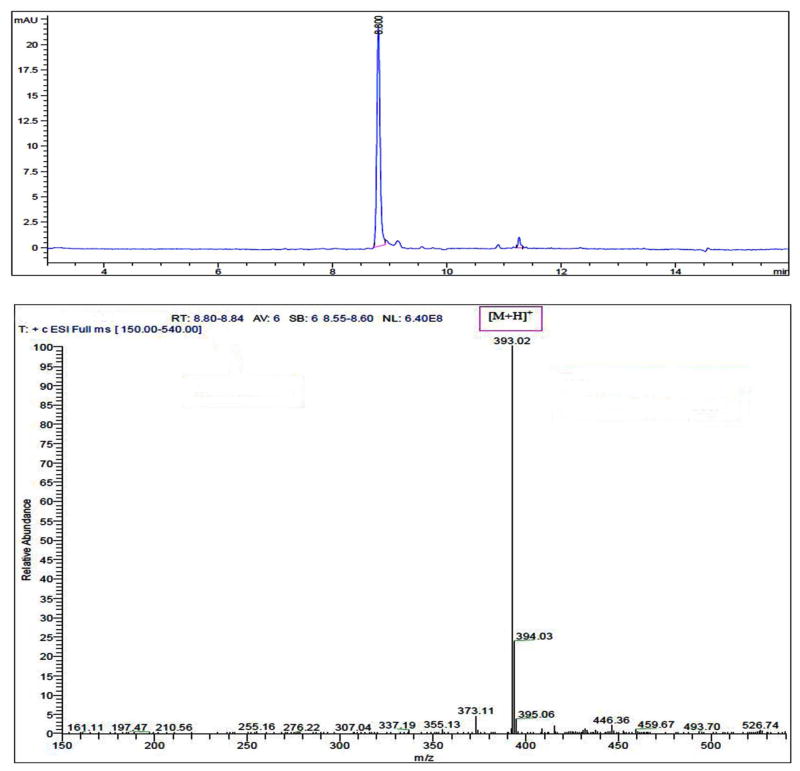
From the in vitro release sample, Dex was identified and quantitated using LC-ESI-MS/MS. Dexamethasone was revealed as a single peak at a retention time of 8.8 minutes (Figure 6, Upper panel), and protonated molecular ion peak at m/z 393 which is the same as the free dexamethasone (Figure 6, lower panel).
Figure 6.
LC spectra (upper panel) and ESI-MS spectra (lower panel) of dexamethasone released from cov-OPS-COO-DEX.
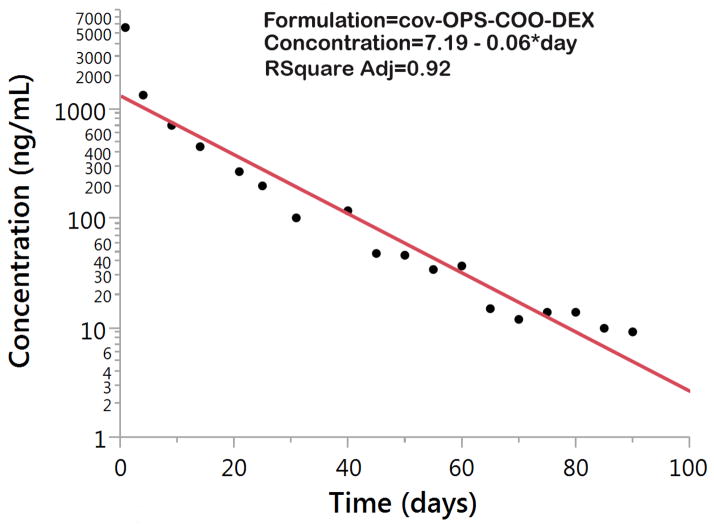
The in vitro drug concentrations from the particles of cov-OPS:DEX (loading time of 7 days), of ads-OPS:DEX, and free drug were standardized using initial dose and release profiles were plotted together for demonstration in Figure 7. It revealed that cov-OPS:DEX demonstrated sustained degradation of OPS with Dex release for 3 months, and that Dex concentration was maintained at 9 ng/mL on 90th day of release. From a perspective of in vitro drug dissolution, Dex release from the particles of cov-OPS:DEX fits well to a first-order regression model (Figre 8) with the first order rate constant (K1) of −0.06 and the adjusted coefficient determination (R2 adjusted) of 0.92. In contrast to the particles of cov-OPS:DEX, ads-OPS:DEX and free drug showed a rapid release profile.
Figure 7.
Dexamethasone release in vitro from cov-OPS-COO-DEX, ads-OPS-COOH:DEX and free drug in cell culture grade water.
Figure 8.
Dexamethasone release or dissolution in vitro from cov-OPS:DEX fits well to a first-order regression model, with R2adjusted (adjusted coefficient determination) = 0.92.
3.5. In vivo study
No toxicity was noted with any of the injected eyes. For each animal only one eye was injected and the contralateral eye was served as control. For IOP and ERG, paired t test was performed between the right eye and the left eye. The average IOP of the injected eyes was 10.56±1.63 versus 10.70±2.18 mmHg of the control eyes (p=0.68, paired t-test). For ERG data, 1-week and 2-week data were stacked and dark-adapted ERGs b-wave amplitudes were compared between the right eye and the left eye using paired t-test. The right eye versus the left eye for amplitude was 120.8±29 micro volts versus 117.7±44.8 micro volts (p=0.77, paired t-test). The vitreous was clear, and the retina looked normal, with the particles suspended in the inferior vitreous cavity (Figure 9). The average free Dex level in rabbit vitreous supernatant at day 7 and 14 was 193.69+/−93.62 and 107.23+/−10.54 ng/mL, respectively.
Figure 9.
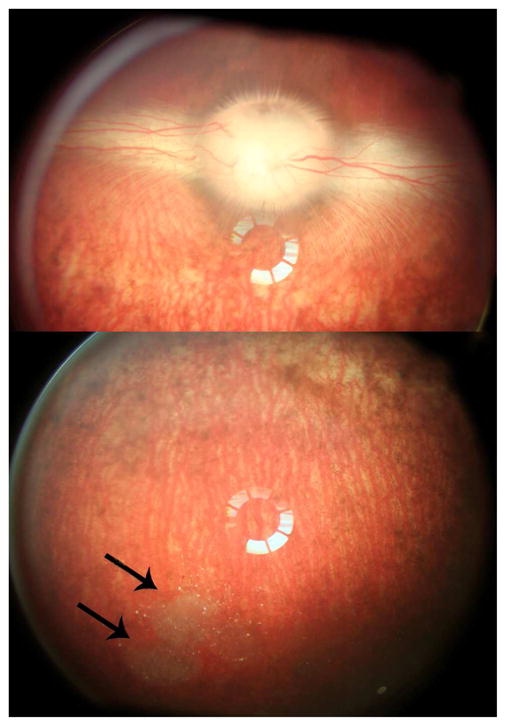
Color fundus photograms taken on day 14 before sacrifice. The upper panel photo demonstrated normal clear media, normal optic nerve head and medullary ray. The lower panel photo showed clear media, normal inferior retina, as well as the depot of cov-OPS-COO-DEX particles away from visual axis and at the inferior vitreous cavity (arrows). The rings at the centers of the images were from a light reflex from the pre-place 20 diopter lens in the front of the camera lens for wider viewing purpose.
Discussion
In this study, we conjugated dexamethasone to oxidized porous silicon particles. In the loading reaction, the porous silicon particles were modified by amine-functionalized groups, and carboxylic groups respectively. Due to the ample surface terminated with carboxylic acid groups, carboxyl groups reacted with the hydroxyl groups of dexamethasone by the Steglich Esterificaion Reaction. This is a reversible reaction that reaches a balance at a time. Indeed, this reactive characteristic was reflected in the drug loading efficiency, and length of reaction time. The drug loading efficiency improved from almost 50% from 24 hours of reaction to 72 hours of reaction; however, improvement from 72 hours of reaction to 1 week was much limited due to approaching the reaction balance. This finding is important for this porous silicon based drug delivery system, which is subject to the limits in the amount of silicon particles that can be injected due to the limited vitreous volume. In addition, higher drug loading will provide higher drug release, which may be critical for a pharmacodynamics study. In the current study, controlled release of Dex was achieved by creation of ester bonds between the functionalized pSiO2 and Dex. In our previous study, daunorubicin was loaded to pSiO2 by amide bonding. (Chhablani et al., 2013) In that study the in vitro release showed that the release of daunorubicin from the pSiO2 was very slow (Chhablani et al., 2013) and yielded a very low vitreous daunorubicin levels in a subsequent in vivo study (less than 2ng/mL when the pore size of pSiO2 was around 15 nm). (Hou et al., 2014) In that report, we used pore size enlargement to enhance the release of daunorubicin. (Hou et al., 2014) In general, amide bonding is stronger than ester bonding which, we hypothesized, may enhance release of payload. The current experimental data supported this hypothesis in that the detected Dex at 2 weeks was 107.23+/−10.54 ng/mL versus 1.05 ± 0.78 ng/mL for daunorubicin following the injection of similar pore sizes of 3mg of the particles with the similar drug loading efficiency. (Hou et al., 2014) There maybe are two possible ways for dexamethasone releasing from the engineered microparticles. One may be the direct hydrolysis of carboxylic ester, which is the reverse reaction of dexamethasone loading reaction. And the other may be that dexamethasone together with the linker disassociated with the pSi particles due to its degradation and then separated from the linker through hydrolysis reaction at the carboxylic ester bond. Though this drug delivery system needs to be further characterized through a standalone pharmacodynamics study, current study cleared the safety concern by demonstrating good preclinical safety as shown in repeated clinical exams as well as ocular tonometry and electroretinography. Dexamethasone is a potent glucocorticoid and is five times more potent than triamcinolone acetonide, which is a widely used intravitreal injection agent for various chronic chorioretinal inflammations due to its long-lasting drug depot in the vitreous. (Sivaprasad et al., 2006; Yilmaz et al., 2009) Dexamethasone, unlike triamcinolone, has a short vitreous half-life of 5.5 hours and can be rapidly eliminated after a single intravitreal injection. (Gan et al., 2005) Therefore, various sustained delivery system have been under development. (Kuppermann et al., 2007; Murata et al., 2013; Silva-Cunha et al., 2009; Xu et al., 2007; Zhang et al., 2009) Currently, Ozurdex is the only FDA approved dexamethasone intraocular implant that is placed on the vitreous base and is closer to the aqueous pathway and lens than the macula. The most recent reports showed that roughly 50% cataract formation and IOP rise from Ozurdex implantation. (Mayer et al., 2013) We are developing a porous silicon based ocular drug delivery system that uses a fine needle intravitreal injection route. The cov-OPS:DEX particles used in this study can easily pass through a 27-gauge needle, and can place the drug loaded particles into vitreous at the posterior. Due to the fact that vitreous water exits mainly through the retina into the choroid (over 80%), (Moseley et al., 1984) the drug placed in the posterior vitreous would be the most effective for macular diseases and would bring the least complications, such as high IOP and cataract. From the current in vitro drug release, the covalent conjugation of Dex to pSiO2 led to a sustained Dex release for at least 90 days, with a mean concentration of 2480±2549 ng/mL during first 9 days, 224±141 ng/mL from day 14 to day 40, and 24±15 ng/mL during the last segment of 45 days (from day 45 to day 90). All these drug levels are therapeutic, and well above the IC50 of dexamethasone (1.6 ng/mL) for trans-repression of many genes encoding pro-inflammatory mediators. (Jaffuel et al., 2001) Comparing the drug release profile of Ozurdex with the current cov-OPS:DEX formulation, the former had a 60 day steady release (vitreous concentration of ~200 ng/mL in monkey eyes or ~560 ng/mL in rabbit eyes) and then sharply dropped below therapeutic.(Chang-Lin et al., 2011a) (Chang-Lin et al., 2011b). In contrast, the current pSiO2 based formulation demonstrated a sustained release for 90 days, and an in vivo study confirmed that the formulation was not toxic and provided a sustained release mode (took one week for the concentration drop from 193.69+/−93.62 at 1-week time point to 107.23+/−10.54 ng/mL at 2-week time point) and a therapeutic level in vitreous (107.23+/−10.54 ng/mL at two weeks) which was only half to one fifth of vitreous Dex level in the Ozurdex implanted eyes. (Chang-Lin et al., 2011a) (Chang-Lin et al., 2011b) A lower but therapeutic drug level would reduce the side effects. Current study demonstrated that dexamethasone was able to covalently load to the porous silica particles in the presence of dicyclohexylcarbodiimide and 4-N,N-dimethylamminopyridine. Furthermore, the study found that the drug loading amount and loading reactive time was positively associated until it reached the balance at day 7 of reaction. With a reactive time of 7 days, 134 ± 13 μg dexamethasone was loaded into each milligram of the pSiO2 particles while only 44 ± 1 μg dexamethasone was loaded per milligram of the particles if using physical adsorption. This was a pilot study to develop a porous silicon based sustained delivery of Dex to the posterior segment of the eye globe. Further in vivo studies are needed to fully characterize this delivery system. Nonetheless, the preliminary results demonstrated a good safety profile and sustained release capability, which may be useful for many refractory chorioretinal diseases.
Highlights.
Dexamethasone can be covalently grafted to amine and carboxyl groups functionalized Porous silicon dioxide (pSiO2) by the Steglich Esterificaion Reaction.
Dexamethasone in vitro release from pSiO2 particles demonstrated good sustainability which was 80 days longer than free dexamethasone or infiltration loaded pSiO2 particle formulation in the same setting.
Intravitreal 3 mg of covalently dexamethasone-loaded pSiO2 particles in rabbit eyes demonstrated good ocular safety profile and intravitreal free dexamethasone at week 2 was 66 times higher than the IC50 of dexamethasone (1.6 ng/mL) for trans-repression of many genes encoding pro-inflammatory mediators.
Acknowledgments
Financial support: This study was supported by the National Institutes of Health under grant number NIH EY020617; P30EY022589.
The work was also partially supported by Research to Prevent Blindness, UCSD.
Footnotes
Disclosure: W.R. Freeman, Spinnaker Biosciences (C, I); M.J. Sailor, Spinnaker Biosciences (I); L. Cheng, Spinnaker Biosciences (C, I).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.A review of evidence guiding the use of corticosteroids in the treatment of intraocular inflammation. Ocular Immunology and Inflammation. 12:169–192. doi: 10.1080/092739490500192. [DOI] [PubMed] [Google Scholar]
- 2.Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration. New England Journal of Medicine. 364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganciclovir Ophthalmic Gel 0.15%: Safety and Efficacy of a New Treatment for Herpes Simplex Keratitis. Current Eye Research. 37:654–660. doi: 10.3109/02713683.2012.692846. [DOI] [PubMed] [Google Scholar]
- 4.Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB, III, Miller M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110:681–686. doi: 10.1016/S0161-6420(02)01969-3. [DOI] [PubMed] [Google Scholar]
- 5.Bressler NM. AGe-related macular degeneration is the leading cause of blindness. JAMA. 2004;291:1900–1901. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 6.Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, Welty D. Pharmacokinetics and Pharmacodynamics of a Sustained-Release Dexamethasone Intravitreal Implant. Investigative ophthalmology & visual science. 2011a;52:80–86. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 7.Chang-Lin JE, Burke JA, Peng Q, Lin T, Orilla WC, Ghosn CR, Zhang KM, Kuppermann BD, Robinson MR, Whitcup SM, Welty DF. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Investigative ophthalmology & visual science. 2011b;52:4605–4609. doi: 10.1167/iovs.10-6387. [DOI] [PubMed] [Google Scholar]
- 8.Cheng L, Anglin E, Cunin F, Kim D, Sailor MJ, Falkenstein I, Tammewar A, Freeman WR. Intravitreal properties of porous silicon photonic crystals: a potential self-reporting intraocular drug-delivery vehicle. The British journal of ophthalmology. 2008;92:705–711. doi: 10.1136/bjo.2007.133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng LY, Hostetler KY, Gardner MF, Avila CP, Bergeron-Lynn G, Severson GM, Freeman WR. Intravitreal pharmacokinetics in rabbits of the foscarnet lipid prodrug: 1-O-octadecyl-sn-glycerol-3-phosphonoformate (ODG-PFA) Current Eye Research. 1999;18:161–167. doi: 10.1076/ceyr.18.3.161.5366. [DOI] [PubMed] [Google Scholar]
- 10.Chew EY, Glassman AR, Beck RW, Bressler NM, Fish GE, Ferris FL, Kinyoun JL, Res DRC. Ocular Side Effects Associated with Peribulbar Injections of Triamcinolone Acetonide for Diabetic Macular Edema. Retina-J Ret Vit Dis. 2011;31:284–289. doi: 10.1097/IAE.0b013e3181f049a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chhablani J, Nieto A, Hou H, Wu EC, Freeman WR, Sailor MJ, Cheng L. Oxidized porous silicon particles covalently grafted with daunorubicin as a sustained intraocular drug delivery system. Investigative ophthalmology & visual science. 2013;54:1268–1279. doi: 10.1167/iovs.12-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan IM, Ugahary LC, van Dissel JT, van Meurs JC. Effect of intravitreal dexamethasone on vitreous vancomycin concentrations in patients with suspected postoperative bacterial endophthalmitis. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle. Ophthalmologie. 2005;243:1186–1189. doi: 10.1007/s00417-005-1182-1. [DOI] [PubMed] [Google Scholar]
- 13.Haller JA, Bandello F, Belfort R, Jr, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jiao J, Li XY, Whitcup SM. Dexamethasone Intravitreal Implant in Patients with Macular Edema Related to Branch or Central Retinal Vein Occlusion: Twelve-Month Study Results. Ophthalmology. 2011;118:2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann KI, Nieto A, Wu EC, Freeman WR, Kim JS, Chhablani J, Sailor MJ, Cheng L. Hydrosilylated porous silicon particles function as an intravitreal drug delivery system for daunorubicin. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2013;29:493–500. doi: 10.1089/jop.2012.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Hou H, Nieto A, Ma F, Freeman WR, Sailor MJ, Cheng L. Tunable sustained intravitreal drug delivery system for daunorubicin using oxidized porous silicon. Journal of Controlled Release. 2014;178:46–54. doi: 10.1016/j.jconrel.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffuel D, Roumestan C, Balaguer P, Henriquet C, Gougat C, Bousquet J, Demoly P, Mathieu M. Correlation between different gene expression assays designed to measure trans-activation potencies of systemic glucocorticoids. Steroids. 2001;66:597–604. doi: 10.1016/s0039-128x(00)00235-x. [DOI] [PubMed] [Google Scholar]
- 18.Jonas JB, Degenring RF, Kreissig I, Akkoyun I, Kamppeter BA. Intraocular Pressure Elevation After Intravitreal Triamcinolone Acetonide Injection. Ophthalmology. 2005;112:593–598. doi: 10.1016/j.ophtha.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 19.Kuppermann BD, Blumenkranz MS, Haller JA, Williams GA, Weinberg DV, Chou C, Whitcup SM, Dexamethasone DDSPIISG. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Archives of ophthalmology. 2007;125:309–317. doi: 10.1001/archopht.125.3.309. [DOI] [PubMed] [Google Scholar]
- 20.Lowder C, Belfort R, Jr, Lightman S, Foster CS, Robinson MR, Schiffman RM, Li XY, Cui H, Whitcup SM, Ozurdex HSG. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Archives of ophthalmology. 2011;129:545–553. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- 21.Mayer WJ, Wolf A, Kernt M, Kook D, Kampik A, Ulbig M, Haritoglou C. Twelve-month experience with Ozurdex for the treatment of macular edema associated with retinal vein occlusion. Eye. 2013;27:816–822. doi: 10.1038/eye.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moseley H, Foulds WS, Allan D, Kyle PM. Routes of clearance of radioactive water from the rabbit vitreous. The British journal of ophthalmology. 1984;68:145–151. doi: 10.1136/bjo.68.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murata M, Sanbe A, Lee JW, Nishigori H. Laser-induced intrachoroidal dexamethasone drug delivery system to posterior eye segment. Investigative ophthalmology & visual science. 2013;54:8317–8324. doi: 10.1167/iovs.13-13078. [DOI] [PubMed] [Google Scholar]
- 24.Nieto A, Hou H, Freeman WR, Sailor MJ, Cheng L. Ocular silicon distribution and clearance following intravitreal injection of porous silicon microparticles. Experimental Eye Research. 2013 doi: 10.1016/j.exer.2013.09.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert opinion on drug delivery. 2004;1:99–114. doi: 10.1517/17425247.1.1.99. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki H, Yamamura K, Mukai T, Nishida K, Nakamura J, Nakashima M, Ichikawa M. Pharmacokinetic prediction of the ocular absorption of an instilled drug with ophthalmic viscous vehicle. Biological & pharmaceutical bulletin. 2000;23:1352–1356. doi: 10.1248/bpb.23.1352. [DOI] [PubMed] [Google Scholar]
- 27.Shen L, You Y, Sun S, Chen Y, Qu J, Cheng L. Intraocular and Systemic Pharmacokinetics of Triamcinolone Acetonide after a Single 40-mg Posterior Subtenon Application. Ophthalmology. 2010;117:2365–2371. doi: 10.1016/j.ophtha.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Silva-Cunha A, Fialho SL, Naud MC, Behar-Cohen F. Poly-ε-Caprolactone Intravitreous Devices: An In Vivo Study. Investigative ophthalmology & visual science. 2009;50:2312–2318. doi: 10.1167/iovs.08-2969. [DOI] [PubMed] [Google Scholar]
- 29.Sivaprasad S, McCluskey P, Lightman S. Intravitreal steroids in the management of macular oedema. Acta ophthalmologica Scandinavica. 2006;84:722–733. doi: 10.1111/j.1600-0420.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 30.Stewart MP, Buriak JM. Chemical and biological applications of porous silicon technology. Adv Mater. 2000;12:859–869. [Google Scholar]
- 31.Vemulakonda GA, Hariprasad SM, Mieler WF, Prince RA, Shah GK, Van Gelder RN. Aqueous and vitreous concentrations following topical administration of 1% voriconazole in humans. Archives of ophthalmology. 2008;126:18–22. doi: 10.1001/archophthalmol.2007.8. [DOI] [PubMed] [Google Scholar]
- 32.Weijtens O, Schoemaker RC, Romijn FP, Cohen AF, Lentjes EG, van Meurs JC. Intraocular penetration and systemic absorption after topical application of dexamethasone disodium phosphate. Ophthalmology. 2002;109:1887–1891. doi: 10.1016/s0161-6420(02)01176-4. [DOI] [PubMed] [Google Scholar]
- 33.Williams GA, Haller JA, Kuppermann BD, Blumenkranz MS, Weinberg DV, Chou C, Whitcup SM. Dexamethasone Posterior-Segment Drug Delivery System in the Treatment of Macular Edema Resulting from Uveitis or Irvine-Gass Syndrome. American Journal of Ophthalmology. 2009;147:1048–1054. e1042. doi: 10.1016/j.ajo.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 34.Williams R, Airey M, Baxter H, Forrester J, Kennedy-Martin T, Girach A. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye. 2004;18:963–983. doi: 10.1038/sj.eye.6701476. [DOI] [PubMed] [Google Scholar]
- 35.Wu EC, Andrew JS, Buyanin A, Kinsella JM, Sailor MJ. Suitability of porous silicon microparticles for the long-term delivery of redox-active therapeutics. Chemical communications. 2011;47:5699–5701. doi: 10.1039/c1cc10993f. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Wang Y, Li Y, Yang X, Zhang P, Hou H, Shi Y, Song C. Inhibitory efficacy of intravitreal dexamethasone acetate-loaded PLGA nanoparticles on choroidal neovascularization in a laser-induced rat model. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2007;23:527–540. doi: 10.1089/jop.2007.0002. [DOI] [PubMed] [Google Scholar]
- 37.Yilmaz T, Weaver CD, Gallagher MJ, Cordero-Coma M, Cervantes-Castaneda RA, Klisovic D, Lavaque AJ, Larson RJ. Intravitreal Triamcinolone Acetonide Injection for Treatment of Refractory Diabetic Macular Edema: A Systematic Review. Ophthalmology. 2009;116:902–913. doi: 10.1016/j.ophtha.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Li Y, Zhang C, Wang Y, Song C. Pharmacokinetics and tolerance study of intravitreal injection of dexamethasone-loaded nanoparticles in rabbits. International journal of nanomedicine. 2009;4:175–183. doi: 10.2147/ijn.s6428. [DOI] [PMC free article] [PubMed] [Google Scholar]