Abstract
GNE myopathy is a rare autosomal recessive muscle disease caused by mutations in GNE, the gene encoding the rate-limiting enzyme in sialic acid biosynthesis. GNE myopathy usually manifests in early adulthood with distal myopathy that progresses slowly and symmetrically, first involving distal muscles of the lower extremities, followed by proximal muscles with relative sparing of the quadriceps. Upper extremities are typically affected later in the disease. We report a patient with GNE myopathy who presented with asymmetric hand weakness. He had considerably decreased left grip strength, atrophy of the left anterior forearm and fibro-fatty tissue replacement of left forearm flexor muscles on T1-weighted magnetic resonance imaging. The patient was an endoscopist and thus the asymmetric hand involvement may be associated with left hand overuse in daily repetitive pinching and gripping movements, highlighting the possible impact of environmental factors on the progression of genetic muscle conditions.
Keywords: GNE myopathy, inclusion body myopathy 2, hereditary inclusion body myopathy (HIBM), Nonaka myopathy, distal myopathy with rimmed vacuoles (DMRV), N-acetylmannosamine (ManNAc), sialic acid
Introduction
GNE myopathy results from biallelic mutations in GNE, which encodes the rate-limiting, bifunctional enzyme in the sialic acid biosynthetic pathway [1]. This panethnic disorder has an estimated prevalence of ~4–21/1,000,000 worldwide [2], and of 1:1500 in the Iranian Jewish population [1]. The disease has been previously referred to as Hereditary Inclusion Body Myopathy (HIBM), Inclusion Body Myopathy 2 (IBM2; OMIM 600737), Distal Myopathy with Rimmed Vacuoles (DMRV) or Nonaka Myopathy (OMIM 605820) [3].
GNE myopathy usually manifests in early adulthood with foot drop secondary to anterior tibialis muscle weakness [4]. The disease typically begins with symmetric, distal lower extremity weakness and atrophy, which slowly progresses to involve proximal muscles, with relative sparing of the quadriceps [5]. Upper extremity involvement appears approximately 10 years after the onset of symptoms and typically presents with grip weakness [6]. Hand involvement has been previously described in GNE myopathy as atrophy of interosseus and thenar muscles [7, 8] and finger flexors [9]. Muscle pathology is characterized by ‘rimmed’ vacuoles evident on Gomori trichrome staining, fiber size variation, and typically a lack of inflammation [10]. Muscle magnetic resonance imaging (MRI) of patients with GNE myopathy is characterized by T2-weighted short tau inversion recovery (STIR) hyperintensity of affected muscles in early stages, followed by fatty-fibrous replacement on T1-weighted imaging [11]. The diagnosis is confirmed by identification of biallelic GNE gene mutations [2]. The diagnosis of GNE myopathy can be challenging because of the disorder’s rarity [10], and can become more difficult in patients with an atypical presentation. Phenotypic variability in GNE myopathy has been observed even among members of the same family [12], and genetic and environmental factors are thought to play a role in the different rates of progression among patients.
We report the case of a patient with GNE myopathy with an atypical presentation of unilateral hand weakness.
Case Report
Presentation and diagnosis
A right-handed male patient of Indian ethnicity presented at 27 years of age with progressive left hand weakness impacting his activities of daily living. Although he performed activities of daily living predominantly with his right hand, his profession involved performing 12–15 endoscopy procedures per day, which requires repetitive pinching and gripping movements, with preferential use of the left hand. He described muscle pain and fatigue of the left hand after prolonged use and eventually the inability to perform endoscopy procedures, due to pain and weakness. He also noted left hand weakness while lifting weights, progressing from being able to lift 27 kg to only 9 kg a year later. During his initial evaluation at 29 years of age, he denied numbness, tingling, difficulties swallowing or symptoms of lower extremity, pharyngeal or respiratory weakness. On examination, he had left grip and finger abduction weakness and atrophy of left hand, wrist and forearm. Grip strength was considerably lower on the left (6.8 kg) when compared to the right (20.4 kg). He had normal gait and strength of lower extremities was preserved. Cervical MRI was normal. There was no electrophysiologic evidence of a co-existing cervical radiculopathy or mononeuropathy to otherwise explain the left hand weakness, specifically nerve conduction studies showed normal left median-D2, ulnar-D5 and radial sensory nerve action potentials and normal left median-Abductor Pollicis Brevis and ulnar-Abductor Digiti Minimi compound action potentials. Electromyogram showed evidence of myopathy, distal more than proximal, with increased insertional activity, fibrillation and positive sharp waves in several flexor muscles more than extensors. Creatine kinase was elevated at 1053 U/L (normal range: 52–386 U/L). A muscle biopsy of the left biceps was characteristic of GNE myopathy (Figure 1). Genetic testing revealed two mutations in GNE, a previously unreported nonsense mutation in the epimerase domain (p.R160X), and a missense mutation in the kinase domain (p.V727M), previously described in patients of Indian ethnicity [1]. Mutations in this report are named according to current nomenclature [13].
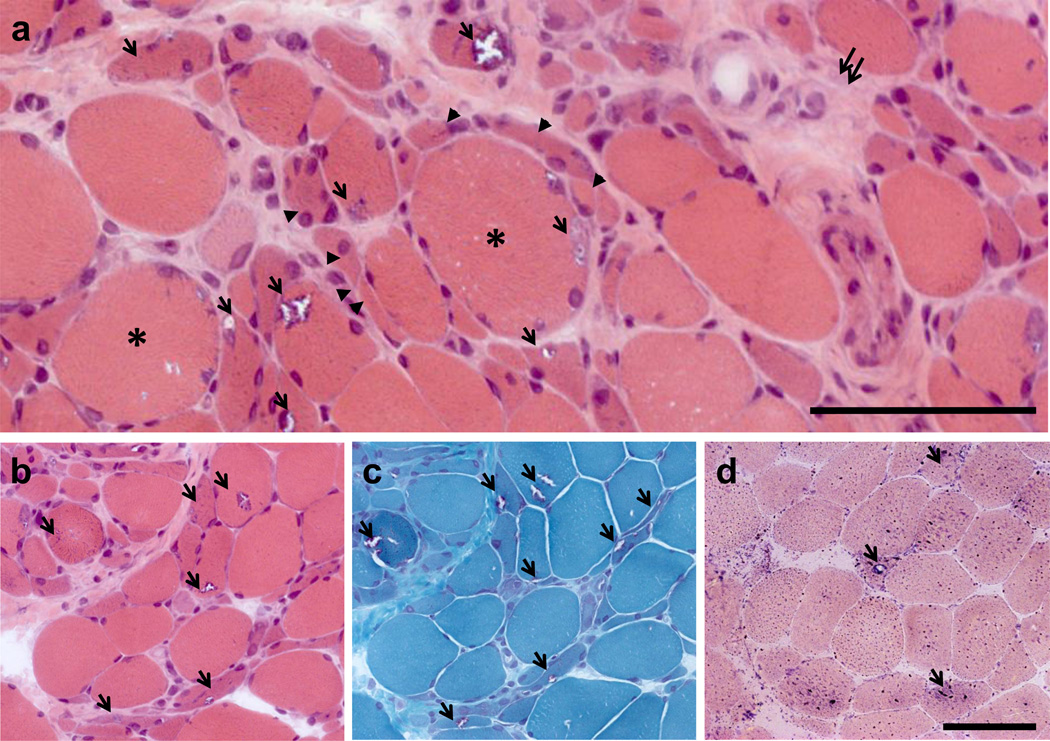
Figure 1.
Muscle biopsy sections taken from left biceps. (a) Hematoxylin and eosin staining show marked variation in fiber size. Groups of atrophic fibers (arrowheads) surround large fibers (asterisk). Characteristic rimmed vacuoles are denoted by arrows. Fibrosis is seen in scattered areas (double arrows). (b) Hematoxylin and eosin and (c) modified Gomori trichrome serial sections demonstrating rimmed vacuoles (arrows) in both atrophic and non-atrophic fibers. (d) Fibers with rimmed vacuoles (arrows) stain intensely with acid phosphatase, indicating increased lysosomal activity.
Follow-up Evaluation
The patient was subsequently evaluated at 30 years of age under NIH clinical protocol 11-HG-0218 “A Natural History Study of Patients with GNE myopathy” (ClinicalTrials identifier: NCT01417533). This study was approved by the NHGRI Institutional Review Board. The patient signed a written informed consent to participate in the study, including consent for reproduction of images. Since the onset of symptoms, three years before his evaluation at the NIH, left hand weakness had progressed, causing difficulties with buttoning shirts, opening jars, and grasping objects with the left hand. He described no symptoms of lower extremity weakness, no difficulties with walking or changes in gait.
Physical Examination
Examination revealed asymmetric weakness and atrophy of the distal upper extremities, more on the left than right, affecting mainly the first dorsal interosseus, hypothenar and forearm flexor muscles (Figure 2A, 2B). Strength was 4/5 for bilateral wrist flexors, finger abductors and abductor digiti minimi and the left finger adductors. In the lower extremities, there was questionable ankle dorsiflexion weakness (4+/5) bilaterally. Strength of other lower extremity muscles was 5/5. Gait, including toe and heel walking, was normal. His 6-minute walk distance was 664 meters, which is normal for his age and gender [14].
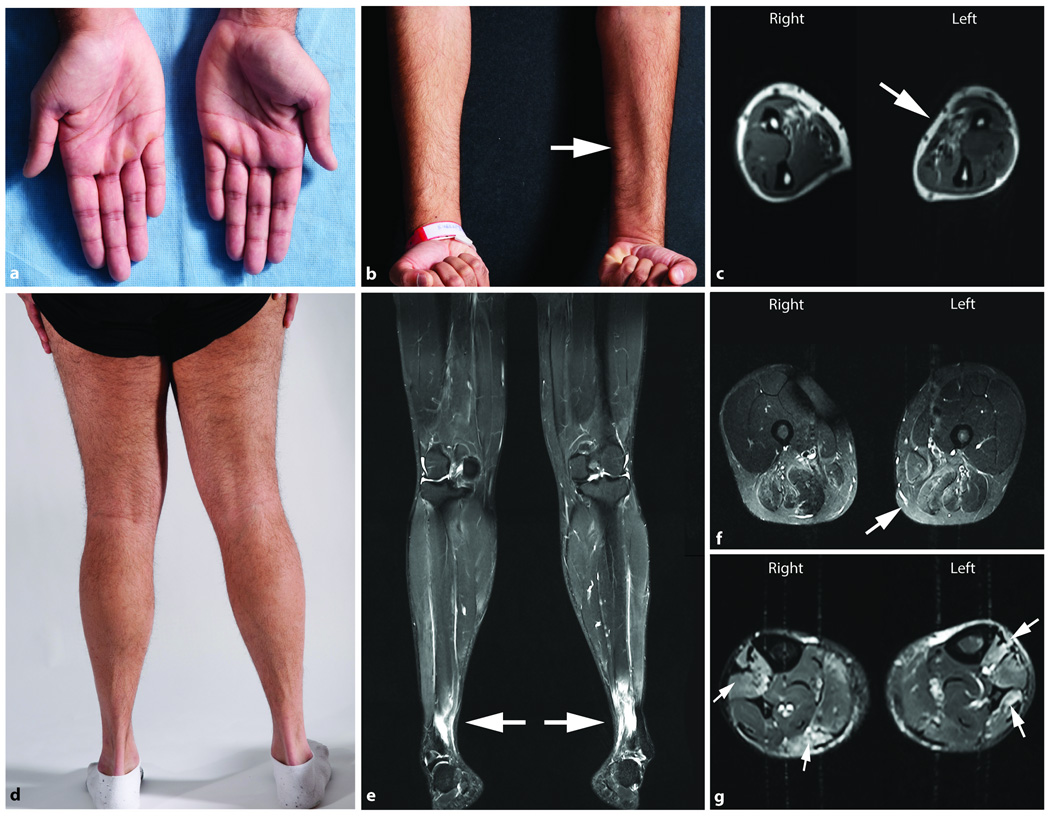
Figure 2.
Physical and MRI findings in a patient with GNE myopathy presenting with left hand weakness. (a) Photograph of hands showing atrophy of the hypothenar area, more pronounced on the left. (b) Photograph and (c) T1-weighted axial muscle MRI of the forearms show atrophy of forearm flexor muscles, more prominent on the left arm (arrows). Involvement of the distal lower extremities is evident on (d) a photograph of posterior lower extremities and (e) the T2-weighted STIR coronal muscle MRI image of the lower extremities.
T2-weighted STIR muscle MRI images of the lower extremities including coronal (e) and axial views of the thigh (f), and lower leg (g), show T2 hyperintense areas (arrows) noted bilaterally within the anterior tibialis muscles (e and g) gastrocnemius, flexor hallux longus and the soleus muscles (g), and patchy T2 signal hyperintense areas on the posterior thigh involving the adductor and hamstring muscle groups bilaterally (f).
The creatine kinase was 1152 U/L (normal, 52–386 U/L), alanine aminotransferase was 67 (6–41 U/L), and other liver function tests, chemistries and complete blood counts were normal. ECG, echocardiogram and pulmonary function tests were also normal.
MRI
T1-weighted muscle MRI imaging of the upper extremities showed atrophy and fibro-fatty tissue replacement of the anterior compartment of the forearm, especially the pronator teres muscle, more pronounced on the left than on the right (Figure 2C). T2-weighted STIR images showed mild hyperintensity in muscles of the posterior compartment of the arm and anterior compartment of the forearm, involving the lateral head of the triceps muscle, brachioradialis, and flexor forearm muscle groups. In the lower extremities, T2-weighted STIR imaging revealed hyperintensity of the anterior tibialis muscles (Figure 2E, 2G) the adductor and hamstring muscle groups (Figure 2F) bilaterally, despite the absence of significant abnormalities on the T1-weighted images.
Quantitative muscle strength
Muscle strength of the patient was evaluated utilizing the Quantitative Muscle Strength Assessment (QMA) which measures maximal voluntary isometric contraction (in kilograms) using a fixed frame dynamometer, a strain gauge tensiometer, and a computer-aided acquisition system (Aeverl Medical, Gainesville GA, USA). The results were compared to predicted strength [15–18] for age, gender, height and weight. The values on upper extremity quantitative muscle strength show an asymmetric upper left distal extremity weakness with more than 15% difference in the sum of distal strength between the left (20.4%) and right (36%) upper extremities. Specifically, left hand grip was considerably weaker on the left (17.7%) when compared to the right (34%), as well as lateral pinch (16.5% on the left and 29.2% on the right), tip pinch (5.6% on the left and 13.2% on the right) and wrist extension (39.2% on the left and 57.7% on the right). Shoulder abduction strength was similar on the left (85.1%) and right (89.1%). Lower extremity muscles were affected symmetrically; ankle dorsiflexion and knee flexion were close to 50% of predicted, even in the absence of lower extremity symptoms, and knee extension was above 70% of predicted.
Discussion
GNE myopathy is caused by biallelic mutations in GNE leading to decreased activity of GNE (N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase), the key enzyme in sialic acid biosynthesis [1]. This defect is considered to cause decreased sialylation of muscle proteins [6, 19] and it has been shown that muscle weakness and atrophy can be prevented by administration of sialic acid or its precursor ManNAc in a transgenic mouse model of GNE myopathy [20]. Sialic acids are the most abundant terminal monosaccharides on glycoconjugates in eukaryotic cells where they have many biological functions [21]. The exact mechanism by which GNE alterations lead to muscle atrophy and deterioration still needs to be determined but environmental factors are thought to play a role.
The patient was compound heterozygous for a missense GNE mutation, p.V727M (GenBank accession NP_001121699), which has been previously described in patients of Indian descent [1] as well as patient of Algerian, Asian, Chinese, Middle-Eastern, Indian, Pakistani, Thai and Portuguese descent. The other is a novel nonsense mutation p.R160X, which we have seen in another patient of Indian descent in our cohort [2] and that is one of the few nonsense mutations described for GNE myopathy. The large majority of patients harbor missense mutations and no patient is known to have biallelic null mutations [2], which is consistent with embryonic lethality in the Gne knockout mouse model [22]. It is not clear whether patients harboring nonsense mutations of GNE have a more severe phenotype or a faster progression of the disease.
Upper extremity weakness as an initial presentation of GNE myopathy has not been previously reported. GNE myopathy typically presents with foot drop, and hand involvement does not typically occur until approximately 10 years after the first onset of lower extremity symptoms [6]. Here we describe a patient with GNE myopathy and asymmetric left hand involvement that was evident clinically, by muscle MRI and by quantitative muscle strength, raising concern that patients with GNE myopathy may present with distal upper extremity weakness and currently be undiagnosed.
The patient had asymptomatic and almost clinically undetectable involvement of the lower extremities; however, there were hyperintense areas on T2-STIR muscle imaging and decreased strength of lower extremity muscles, particularly of the anterior tibialis, suggesting that muscle MRI and QMA are sensitive measures that can be used to evaluate pre-symptomatic patients.
Muscle overuse may play a role in the atypical presentation of this patient. Endoscopies are known to be associated with work-related strain injury including repetitive pinching and gripping of the endoscope and manipulation of the insertion tube [23]. Overuse syndrome related to performing endoscopies has been reported as thumb and hand pain occurring more commonly in the left hand. The endoscopic technique involves repetitive use of the left thumb, with repeated flexion and extension of the left wrist for prolonged periods of time. The left hand holds the endoscope while the right hand advances the endoscope [24]. In this case, the patient had considerably weaker pinch, grip, wrist extension and flexion on the left hand compared to the right, while elbow flexion and shoulder abduction were comparable on both sides, consistent with the pattern seen in endoscopists with strain injuries. Future studies should address the role of muscle overuse and other environmental factors in the presentation and progression of GNE myopathy.
Highlights.
GNE myopathy typically presents with distal lower extremity weakness.
We report a case of GNE myopathy presenting as asymmetric hand weakness.
Our findings suggest that the contribution of muscle overuse as an environmental factor in GNE myopathy and other genetic muscle diseases should be further studied.
Acknowledgments
This study was supported by the Intramural Research Programs of the National Human Genome Research Institute (NHGRI), the National Center for Advancing Translational Sciences (NCATS), and the NIH Clinical Center, all of National Institutes of Health, Bethesda, Maryland, United States. Clinical protocol was approved by the NHGRI Institutional Review Board. Patient provided consent for reproduction of images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eisenberg I, Avidan N, Potikha T, et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001;29:83–87. doi: 10.1038/ng718. [DOI] [PubMed] [Google Scholar]
- 2.Celeste FV, Vilboux T, Ciccone C, et al. Mutation Update for GNE Gene Variants Associated with GNE Myopathy. Human mutation. 2014 doi: 10.1002/humu.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishino I, Noguchi S, Murayama K, et al. Distal myopathy with rimmed vacuoles is allelic to hereditary inclusion body myopathy. Neurology. 2002;59:1689–1693. doi: 10.1212/01.wnl.0000041631.28557.c6. [DOI] [PubMed] [Google Scholar]
- 4.Huizing M, Krasnewich DM. Hereditary inclusion body myopathy: a decade of progress. Biochim Biophys Acta. 2009;1792:881–887. doi: 10.1016/j.bbadis.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argov Z, Yarom R. "Rimmed vacuole myopathy" sparing the quadriceps. A unique disorder in Iranian Jews. J Neurol Sci. 1984;64:33–43. doi: 10.1016/0022-510x(84)90053-4. [DOI] [PubMed] [Google Scholar]
- 6.Huizing M, Malicdan MV, Krasnewich DM, Manoli I, Carrillo-Carrasco N. GNE Myopathy. In: Scriver CR, Childs B, Sly WS, Valle D, Beaudet AL, Vogelstein B, Kinsler KW, editors. Scriver's Online Metabolic and Molecular Bases of Inherited Disease. http://www.ommbid.com., ed. Valle D, et al. Vol. Chapter 216.1. New York: McGraw-Hill; 2014. [Google Scholar]
- 7.Sparks S, Rakocevic G, Joe G, et al. Intravenous immune globulin in hereditary inclusion body myopathy: a pilot study. 2007;7:3. doi: 10.1186/1471-2377-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalaydjieva L, Lochmuller H, Tournev I, et al. 125th ENMC International Workshop: Neuromuscular disorders in the Roma (Gypsy) population, 23–25 April 2004, Naarden, The Netherlands. Neuromuscul Disord. 2005;15:65–71. doi: 10.1016/j.nmd.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Chaouch A, Brennan KM, Hudson J, et al. Two recurrent mutations are associated with GNE myopathy in the North of Britain. Journal of neurology, neurosurgery, and psychiatry. 2014 doi: 10.1136/jnnp-2013-306314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishino I, Carrillo-Carrasco N, Argov Z. GNE myopathy: current update and future therapy. Journal of neurology, neurosurgery, and psychiatry. 2014 doi: 10.1136/jnnp-2013-307051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tasca G, Ricci E, Monforte M, et al. Muscle imaging findings in GNE myopathy. J Neurol. 2012;259:1358–1365. doi: 10.1007/s00415-011-6357-6. [DOI] [PubMed] [Google Scholar]
- 12.Ro LS, Lee-Chen GJ, Wu YR, Lee M, Hsu PY, Chen CM. Phenotypic variability in a Chinese family with rimmed vacuolar distal myopathy. J Neurol Neurosurg Psychiatry. 2005;76:752–725. doi: 10.1136/jnnp.2004.048876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huizing M, Carrillo-Carrasco N, Malicdan MC, et al. GNE myopathy: new name and new mutation nomenclature. Neuromuscular disorders : NMD. 2014;24:387–389. doi: 10.1016/j.nmd.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chetta A, Zanini A, Pisi G, et al. Reference values for the 6-min walk test in healthy subjects 20–50 years old. Respir Med. 2006;100:1573–1578. doi: 10.1016/j.rmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Muscular weakness assessment: use of normal isometric strength data. The National Isometric Muscle Strength (NIMS) Database Consortium. Arch Phys Med Rehabil. 1996;77:1251–1255. doi: 10.1016/s0003-9993(96)90188-4. [DOI] [PubMed] [Google Scholar]
- 16.Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76:248–259. doi: 10.1093/ptj/76.3.248. [DOI] [PubMed] [Google Scholar]
- 17.Meldrum D, Cahalane E, Conroy R, Fitzgerald D, Hardiman O. Maximum voluntary isometric contraction: reference values and clinical application. Amyotroph Lateral Scler. 2007;8:47–55. doi: 10.1080/17482960601012491. [DOI] [PubMed] [Google Scholar]
- 18.Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- 19.Noguchi S, Keira Y, Murayama K, et al. Reduction of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase activity and sialylation in distal myopathy with rimmed vacuoles. J Biol Chem. 2004;279:11402–11407. doi: 10.1074/jbc.M313171200. [DOI] [PubMed] [Google Scholar]
- 20.Malicdan MC, Noguchi S, Hayashi YK, Nonaka I, Nishino I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat Med. 2009;15:690–695. doi: 10.1038/nm.1956. [DOI] [PubMed] [Google Scholar]
- 21.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarzkopf M, Knobeloch KP, Rohde E, et al. Sialylation is essential for early development in mice. Proc Natl Acad Sci U S A. 2002;99:5267–5270. doi: 10.1073/pnas.072066199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansel SL, Crowell MD, Pardi DS, Bouras EP, DiBaise JK. Prevalence and impact of musculoskeletal injury among endoscopists: a controlled pilot study. J Clin Gastroenterol. 2009;43:399–404. doi: 10.1097/MCG.0b013e31817b0124. [DOI] [PubMed] [Google Scholar]
- 24.Buschbacher R. Overuse syndromes among endoscopists. Endoscopy. 1994;26:539–544. doi: 10.1055/s-2007-1009030. [DOI] [PubMed] [Google Scholar]