Abstract
Stromal transparency is a critical factor contributing to normal function of the visual system. Corneal injury, surgery, disease and infection elicit complex wound healing responses that serve to protect against insults and maintain the integrity of the cornea, and subsequently to restore corneal structure and transparency. However, in some cases these processes result in prolonged loss of corneal transparency and resulting diminished vision. Corneal opacity is mediated by the complex actions of many cytokines, growth factors, and chemokines produced by the epithelial cells, stromal cells, bone marrow-derived cells, lacrimal tissues, and nerves. Myofibroblasts, and the disorganized extracellular matrix produced by these cells, are critical determinants of the level and persistence of stromal opacity after corneal injury. Decreases in corneal crystallins in myofibroblasts and corneal fibroblasts contribute to cellular opacity in the stroma. Regeneration of a fully functional epithelial basement membrane (BM) appears to have a critical role in the maintenance of corneal stromal transparency after mild injuries and recovery of transparency when opacity is generated after severe injuries. The epithelial BM likely has a regulatory function whereby it modulates epithelium-derived growth factors such as transforming growth factor (TGF) β and platelet-derived growth factor (PDGF) that drive the development and persistence of myofibroblasts from precursor cells. The purpose of this article is to review the factors involved in the maintenance of corneal transparency and to highlight the mechanisms involved in the appearance, persistency and regression of corneal opacity after stromal injury.
Keywords: Cornea, epithelial basement membrane, wound healing, myofibroblasts, corneal fibroblasts, bone marrow-derived cells, fibrocytes, stroma, haze, transforming growth factor-beta, platelet-derived growth factor
1. Introduction
Corneal transparency is maintained by many factors, including the ultrastructural anatomy and the physiology of the cornea and its cellular and extracellular components. Corneal injuries, surgeries, diseases and infections trigger complex stromal responses that function to maintain corneal integrity and then restore corneal transparency once the threat has subsided. In severe injuries, however, depending on the depth and overall extent of the insult, persistent corneal opacity may result. Under normal conditions, keratocytes are relatively quiescent and their primary functions are to maintain collagen and other extracellular matrix components in the stroma (West-Mays and Dwivedi, 2006; Singh et al., 2012). During corneal wound healing responses, however, stromal and bone marrow-derived precursor cells may give rise to alpha-smooth muscle actin (α-SMA) expressing myofibroblasts. Deposition of large quantities of disorganized extracellular matrix by these cells, in addition to decreased crystallin protein expression by the cells themselves, are important contributors to loss of corneal transparency (Jester et al., 1999b; Mohan et al., 2003; West-Mays and Dwivedi, 2006).
Previous studies have shown that epithelial–stromal interactions are critical to myofibroblast generation and the development of corneal opacity (Netto et al., 2006a). Myofibroblast development occurs when structural and functional defects in the regenerated epithelial basement membrane (BM) allows penetration of transforming growth factor beta (TGF-β) and platelet-derived growth factor (PDGF) from the epithelium into the stroma at sufficient levels to drive precursor development (Singh et al., 2014a; Kaur et al., 2009a; Netto et al., 2006a; Torricelli et al., 2013a).
The purpose of this article is to review the factors involved in the maintenance of corneal transparency and to highlight the mechanisms involved in the appearance, persistence and regression of corneal opacity after corneal injury.
2. Corneal anatomy
The cornea is a transparent and centrally avascular tissue responsible for two-thirds of the refractive power of the eye (Land and Fernald, 1992). The shape of the normal cornea is “prolate”—meaning it is steeper centrally and flatter in the periphery—which creates an aspheric optical system (DelMonte and Kim, 2011). Several distinct layers compose this structure that is critical to normal vision.
The outermost layer, and the primary barrier to threats from the outside environment, is the corneal epithelium that is composed of non-keratinized, stratified squamous epithelial cells (Rufer et al., 2005). The thickness of the corneal epithelium is remarkably constant in different species, ranging from 45 to 50 µm in human, mouse and rabbit (Cavanagh et al., 2002; Moller-Pedersen et al., 1998c; Robertson et al., 2006). Underlying the corneal epithelium, the epithelial BM is a highly specialized extracellular matrix that forms a thin acellular layer that has a critical role in corneal homeostasis, wound healing and disease. The epithelial BM is composed of a wide assemblage of extracellular molecules, but in general terms it is generated from four primary components: collagens (for example, collagen type IV isoforms), laminins (for example, laminin 332), heparan sulfate proteoglycans (for example, perlecan) and nidogens (Torricelli et al., 2013b).
Bowman’s layer is classically described as an acellular condensation of the anterior stroma of the cornea and is usually absent in non-primate species such as rabbits (Wilson and Hong, 2000). Studies have found that the fibrils of Bowman’s layer are only 1/2 or 2/3 as thick as the fibrils that comprise the underlying stroma and these fibrils are randomly interwoven to form a dense, felt-like sheet (Jacobsen et al., 1984; Komai and Ushiki, 1991). The authors believe that Bowman’s layer is formed during development and maintained during adult life by ongoing epithelial-derived negative chemotactic factors that participate in the development and maintenance of corneal tissue organization (Wilson and Hong, 2000).
The corneal stroma provides the bulk of the structural framework of the cornea and comprises about 90% of the total volume of the cornea (Moller-Pedersen, 2004). The stromal thickness varies considerably depending on the species and can be very thin in mice (70 to 90 µm), thicker in rabbits (300 to 400 µm) and humans (450 to 700 µm), and very thick in pig and cows (approximately 1 mm) (Moller-Pedersen et al., 1998c; Moller-Pedersen et al., 1997; Robertson et al., 2006). The stroma has bundles (lamellae) of highly ordered collagenous fibers and ground substance— with water, inorganic salts, proteoglycans, and glycoproteins being the main structural components of the extracellular matrix (Moller-Pedersen, 2004; Hassell and Birk, 2010). Corneal stromal collagen fibrils are composed of type I collagen in a heteridimeric complex with type V collagen to yield a uniquely narrow diameter (Fini and Stramer, 2005). These complexes are surrounded by specialized proteoglycans such as lumican, keratocan, mimecan, and decorin (Kao and Liu, 2002). Keratocytes are the major cells of the stroma and serve to maintain the extracellular environment. They function to reabsorb and resynthesize collagen molecules and glycosaminoglycans, and also produce matrix metalloproteases (MMPs) that are crucial to stromal homeostasis (DelMonte and Kim, 2011). There is also a population of transient bone marrow-derived cells, monocytes (macrophages), and dentritic cells that reside in the cornea (Hassell and Birk, 2010).
The cornea is one of the most innervated tissues in the body. Sensory nerves enter into the corneal stroma and are derived from the nasociliary branch of the ophthalmic division of the trigeminal nerve (DelMonte and Kim, 2011). These sensory nerves run centrally and anteriorly in a radial fashion toward the central cornea and give rise to branches that innervate the anterior and mid stroma. In the interface between Bowman’s layer and the anterior stroma, the stromal nerves form a subepithelial nerve plexus and perforate Bowman’s layer to form the subbasal epithelial nerve plexus. Thus, these sensory nerve fibers provide innervation to the basal epithelial cell layer and terminate within the superficial epithelial layers. It is controversial whether there is innervation to the posterior stroma. However, it is believed that Descemet’s membrane and the endothelium are not innervated in humans (Oliveira-Soto and Efron, 2001). The cornea also contains autonomic nerve fibers (Marfurt, Jones, Thrasher, 1998; Ivanusic, Wood and Brock, 2013).
Beneath the stroma is Descemet’s membrane—a modified basement membrane that is continuously secreted by corneal endothelial cells. This layer is composed primarily of collagen type IV fibrils and is three µm thick prior to birth in humans and can grow with age up to a thickness of greater than 10 µm (Murphy, Alvarado, and Juster, 1984).
The endothelium of the cornea is a cuboidal monolayer of mitochondria-rich cells approximately five micrometers thick. These cells are responsible for the relatively deturgescent state (78% water content) of the cornea (Geroski et al., 1985). Corneal endothelial cells may also produce growth factors that modulate the functions of lens, iris and trabecular meshwork cells (Wordinger, et al., 1999; Weng, et al., 1997; Wilson, et al., 1993). Endothelial cells have a very limited ability for regeneration in human corneas but substantial capacity for proliferation in other species (Schultz et al., 1992).
3. Maintenance of corneal transparency
Transparency is the capacity of a structure to transmit light without appreciable scattering so that structures lying beyond are clearly seen. Although the cornea is not perfectly clear, it scatters only a small portion of the light that enters and allows light transmission needed for quality vision. (Moller-Pedersen, 2004) This fundamental property of the cornea has been well researched and many factors and components are involved in corneal clarity—including the uniform diameter of the collagen fibrils and their regular packing within the stroma (Hassell and Birk, 2010).
The first theories to explain corneal transparency were based on light propagation in the stromal extracellular matrix. Maurice (1957) theorized in his “lattice theory” that the regular arrangement of collagen fibrils which present a smaller diameter than the wavelength of light causes destructive interference of any scattered waves except for those in the direction of the incident beam. Later this theory was modified when Goldman and coworkers (1968) proposed that the periodic fluctuation in the index of refraction is the fundamental mechanism explaining light scattering and the relatively miniscule distance between collagen fibrils relative to the wavelength of the light minimized the possibility of scatter regardless of fibril arrangement. The refractive index changes from 1.380 to 1.373 across the stroma’s anterior to posterior dimension and less than 1% of the total light is scattered (Jalbert and Stapleton, 2005; Qazi et al., 2010). Meek at al (2003) demonstrated, using a mathematical model, that the transparency of the cornea is critically dependent on hydration. Thus, if the cornea swells, light scattering increases. The epithelium and endothelium have significant roles in maintaining corneal transparency by serving as barriers to fluid diffusion and by creating gradients that allow the transport of water out of the stroma.
The lumican-deficient mouse model is a good in vivo example of the relationship between abnormal collagen architecture and loss of corneal transparency (Chakravarti et al., 1998, 2000). Small, leucine-rich proteoglycans, including lumican, are major proteoglycan components of the corneal stroma. These molecules are thought to regulate collagenous matrix assembly in connective tissues via their bi-functional character. Thus, the protein moiety of lumican binds collagen fibrils at strategic loci and the highly charged hydrophilic glycosaminoglycans regulate interfibrillar spacing (Funderburgh et al., 1995; Hassell et al., 1983; Kao and Liu, 2002). Corneal opacity (recognized with a slit lamp and/or in vivo confocal microscopy) were reported in Lum−/− mice and electron microscopic examination revealed abnormally thick collagen fibers, especially in the posterior stroma. This haze is associated with the presence of a disorganized collagenous matrix with larger fibril diameters and disorganized fibril spacing (Chakravarti et al., 1998; Kao and Liu, 2002).
Although these theories explain in part the transparency of the cornea, they do not explanation how other corneal components, including cells, contribute to tissue clarity. Moller-Pederson (2004) suggested that keratocyte nuclei, cell bodies, cell processes, and other structures (such as mitochondria) have different refractive indexes and may influence light scattering. Thus, keratocytes contribute to specular or backward scattered light and are a source of corneal light scattering (Andreo and Farrell, 1982; Gallagher and Maurice, 1977). Jester et al (1999b) showed that keratocyte nuclei are a significant source of light scattering in the normal stroma using in vivo confocal microscopy. Importantly, these authors showed that the expression of corneal crystallins in keratocytes minimizes light scattering by the cells—similar to crystallin protein function in the lens (Delaye and Tardieu, 1983; Tardieu, 1998). Water-soluble proteins in high abundance in keratocytes were identified as crystallins in rabbit corneas (Jester et al., 1999b). The first corneal crystallin identified was aldehyde dehydrogenase class 3 (ALDH3A1) (Alexander et al., 1981). Other corneal crystallins have also been reported—including transketolase (TKT) in mouse, rabbit and human corneas (Jester et al., 1999b; Sax et al., 2000), aldehyde dehydrogenase 1A1 (ALDH1A1) in rabbit corneas (Jester et al., 1999b), and α-enolase in human, mouse and chicken corneas (Cuthbertson et al., 1992). There is an important link between the abundant expression of these proteins and cellular light scattering for both the corneal epithelial cells and keratocytes (Jester, 2008). One study showed that ALHDH3A1 deficient knockout mice had normal corneal transparency (Nees et al., 2002). However, it is likely there is redundancy in this important function and other crystallin proteins can be up-regulated when expression of another is lacking so the transparency critical to vision is maintained.
Corneal avascularity is another essential element of corneal transparency (Ambati et al., 2006; Chang et al., 2001) and the maintenance of the avascular state of the cornea has been termed “angiogenic privilege” (Azar, 2006; Beebe, 2008). Several molecules have been shown to contribute to corneal avascularity. One of the first molecules thought to have a major role in maintaining corneal avascularity was pigment epithelium-derived factor (PEDF) (Tombran-Tink et al., 1991). Alternatively spliced versions of VEGF receptor-1 (sFlt1) and thrombospondins (TSPs) 1–4 have also been implicated in the suppression of vascularization (Ambati et al., 2006; Cursiefen et al., 2004; Tucker et al., 1997). Several other molecules have been proposed as candidates to maintain corneal avascularity or prevent pathologic neovascularization. These include the angiopoietin-like molecule, cornea-derived transcript −6 (CDT6, also called AngX) (Peek et al., 1998) and the inhibitory PAS domain transcription factor, IPAS—which has been reported to prevent the hypoxic induction of VEGF-A (Makino et al., 2001). Factors leading to corneal neovascularization, including persistent hypoxia, chronic inflammation, and corneal injury, have been previously reviewed (Azar, 2006; Beebe, 2008).
4. Corneal opacity — development, persistence and regression
Immediately after corneal injury, a complex cascade of events mediated by autocrine and paracrine interactions of cytokines, growth factors and chemokines produced by epithelial, stromal, bone marrow-derived, lacrimal, and nerve cells is triggered to respond to the threat and then, when the insult is controlled, restore corneal structure and function. Lesions that are restricted to the epithelium, especially if the epithelial BM is not injured, normally heal rapidly through migration, mitosis, and differentiation of transient amplifying cells and limbal stem cells without corneal scar formation. However, if the injury reaches the epithelial BM and the underlying stroma then corneal transparency may be compromised.
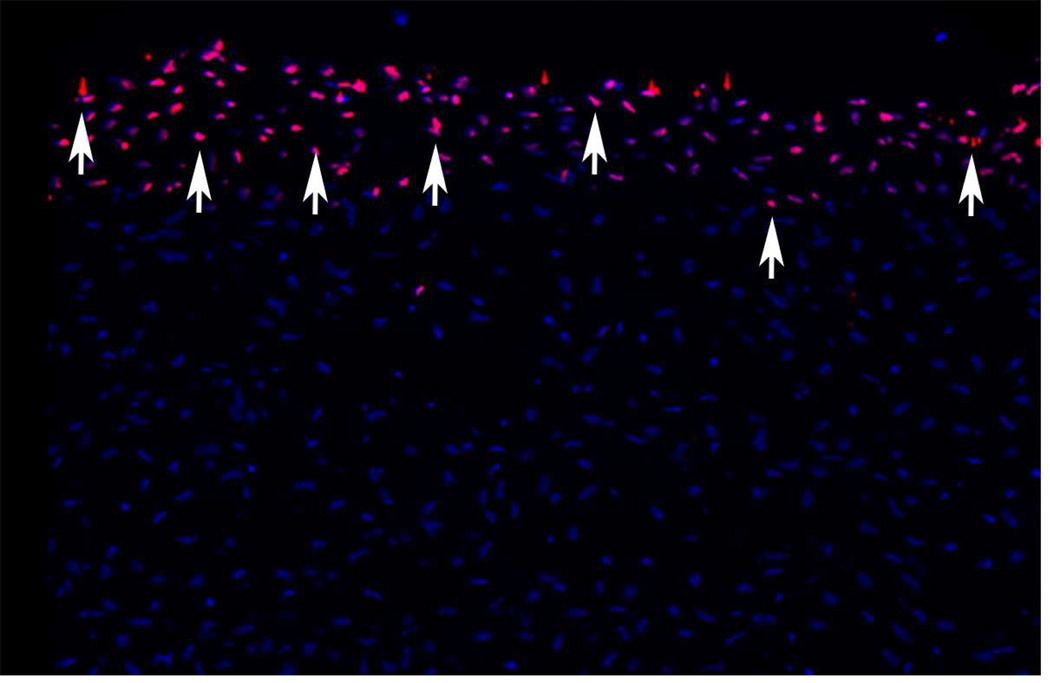
The earliest detectable stromal cellular response to corneal epithelial wounding is the disappearance of anterior stromal keratocytes (Wilson, 1998, 1996) by apoptosis (Fig. 1). Apoptosis is a gentle, involution form of cell death that occurs with little release of lysosomal enzymes and other intracellular components that produce damage to surrounding cells and extracellular matrix that can occur with necrotic cell death (Wilson, 2002). Cytokines released from the injured epithelium, including interleukin-1 (IL-1) and tumor necrosis factor alpha, mediate apoptosis via the Fas-Fas ligand system (Mohan et al., 1998, 1997). Studies with terminal deoxyribonucleotidyl trasferase-mediated dUTP-digoxigenin nick end label (TUNEL) assay, confirmed by transmission electron microscopy, detected keratocyte apoptosis within moments of epithelial injury (Helena et al., 1998; Wilson et al., 1996). Apoptosis of cells continues in the stroma for a week or longer, and includes bone marrow-derived cells that are attracted into the stroma after the injury (Mohan et al., 1998; Hong et al., 2001; Wilson et al., 2004).
Fig. 1.
Keratocyte apoptosis (red, arrows) detected with the TUNEL assay 4 hours after −9D PRK. DAPI stains intact nuclei in deeper keratocytes. 400x
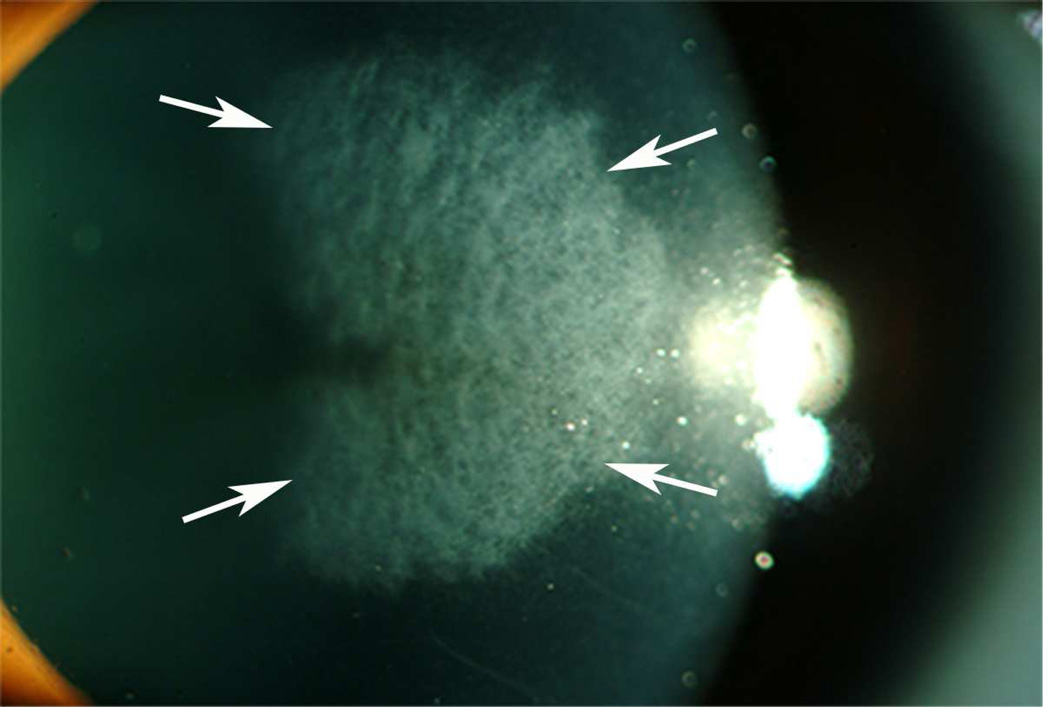
Many remaining keratocytes in the posterior and peripheral stroma begin to undergo mitosis at 12 to 24 hours after injury. This mitosis is best detected by deoxyridine incorporation or immunocytochemical staining for mitosis-specific antigen ki-67 (Zieske et al., 2001). Previous studies suggested that the keratocyte proliferation after injury also generates precursors to myofibroblasts that develop and persist after severe injuries that produce stromal scarring (Fig. 2) due to the opacity of the cells themselves and the disorganized extracellular matrix they produce (Jester et al., 1999c; Masur et al., 1996; Moller-Pedersen et al., 1998b).
Fig. 2.
Corneal haze at 4 months after −7D PRK in a human cornea that was not treated with mitomycin C. 15X.
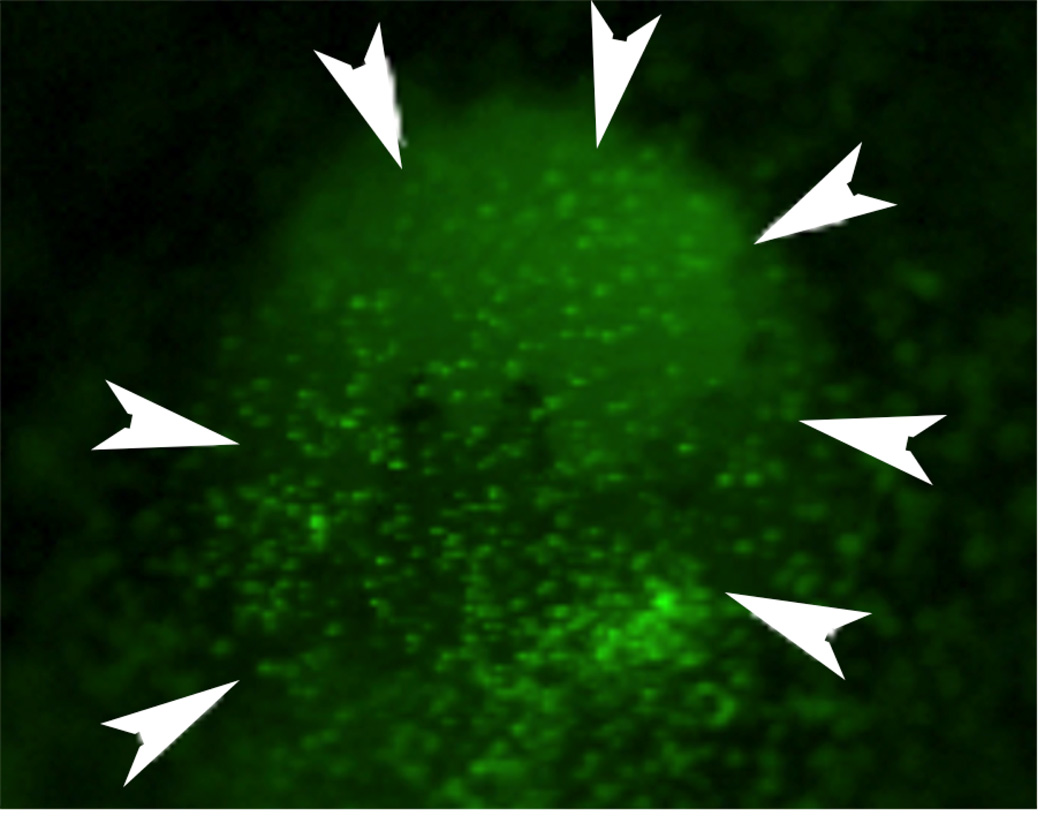
Concurrently, thousands of bone marrow-derived cells migrate into the corneal stroma (Fig. 3) from the limbal blood vessels (Barbosa et al., 2010a; Wilson et al., 2004) attracted by proinflammatory cytokines and chemokines released by the injured epithelium and up-regulated in keratocytes (Hong, et al., 2001). In vitro studies with corneal fibroblasts and bone marrow-derived cells isolated from both normal and green fluorescent mice demonstrated that both bone marrow-derived cells and corneal fibroblasts can transform into myofibroblasts in vitro and demonstrated that the presence of one cell type augments myofibroblast development from the other (Singh et al., 2014b; Singh et al., 2012). In vivo studies ( Singh et al., 2014a; Singh et al., 2013) using chimeric mice with green fluorescent protein (GFP)+ bone marrow transplants have confirmed myofibroblast development from bone marrow-derived cell precursors that likely are fibrocytes (Bucala et al., 1994; Abe et al., 2001). Thus, in a particular injured cornea with haze, 30% to 70% of myofibroblasts develop from keratocyte-derived precursors and 30% to 70% of myofibroblasts develop from bone marrow-derived precursors. It appears this is redundancy in an important function since in vitro studies of gel contraction and other functions found no differences between myofibroblasts generated from corneal fibroblast precursors compared to bone marrow-derived precursors (V. Singh and S.E. Wilson, unpublished data, 2012). Furthermore, it is likely that the type and/or depth of the injury, as well as variation in the wound healing response between different species, and even between strains in the same species, may impact the level of myofibroblasts generation from bone marrow-derived precursors relative to keratocyte-derived precursors.
Fig. 3.
Bone marrow-derived cells (green, arrowheads) infiltrate a mouse cornea at 24 hours after epithelial injury in a chimeric mouse that had a total body irradiation and a bone marrow transplant from a mouse expressing green fluorescent protein in all of its cells several months earlier. (see Wilson et al., 2004) Magnification 10X
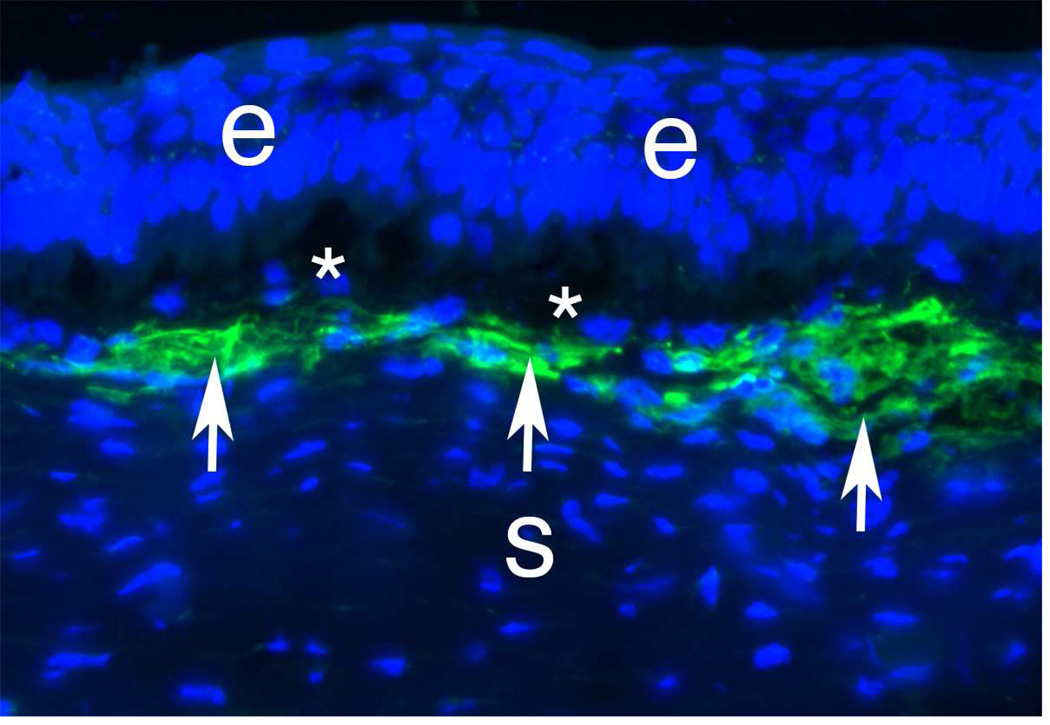
Myofibroblasts are fibroblastic cells that have ultrastructural and physiological characteristics of smooth muscles cells, such as prominent intracellular microfilament bundles and contractile response to smooth muscle agonists (Jester et al., 1999a; Luttrull et al., 1985; Wilson, 2012). The immunocytochemical detection of α-SMA is the most common marker used to detect myofibroblast in vitro and in situ (Fig. 4), although during their early development corneal myofibroblasts express vimentin (V) (Fig. 5) but no α-SMA (A) or desmin (D) (Chaurasia et al., 2009), and, thus, are V+A-D- myofibroblast precursors. Mature corneal myofibroblasts also express vimentin and desmin (Fig. 6) as they complete their development under the influence of TGF-β and PDGF and become mature V+A+D+ myofibroblasts (Chaurasia et al., 2009). These cells are particularly suited to restore the integrity of the cornea after a penetrating injury because of their capacity to contract wounds, secrete extracellular matrix, and generate adhesion structures with the surrounding substrate. Thus, myofibroblast generation can be beneficial in corneal healing associated with lacerations or stromal incisions (Wilson, 2012). However, after surgeries such as photorefractive keratectomy, development of these cells is detrimental and creates central stromal opacity due to the production of large amounts of collagen, hyaluronate and biglycan that contribute to a disorganized and opaque extracellular matrix (Jester et al., 1999c; Mohan et al., 2003). It is thought that the proteoglycans found in the normal cornea, such as keratan sulfate proteoglycan, regulate the diameter and spacing of the collagen fibrils associated with stromal transparency (Hassell and Birk, 2010). Jester et al (2005) have also shown that myofibroblasts have a marked reduction in the expression of corneal crystallins—transketolase and aldehyde dehydrogenase 1, for example, compared to normal keratocytes. Diminished corneal crystallin production is associated with increased reflectivity of the cells and contributes to stromal opacity.
Fig. 4.
α-SMA+ cells (green, arrows) in the anterior stroma at one month after −9D PRK in a rabbit. * indicates artifact separation of the epithelium from the stroma that occurs during cutting of the section with a cryolathe. Blue is DAPI staining for cell nuclei. e is epithelium and s is the stroma. 400X.
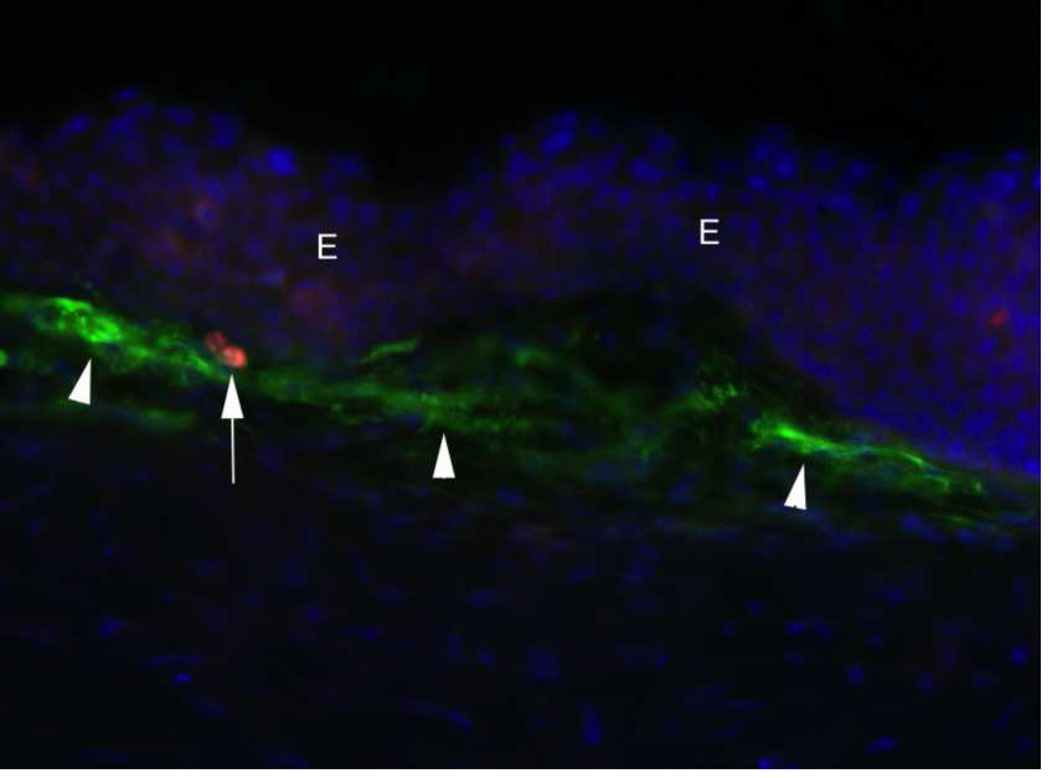
Fig. 5.
Vimentin+ cells (arrows) in the anterior stroma at one week after −9D PRK in a rabbit. e indicates epithelium. This is a rabbit cornea one week after −9D PRK that underwent double immunohistochemistry for vimentin (orange) and α-SMA (green). At this time point after surgery, none of the cells in the anterior stroma express α-SMA. However, many of these vimentin+ cells are likely myofibroblasts in early development that will begin to express α-SMA with further maturation at about two weeks after surgery. Note keratocytes (arrowheads) that have been shown in prior studies to also express vimentin, but at much lower levels, and this expression was not detected with the concentration of primary antibody for vimentin used in this staining (see Chaurasia et al., 2009). Thus, these early myofibroblasts that are vimentin+SMA-express vimentin at high levels. 400X
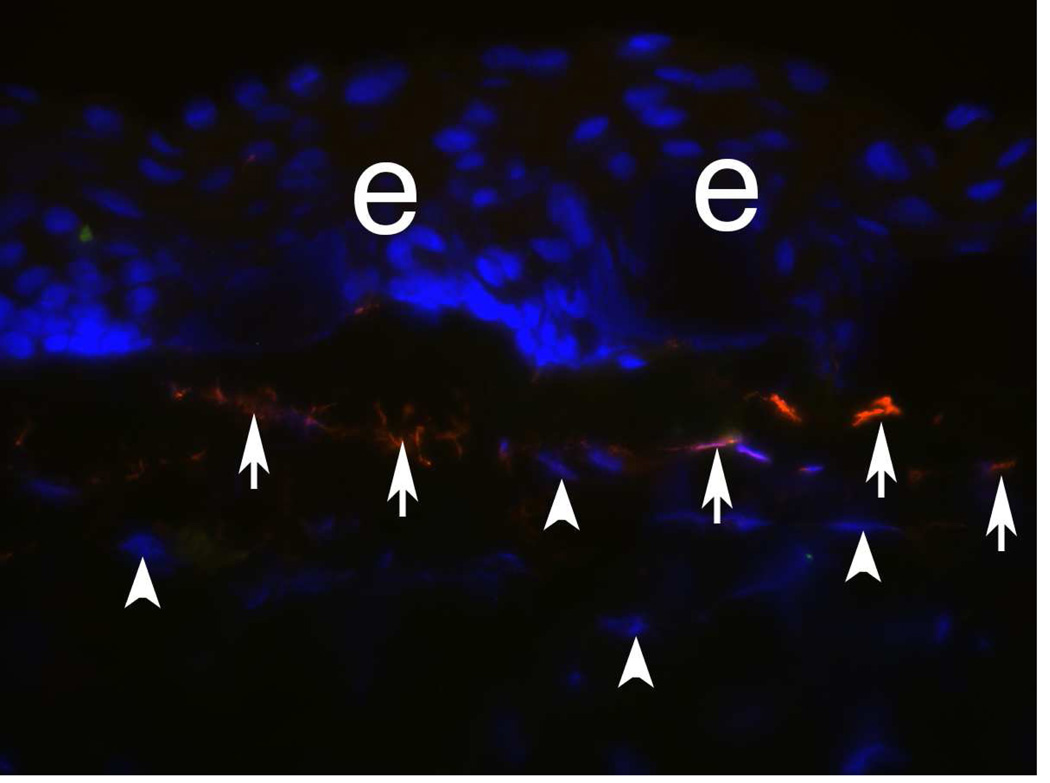
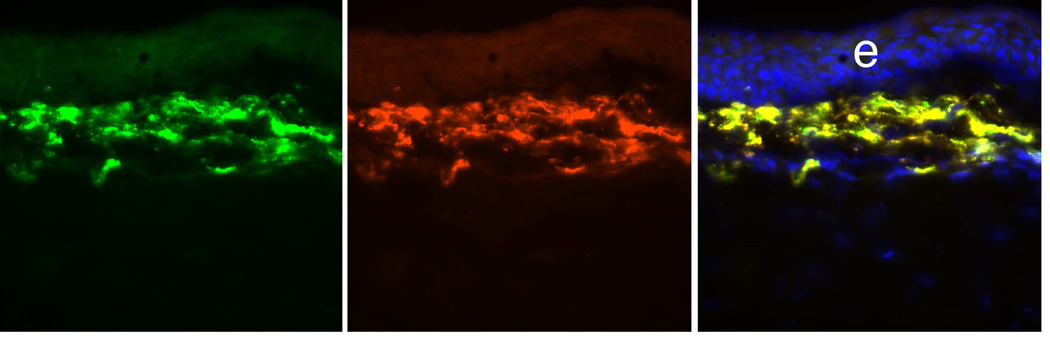
Fig. 6.
α-SMA+ Desmin+ myofibroblasts in a rabbit cornea at 1 month after −9D PRK. At this point after surgery, corneal myofibroblasts express vimentin (not shown), α-SMA (left panel, green), and desmin (center panel, red). In this double-stained section there is nearly 100% concurrence of α-SMA and desmin expression (right panel, overlay). Blue is DAPI staining of cell nuclei. 400X. After Chaurasia et al., 2009.
The development of myofibroblasts has been well characterized with in vitro models and in animal models following photorefractive keratotomy (PRK) (Jester et al., 1999c; Masur et al., 1996; Mohan et al., 2003; Mohan et al., 2008; Singh et al., 2011; Singh et al., 2014b). In vitro studies have demonstrated that TGFβ is a key cytokine in the development of corneal myofibroblasts (Jester et al., 1999a; Masur et al., 1996; Singh et al., 2014b). TGF-β stimulates the production of extracelullar matrix proteins, inhibits matrix metalloproteinases, and modulates many other components of the corneal wound healing response. However, in fibrotic diseases, and other pathophysiological conditions, excessive TGF-β production and signaling promotes extensive tissue fibrosis (Finnson et al., 2013). There are three TGF-β isoforms (−β1, −β2, and −β3) and they appear to play distinct roles in wound healing, with TGFβ1 and 2 having predominantly pro-scarring effects and TGFβ3 having mainly anti-scarring effects (Finnson et al., 2013). TGF-β is released from epithelial cells in a latent complex formed by three proteins: TGFβ, the processed TGF-β pro-peptide, and a member of the latent TGF-β binding protein (LTBP) family. LTBPs are microfibril-associated proteins that bind latent TGFβ to the extracellular matrix. TGF-β activation appears to be a critical checkpoint controlling TGF-β’s actions, and has been intensely investigated (Munger and Sheppard, 2011). Latent TGF-β activators include proteases, thrombospondin-1, and integrins (Horiguchi et al., 2012). Mature TGFβ is a covalent 25-kD homodimer produced after intracellular proteolytic cleavage from its propeptide dimer [latency associated peptide (LAP)]. It is likely that this large TGFβ latent complex cannot penetrate the normal epithelial basement membrane in the cornea.
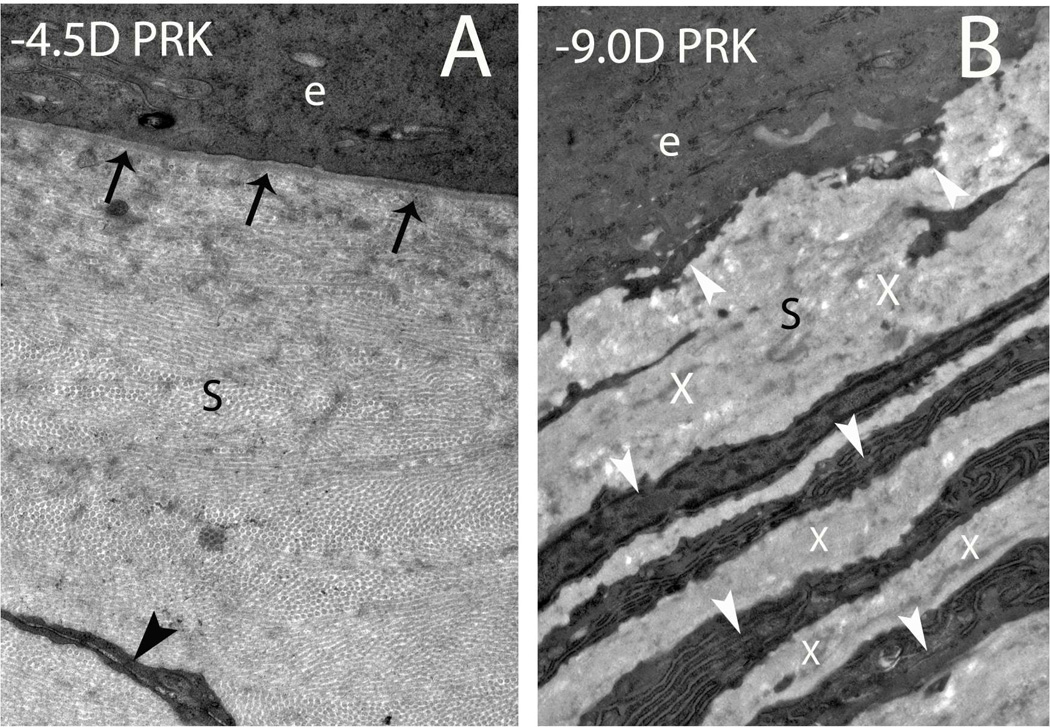
Other studies have also supported an important role for PDGF in corneal myofibroblast development (Jester et al., 2002; Kaur et al., 2009b). Singh et al (2012) hypothesized that the normally functioning epithelial BM critically modulates myofibroblast development through its barrier function that prevents penetration of epithelium-derived TGFβ and PDGF into the stroma at sufficient levels to drive myofibroblast development and maintains viability once mature myofibroblast are generated in the subepithelial stroma. Corneal injury of sufficient magnitude often leads to persistent structural and functional defects in the epithelial BM—which increases and prolongs penetration of epithelial TGFβ and PDGF into the stroma to drive myofibroblast development from either keratocyte-derived or bone marrow-derived precursor cells (Barbosa et al., 2010a; Singh et al., 2011). Torricelli et al (2013a) used transmission electron microscopy to demonstrate defective regeneration of the epithelial BM (no regeneration of lamina lucida or lamina densa) of rabbit corneas with anterior stromal opacity (haze) at one month after high-correction (−9D) PRK (Fig. 7). These corneas with haze were also found to have highly disorganized extracellular matrix and prominent myofibroblasts in the subepithelial stroma beneath the epithelial BM defects, which likely interfere with keratocyte and corneal fibroblast contributions of critical epithelial BM components such as nidogen-1, nidogen-2 and perlecan, resulting in the defective BM regeneration (A. Santhanam, J. Wu, A.A.M. Torricelli and S.E. Wilson, unpublished data, 2014). Unwounded control corneas or corneas without haze at one month after low-correction (−4.5D) PRK had a normal, continuous epithelial BM and few myofibroblasts and no disorganized extracellular matrix (Fig. 7). Thus, the epithelial BM likely functions as a critical corneal regulatory structure that limits the fibrotic response in the stroma by modulating the availability of epithelium-derived TGFβ, PDGF, and perhaps other growth factors, that will promote myofibroblast development from precursors. Once myofibroblast-associated anterior stromal opacity develops, it persists until the normal structure and function of the epithelial BM is regenerated—which often takes years in corneas with severe haze. After epithelial BM regeneration, epithelium-derived TGFβ and PDGF levels in the stroma fall and the myofibroblasts, that are dependent on these growth factors for survival, undergo apoptosis (Wilson, Chaurasia and Medeiros, 2007). Myofibroblasts undergoing apoptosis can be detected using the TUNEL assay even at one week or one month (Fig. 8) after PRK, but at these time points after injury myofibroblast generation outstrips apoptosis in corneas that develop severe haze (Wilson unpublished data, 2004). Thus, during the corneal wound healing response, the balance between myofibroblast precursor apoptosis and myofibroblast development is a critical determinate of whether opacity develops. Thus, myofibroblast precursors develop early in the wound healing response even after mild injuries to the cornea that do not cause haze, but these precursors are eliminated by apoptosis once the epithelial BM regenerates to its normal structure and function. At some point in corneas with severe haze, which could be months or years after PRK, apoptosis of myofibroblasts also exceeds generation of myofibroblasts and these cells slowly disappear from the cornea. Repopulating keratocytes subsequently reabsorb disorganized extracellular matrix and thereby restore transparency (Fini and Stramer, 2005; Singh et al., 2012; Stramer et al., 2003). Other studies have also shown that IL-1 has opposing effects to TGF-β in regulating myofibroblast function (Barbosa et al., 2010b; Kaur et al., 2009a). In those studies, it was found that autocrine or paracrine IL-1α and IL-1β act as inducers of myofibroblast apoptosis if the cell is not continuously exposed to high levels of TGF-β.
Fig. 7.
Defective epithelial basement membrane regeneration in corneas with stromal haze after PRK. (A) In a rabbit cornea at one month after −4.5D PRK that did not develop significant stromal haze there is normal regeneration of the epithelial basement membrane (arrows) with the lamina lucida and lamina densa clearly visible with transmission electron microscopy, as it is in the normal unwounded corneas (not shown). The black arrowhead indicates a keratocyte in the stroma (s). (B) At one month after PRK for high myopia (−9D), in a rabbit cornea that developed severe stromal haze, there is defective regeneration of the epithelial basement membrane with no visible lamina lucida or lamina densa. White arrowheads indicate layers of myofibroblasts with large amounts of rough endoplasmic reticulum and the Xs indicate disordered extracellular matrix these cells secrete in the anterior stroma (s). e indicates epithelium in both panels. Magnification 23,000X
Fig. 8.
Apoptosis of myofibroblasts at one month after −9D PRK. TUNEL assay was used to detect apoptosis and immunohistochemistry to detect α-SMA in myofibroblasts (arrowheads) revealed one myofibroblast undergoing apoptosis (arrow). E indicates epithelium. Blue stain is DAPI for cell nuclei. 400X. The balance between myofibroblast generation and myofibroblast apoptosis in a particular cornea after injury determines whether haze is increasing, persisting, or disappearing over time. After Wilson, Chaurasia, and Medeiros, F.W. 2007 with permission.
5. Loss of corneal transparency related to corneal surgery, injury or infection
5.1. Photorefractive keratectomy-induced corneal haze
Photorefractive keratectomy (PRK) is a surgical technique that is performed by removal of the epithelium followed by excimer laser ablation of the superficial corneal stroma to alter corneal curvature to correct the refractive errors of the eye. PRK continues to represent an alternative to laser in situ keratomileusis (LASIK) for many patients, including those with thinner corneas or corneal topographic abnormalities (Netto et al., 2006b; Rajan et al., 2004). The corneal wound healing process following PRK involves a very complex, and sometime unpredictable, biological response. After surgery, the organization of the extracellular matrix can be altered and, along with changes in cellular density and phenotype, may result in a decrease in stromal transparency—referred to as corneal haze or opacity (Netto et al., 2006a). In most corneas, the level of haze is minimal and transient, and associated with the generation of corneal fibroblasts. Rare corneas, especially those with corrections greater than six diopters of myopia that are not treated with mitomycin C, develop severe, clinically significant haze (Fig. 2). Myofibroblast generation and persistence has been identified as the main biological factor responsible for the formation of corneal haze after PRK surgery ( Jester et al., 1999a; Jester and Ho-Chang, 2003; Mohan, et al., 2003).
Many factors contribute to haze formation, such as length of time required for epithelial defects to heal, the depth of the ablation into the stroma, irregularity of the postoperative stromal surface, or damage to or removal of the epithelial BM (Kuo et al., 2004; Moller-Pedersen et al., 1998a; Stramer et al., 2003; Tang and Liao, 1997; Torricelli et al., 2013a; Vinciguerra et al., 1998).
Mohan et al (2003) showed significant differences in wound healing processes between PRK for low and high myopia in rabbit corneas, including greater keratocyte apoptosis, keratocyte proliferation, and myofibroblast generation in the anterior stroma. Netto and coworkers (2006a) also demonstrated a direct relationship between the level of stroma surface irregularity after PRK and myofibroblast generation, along with the development of corneal haze, that we now understand from transmission electron microscopic studies (Fig. 7) is a function of increasing difficulty in regeneration of a structurally and functionally normal epithelial BM that modulates epithelial cytokines such as TGF-β that drive development of myofibroblasts from precursors and maintain mature myofibroblasts in the anterior stroma after they are established (Torricelli et al., 2013a).
It is important to distinguish pathological late haze associated with myofibroblast generation from the mild haze that occurs in the first few weeks to months after almost all PRK surgeries, including those with perfect clinical outcomes (Wilson, 2012). This more common, clinically insignificant, haze is not attributable to mature myofibroblasts, and the excessive disordered extracellular matrix they produce, but to corneal fibroblasts that are opaque due to decreased corneal crystallin production (Jester et al., 1999b) and less disordered alterations in the anterior stromal extracellular matrix. Corneal fibroblasts produce collagens, as well as keratocan and lumican with keratan sulfate chains, to form a more organized extracellular matrix that has only a limited effect in decreasing corneal transparency (Citron and Kublin, 1977; Hassell et al. 1983; Funderburgh at el, 1998, Ljubimov et al, 1998; Dawson et al, 2005). This can be noted in almost all human and rabbit corneas that have a normal healing response after PRK. Conversely, myofibroblasts secrete high levels of disorganized collagens, hyaluronan, and biglycan, but only low levels of keratan sulfate proteoglycans, which results in a disorganized and opaque extracellular matrix (Hassell and Kirk, 2010). This highly opaque matrix is seen in corneas that develop late haze after PRK (Fig. 2).
Another significant factor in haze generation after PRK is the time of appearance of the haze and associated SMA+ myofibroblasts. In humans, severe, pathological PRK-associated haze is typically detectable by slit lamp examination at around two to three month after surgery and reaches a peak at approximately three to four months after surgery (Raviv et al., 2000). In rabbits, the appearance of haze after PRK follows a similar course, although some SMA+ myofibroblasts that give rise to haze can be detected as early as two weeks after surgery (Chaurasia et al., 2009). A study of intermediate filament expression in rabbits provided important insights regarding the timing of appearance of haze and myofibroblast formation (Chaurasia et al., 2009). That study found that the earliest stromal precursors to myofibroblasts in the cornea express vimentin (V), but not α-SMA (A) or desmin (D)—which are later markers of myofibroblast development (Chaurasia et al., 2009). Thus, corneal myofibroblasts in the cornea go through a sequential developmental change in phenotype from V+A-D- to V+A+D- to V+A+D+ cells.
After PRK surgery, myofibroblast precursor cells (both keratocyte-derived and bone marrow-derived) begin their differentiation mediated by TGF-β and PDGF that penetrate into the stroma from the epithelium after injury to the epithelium and epithelial BM. α-SMA expression is not detected in the early post-injury period since in situ the precursors only begin to express α-SMA after about one to two weeks of exposure to increased levels of TGF-β (Singh et al., 2014b). In most corneas that undergo PRK, the epithelial BM regenerates completely, epithelium-derived TGF-β and PDGF levels in the stroma fall, and the myofibroblast precursors halt their development, and likely undergo apoptosis. In corneas where the epithelial BM fails to regenerate after PRK, which can occur in a localized area or over the entire excimer laser-ablated zone, TGF-β and PDGF continue to penetrate into the stroma at sufficient levels to drive myofibroblast precursor cells to development into mature V+A+D+ myofibroblasts—which secrete large amounts of disorganized extracellular matrix that contributes to subepithelial opacity or haze (Wilson, 2012).
Studies have shown that either keratocyte-derived precursors or bone marrow-derived precursors may give rise to corneal myofibroblasts after PRK (Singh et al., 2014a; Barbosa et al., 2010a; Singh et al., 2012). In addition, the presence of both precursor cell types augments mature myofibroblast generation (Singh et al., 2014b). Thus, both precursors cells appear to have identical roles in haze formation and represent duplication of an important cellular function, although some interaction between the different precursor cells appears to occur to heighten the response.
5.2. Corneal scars at the LASIK flap margin
LASIK is the most common form of corneal surgery to correct myopia, hyperopia, and astigmatism (Wilson, 2004). LASIK entails the creation of an epithelial-stromal flap and subsequent anterior stromal ablation. Preservation of the integrity of the central corneal epithelium and its BM result in less epithelial-stromal cell interactions in the central cornea and consequently lower rates of keratocyte apoptosis and necrosis, and virtually no myofibroblast generation in the central cornea—even after high ablations for myopia (Mohan et al., 2003; O'Brien et al., 1998). At the margin of the flap, however, a well-demarked circular scar can be noted in most corneas that undergo LASIK surgery. In a rabbit model, Ivarsen et al (2003) showed on slit lamp examination that a circular scar started to appear at the flap edge during the first week after LASIK surgery with a mechanical microkeratome. In the following weeks, this scar became increasingly reflective in most eyes. The course of this scar formation follows a similar pattern after femtosecond LASIK flap formation and is restricted to a circumferential band in the anterior stroma at the site of the incisional breaks in the epithelial BM where myofibroblasts develop (Netto et al., 2007). Thus, the fibrotic wound healing response at the LASIK flap margin is also associated with myofibroblast generation and involves the same epithelium-derived growth factors, TGF-β and PDGF, as in PRK, that reach the anterior peripheral stroma through breaks in the epithelial BM. Dawson et al (2005) verified that the extracellular matrix of this scar type is composed of dense network of normal diameter collagen fibers with increased collagen type 3.
Femtosecond lasers use ultrashort laser pulses and photodisruption to cut corneal tissue. The earlier models of the femtosecond lasers, such as the 6-kHz or 15-kHz IntraLase (Advanced Medical Optics, Irvine, CA), generated greater epithelial injury at the site of the flap edge cut compared to more recent 30-kHz Intralase and 60-kHz IntraLase models, or mechanical microkeratomes, because the laser incision used to make the side-cut had greater beam diameter and was produced with higher energy levels with the earlier models. This resulted in greater stromal cell proliferation and more myofibroblast generation at the margin of the flap and greater opacity at the flap edge at a month or more after LASIK (Netto et al., 2007). With later femtosecond laser models, such as the 60-kHz or 150-kHz IntraLase lasers, less energy is applied and incisional beams are narrower, so the stromal inflammatory and wound-healing processes are similar to those generated by microkeratome (Netto et al., 2007; Santhiago and Wilson, 2012).
5.3. Wound repair after corneal radial keratotomy, penetrating keratoplasty and lamellar keratoplasty
Radial keratotomy (RK) employs radial corneal incisions to correct myopia. RK achieves its effects by incising deep into the stroma to allow the mid-peripheral cornea to bulge—which concomitantly flattens the central cornea (Binder, 1987; Melles and Binder, 1990). A prior study showed that the corneal wound healing process after RK undergoes a biphasic change in wound gape (Garana et al., 1992). In the first days after injury, there is migration of epithelial cells into the wound to form an epithelial plug. Then, slowly, over weeks or months, the epithelial plug may be replaced by fibrotic tissue—which involves the transformation of keratocytes, and probably bone marrow-derived cells (Barbosa et al. 2010a), into myofibroblasts responsible for wound contraction. In some incisions, the orientation of myofibroblasts across the keratotomy wound is blocked by the epithelial plug and myofibroblasts orient parallel to the incision where they cannot contribute to wound closure (Melles and Binder, 1990). This may lead to incomplete wound healing and wound gape and variations of surface contour that underlie vision fluctuations that are characteristic of RK even decades after surgery.
In penetrating keratoplasty or lamellar keratoplasty, damaged or diseased cornea is replaced with donor corneal tissue. In these procedures, a more complete wound healing response is usually noted at donor-recipient interface due to suturing of the wound and the absence of wound gape that facilitates myofibroblast orientation across the interface from stroma to stroma without intervening epithelium and collagen deposition parallel to corneal surface (Connon and Meek, 2003).
Posterior lamellar keratoplasty transplant procedures are commonly performed for corneal endotheial disorders (Bachmann et al., 2010; Melles et al., 2008; Price et al., 2009). In Descemet’s stripping automated endothelial keratoplasty (DSAEK) a thin graft consisting of donor Descemet’s membrane and endothelium is transplanted after striping the recipient Descemet’s membrane and diseased endothelium. Zhang et al (2010), using immunohistochemical assays and transmission electron microscopy, evaluated 47 corneal specimens from patients who underwent either penetrating keratoplasty or repeated DSAEK for failed DSAEK. In that study, the authors reported in 19% of the cases (9 of 47) the presence of fibrocellular tissue at the margin of the lenticule extending into the interface. Immunohistochemical staining for α-SMA was positive and ultrastructural examination showed intracytoplasmic filaments with fusiform densities—indicating myofibroblastic differentiation. These findings are similar to the hypercellular scar formation that can be found at the margin of the LASIK flap (Dawson et al., 2005). In contrast, in the central portion of the DSAEK scar, there was a hypocelular wound with few keratocytes and no myofibroblasts, similar to the central portion of a LASIK wound (Zhang et al., 2010). Several others histopathological studies have found fibrocellular tissue, likely from myofibroblast generation, in the graft-host interface in corneas that had failed DSAEK (Shulman et al., 2009; Suh et al., 2009).
6. Corneal scars due to corneal infections and other disorders
Microbial corneal ulcers are characterized by corneal epithelial defects with underlying stromal inflammation and potential stromal tissue loss (Tuli et al., 2007). Severe corneal ulcers can lead to corneal perforation that threatens the eye, and even with aggressive treatment commonly cause corneal scarring and visual impairment. Many factors are involved in the etiology and progression of corneal ulcers, including cytokines, proteases, and other modulators (Fleiszig and Evans, 2002). Corneal ulcers can be broadly categorized as infectious (bacterial, viral, fungal, and protozoal) and noninfectious (neurotrophic, chemical, and immune-mediated).
In infectious corneal ulcers, the infectious agent usually penetrates into the stroma through a defect in the corneal epithelium, although some organisms can penetrate intact epithelium, and the eye reacts with an inflammatory response. Chemokines, proteases and other factors produced by epithelial, keratocyte and inflammatory cells attract more inflammatory cells and fight the infection. However, they may also degrade corneal stromal structural proteins and induce ulceration and opacity due to myofibroblast development and production of disordered extracellular matrix (Fleiszig and Evans, 2002; Tuli et al., 2007). The etiology of noninfectious ulcers is less well understood than that of infectious ulcers. However, in both infectious and non-infectious corneal ulcers myofibroblasts and corneal stromal opacity are often generated.
7. Final considerations
The development of corneal opacity involves complex processes mediated by cytokines, growth factors, and chemokines—and corneal epithelial-stromal interactions that involve the epithelial basement membrane—that may lead to myofibroblast generation, a decrease in cellular corneal crystallins, and loss of stromal structural components. A better understanding of cells and molecules involved in this process may lead to new treatment options to restore corneal transparency and prevent corneal scar formation.
Highlights.
Myofibroblast development vs. apoptosis is critical in opacity development
The corneal epithelial basement membrane critically regulates healing
Cytokines, growth factors and chemokines control cellular events in healing
Acknowledgments
Supported in part by US Public Health Service grants EY10056 and EY015638 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proprietary interest statement: None of the authors have any proprietary or financial interests in the topics discussed in this manuscript.
REFERENCES
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Alexander RJ, Silverman B, Henley WL. Isolation and characterization of BCP 54, the major soluble protein of bovine cornea. Exp Eye Res. 1981;32:205–216. doi: 10.1016/0014-4835(81)90009-9. [DOI] [PubMed] [Google Scholar]
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreo RH, Farrell RA. Corneal small-angle light-scattering theory: wavy fibril models. J Opt Soc Am. 1982;72:1479–1492. doi: 10.1364/josa.72.001479. [DOI] [PubMed] [Google Scholar]
- Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing. Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- Bachmann BO, Laaser K, Cursiefen C, Kruse FE. A method to confirm correct orientation of descemet membrane during descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2010;149:922–925. doi: 10.1016/j.ajo.2010.01.005. e922. [DOI] [PubMed] [Google Scholar]
- Barbosa FL, Chaurasia SS, Cutler A, Asosingh K, Kaur H, de Medeiros FW, Agrawal V, Wilson SE. Corneal myofibroblast generation from bone marrow-derived cells. Exp Eye Res. 2010a;91:92–96. doi: 10.1016/j.exer.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FL, Chaurasia SS, Kaur H, de Medeiros FW, Agrawal V, Wilson SE. Stromal interleukin-1 expression in the cornea after haze-associated injury. Exp Eye Res. 2010b;91:456–461. doi: 10.1016/j.exer.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008;19:125–133. doi: 10.1016/j.semcdb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder PS. Surgical correction of astigmatism. Trans New Orleans Acad Ophthalmol. 1987;35:1–19. [PubMed] [Google Scholar]
- Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- Cavanagh HD, Ladage PM, Li SL, Yamamoto K, Molai M, Ren DH, Petroll WM, Jester JV. Effects of daily and overnight wear of a novel hyper oxygen-transmissible soft contact lens on bacterial binding and corneal epithelium: a 13-month clinical trial. Ophthalmology. 2002;109:1957–1969. doi: 10.1016/s0161-6420(02)01278-2. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Petroll WM, Hassell JR, Jester JV, Lass JH, Paul J, Birk DE. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci. 2000;41:3365–3373. [PMC free article] [PubMed] [Google Scholar]
- Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Chaurasia SS, Kaur H, de Medeiros FW, Smith SD, Wilson SE. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp Eye Res. 2009;89:133–139. doi: 10.1016/j.exer.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintron C, Kublin CL. Regeneration of corneal tissue. Dev Biol. 1977;61:346–357. doi: 10.1016/0012-1606(77)90304-9. [DOI] [PubMed] [Google Scholar]
- Connon CJ, Meek KM. Organization of corneal collagen fibrils during the healing of trephined wounds in rabbits. Wound Repair Regen. 2003;11:71–78. doi: 10.1046/j.1524-475x.2003.11111.x. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Masli S, Ng TF, Dana MR, Bornstein P, Lawler J, Streilein JW. Roles of thrombospondin-1 and −2 in regulating corneal and iris angiogenesis. Invest Ophthalmol Vis Sci. 2004;45:1117–1124. doi: 10.1167/iovs.03-0940. [DOI] [PubMed] [Google Scholar]
- Cuthbertson RA, Tomarev SI, Piatigorsky J. Taxon-specific recruitment of enzymes as major soluble proteins in the corneal epithelium of three mammals, chicken, and squid. Proc Natl Acad Sci U S A. 1992;89:4004–4008. doi: 10.1073/pnas.89.9.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DG, Kramer TR, Grossniklaus HE, Waring GO, 3rd, Edelhauser HF. Histologic, ultrastructural, and immunofluorescent evaluation of human laser-assisted in situ keratomileusis corneal wounds. Arch Ophthalmol. 2005;123:741–756. doi: 10.1001/archopht.123.6.741. [DOI] [PubMed] [Google Scholar]
- Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37:588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24:S2–S11. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- Finnson KW, McLean S, Di Guglielmo GM, Philip A. Dynamics of transforming growth factor beta signaling in wound healing and scarring. Adv Wound Care. 2013;2:195–214. doi: 10.1089/wound.2013.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SM, Evans DJ. The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa. Clin Exp Optom. 2002;85:271–278. doi: 10.1111/j.1444-0938.2002.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Hevelone ND, Stech ME, Justice MJ, Liu CY, Kao WW, Conrad GW. Sequence, molecular properties, and chromosomal mapping of mouse lumican. Invest Ophthalmol Vis Sci. 1995;36:2296–2303. [PubMed] [Google Scholar]
- Funderburgh JL, Hevelone ND, Roth MR, Funderburgh ML, Rodrigues MR, Nirankari VS, Conrad GW. Decorin and biglycan of normal and pathologic human corneas. Invest Ophthalmol Vis Sci. 1998;39:1957–1964. [PubMed] [Google Scholar]
- Gallagher B, Maurice D. Striations of light scattering in the corneal stroma. J Ultrastruct Res. 1977;61:100–114. doi: 10.1016/s0022-5320(77)90009-0. [DOI] [PubMed] [Google Scholar]
- Garana RM, Petroll WM, Chen WT, Herman IM, Barry P, Andrews P, Cavanagh HD, Jester JV. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest Ophthalmol Vis Sci. 1992;33:3271–3282. [PubMed] [Google Scholar]
- Geroski DH, Matsuda M, Yee RW, Edelhauser HF. Pump function of the human corneal endothelium. Effects of age and cornea guttata. Ophthalmology. 1985;92:759–763. doi: 10.1016/s0161-6420(85)33973-8. [DOI] [PubMed] [Google Scholar]
- Goldman JN, Benedek GB, Dohlman CH, Kravitt B. Structural alterations affecting transparency in swollen human corneas. Invest Ophthalmol. 1968;7:501–519. [PubMed] [Google Scholar]
- Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Cintron C, Kublin C, Newsome DA. Proteoglycan changes during restoration of transparency in corneal scars. Arch Biochem Biophys. 1983;222:362–369. doi: 10.1016/0003-9861(83)90532-5. [DOI] [PubMed] [Google Scholar]
- Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39:276–283. [PubMed] [Google Scholar]
- Horiguchi M, Ota M, Rifkin DB. Matrix control of transforming growth factor-β function. J Biochem. 2012;152:321–329. doi: 10.1093/jb/mvs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanusic JJ, Wood RJ, Brock JA. Sensory and sympathetic innervation of the mouse and guinea pig corneal epithelium. J Comp Neurol. 2013;521:877–893. doi: 10.1002/cne.23207. [DOI] [PubMed] [Google Scholar]
- Ivarsen A, Laurberg T, Moller-Pedersen T. Characterisation of corneal fibrotic wound repair at the LASIK flap margin. Br J Ophthalmol. 2003;87:1272–1278. doi: 10.1136/bjo.87.10.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen IE, Jensen OA, Prause JU. Structure and composition of Bowman's membrane. Study by frozen resin cracking. Acta Ophthalmol (Copenh) 1984;62:39–53. doi: 10.1111/j.1755-3768.1984.tb06755.x. [DOI] [PubMed] [Google Scholar]
- Jalbert I, Stapleton F. The corneal stroma during contact lens wear. Cont Lens Anterior Eye. 2005;28:3–12. doi: 10.1016/j.clae.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Jester JV. Corneal crystallins and the development of cellular transparency. Semin Cell Dev Biol. 2008;19:82–93. doi: 10.1016/j.semcdb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Budge A, Fisher S, Huang J. Corneal keratocytes: phenotypic and species differences in abundant protein expression and in vitro light-scattering. Invest Ophthalmol Vis Sci. 2005;46:2369–2378. doi: 10.1167/iovs.04-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999a;40:1959–1967. [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, Cavanagh HD. TGFbeta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFbeta, PDGF and integrin signaling. Exp Eye Res. 2002;75:645–657. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for 'corneal crystallins'. J Cell Sci. 1999b;112(Pt 5):613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999c;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Kao WW, Liu CY. Roles of lumican and keratocan on corneal transparency. Glycoconj J. 2002;19:275–285. doi: 10.1023/A:1025396316169. [DOI] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Agrawal V, Suto C, Wilson SE. Corneal myofibroblast viability: opposing effects of IL-1 and TGF beta1. Exp Eye Res. 2009a;89:152–158. doi: 10.1016/j.exer.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, de Medeiros FW, Agrawal V, Salomao MQ, Singh N, Ambati BK, Wilson SE. Corneal stroma PDGF blockade and myofibroblast development. Exp Eye Res. 2009b;88:960–965. doi: 10.1016/j.exer.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991;32:2244–2258. [PubMed] [Google Scholar]
- Kuo IC, Lee SM, Hwang DG. Late-onset corneal haze and myopic regression after photorefractive keratectomy (PRK) Cornea. 2004;23:350–355. doi: 10.1097/00003226-200405000-00007. [DOI] [PubMed] [Google Scholar]
- Land MF, Fernald RD. The evolution of eyes. Annu Rev Neurosci. 1992;15:1–29. doi: 10.1146/annurev.ne.15.030192.000245. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Alba SA, Burgeson RE, Ninomiya Y, Sado Y, Sun TT, Nesburn AB, Kenney MC, Maguen E. Extracellular matrix changes in human corneas after radial keratotomy. Exp Eye Res. 1998;67:265–272. doi: 10.1006/exer.1998.0511. [DOI] [PubMed] [Google Scholar]
- Luttrull JK, Smith RE, Jester JV. In vitro contractility of avascular corneal wounds in rabbit eyes. Invest Ophthalmol Vis Sci. 1985;26:1449–1452. [PubMed] [Google Scholar]
- Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H, Cao Y, Berkenstam A, Poellinger L. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414:550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Jones MA, Thrasher K. Parasympathetic innervation of the rat cornea. Exp Eye Res 1998. 1998;66:437–48. doi: 10.1006/exer.1997.0445. [DOI] [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci U S A. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice DM. The structure and transparency of the cornea. J Physiol. 1957;136:263–286. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, Dennis S, Khan S. Changes in the refractive index of the stroma and its extrafibrillar matrix when the cornea swells. Biophys J. 2003;85:2205–2212. doi: 10.1016/s0006-3495(03)74646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melles GR, Binder PS. A comparison of wound healing in sutured and unsutured corneal wounds. Arch Ophthalmol. 1990;108:1460–1469. doi: 10.1001/archopht.1990.01070120108039. [DOI] [PubMed] [Google Scholar]
- Melles GR, Ong TS, Ververs B, van der Wees J. Preliminary clinical results of Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2008;145:222–227. doi: 10.1016/j.ajo.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Kim WJ, Mohan RR, Chen L, Wilson SE. Bone morphogenic proteins 2 and 4 and their receptors in the adult human cornea. Invest Ophthalmol Vis Sci. 1998;39:2626–2636. [PubMed] [Google Scholar]
- Mohan RR, Liang Q, Kim WJ, Helena MC, Baerveldt F, Wilson SE. Apoptosis in the cornea: further characterization of Fas/Fas ligand system. Exp Eye Res. 1997;65:575–589. doi: 10.1006/exer.1997.0371. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Stapleton WM, Sinha S, Netto MV, Wilson SE. A novel method for generating corneal haze in anterior stroma of the mouse eye with the excimer laser. Exp Eye Res. 2008;86:235–240. doi: 10.1016/j.exer.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller-Pedersen T. Keratocyte reflectivity and corneal haze. Exp Eye Res. 2004;78:553–560. doi: 10.1016/s0014-4835(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998a;17:627–639. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Neutralizing antibody to TGFbeta modulates stromal fibrosis but not regression of photoablative effect following PRK. Curr Eye Res. 1998b;17:736–747. [PubMed] [Google Scholar]
- Moller-Pedersen T, Li HF, Petroll WM, Cavanagh HD, Jester JV. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 1998c;39:487–501. [PubMed] [Google Scholar]
- Moller-Pedersen T, Vogel M, Li HF, Petroll WM, Cavanagh HD, Jester JV. Quantification of stromal thinning, epithelial thickness, and corneal haze after photorefractive keratectomy using in vivo confocal microscopy. Ophthalmology. 1997;104:360–368. doi: 10.1016/s0161-6420(97)30307-8. [DOI] [PubMed] [Google Scholar]
- Munger JS, Sheppard D. Cross talk among TGF-β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol. 2011;3:a005017. doi: 10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Alvarado J, Juster R. Prenatal and postnatal growth of the human Descemet's membrane. Invest Ophthalmol Vis Sci. 1984;25:1402–1415. [PubMed] [Google Scholar]
- Nees DW, Wawrousek EF, Robison WG, Jr, Piatigorsky J. Structurally normal corneas in aldehyde dehydrogenase 3a1-deficient mice. Mol Cell Biol. 2002;22:849–855. doi: 10.1128/MCB.22.3.849-855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Medeiros FW, Dupps WJ, Jr, Sinha S, Krueger RR, Stapleton WM, Rayborn M, Suto C, Wilson SE. Femtosecond laser and microkeratome corneal flaps: comparison of stromal wound healing and inflammation. J Refract Surg. 2007;23:667–676. doi: 10.3928/1081-597x-20070901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006a;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Gupta PC, Wilson SE. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, cellular proliferation, haze, and long-term keratocyte density in rabbits. J Refract Surg. 2006b;22:562–574. doi: 10.3928/1081-597x-20060601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien TP, Li Q, Ashraf MF, Matteson DM, Stark WJ, Chan CC. Inflammatory response in the early stages of wound healing after excimer laser keratectomy. Arch Ophthalmol. 1998;116:1470–1474. doi: 10.1001/archopht.116.11.1470. [DOI] [PubMed] [Google Scholar]
- Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–384. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- Peek R, van Gelderen BE, Bruinenberg M, Kijlstra A. Molecular cloning of a new angiopoietinlike factor from the human cornea. Invest Ophthalmol Vis Sci. 1998;39:1782–1788. [PubMed] [Google Scholar]
- Price MO, Giebel AW, Fairchild KM, Price FW., Jr Descemet's membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009;116:2361–2368. doi: 10.1016/j.ophtha.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Qazi Y, Wong G, Monson B, Stringham J, Ambati BK. Corneal transparency: genesis, maintenance and dysfunction. Brain Res Bull. 2010;81:198–210. doi: 10.1016/j.brainresbull.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan MS, Jaycock P, O'Brart D, Nystrom HH, Marshall J. A long-term study of photorefractive keratectomy; 12-year follow-up. Ophthalmology. 2004;111:1813–1824. doi: 10.1016/j.ophtha.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Raviv T, Majmudar PA, Dennis RF, Epstein RJ. Mytomycin-C for post-PRK corneal haze. J Cataract Refract Surg. 2000;26:1105–1106. doi: 10.1016/s0886-3350(00)00625-8. [DOI] [PubMed] [Google Scholar]
- Robertson DM, Ladage PM, Yamamoto N, Jester JV, Petroll WM, Cavanagh HD. Bcl-2 and Bax regulation of corneal homeostasis in genetically altered mice. Eye Contact Lens. 2006;32:3–7. doi: 10.1097/01.icl.0000156216.37737.b3. [DOI] [PubMed] [Google Scholar]
- Rufer F, Schroder A, Erb C. White-to-white corneal diameter: normal values in healthy humans obtained with the Orbscan II topography system. Cornea. 2005;24:259–261. doi: 10.1097/01.ico.0000148312.01805.53. [DOI] [PubMed] [Google Scholar]
- Santhiago MR, Wilson SE. Cellular effects after laser in situ keratomileusis flap formation with femtosecond lasers: a review. Cornea. 2012;31:198–205. doi: 10.1097/ICO.0b013e3182068c42. [DOI] [PubMed] [Google Scholar]
- Sax CM, Kays WT, Salamon C, Chervenak MM, Xu YS, Piatigorsky J. Transketolase gene expression in the cornea is influenced by environmental factors and developmentally controlled events. Cornea. 2000;19:833–841. doi: 10.1097/00003226-200011000-00014. [DOI] [PubMed] [Google Scholar]
- Schultz G, Cipolla L, Whitehouse A, Eiferman R, Woost P, Jumblatt M. Growth factors and corneal endothelial cells: III. Stimulation of adult human corneal endothelial cell mitosis in vitro by defined mitogenic agents. Cornea. 1992;11:20–27. doi: 10.1097/00003226-199201000-00003. [DOI] [PubMed] [Google Scholar]
- Shulman J, Kropinak M, Ritterband DC, Perry HD, Seedor JA, McCormick SA, Milman T. Failed descemet-stripping automated endothelial keratoplasty grafts: a clinicopathologic analysis. Am J Ophthalmol. 2009;148:752–759. doi: 10.1016/j.ajo.2009.06.023. e752. [DOI] [PubMed] [Google Scholar]
- Singh V, Agrawal V, Santhiago MR, Wilson SE. Stromal fibroblast-bone marrow-derived cell interactions: implications for myofibroblast development in the cornea. Exp Eye Res. 2012;98:1–8. doi: 10.1016/j.exer.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Barbosa FL, Torricelli AA, Santhiago MR, Wilson SE. Transforming growth factor β and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro. Exp. Eye Res. 2014b;120:152–160. doi: 10.1016/j.exer.2014.01.003. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Jaini R, Torricelli AA, Santhiago M, Singh N, Ambati BK, Wilson SE. TGFβ and PDGF-B signaling blockade inhibits myofibroblast development from both bone marrow-derived and keratocyte-derived precursor cells in vivo. Exp. Eye Res. 2014a;121:35–40. doi: 10.1016/j.exer.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Jaini R, Torricelli AA, Tuohy VK, Wilson SE. A method to generate enhanced GFP+ chimeric mice to study the role of bone marrow-derived cells in the eye. Exp Eye Res. 2013;116:366–370. doi: 10.1016/j.exer.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Santhiago MR, Barbosa FL, Agrawal V, Singh N, Ambati BK, Wilson SE. Effect of TGF beta and PDGF-B blockade on corneal myofibroblast development in mice. Exp Eye Res. 2011;93:810–817. doi: 10.1016/j.exer.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Suh LH, Dawson DG, Mutapcic L, Rosenfeld SI, Culbertson WW, Yoo SH, O'Brien TP, Dubovy SR. Histopathologic examination of failed grafts in descemet's stripping with automated endothelial keratoplasty. Ophthalmology. 2009;116:603–608. doi: 10.1016/j.ophtha.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Tang X, Liao Z. [A clinical study of correlation between ablation depth and corneal subepithelial haze after photorefractive keratectomy] Zhonghua Yan Ke Za Zhi. 1997;33:204–206. [PubMed] [Google Scholar]
- Tardieu A. alpha-Crystallin quaternary structure and interactive properties control eye lens transparency. Int J Biol Macromol. 1998;22:211–217. doi: 10.1016/s0141-8130(98)00018-x. [DOI] [PubMed] [Google Scholar]
- Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53:411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Agrawal V, Santhiago MR, Wilson SE. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest Ophthalmol Vis Sci. 2013a;54:4026–4033. doi: 10.1167/iovs.13-12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci. 2013b;54:6390–6400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RP, Hagios C, Chiquet-Ehrismann R, Lawler J. In situ localization of thrombospondin-1 and thrombospondin-3 transcripts in the avian embryo. Dev Dyn. 1997;208:326–337. doi: 10.1002/(SICI)1097-0177(199703)208:3<326::AID-AJA4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Tuli SS, Schultz GS, Downer DM. Science and strategy for preventing and managing corneal ulceration. Ocul Surf. 2007;5:23–39. doi: 10.1016/s1542-0124(12)70050-2. [DOI] [PubMed] [Google Scholar]
- Vinciguerra P, Azzolini M, Radice P, Sborgia M, De Molfetta V. A method for examining surface and interface irregularities after photorefractive keratectomy and laser in situ keratomileusis: predictor of optical and functional outcomes. J Refract Surg. 1998;14:S204–S206. doi: 10.3928/1081-597X-19980401-13. [DOI] [PubMed] [Google Scholar]
- West-Mays JA, Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol. 2006;38:1625–1631. doi: 10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE. Everett Kinsey Lecture. Keratocyte apoptosis in refractive surgery. CLAO J. 1998;24:181–185. [PubMed] [Google Scholar]
- Wilson SE. Analysis of the keratocyte apoptosis, keratocyte proliferation, and myofibroblast transformation responses after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2002;100:411–433. [PMC free article] [PubMed] [Google Scholar]
- Wilson SE. Clinical practice. Use of lasers for vision correction of nearsightedness and farsightedness. N Engl J Med. 2004;351:470–475. doi: 10.1056/NEJMcp033210. [DOI] [PubMed] [Google Scholar]
- Wilson SE. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp Eye Res. 2012;99:78–88. doi: 10.1016/j.exer.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response. Exp. Eye Res. 2007;85:305–311. doi: 10.1016/j.exer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, He YG, Weng J, Li Q, McDowall AW, Vital M, Chwang EL. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62:325–327. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Hong JW. Bowman's layer structure and function: critical or dispensable to corneal function? A hypothesis. Cornea. 2000;19:417–420. doi: 10.1097/00003226-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Hutcheon AE, Mohan RR, Ambrosio R, Zieske JD, Hong J, Lee J. Effect of ectopic epithelial tissue within the stroma on keratocyte apoptosis, mitosis, and myofibroblast transformation. Exp Eye Res. 2003;76:193–201. doi: 10.1016/s0014-4835(02)00277-4. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Netto M, Perez V, Possin D, Huang J, Kwon R, Alekseev A, Rodriguez-Perez JP. RANK, RANKL, OPG, and M-CSF expression in stromal cells during corneal wound healing. Invest Ophthalmol Vis Sci. 2004;45:2201–2211. doi: 10.1167/iovs.03-1162. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Randleman JB, Stulting RD, Lee WB, Stone DU, Kozarsky AM, Grossniklaus HE. Clinicopathologic findings in failed descemet stripping automated endothelial keratoplasty. Arch Ophthalmol. 2010;128:973–980. doi: 10.1001/archophthalmol.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD, Guimaraes SR, Hutcheon AE. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res. 2001;72:33–39. doi: 10.1006/exer.2000.0926. [DOI] [PubMed] [Google Scholar]