Abstract
Previous studies in our laboratory discovered that SIX1 mRNA expression increased during in vitro progression of HPV16-immortalized human keratinocytes (HKc/HPV16) toward a differentiation-resistant (HKc/DR) phenotype. In this study, we explored the role of Six1 at early stages of HPV16-mediated transformation by overexpressing Six1 in HKc/HPV16. We found that Six1 overexpression in HKc/HPV16 increased cell proliferation and promoted cell migration and invasion by inducing epithelial-mesenchymal transition (EMT). Moreover, the overexpression of Six1 in HKc/HPV16 resulted in resistance to serum and calcium-induced differentiation, which is the hallmark of the HKc/DR phenotype. Activation of MAPK in HKc/HPV16 overexpressing Six1 is linked to resistance to calcium-induced differentiation. In conclusion, this study determined that Six1 overexpression resulted in differentiation resistance and promoted EMT at early stages of HPV16-mediated transformation of human keratinocytes.
Keywords: Six1, human papillomavirus, epithelial-mesenchymal transition, cervical cancer, differentiation resistance, MAPK
Introduction
Although screening is very effective in preventing cervical cancer, approximately 500,000 new cases of cervical cancer are diagnosed and 280,000 deaths result from this disease annually (Peralta-Zaragoza et al., 2012). The etiological agent for cervical cancer is an infection with a high risk human papillomavirus (HPV). HPV16 accounts for 55-60% of all cervical cancer while HPV18 accounts for another 10-15% of cervical cancers (de Sanjose et al., 2010; Munoz et al., 2003; Walboomers et al., 1999). While most HPV infections are asymptomatic and are cleared naturally, in some individuals the HPV infection remains persistent, which can then lead to cervical intraepithelial neoplasia (CIN) and over time cervical cancer (Saslow et al., 2012).
To explore the cellular and molecular events associated with HPV16-mediated transformation and progression, we have established an in vitro model, which in many respects mimics the distinct stages of premalignant progression. In this model normal human foreskin keratinocytes (HKc) are immortalized by transfection with a plasmid containing a head-to-tail dimer of HPV16 DNA (HKc/HPV16). HKc/HPV16 are cultured in serum-free medium supplemented with epidermal growth factor (EGF) and bovine pituitary extract (BPE) (Pirisi et al., 1987). Growth factor-independent HKc (HKc/GFI) are then selected by culturing HKc/HPV16 in medium lacking EGF and BPE. Finally, differentiation resistant cells (HKc/DR) are selected from HKc/GFI in medium containing 1 mM calcium chloride and 5% fetal bovine serum (FBS) (Pirisi et al., 1988). By comparing the gene expression profiles of HKc/DR and HKc/HPV16, we identified Six1 as a gene whose expression is significantly increased in HKc/DR (Wan et al., 2008).
Six1 is a member of the Six family of homeodomain transcription factors and is a key regulator essential for the proper development of numerous organs (Ikeda et al., 2010; Xu et al., 2003; Zheng et al., 2003). Six1 overexpression has been found in various human cancers. (Behbakht et al., 2007; Coletta et al., 2008; Ng et al., 2010; Zheng et al., 2010). The overexpression of Six1 is usually associated with increased tumor progression and metastasis, and decreased survival (Christensen et al., 2008). For example, Six1 overexpression in cervical cancer cell lines and cervical cancer tissues is correlated to increased malignancy and lymph node metastasis (Tan, Zhang, and Qian, 2011; Zheng et al., 2010). While our initial work determined that the expression of SIX1 mRNA increased in HKc/DR compared to HKc/HPV16 (Wan et al., 2008), we more recently reported that overexpression of Six1 in HKc/DR increased cell motility, induced epithelial-mesenchymal transition (EMT), and resulted in malignant conversion (Xu et al., 2014). Furthermore, induction of EMT by Six1 overexpression in HKc/DR was associated with activation of transforming growth factor beta receptor type 2 (TβRII)-p38 signaling (Xu et al., 2014). The fact that Six1 expression increased during in vitro progression of HPV16-immortalized cells and Six1 was overexpressed in cervical cancer tissues (Wan et al., 2008; Zheng et al., 2010), strongly suggested that Six1 might also be involved in premalignant progression. Therefore, in this study, we explored the role of Six1 during the early stages of HPV16-mediated transformation by overexpressing Six1 in HKc/HPV16. We found that Six1 overexpression in HKc/HPV16 increased cell proliferation and induced cell migration and invasion by promoting EMT. Importantly, the overexpression of Six1 in HKc/HPV16 resulted in resistance to serum and calcium-induced differentiation. Finally, we found that activation of MAPK signaling is linked to resistance to calcium-induced differentiation in HKc/HPV16 overexpressing Six1. Overall our results support the conclusion that Six1 overexpression promotes differentiation resistance and EMT at early stages of HPV16-mediated transformation of HKc.
Materials and methods
Cell culture and treatment
The HKc/HPV16 and HKc/DR cell lines used in this work have been described in detail previously (Pirisi et al., 1988; Pirisi et al., 1987). HKc/HPV16 were cultured in keratinocyte serum free medium supplemented with EGF and BPE (Invitrogen, Carlsbad, CA). This medium is referred to as complete medium. HKc/HPV16 were transfected, using TransFast reagent (Promega, Madison, WI), with the mammalian expression vector pcDNA3.1 (Invitrogen) alone (HKc/HPV16-Ctrl) or pcDNA3.1 containing full-length human Six1 cDNA that was cloned into the NheI and BamHI site (HKc/HPV16-Six1) (Xu et al., 2014). Stable transfectants of HKc/HPV16-Ctrl and HKc/HPV16-Six1 (originating from our HKc/HPV16D-4 line) were selected in complete medium containing 50 μg/ml Zeocin (Invitrogen) (Pirisi et al., 1988). All experiments were conducted using the same clonal HKc/HPV16-Six1 cell line. For differentiation resistance experiments, HKc/HPV16-Ctrl and HKc/HPV16-Six1 were cultured with either complete medium or complete medium supplemented with 1 mM calcium chloride or 5% fetal bovine serum (FBS) for the indicated times. All cells were maintained in a humidified atmosphere of 5% CO2 at 37 °C.
Real time PCR
Total RNA was isolated from cells using the Total RNA Isolation Mini Kit (Agilent, Wilmington, DE). Reverse transcription was carried out with 1 μg of total RNA using the iScript cDNA Synthesis Kit (BioRad, Hercules, CA). Real time PCR was performed using iQ SYBR Green Supermix (BioRad). All the procedures were according to the manufacturer's instructions. The DNA sequence of the primers used for real time PCR have previously been reported (Xu et al., 2014). β-actin was used as an internal control. All samples were analyzed in triplicate.
Antibodies and Western blot analysis
Cells lysates were prepared using RIPA buffer (Pierce, Rockford, IL). Antibodies against the following proteins were used: Six1, transforming growth factor-beta (TGF-β) type II receptor (TβRII), TGF-β type III receptor (TβRIII) (all at 1:1000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA), E-cadherin, fibronectin (at 1:10,000 dilution, BD Biosciences, San Jose, CA), ERK, p-ERK, JNK, p-JNK, p38, p-p38, p-STAT3, keratin 10 (all at 1:3000 dilution, Cell Signaling Technology, Danvers, MA). The blots were incubated with primary antibody overnight at 4°C, washed three times with PBST, followed by incubation with HRP-conjugated secondary antibody (at 1:10,000 dilution, Millipore, Temecula, CA). The blots were visualized using the ECL Prime Western Blotting Detection System (GE Healthcare, Buckinghamshire, UK). β-actin was used as an internal control for equal protein loading. For Triton X-100-soluble and -insoluble protein fraction experiments, cell extracts were prepared as previously described (Sadot et al., 1998).
Cell proliferation assay
To compare the growth rate of HKc/HPV16, HKc/HPV16-Ctrl and HKc/HPV16-Six1, 50,000 cells in 2 ml of their respective media were plated per well in 6-well plates. Cells plated in triplicate wells were counted daily for 5 days using a hemocytometer.
Cell invasion and migration assay
The invasive ability of HKc/HPV16-Ctrl and HKc/HPV16-Six1 was determined using transwell chambers (24 well plate) (Costar, Cambridge, MA) with polycarbonate membranes (8.0-μm pore size) coated with 100 μl Matrigel (BD Biosciences, Franklin Lakes, NJ) on the top side of the membrane. Then, 20,000 cells per well in basal medium containing 0.1% bovine serum albumin were plated in upper chamber. The lower chamber contained complete medium. After 24 h the cells were fixed with methanol and stained with 0.1% crystal violet. Cells and Matrigel on the upper surface of the membrane were carefully removed with a cotton swab. Cells were quantified by visually counting cells in five randomly chosen areas. The migration of cells was measured by counting cells migrated to the lower chamber. Each experiment was performed in triplicate wells and repeated three times.
Immunofluorescence
HKc/HPV16-Ctrl and HKc/HPV16-Six1 were plated in Lab-Tek II chambers (Nalge Nunc International, Rochester, NY) for 24 h. Following treatment, fixation (with 2% paraformaldehyde in PBS, pH 7.2) and permeabilization (with 0.1% Triton X-100 in PBS), samples were incubated with antibodies against E-cadherin (at 1:200 dilution), fibronectin (at 1:200 dilution) and keratin 10 (at 1:100 dilution) overnight at 4°C. Samples were then washed three times with PBST, followed by incubation with FITC- and Alexa 568- conjugated secondary antibodies (at 1:1000 dilution, Invitrogen). Nuclei were stained with a 1:20,000 dilution of 4’, 6-diamidino-2-phenylindole (DAPI) (Invitrogen) before cells were mounted. Samples were observed using an Olympus X81 fluorescence microscope.
Luciferase assays
Cells were plated into 6-well plates. Transfections were performed using TransFast Transfection Reagent (Promega) according to the manufacturer's instructions. The luciferase construct p6SBE-luc, which contains six copies of the Smad binding element (SBE) and p6SME-luc, which contains Smad mutated elements (SME) were obtained from Dr. Scott Kern (Dai et al., 1998). For each well, cells were transfected with either 4 μg p6SBE-luc or 4 μg p6SME-luc, along with 4 ng pRL-SV40 (Promega) Renilla luciferase as a control for transfection efficiency. Cells were treated with human TGF-β1 (R&D Systems, Minneapolis, MN) or vehicle 24 h after transfection. Both firefly and Renilla luciferase activity were measured using the Dual Luciferase Assay Kit (Promega) 24 h after treatment. Firefly luciferase values were normalized to Renilla luciferase values.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). Statistical analysis was performed using a student's t-test when only two value sets were compared, and by one-way ANOVA followed by the Dunnett's test when the data involved three or more groups. P < 0.05, P < 0.01 or P < 0.001 were considered statistically significant and indicated in the figures by *, ** or *** respectively.
Results
The expression of Six1 increases during HPV16-mediated transformation of HKc
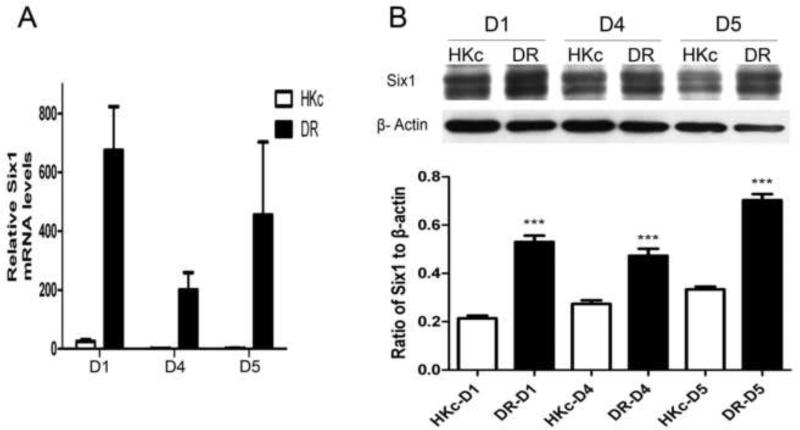
Previous studies in our laboratory discovered that the expression of SIX1 mRNA increased during in vitro progression of HKc/HPV16 toward the HKc/DR phenotype (Wan et al., 2008). To further explore overexpression of Six1 during in vitro progression of HPV16-immortalized HKc, we conducted both RT-PCR and Western blot analysis for Six1 in three independent sets of HKc/HPV16 and HKc/DR lines (designated D1, D4 and D5), derived from normal HKc isolated from three different foreskin donors. The results showed that the expression of Six1 mRNA increased 200 to over 600-fold in HKc/DR compared to their respective HKc/HPV16 lines (Fig. 1A) while protein levels of Six1 increased about 2-fold (Fig. 1B). The biological basis for the much greater increase in Six1 mRNA levels relative to Six1 protein levels is unknown and currently under study. Furthermore, the two bands of Six1 we observed in the Western blots is consistent with published studies and likely the result of posttranslational phosphorylation (Ford et al., 2000; Micalizzi et al., 2010).
Fig.1. The expression of Six1 increases during in vitro progression of HPV16-immortalized human keratinocytes.
(A) Real-time PCR analysis of Six1 mRNA in three independently derived HKc/HPV16 (HKc) lines (D1, D4 and D5) and their corresponding HKc/DR (DR) lines. Six1 expression was normalized to β-actin. (B) Top: western blot for Six1 in HKc/HPV16-D1, -D4 and -D5, (labeled as HKc) and their corresponding HKc/DR (labeled as DR). β-actin was used as control for equal protein loading. Bottom: the expression of Six1 protein was quantified by ImageJ. Bars indicate standard deviation (SD) and *** indicate statistically significant p value < 0.001.
Six1 increases proliferation, migration and invasion in HKc/HPV16
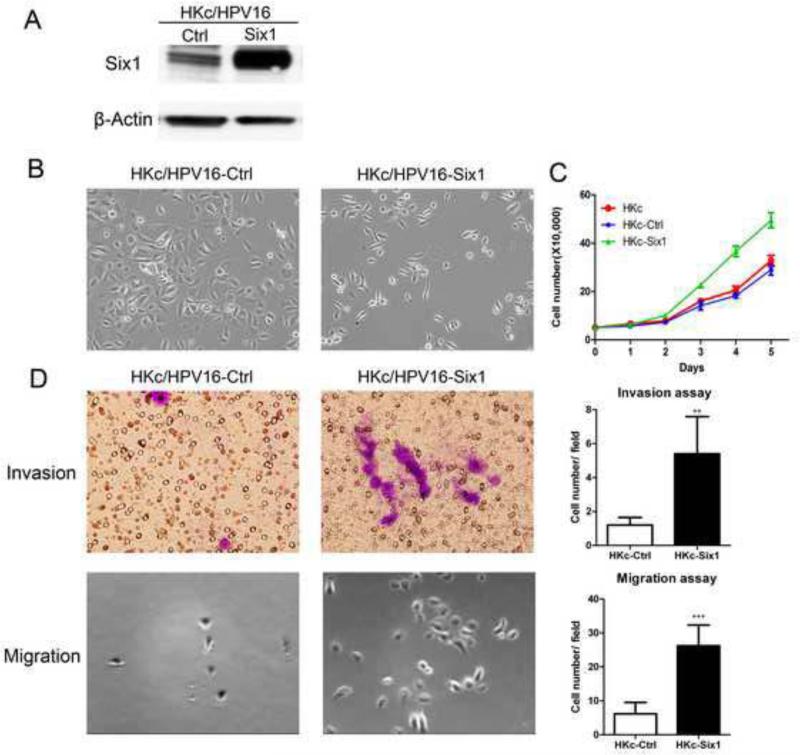
To further explore the function of Six1 in HKc/HPV16, we cloned a full-length human Six1 cDNA into the expression plasmid pcDNA3.1 (pcDNA3.1-Six1). HKc/HPV16-Six1 were established by stable transfection of HKc/HPV16 with pcDNA3.1-Six1. HKc/HPV16-Ctrl were HKc/HPV16 transfected with the pcDNA3.1 expression plasmid alone. Western blots confirmed the overexpression of Six1 in HKc/HPV16-Six1 (Fig. 2A). HKc/HPV16-Six1 exhibited an elongated and more fibroblastic-like appearance rather than the typical cuboidal morphology of HKc/HPV16-Ctrl (Fig. 2B). HKc/HPV16-Six1 also showed increased cell proliferation compared to HKc/HPV16-Ctrl (Fig. 2C). Moreover, Six1 overexpression in HKc/HPV16 increased cell invasion by 4.5-fold and cell migration approximately 4.2-fold (Fig. 2D). These data demonstrate that HKc/HPV16 overexpressing Six1 exhibited a more mesenchymal phenotype with increased cell growth, migration and invasion compared to HKc/HPV16-Ctrl.
Fig.2. Six1 overexpression in HKc/HPV16 changes cell morphology and increases cell proliferation, invasion and migration.
(A) HKc/HPV16 were stably transfected with either pcDNA3.1 (Ctrl) or pcDNA3.1-Six1 (Six1). Six1 protein levels in cell extracts were determined by western blotting. β-actin was used as a loading control. (B) Cell morphology of HKc/HPV16-Ctrl and HKc/HPV16-Six1. Images are shown at 100× magnification. (C) Proliferation of HKc/HPV16-Six1 (HKc-Six1), HKc/HPV16-Ctrl (HKc-Ctrl) and HKc/HPV16 (HKc). Cell numbers were determined by manually counting cells on the days indicated. (D) Invasion (upper panels) and migration (lower panels) assays for HKc/HPV16-Ctrl and HKc/HPV16-Six1. Cell invasion was determined by a Matrigel invasion assay, and cell migration was determined by a transwell migration assay. Images for the invasion assay are shown at 400× and those for the migration assay at 100× magnification. Quantification of the invasion assay and the migration assay are shown in the upper right and lower right panels, respectively. Each column represents the mean of five different fields. Bars indicate SD, and ** and *** indicate statistically significant p values < 0.01 and 0.001 respectively.
Six1 promotes EMT
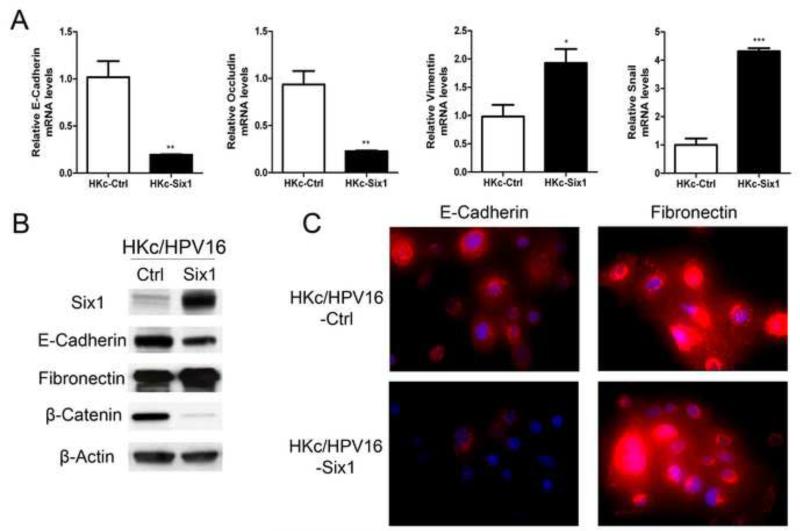
Due to the changes in cell morphology, growth and invasion associated with Six1 overexpression, we next explored the expression of EMT related genes upon Six1 overexpression in HKc/HPV16. As shown in Figure 3A, the overexpression of Six1 resulted in increased expression of mesenchymal markers and decreased expression of epithelial markers. For example, the mRNA level of E-cadherin and occludin decreased by about 81% and 76%, while mRNA levels of vimentin and snail increased by 2.0-, and 4.3-fold respectively in HKc/HPV16-Six1 when compared with HKc/HPV16-Ctrl (Fig. 3A). Moreover, we examined the protein levels of E-cadherin and fibronectin by western blot analysis and immunofluorescence. The overexpression of Six1 in HKc/HPV16 led to decreased protein levels of E-cadherin and slightly increased levels of fibronectin (Fig. 3B, C). We also investigated β-catenin, a key component of adherens junctions in epithelial cells (Gumbiner, 2005), and found that protein levels of β-catenin decreased dramatically in Six1-overexpressing HKc/HPV16 (Fig. 3B). Taken together, our data show that Six1 overexpression promotes EMT in HKc/HPV16.
Fig.3. Six1 overexpression induces EMT in HKc/HPV16.
(A) mRNA expression of E-cadherin, occludin, vimentin and snail were determined by Real-time PCR in HKc/HPV16-Ctrl (HKc-Ctrl) and HKc/HPV16-Six1 (HKc-Six1) cultured in complete medium . Data were normalized to J-actin expression. Bars indicate SD, and *, ** and *** indicate statistically significant p values < 0.05, 0.01 and 0.001 respectively. (B) Western blot for Six1, E-cadherin, fibronectin and β-catenin in HKc/HPV16-Ctrl (Ctrl) and HKc/HPV16-Six1 (Six1) cultured in complete medium. β-actin was used as loading control. (C) Immunofluorescent staining of E-cadherin (red) (left panels) and fibronectin (red) (right panels) in HKc/HPV16-Ctrl and HKc/HPV16-Six1 cultured in complete medium. Nuclei were stained with DAPI (blue). Images are shown at 400× magnification.
The overexpression of Six1 in HKc/HPV16 results in resistance to serum and calcium-induced differentiation
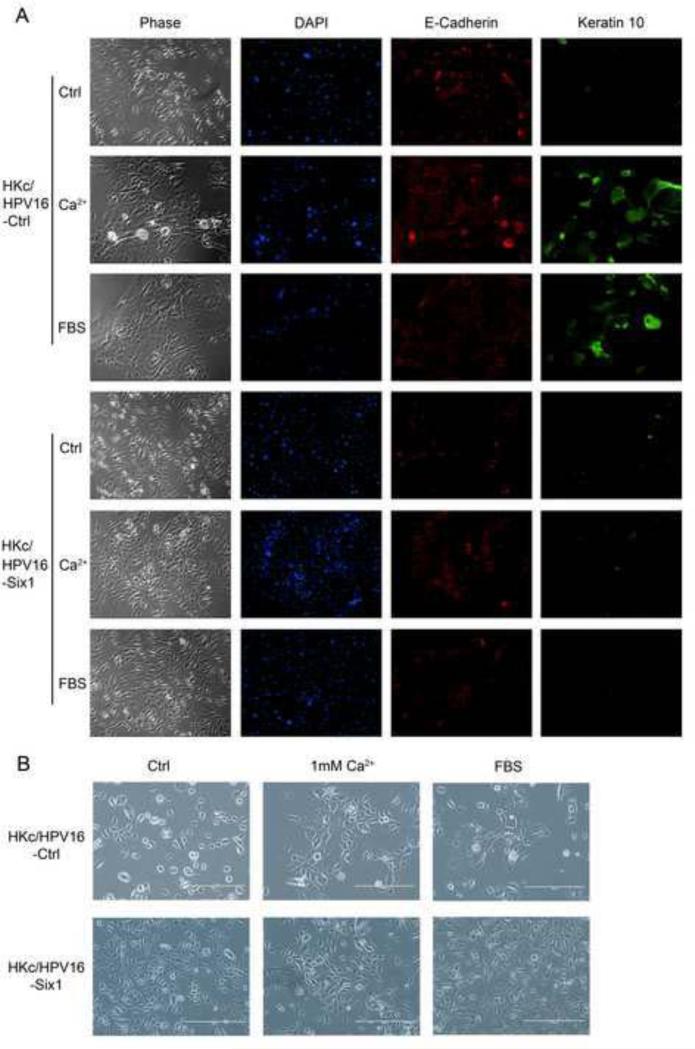
We used Western blots to determined protein levels of Six1 at all stages of our in vitro model for HPV16-mediated transformation. By far, the most dramatic increase in Six1 expression was observed in the final HKc/DR stage compared to the earlier HKc/HPV16 and HKc/GFI stages (supplemental Fig.1). Normal HKc and HKc/HPV16 can be propagated in serum-free media and under low Ca2+ conditions (30 μM), and media containing high-calcium (1 mM) or serum (5%) triggers terminal squamous differentiation (Boyce and Ham, 1983; Pirisi et al., 1988; Pirisi et al., 1987). The hallmark of HKc/DR is resistance to serum and calcium-induced differentiation (Pirisi et al., 1988). Therefore, we explored if the overexpression of Six1 is associated with differentiation resistance. As shown in Figure 4A, HKc/HPV16-Ctrl stratified and expressed keratin 10, a differentiation marker, after being treated with 1 mM Ca2+ or 5% FBS for 5 days. In contrast, HKc/HPV16-Six1 did not stratify or express keratin 10 in response to 1 mM Ca2+ or 5% FBS treatment (Fig. 4A). We consistently found that keratin 10 protein levels dramatically increased at day 5 following 1 mM calcium chloride treatment in HKc/HPV16-Ctrl, but not in HKc/HPV16-Six1 (Fig. 5A). The change in cell morphology in HKc/HPV16-Ctrl could be observed as early as 24 h post 1 mM Ca2+ or 5% FBS treatment (Fig. 4B). HKc/HPV16-Six1 cultured in medium supplemented with 1 mM calcium chloride or 5% FBS showed a mixed morphology of spindle-like cells as well as enlarged cobblestone shaped cells, which is typical of the morphology exhibited by HKc/DR (Fig. 4A, B).
Fig.4. Six1 overexpression in HKc/HPV16 results in resistance to serum and calcium-induced differentiation.
(A) HKc/HPV16-Ctrl and HKc/HPV16-Six1 were cultured with either complete medium or complete medium supplemented with 1 mM calcium chloride or 5% FBS for 5 days. Cell morphology of HKc/HPV16-Ctrl and HKc/HPV16-Six1 under the indicated treatment is shown in the first column (Phase). Immunofluorescent staining of nuclei with DAPI (blue), E-cadherin (red) and keratin 10 (green) in HKc/HPV16-Ctrl (upper panels) and HKc/HPV16-Six1 (lower panels) is shown in columns 2-4. (B) Cell morphology of HKc/HPV16-Ctrl and HKc/HPV16-Six1 cultured in complete medium (Ctrl) or complete medium containing high calcium (1 mM Ca2+) or FBS for 24 h. Images are shown at 100× magnification.
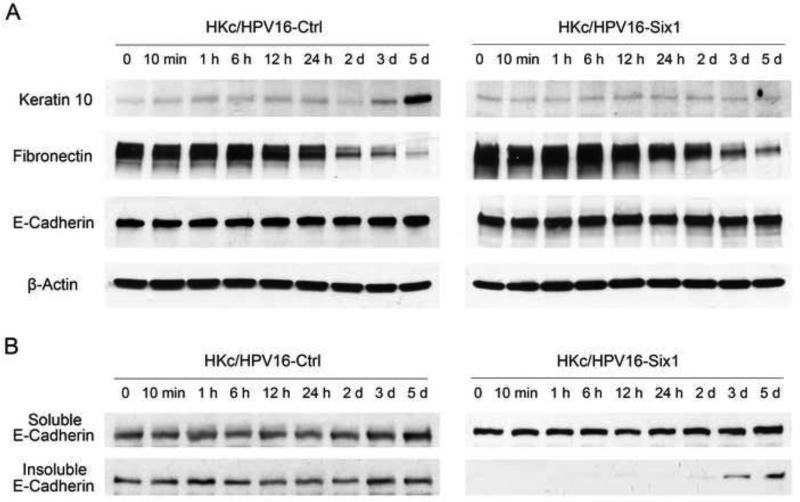
Fig.5. Six1 overexpression in HKc/HPV16 does not impact the redistribution of adhesion junctions when cultured in high calcium media.
(A) Western blot of keratin 10, fibronectin and E-cadherin in whole cell lysates from HKc/HPV16-Ctrl and HKc/HPV16-Six1 that were treated with media containing 1 mM Ca2+ for the indicated times. β-actin was used as a loading control. (B) The expression of E-cadherin in soluble (cytosolic) and insoluble (cytoskeleton-associated) fractions from HKc/HPV16-Ctrl and HKc/HPV16-Six1 that were treated with 1 mM Ca2+ for the indicated times. Fractions were analyzed by western blotting.
The E-cadherin-β-catenin complex at the plasma membrane plays a critical role in differentiation of normal HKc, and knocking-down E-cadherin or β-catenin expression blocks calcium-induced keratinocyte differentiation (Xie and Bikle, 2007). We thus examined E-cadherin by immunofluorescence and we found that both HKc/HPV16-Six1 and HKc/HPV16-Ctrl exhibited increased membrane-associated E-cadherin and intercellular junction formation when exposed in high Ca2+ medium (Fig. 4A). We also used western blotting to detect protein levels of total E-cadherin, fibronectin, as well as soluble (cytoplasmic) and insoluble (cytoskeletal-associated) E-cadherin in HKc/HPV16-Ctrl and HKc/HPV16-Six1 after being exposed to media containing 1 mM Ca2+ for varying times (10 min to 5 days). We found that total E-cadherin remained relatively unchanged and that the expression of fibronectin decreased in both HKc/HPV16-Ctrl and Six1-overexpressing cells upon high calcium treatment (Fig. 5A). Consistent with what we observed by immunofluorescence, HKc/HPV16-Six1 exhibited increased insoluble E-cadherin from 48 h after high calcium treatment, indicating the formation of intercellular junctions (Fig. 5B). Phosphatidylinositol 3-kinase (PI3K)/AKT signaling can be activated by the E-cadherin complex and promotes early keratinocyte differentiation (Xie and Bikle, 2007). However, we did not observe any significant increase in phospho-PI3K or phospho-AKT in response to high calcium treatment (Supplemental Fig. 2). Taken together, our data strongly indicate that overexpression of Six1 in HKc/HPV16 results in resistance to Ca2+ or serum-induced differentiation. The resistance to Ca2+ induced differentiation by Six1 is not induced through activation of the PI3K pathway by E-cadherin-β-catenin.
Smad-dependent TGF-β signaling is decreased in association with Six1 overexpression
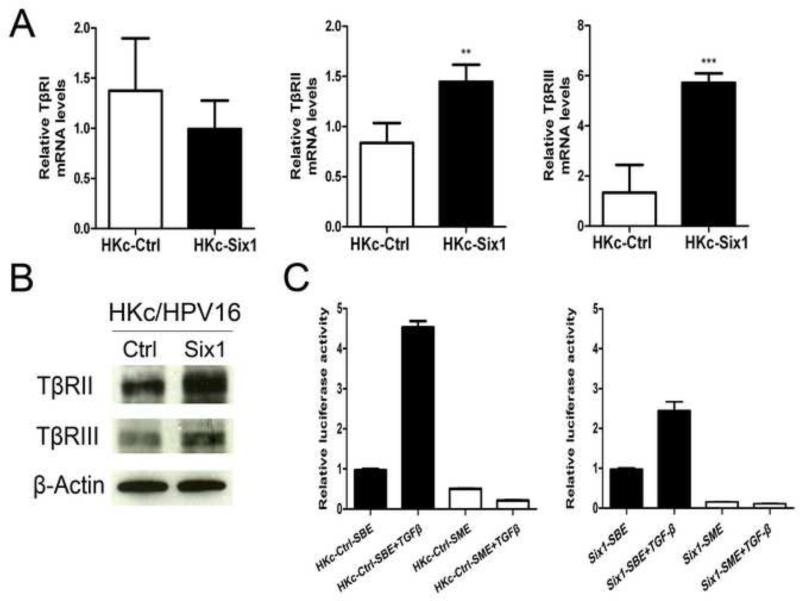
Next, we explored possible mechanisms by which Six1-overexpression in HKc/HPV16 leads to resistance to calcium/serum-induced differentiation. Cell cycle exit is one of the major processes involved in entering the terminally differentiated state (Missero et al., 1995; Missero et al., 1996). TGF-β-Smad2/3 signaling induces cell cycle withdrawal during keratinocyte terminal differentiation (Descargues et al., 2008), and TGF-β1 enhances differentiation of HKc under high Ca2+ conditions (Matsumoto et al., 1990). Moreover, TGF-β signaling is important in Six1-induced EMT (Micalizzi et al., 2009; Micalizzi et al., 2010). Compared to HKc/HPV16-Ctrl, mRNA levels of TβRII and TβRIII in HKc/HPV16-Six1 were increased 1.7-fold and 4.3-fold, respectively, while TβRI mRNA was slightly decreased (Fig. 6A). In addition, increases in the protein levels of TβRII and TβRIII in HKc/HPV16-Six1 were detected by western blotting (Fig. 6B). We next investigated whether Six1-induced EMT and differentiation resistance are linked to altered TGF-β-Smad2/3 signaling. We compared both the basal and TGF-β stimulated activity of a Smad-responsive luciferase reporter construct (p6SBE-Luc) in HKc/HPV16-Ctrl with HKc/HPV16-Six1. Six1 overexpression did not change the basal activity of the Smad-dependent reporter construct (data not shown). However, activation of Smad-dependent signaling in response to TGF-β1 treatment was attenuated by ~ 45% in HKc/HPV16-Six1 as compared with HKc/HPV16-Ctrl (Fig. 6C). These data suggest that Six1overexpression decreases activation of Smad-dependent signaling in response to TGF-β1 treatment despite increasing the levels of TβRII and TβRIII.
Fig.6. Six1 overexpression in HKc/HPV16 alters TGF-β signaling.
(A) mRNA expression of TGF-β receptors TβRI, TβRII and TβRIII were determined by Real time PCR in HKc/HPV16-Ctrl (HKc-Ctrl) and HKc/HPV16-Six1 (HKc-Six1). Data were normalized to β-actin expression. (B) Western blot analysis of TβRII and TβRIII in HKc/HPV16-Ctrl (Ctrl) and HKc/HPV16-Six1(Six1). β-actin was used as a loading control. (C) HKc/HPV16-Ctrl (HKc-Ctrl) or HKc/HPV16-Six1 (HKc-Six1) were transfected with either p6SBE-luc (SBE) or p6SME-luc (SME). Twenty four h after transfection, cells were treated with 40 pM TGF-β1 for 24 h and then luciferase activity determined. Values were normalized to Renilla luciferase. Bars indicate SD, and ** and *** indicate statistically significant p values < 0.01 and < 0.001 respectively.
Six1 activates ERK and p38 MAPK signaling
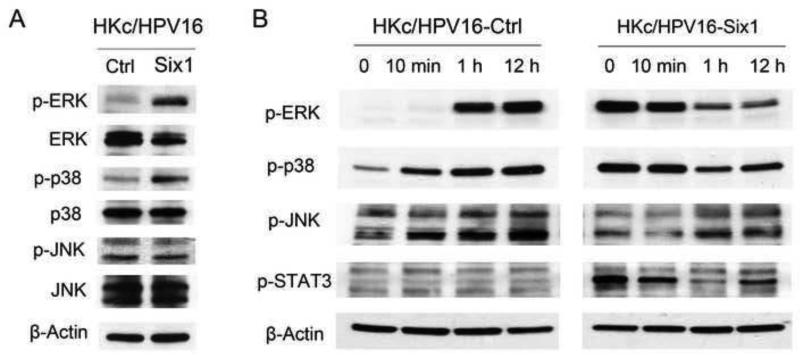
The Mitogen-Activated Protein Kinases (MAPKs) are central players in regulating keratinocyte differentiation (Eckert et al., 2002). MAPK signaling is one of the non-Smad TGF-β pathways (Zhang, 2009) and we have previously found that activation of MAPK is involved in Six1-induced EMT in HKc/DR (Xu et al., 2014). We compared MAPK activity in HKc/HPV16-Ctrl and HKc/HPV16-Six1 by conducting Western blots using phospho-specific p-ERK, p-p38 and p-JNK antibodies. As shown in Figure 7A, phosphorylation of ERK and p38, but not JNK, was dramatically increased in response to Six1 overexpression in HKc/HPV16. After treatment of HKc/HPV16-Ctrl with 1 mM Ca2+, ERK was phosphorylated within 1 h, and increased phosphorylation of p38 was detected as soon as 10 min after Ca2+ treatment. In contrast, in HKc/HPV16-Six1, the phosphorylation of ERK and p38 was not further increased but rather decreased within 1 h after high Ca2+ treatment (Fig. 7B). Signal transducer and activator of transcription 3 (STAT3) also plays an important role in differentiation, migration and apoptosis of keratinocytes (Saeki et al., 2012). The phosphorylation of STAT3 increased in HKc/HPV16-Six1 compared to HKc/HPV16-Ctrl (Fig. 7B). However, there is no STAT3 activation in HKc/HPV16-Ctrl following high Ca2+ treatment and the phosphorylation of STAT3 decreased in HKc/HPV16-Six1 following the addition of high Ca2+ medium (Fig. 7B).
Fig.7. Six1 overexpression in HKc/HPV16 activates MAPK signaling.
(A) Protein levels of p-ERK, ERK, p-p38, p38, p-JNK and JNK were determined by western blotting of cell lysates prepared from HKc/HPV16-Ctrl and HKc/HPV16-Six1. (B) Western blot of p-ERK, p-p38, p-JNK and p-STAT3 in HKc/HPV16-Ctrl and HKc/HPV16-Six1 that were cultured in medium containing 1 mM Ca2+ for the indicated times. β-actin was used as a loading control.
Discussion
There is evidence that the inappropriate expression of homeobox genes contributes to tumorigenesis and tumor progression (Abate-Shen, 2002). The Six1 homeoprotein is essential for embryonic development, and also contributes to cancer cell proliferation and survival. The overexpression of Six1 has been found in various cancers, and is associated with increased tumor progression and metastasis, and decreased survival (Christensen et al., 2008). Our previous work determined that SIX1 mRNA expression increased during in vitro progression of HPV16-immortalized HKc toward a differentiation resistant phenotype (HKc/DR) and that Six1 protein was overexpressed in cervical cancer tissues, which strongly suggests that Six1 might play an important role in the progression of premalignant cervical lesions initiated by HPV infection (Wan et al., 2008). In the current study, we focused on the role of Six1 at early stages of HPV16-mediated transformation. Interestingly, we found that Six1 overexpression in HKc/HPV16 induces resistance to calcium- and serum-induced differentiation, a hallmark of HKc/DR. Resistance to calcium and serum-induced differentiation in HKc/HPV16 overexpressing Six1 was associated with ERK and p38 MAPK activation.
MAPKs play a vital role in regulating keratinocyte differentiation (Eckert et al., 2002). For example: P38 MAPK functions to promote HKc differentiation by regulating the expression of involucrin, a marker of keratinocyte differentiation (Dashti, Efimova, and Eckert, 2001). High calcium induces a transient, peak-like activation of ERK in HKc, and calcium-induced differentiation can be blocked by MEK inhibition, which suggests an important role of the MEK/ERK pathway in the early stages of keratinocyte differentiation (Schmidt et al., 2000). We show that the level of phosphorylation of ERK and p38 increased in HKc/HPV16-Ctrl upon high calcium treatment. In contrast, the basal levels of p-ERK and p-p38 are very high in HKc/HPV16-Six1 and the phosphorylation levels of ERK and p38 actually decreased in HKc/HPV16-Six1 following treatment with high calcium. STAT3 also has been known to play an important role in keratinocyte differentiation. Constitutive activation of STAT3 in mouse epidermis results in partial loss of the keratinocyte differentiation markers K10 and filaggrin and enhances malignant progression (Chan et al., 2008). STAT3 knock-down sensitizes terminal differentiation of HKc at low-calcium condition (Saeki et al., 2012). Our study showed a significant activation of STAT3 in Six1-overexpressing HKc/HPV16. STAT3 overexpression in HKc/HPV16-Six1 may in fact delay differentiation even though these cells express high levels of p-ERK and p-p38.
TGF-β signaling plays a dual role in tumor progression, and dysregulation of TGF-β signaling is thought to be associated with progression of premalignant cervical lesions to cervical cancer (Baritaki et al., 2007; Noordhuis et al., 2011). TGF-β acts as a potent tumor suppressor since it strongly inhibits the proliferation of normal and early neoplastic cells. However, at late stages of tumorigenesis, tumors become resistant to TGF-β mediated growth inhibition and exploit TGF-β signaling to facilitate tumor metastasis and invasion (Meulmeester and Ten Dijke, 2011). Although HKc/HPV16 are initially as sensitive as normal HKc to the antiproliferative effects of TGF-β, HKc/HPV16 become increasingly resistant to the antiproliferative effects of TGF-β during in vitro progression (Mi et al., 2000). In fact, HKc/DR are completely resistant to the antiproliferative effects of TGF-β and exhibit decreased expression of TβRI (Hypes, Pirisi, and Creek, 2009; Mi et al., 2000). However, in HKc/DR the Smad pathway remains relatively intact, although TGF-β activation of Smad-dependent signaling is reduced by about 50% (Altomare et al., 2013; Kowli et al., 2013). Despite a partial loss of Smad-dependent signaling in HKc/DR, TGF-β treatment of HKc/DR results in modulation of the expression of genes associated with EMT (Kowli et al., 2013). In the present study we found that Six1 overexpression in HKc/HPV16 reduced Smad-dependent signaling in response to TGF-β1 to about the same extent (about 45%) as we previously reported in HKc/DR (Altomare et al., 2013; Kowli et al., 2013). It is possible that decreased Smad-dependent signaling may contribute to the enhanced proliferation we observed in HKc/HPV16-Six1 compared to HKc/HPV16-Ctrl. We previously reported that Six1 overexpression in HKc/DR decreased cell proliferation while promoting EMT and malignant conversion (Xu et al., 2014). The basis for the different growth response to Six1 overexpression in HKc/HPV16 and HKc/DR is likely to involve alterations in TGF-β signaling, but the molecular mechanisms of this alteration are not completely understood. For example, the sensitivity of HKc/HPV16-Six1 to the antiproliferative effects of TGF-β still remains to be determined. TGF-β is one of the primary serum factors responsible for inducing keratinocytes to undergo terminal differentiation (Bertolero et al., 1986). Thus, the decreased Smad signaling found in HKc/HPV16-Six1 likely plays some role in the resistance of these cells to serum-induced differentiation.
EMT allows immotile and polarized epithelial cells to acquire a motile, apolar and fibroblastoid phenotype, and is considered a critical step in mediating tumor progression, metastasis as well as cancer stem cell properties (Christiansen and Rajasekaran, 2006; Mani et al., 2008). TGF-β can induce EMT through both Smad-dependent and non-Smad signaling (Lamouille, Xu, and Derynck, 2014; Zhang, 2009). In this study we found increased expression of TβRII and TβRIII in Six1-overexpressing HKc/HPV16, but we did not find any increase in Smad-dependent signaling. After examining typical non-Smad TGF-β pathways, we showed a significant activation of ERK and p38 MAPK in response to Six1 overexpression in HKc/HPV16. Inhibition of ERK and p38 MAPK activity in HKc/HPV16-Six1 significantly decreases the expression of fibronectin (a mesenchymal marker) and the cells also lose their fibroblastic appearance (data not shown). These findings are consistent with our observation in HKc/DR, in which Six1 overexpression activates MAPK, and MAPK activation is critical for Six1-induced EMT (Xu et al., 2014).
We previously reported the cellular responses to Six1 overexpression at late stages of HPV16-mediated transformation (HKc/DR) (Xu et al., 2014) and in this study we report the cellular effects of Six1 over expression at the early stages of HPV16-mediated transformation (HKc/HPV16). When comparing cellular responses to Six1 overexpression in HKc/HPV16 to HKc/DR, many of the responses are similar, including; increased cell invasion and migration, induction of EMT, increases in TβRII and TβRIII, and activation of ERK and p38 MAPK (Xu et al., 2014). Inhibition of MAPK activation or TβRII-mediated signaling results in the reversal of Six1-induced EMT. These results suggest that Six1 promotes EMT through TβRII and MAPK signaling. However, HKc/HPV16 and HKc/DR also show some different responses to Six1 overexpression. For example, HKc/HPV16-Six1 are resistant to calcium-induced differentiation, which is a hallmark of HKc/DR, but are not tumorigenic (data not shown); while HKc/DR-Six1 are tumorigenic and form tumors in the flank of nude mice, indicating that other genetic alterations during HPV16-mediated transformation are also important for malignant conversion (Xu et al., 2014). Additionally, Six1 overexpression increases proliferation of HKc/HPV16 in vitro, but decrease proliferation in HKc/DR. Our previously published studies demonstrated that HKc/DR have decreased expression of TβRI compared to HKc/HPV16, which accompanies resistance to the antiproliferative effects of TGF-β (Mi et al., 2000). The different growth response to Six1 overexpression in HKc/HPV16 and HKc/DR may be explained by the different expression of TGF-β receptors and TGF-β signaling. Finally, in HKc/DR, p38 activation plays a dominant role in Six1-induced EMT; and in HKc/HPV16, both ERK and p38 contribute to the EMT associated with Six1 overexpression. This observation may be explained by the decreased phosphorylation of p38 in HKc/DR compared to HKc/HPV16 (data not shown), which makes the activation of p38 in HKc/DR exhibit a more robust response.
In summary, Six1 overexpression in HKc/HPV16 increases cell proliferation, invasion, migration, and EMT and induces resistance to calcium and serum-induced differentiation. Although TβRII and TβRIII levels are increased in HKc/HPV16-Six1, Smad-dependent TGF-β signaling is reduced, while MAPK is activated. Activation of MAPK in HKc/HPV16 overexpressing Six1 is associated with resistance to calcium-induced terminal differentiation, although the precise mechanism remains to be determined.
Supplementary Material
Supplemental Fig.1. Western blot for Six1 in HKc/HPV16-low passage, HKc/HPV16-high passage, HKc/GFI and HKc/DR (upper: D1 HKc/HPV16 line, lower: D5 HKc/HPV16 line). β-actin was used as a loading control.
Supplemental Fig.2. Western blot of p-PI3-kinase p85 (p-PI3K) and p-AKT in HKc/HPV16-Ctrl and HKc/HPV16-Six1 that were cultured in medium containing1 mM Ca2+ for the indicated times. β-actin was used as a loading control.
Highlights.
Six1 expression increases during HPV16-mediated transformation.
Six1 overexpression causes differentiation resistance in HPV16-immortalized cells.
Six1 overexpression in HPV16-immortalized keratinocytes activates MAPK.
Activation of MAPK promotes EMT and differentiation resistance.
Six1 overexpression reduces Smad-dependent TGF-β signaling.
Acknowledgements
This research was supported in part by grant 5P20MD001770 from the National Institute on Minority Health and Health Disparities (NIMHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMHD or the NIGMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2(10):777–85. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- Altomare D, Velidandla R, Pirisi L, Creek KE. Partial loss of Smad signaling during in vitro progression of HPV16-immortalized human keratinocytes. BMC Cancer. 2013;13:424. doi: 10.1186/1471-2407-13-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baritaki S, Sifakis S, Huerta-Yepez S, Neonakis IK, Soufla G, Bonavida B, Spandidos DA. Overexpression of VEGF and TGF-beta1 mRNA in Pap smears correlates with progression of cervical intraepithelial neoplasia to cancer: implication of YY1 in cervical tumorigenesis and HPV infection. Int J Oncol. 2007;31(1):69–79. [PubMed] [Google Scholar]
- Behbakht K, Qamar L, Aldridge CS, Coletta RD, Davidson SA, Thorburn A, Ford HL. Six1 overexpression in ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and is associated with poor survival. Cancer Res. 2007;67(7):3036–42. doi: 10.1158/0008-5472.CAN-06-3755. [DOI] [PubMed] [Google Scholar]
- Bertolero F, Kaighn ME, Camalier RF, Saffiotti U. Effects of serum and serum-derived factors on growth and differentiation of mouse keratinocytes. In Vitro Cell Dev Biol. 1986;22(7):423–8. doi: 10.1007/BF02623533. [DOI] [PubMed] [Google Scholar]
- Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983;81(1 Suppl):33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- Chan KS, Sano S, Kataoka K, Abel E, Carbajal S, Beltran L, Clifford J, Peavey M, Shen J, Digiovanni J. Forced expression of a constitutively active form of Stat3 in mouse epidermis enhances malignant progression of skin tumors induced by two-stage carcinogenesis. Oncogene. 2008;27(8):1087–94. doi: 10.1038/sj.onc.1210726. [DOI] [PubMed] [Google Scholar]
- Christensen KL, Patrick AN, McCoy EL, Ford HL. The six family of homeobox genes in development and cancer. Adv Cancer Res. 2008;101:93–126. doi: 10.1016/S0065-230X(08)00405-3. [DOI] [PubMed] [Google Scholar]
- Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66(17):8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- Coletta RD, Christensen KL, Micalizzi DS, Jedlicka P, Varella-Garcia M, Ford HL. Six1 overexpression in mammary cells induces genomic instability and is sufficient for malignant transformation. Cancer Res. 2008;68(7):2204–13. doi: 10.1158/0008-5472.CAN-07-3141. [DOI] [PubMed] [Google Scholar]
- Dai JL, Turnacioglu KK, Schutte M, Sugar AY, Kern SE. Dpc4 transcriptional activation and dysfunction in cancer cells. Cancer Res. 1998;58(20):4592–7. [PubMed] [Google Scholar]
- Dashti SR, Efimova T, Eckert RL. MEK6 regulates human involucrin gene expression via a p38alpha - and p38delta -dependent mechanism. J Biol Chem. 2001;276(29):27214–20. doi: 10.1074/jbc.M100465200. [DOI] [PubMed] [Google Scholar]
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS, de Ruiz PA, Lima MA, Guimera N, Clavero O, Alejo M, Llombart-Bosch A, Cheng-Yang C, Tatti SA, Kasamatsu E, Iljazovic E, Odida M, Prado R, Seoud M, Grce M, Usubutun A, Jain A, Suarez GA, Lombardi LE, Banjo A, Menendez C, Domingo EJ, Velasco J, Nessa A, Chichareon SC, Qiao YL, Lerma E, Garland SM, Sasagawa T, Ferrera A, Hammouda D, Mariani L, Pelayo A, Steiner I, Oliva E, Meijer CJ, Al-Jassar WF, Cruz E, Wright TC, Puras A, Llave CL, Tzardi M, Agorastos T, Garcia-Barriola V, Clavel C, Ordi J, Andujar M, Castellsague X, Sanchez GI, Nowakowski AM, Bornstein J, Munoz N, Bosch FX. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- Descargues P, Sil AK, Sano Y, Korchynskyi O, Han G, Owens P, Wang XJ, Karin M. IKKalpha is a critical coregulator ofa Smad4-independent TGFbeta-Smad2/3 signaling pathway that controls keratinocyte differentiation. Proc Natl Acad Sci USA. 2008;105(7):2487–92. doi: 10.1073/pnas.0712044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert RL, Efimova T, Dashti SR, Balasubramanian S, Deucher A, Crish JF, Sturniolo M, Bone F. Keratinocyte survival, differentiation, and death: many roads lead to mitogen-activated protein kinase. J Investig Dermatol Symp Proc. 2002;7(1):36–40. doi: 10.1046/j.1523-1747.2002.19634.x. [DOI] [PubMed] [Google Scholar]
- Ford HL, Landesman-Bollag E, Dacwag CS, Stukenberg PT, Pardee AB, Seldin DC. Cell cycle-regulated phosphorylation of the human SIX1 homeodomain protein. J Biol Chem. 2000;275(29):22245–54. doi: 10.1074/jbc.M002446200. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6(8):622–34. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Hypes MK, Pirisi L, Creek KE. Mechanisms of decreased expression of transforming growth factor-beta receptor type I at late stages of HPV16-mediated transformation. Cancer Lett. 2009;282(2):177–86. doi: 10.1016/j.canlet.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Kageyama R, Suzuki Y, Kawakami K. Six1 is indispensable for production of functional progenitor cells during olfactory epithelial development. Int J Dev Biol. 2010;54(10):1453–64. doi: 10.1387/ijdb.093041ki. [DOI] [PubMed] [Google Scholar]
- Kowli S, Velidandla R, Creek KE, Pirisi L. TGF-beta regulation of gene expression at early and late stages of HPV16-mediated transformation of human keratinocytes. Virology. 2013;447(1-2):63–73. doi: 10.1016/j.virol.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Hashimoto K, Hashiro M, Yoshimasa H, Yoshikawa K. Modulation of growth and differentiation in normal human keratinocytes by transforming growth factor-beta. J Cell Physiol. 1990;145(1):95–101. doi: 10.1002/jcp.1041450114. [DOI] [PubMed] [Google Scholar]
- Meulmeester E, Ten Dijke P. The dynamic roles of TGF-beta in cancer. J Pathol. 2011;223(2):205–18. doi: 10.1002/path.2785. [DOI] [PubMed] [Google Scholar]
- Mi Y, Borger DR, Fernandes PR, Pirisi L, Creek KE. Loss of transforming growth factor-beta (TGF-beta) receptortype I mediates TGF-beta resistance in human papillomavirus type 16-transformed human keratinocytes at late stages of in vitro progression. Virology. 2000;270(2):408–16. doi: 10.1006/viro.2000.0283. [DOI] [PubMed] [Google Scholar]
- Micalizzi DS, Christensen KL, Jedlicka P, Coletta RD, Baron AE, Harrell JC, Horwitz KB, Billheimer D, Heichman KA, Welm AL, Schiemann WP, Ford HL. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. J Clin Invest. 2009;119(9):2678–90. doi: 10.1172/JCI37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micalizzi DS, Wang CA, Farabaugh SM, Schiemann WP, Ford HL. Homeoprotein Sixl increases TGF-beta type I receptor and converts TGF-beta signaling from suppressive to supportive for tumor growth. Cancer Res. 2010;70(24):10371–80. doi: 10.1158/0008-5472.CAN-10-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C, Calautti E, Eckner R, Chin J, Tsai LH, Livingston DM, Dotto GP. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc Natl Acad Sci U S A. 1995;92(12):5451–5. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto GP. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 1996;10(23):3065–75. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Ng KT, Lee TK, Cheng Q, Wo JY, Sun CK, Guo DY, Lim ZX, Lo CM, Poon RT, Fan ST, Man K. Suppression of tumorigenesis and metastasis of hepatocellular carcinoma by shRNA interference targeting on homeoprotein Six1. Int J Cancer. 2010;127(4):859–72. doi: 10.1002/ijc.25105. [DOI] [PubMed] [Google Scholar]
- Noordhuis MG, Fehrmann RS, Wisman GB, Nijhuis ER, van Zanden JJ, Moerland PD, Ver Loren van Themaat E, Volders HH, Kok M, ten Hoor KA, Hollema H, de Vries EG, de Bock GH, van der Zee AG, Schuuring E. Involvement of the TGF-beta and beta-catenin pathways in pelvic lymph node metastasis in early-stage cervical cancer. Clin Cancer Res. 2011;17(6):1317–30. doi: 10.1158/1078-0432.CCR-10-2320. [DOI] [PubMed] [Google Scholar]
- Peralta-Zaragoza O, Bermudez-Morales VH, Perez-Plasencia C, Salazar-Leon J, Gomez-Ceron C, Madrid-Marina V. Targeted treatments for cervical cancer: a review. Onco Targets Ther. 2012;5:315–28. doi: 10.2147/OTT.S25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirisi L, Creek KE, Doniger J, DiPaolo JA. Continuous cell lines with altered growth and differentiation properties originate after transfection of human keratinocytes with human papillomavirustype 16 DNA. Carcinogenesis. 1988;9(9):1573–9. doi: 10.1093/carcin/9.9.1573. [DOI] [PubMed] [Google Scholar]
- Pirisi L, Yasumoto S, Feller M, Doniger J, DiPaolo JA. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987;61(4):1061–6. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadot E, Simcha I, Shtutman M, Ben-Ze'ev A, Geiger B. Inhibition of beta-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci USA. 1998;95(26):15339–44. doi: 10.1073/pnas.95.26.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki Y, Nagashima T, Kimura S, Okada-Hatakeyama M. An ErbB receptor-mediated AP-1 regulatory network is modulated by STAT3 and c-MYC during calcium-dependent keratinocyte differentiation. Exp Dermatol. 2012;21(4):293–8. doi: 10.1111/j.1600-0625.2012.01453.x. [DOI] [PubMed] [Google Scholar]
- Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS, Jr., Spitzer M, Moscicki AB, Franco EL, Stoler MH, Schiffman M, Castle PE, Myers ER. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–72. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Goebeler M, Posern G, Feller SM, Seitz CS, Brocker EB, Rapp UR, Ludwig S. Ras-independent activation of the Raf/MEK/ERK pathway upon calcium-induced differentiation of keratinocytes. J Biol Chem. 2000;275(52):41011–7. doi: 10.1074/jbc.M003716200. [DOI] [PubMed] [Google Scholar]
- Tan J, Zhang C, Qian J. Expression and significance of Six1 and Ezrin in cervical cancer tissue. Tumour Biol. 2011;32(6):1241–7. doi: 10.1007/s13277-011-0228-8. [DOI] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Wan F, Miao X, Quraishi I, Kennedy V, Creek KE, Pirisi L. Gene expression changes during HPV-mediated carcinogenesis: a comparison between an in vitro cell model and cervical cancer. Int J Cancer. 2008;123(1):32–40. doi: 10.1002/ijc.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282(12):8695–703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhang Y, Altomare D, Pena MM, Wan F, Pirisi L, Creek KE. Six1 promotes epithelial-mesenchymal transition and malignant conversion in human papillomavirus type 16-immortalized human keratinocytes. Carcinogenesis. 2014 doi: 10.1093/carcin/bgu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development. 2003;130(14):3085–94. doi: 10.1242/dev.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19(1):128–39. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130(17):3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XH, Liang PH, Guo JX, Zheng YR, Han J, Yu LL, Zhou YG, Li L. Expression and clinical implications of homeobox gene Six1 in cervical cancer cell lines and cervical epithelial tissues. Int J Gynecol Cancer. 2010;20(9):1587–92. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig.1. Western blot for Six1 in HKc/HPV16-low passage, HKc/HPV16-high passage, HKc/GFI and HKc/DR (upper: D1 HKc/HPV16 line, lower: D5 HKc/HPV16 line). β-actin was used as a loading control.
Supplemental Fig.2. Western blot of p-PI3-kinase p85 (p-PI3K) and p-AKT in HKc/HPV16-Ctrl and HKc/HPV16-Six1 that were cultured in medium containing1 mM Ca2+ for the indicated times. β-actin was used as a loading control.