Abstract
Phenotypical analysis of the lipid interacting residues in the closed state of the mechanosensitive channel of small conductance (MscS) from Escherichia coli (E. coli) has previously shown that these residues are critical for channel function. In the closed state, mutation of individual hydrophobic lipid lining residues to alanine, thus reducing the hydrophobicity, resulted in phenotypic changes that were observable using in vivo assays. Here, in an analogous set of experiments, we identify eleven residues in the first transmembrane domain of the open state of MscS that interact with the lipid bilayer. Each of these residues was mutated to alanine and leucine to modulate their hydrophobic interaction with the lipid tail-groups in the open state. The effects of these changes on channel function were analyzed using in vivo bacterial assays and patch clamp electrophysiology. Mutant channels were found to be functionally indistinguishable from wildtype MscS. Thus, mutation of open-state lipid interacting residues does not differentially stabilize or destabilize the open, closed, intermediate, or transition states of MscS. Based on these results and other data from the literature, we propose a new gating paradigm for MscS where MscS acts as a “Jack-In-The-Box” with the intrinsic bilayer lateral pressure holding the channel in the closed state. In this model, upon application of extrinsic tension the channel springs into the open state due to relief of the intrinsic lipid bilayer pressure.
Keywords: Mechanosensitive channel of small conductance (MscS), lipid interactions, bacterial ion channels, gating mechanism, Jack-In-The-Box
1. Introduction
The mechanosensitive channel of small conductance (MscS), from Escherichia coli (E. coli), gates in direct response to membrane tension [1–6]. Upon osmotic downshock, MscS is activated by tension in the membrane and opens to relieve excess turgor generated by the hypoosmotic shock [7, 8]. The response of MscS to membrane tension has been characterized by a variety of techniques and molecular interactions that alter channel gating have been identified through mutagenesis [2, 9–11].
E. coli MscS is an ideal system for studying tension transduction in mechanosensitive ion channels, since structural models exist for the open, closed, and desensitized state of this channel [1, 2, 10, 12–14]. X-ray crystal structures of MscS have been resolved for the desensitized and open states of the channel [10, 13, 14]. While there is not a closed state crystal structure of MscS, there are several models that have been developed based on experimentally obtained constraints [1, 2, 12]. Collectively, these structures make it possible to interpret functional data from mutated channels at the molecular level.
Analysis of the effects of specific MscS mutations has provided a powerful tool for understanding channel function in the context of a wealth of structural data. This has led to the identification of molecular interactions that play critical roles in gating transitions [2, 5, 9, 11, 15–17]. For example, the triad of residues, D62, R128, and R131, are predicted to work, in concert, during the transition from the closed state of the channel to the open state with one salt-bridge between D62 and R128 stabilizing the closed state and another between D62 and R131 stabilizing the open state [9]. Mutational analysis has also been used to understand the movement of the transmembrane domains away from the central gating axis during the transition from the closed state to the open state. In this case, interactions between I37 and L86, and A51and F68 are thought to stabilize this transition [17]. Furthermore, mutations at the interface of third transmembrane domain and the β-domain have been shown to inhibit wildtype MscS function [15]. Hydrophobic substitutions at V65 and N167 promote the desensitization of MscS under tension.
In the closed state of MscS, seven residues (L35, I39, L42, I43, N50, I78, L82) have been identified as critical for tension sensing [2]. These residues form hydrophobic interactions with the lipid tail groups, thus stabilizing the closed state of the channel. The seven residues are clustered near the periplasmic face of the channel in the first and second transmembrane domains and are analogous to hydrophobic residues that stabilize the closed state of the mechanosensitive channel of large conductance (MscL) [18]. These residues were identified through molecular dynamic studies of the closed state EPR based structure [12] embedded within a lipid bilayer and then the ability of each residue to interact with the lipid bilayer was determined [2]. To access the significance of the energetic contributions of these lipid bilayer interacting residues to channel function, individual hydrophobic residues were mutated to alanine to reduce the hydrophobic contact with the lipids. Osmotically sensitive bacterial expressing MscS variants containing single alanine point mutations resulted in partial loss of function phenotypes, presumably due to the reduction of hydrophobic interactions with the lipid bilayer. Moreover, while these residues interact with the lipid bilayer in the model for the closed state of the channel, with the exception of I43, they are occluded from the lipid tails in both the open and desensitized states of the channel (Figure 1). This work and similar studies on MscL suggest that the hydrophobic interactions between membrane proteins and lipids are not “flexible” and hydrophobic contact area is critical for membrane coupling [18–20]. Additionally, quantitative models of lipid-protein interactions have suggested the importance of lipid-protein interactions to the free energy of gating [21–24].
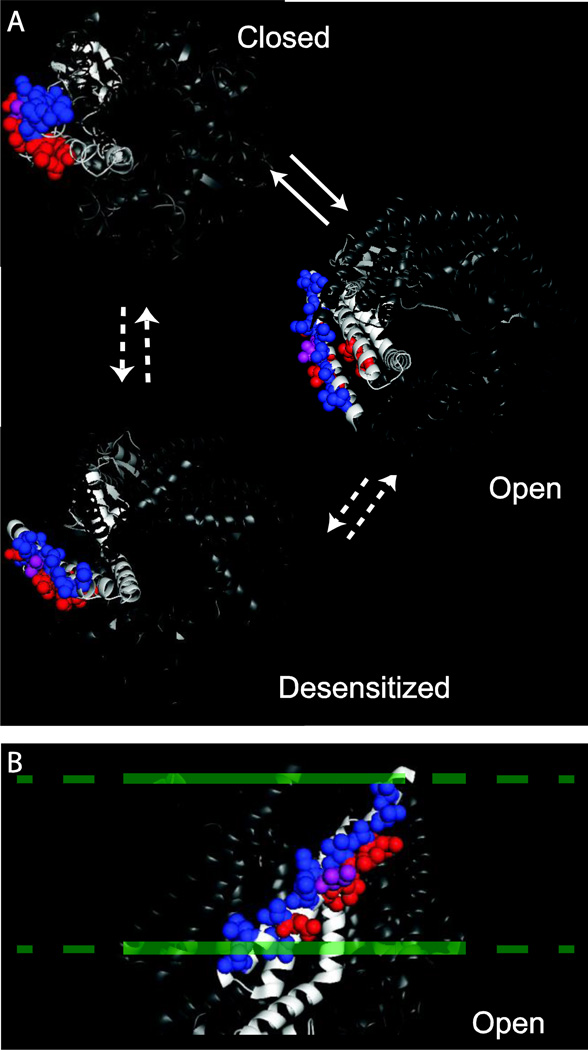
Figure 1.
Lipid lining amino acids in MscS. A) The positions of the previously identified closed state lipid interacting residues (red, L35, I39, L42, N50, I78, L82) and the newly identified open state lipid lining residues are shown (blue, V29, V32, A36, V40, I44, M47, A51, N53, L55, S58) on the closed [2], open (2VV5, [10]), and desensitized (2OAU, [13, 14]) structures of MscS. I43, which is lipid exposed in both the open and closed state of MscS, is shown in purple. B) A side view of the open state crystal structure (2VV5, [10]) showing the lipid interacting residues. The approximate location of the lipid bilayer is shown as a dashed line.
Single point mutations to residues that interact with the lipids in the closed state of MscS show no additional changes to the phenotype when adjacent mutations are combined [2]. Similar behavior has also been observed in large mutational screens of MscL, where a phenotype can be traced back to a single mutation when the phenotype is first observed in a group of mutations [19, 25]. This suggests that a single point mutation is sufficient to enact a phenotype that can be observed in in vivo assays.
Given that we were able to identify closed state stabilizing residues, we now address the question of whether similar interactions are energetically important for the open state of the channel. Thus, an analogous set of experiments have been carried out for the open state of MscS, by mutating the residues presented to the lipids in this state. Lipid interacting residues have been mutated to both alanine and leucine. These mutations decrease or increase the hydrophobic contact between MscS and the lipid bilayer in the open state. If these interactions are energetically important, one would expect them to altering the amount of time the channel resides in the open state or the ability of the channel to access a fully open state. These changes in hydrophobic contact with the lipid bilayer should not massively alter channel structure or the conformational transition between states. As a result, these mutations provide for a clear and interpretable analysis of the role hydrophobic contacts play in channel gating and tension transduction.
Both the open and closed states of an ion channel reflect thermodynamic minima, with the closed state of the channel typically being more stable than the open state in the resting configuration. Application of a stimulus provides the kinetic energy for a channel to transition over an activation barrier from the closed to the open state. For MscS, lipid interactions have been shown to play a critical role in the closed state of the channel [2]; as a result, one might expect similar interactions, with different residues, to be important for open state stability. In this model of gating, the thermodynamic difference between lipid-protein interactions in the open and closed states of MscS would at least partially account for the relative energetics of the open and closed states and, thus, channel gating. An alternative model for channel gating is that lipid interactions are only important for closed state stability and do not impact the open state of the channel. In this model, the closed state of MscS would be stabilized by intrinsic bilayer pressure, thus having important lipid interactions in the closed state, and upon application of tension, relief of these interactions would give rise to channel opening. In other words, MscS would function as a spring-loaded channel that is compressed by intrinsic bilayer pressure, like a Jack-In-The-Box. Application of extrinsic tension would then result in relief of the intrinsic lipid bilayer pressure, causing the channel to spring open. To differentiate between these two gating models, we have identified the lipid lining residues in the open state of the channel and made mutations that would alter any important hydrophobic interactions to determine if lipid interactions are critical for open state stability.
2. Methods
2.1 Strains and Plasmids
The E. coli strain, MJF465 (MscS, MscL, MscK null), was used for osmotic downshock assays and expression experiments [6, 26]. Patch clamp electrophysiology was conducted in MJF429 (MscS and MscK null) [6]. Mutational cloning was conducting using the Top10F` E. coli strain (Invitrogen, Carlsbad, CA). All mutants were created in the pB10b [25, 27, 28] vector with a C-terminal six-His tag and a LacUV5 promoter.
2.2 Site-Directed Mutagenesis
Residues predicted to be involved in protein-lipid interactions were mutated to alanine or leucine using Megaprimer mutagenesis [29]. All mutations in MscS were cloned into the pB10b vector [27]. For Megaprimer mutagenesis, forward and reverse primers located in the vector were used as the exterior primers and primers for mutagenesis were designed using Stratagene’s QuikChange Primer design tool. Primer sequences are given in Table S1 in the Supporting Material. Mutations were verified by enzymatic digestion and sequences confirmed using automated sequencing (Big Dye v3.1, Applied Biosystems, Carlsbad, CA).
2.3 Loss of Function Analysis
Downshock experiments were conducted as previously described [2, 30, 31]. A single colony was used to inoculate an overnight culture in Luria-Bertani Broth (LB Broth, BD Biosciences, San Jose, CA) supplemented with ampicillin (100µg/mL), the overnight culture was subsequently used to inoculate (1:20) a culture in LB Broth with 250 mM NaCl and ampicillin. The resulting culture was grown to an OD600 of approximately 0.5 and induced with 0.1 mM isopropyl-beta-D-thiogalactopyranoside (IPTG) for 30 min. Following 30 min of induction, the culture was diluted (1:40) into 1:1 LB Broth and deionized water or isotonically into LB Broth with 250 mM NaCl, and allowed to recover for 30 m in a shaking incubator. After hypoosmotic downshock or isotonic dilution, bacterial cultures were serially diluted and plated on LB plates supplemented with ampicillin (100µg/mL). Plates containing between 25 and 250 colonies were used to determine the colony forming units (CFU) per millimeter of media. Percent recovery was defined as the CFU of the downshocked culture divided by the CFU of the isotonic dilution. Six trials for each mutation were conducted.
2.4 Steady State Analysis
A single colony was used to inoculate an overnight culture in LB Broth supplemented with ampicillin (100µg/mL), the overnight culture was subsequently used to inoculate (1:25) a culture in LB Broth with ampicillin. The resulting culture was grown to an OD600 of approximately 0.5 and induced with 0.1 mM IPTG for 8 hours. After induction, the OD600 was measured. Four trials for each mutation were carried out.
2.5 Gain of Function Analysis
Gain of function analysis was carried out as previously described [32]. A single colony was used to inoculate an overnight culture in LB Broth supplemented with ampicillin (100µg/mL), the overnight culture was subsequently used to inoculate (1:75) a culture in LB Broth with ampicillin. The resulting culture was grown to an OD600 between 0.5 and 0.8 and diluted to an OD600 of 0.2±0.02. This culture was serially diluted into prewarmed LB with ampicillin, 5µL of the 10−2, 10−3, 10−4, 10−5, 10−6, and 10−7 dilutions were plated onto LB agar plates supplemented with ampicillin and LB agar plates supplemented with ampicillin and IPTG to a final concentration of 1mM. The resulting plates were scored after 20 h of growth by giving a score of one for each concentration containing growth (maximum score of 6). Four trials for each mutation were carried out.
2.6 Bacterial Expression Analysis
To verify bacterial expression of the MscS mutants, cultures were grown as described above for the loss of function analysis and pelleted for 15 min at 16,000 × g after 30 min of induction. The supernatant was removed and the pellets resuspended in a buffer containing 50 mM Tris, 75 mM NaCl, 0.1% Fos-Choline-14 (Affymetrix, Cleveland, OH) at pH 7.5, and protease complete inhibitor (Roche, Basel, Switzerland) at 10µL of buffer per 10 mg of bacteria. Samples were detergent-solubilized using a probe sonicator for three cycles of 15 s on and 45 s off. After lysis and solubilization, insoluble material was removed by pelleting for 30 m at 16,000 × g. The supernatant was combined with sodium dodecyl sulfate polyacrylamide-gel electrophoresis (SDS-PAGE) loading dye, and the samples were boiled for 5 m. Samples were then run on a 12.5% poly-acrylamide gel using a TRIS-glycine running buffer. Equivalent volumes of the supernatant were loaded to allow for comparisons of protein expression levels. Proteins were transferred to a 0.2 µm nitrocellulose membrane using a semidry transfer system and Cashini’s Buffer (0.1 M Tris, 0.02% SDS, 0.2 M glycine, 5% methanol). The protein expression of the C-terminally 6-His-tagged MscS mutants was probed using a primary mouse-anti-His monoclonal IgG (Covance, Emeryville, CA) and a secondary HRP-conjugated goat anti-mouse antibody (Jackson ImunoResearch Laboratories, West Grove, PA).
2.7 Spheroplast Preparation
E. coli spheroplasts of MJF429 cells containing the indicated pB10b constructs were prepared as previously described [33]. Briefly, cells were grown in LB containing ampicillin and 0.06 mg/mL Cephalexin at 37°C with shaking until ‘snakes’ were approximately 50–150 µm. Cells were induced with 1mM IPTG for 5 minutes and then harvested at 2500 × g. Pellets were resuspended in 2.5 mL 0.8 M sucrose prior to sequential addition of 125µL 1 M Tris pH 8.0, 120 µL 5 mg/mL Lysozyme, 30 µL 5 mg/mL DNase, and 150 µL 125 mM EDTA at pH 7.8. Lysozyme digestion proceeded for 5 minutes prior to the addition of 1 mL stop solution (0.67 M Sucrose, 19.4 mM MgCl2, 9.7 mM Tris at pH 8.0). The reaction mixture was layered over 7 mL chilled dilution solution (0.8 M Sucrose, 10 mM MgCl2, 10 mM Tris at pH 8.0) in test tubes. Spheroplasts were pelleted by spinning at 1600 × g for 2 minutes at 4°C and the pellets were gently resuspended in the dilution solution and stored in aliquots at −20°C.
2.8 Patch Clamp Electrophysiology
Excised, inside out patches from E. coli giant spheroplasts were studied at room temperature as previously described [34, 35]. Patch buffer contained 200 mM KCl, 90 mM MgCl2, 10 mM CaCl2, 5 mM HEPES at pH 7.5. Data was acquired using an AxoPatch 200B amplifier in conjunction with Clampex 10.3 (Molecular Devices) at −20mV and a sampling rate of 30303 Hz, with a 5 kHz lowpass filter. The pressure applied to the patch throughout the experiment was monitored with a piezoelectric pressure transducer (WPI). Measurements were analyzed using Clampfit 10.3 (Molecular Devices). E. coli mechanosensitive channel of large conductance (MscL) was used as the internal standard for determining the pressure thresholds, as previously described [25, 27, 36]. Pressure thresholds were obtained by dividing the pressure at which the second MscS opens by the pressure at which the first MscL opens (PS/PL) [37]. PS/PL ratios were calculated from at least six patches from a minimum of two independent spheroplast preparations for each mutation.
3. Results and Discussion
3.1 Identification of Lipid Interactions
To determine the lipid lining residues in the open state of MscS, the open state crystal structure of MscS (2VV5) [10] was embedded into a palmitoyl oleoyl phosphatidylethanolamine (POPE) lipid bilayer as previously described [2]. Residues predicted to have significant lipid interactions with the lipid bilayer were identified by assessing the ability of an individual side chain to interact with the lipid tail groups. To identify these residues, using the embedded 2VV5 structure (Figure S1), the ability of each residue on the outer surface of the protein to interact with the lipid tail groups of the bilayer was determined by visual inspection. Each residue on the outer face of the protein was selected, as a space-filling model, and residues that were within close proximity to the hydrophobic lipid tails (Van der Waals distance) were identified as lipid-lining residues. While we previously used energetic constraints form molecular dynamics simulations to identify lipid-lining residues in the closed state of the channel (2), the results of visual inspection for the closed state of the channel are consistent with the results obtained using energetic constrains. Moreover, the use of molecular dynamic simulations to identify lipid-lining residues in the open state of MscS would suffer from potential simulation artifacts due to the need to apply excessive tension or significant energetic restraints to the channel in order to maintain the open state conformation. From our analysis we determined that lipid-lining residues are V29, V32, A36, V40, I43, I44, M47, A51, N53, L55, and S58 (Figure 1B and S1). These residues span the entire outer face of the first transmembrane domain from the periplasm to the cytoplasm. With the exception of I43, the predicted open state lipid interacting residues do not interact with the lipids in the closed state [2] and are predicted to be occluded from the hydrophobic tails in the desensitized state (Figure 1). This distribution of residues sharply contrasts with the lipid lining residues in the closed state of the channel, where the lipid-interacting residues were found clustered toward the periplasmic face of the channel in both the first and second transmembrane domains [2]. In the open state of the channel, all of the residues in the second transmembrane domain are involved in protein-protein interactions and are excluded from the lipid bilayer. Additionally, the predicted open state lipid interacting residues do not interact with the lipids in the closed state [2] and are structurally predicted to be occluded from the hydrophobic tails in the desensitized state.
While the majority of the residues identified as interacting with the lipid bilayer in the closed state of the channel were large hydrophobic amino acids (leucine or isoleucine), the lipid lining residues in the open state are a mixture of large hydrophobic residues (leucine, isoleucine, and methionine) and small hydrophobic amino acids (valine and alanine). This mixture of small and large hydrophobic amino acids fits a gating model in which the thermodynamic interchange of the open and closed states of MscS is driven by an equilibrium of hydrophobic interactions between lipid lining residues and the lipid tail groups of the bilayer. The hydrophobic stabilization resulting from a mixture of small and large hydrophobic amino acids would be smaller than the hydrophobic stabilization resulting from only larger hydrophobic residues. This in turn would gives rise to stronger protein-lipid interactions in the closed state of the channel when compared to the open state. Thus, a thermodynamic interchange between the protein and the hydrophobic core of the lipid bilayer is set up in which the closed state of the channel is more stable than the open state. Since in vivo MscS is found in its closed state, except during application of hypoosmotic stress, the closed state must be lower in energy than the open state, which fits with the observation of more significant hydrophobic interaction in the closed state of the channel compared to the open state of the channel. The mixture of small and large hydrophobic amino acids would have a smaller energetic contribution than the larger hydrophobic residues that stabilize the closed state of the channel. As required by such a model, the protein-lipid interactions would be stronger in the closed state than in the open state, since the closed state is more stable. We have previously demonstrated that the stability of the closed state is achieved by interactions of large hydrophobic residues on the surface of the channel with the lipid bilayer [2]. While the predicted lipid interactions for the open state would suggest that the closed state would be lower in energy than the open state, the presence of some large hydrophobic lipid-interacting amino acids in the open state would provide the needed stability for the open state of the channel.
3.2 Analysis of Lipid Interactions
To determine the role of open state lipid lining residues on channel function, each residue was mutated to leucine and alanine (unless the residues were leucine or alanine in the wildtype channel). Based on previous mutagenic analyses, we anticipated that if lipid interactions were important for stabilizing the open state of the channel, then altered function would be observed for the mutated channels. Since for bacterial mechanosensitive channels, single point mutations are typically responsible for altered channel function [19, 25]. If a careful balance of hydrophobic lipid interactions is critical for channel gating, then increasing the hydrophobicity of the lipid lining residues in the open state should stabilize the open state of the channel. Conversely, decreasing the hydrophobicity of the lipid lining residues in the open state should destabilize the open state of the channel. However, if hydrophobic interactions are not important in the open state of the channel, and instead channel gating is driven by a reduction in intrinsic bilayer tension, then mutation of putative open state lipid interacting residues should yield channels that are functionally analogous to wildtype MscS.
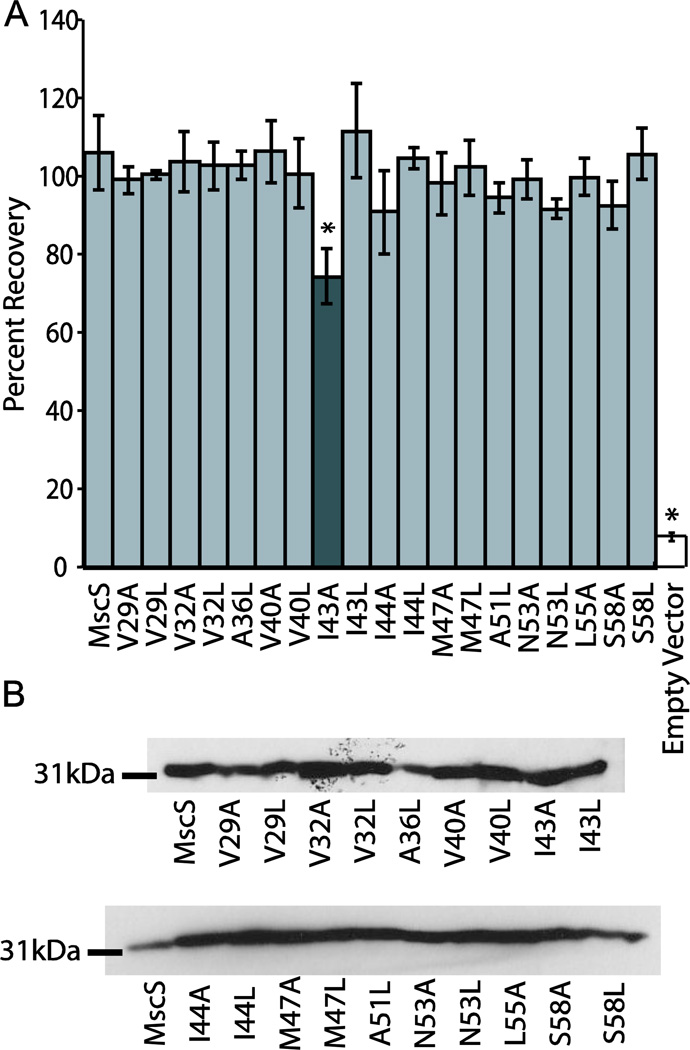
Osmotic downshock analysis of the mutant channels in an osmotically sensitive strain of E. coli (MscL, MscS, and MscK null; MJF465) was used to assay for changes in bacterial survival under osmotic stress. Previous studies have shown that deletion of MscL, MscS, and the potassium efflux system (MscK or KefA) results in an osmotically sensitive strain of E. coli that exhibits reduced survival upon osmotic downshock [6]. Reintroduction of the MscS gene on a plasmid under the control of the LacUV5 promoter allows for the production of MscS upon induction with IPTG. In the case of MscS, the resulting bacteria display an analogous phenotype in downshock assays to native E. coli. Mutations to MscS that also rescue the deletion strain of E. coli are considered functionally wildtype in this assay, while mutations that either fail to rescue the deletion strain of E. coli or only partially rescue it are for historical reasons termed functionally “loss of function” (LOF) or functionally partial LOF, respectively [38–40]. For each mutated channel, six trials of the osmotic downshock assay were conducted and the percent recovery values were compared using a Student’s T-test (Figure 2A). The function of most of the mutated proteins was not statistically different from wildtype MscS, with only I43A being functionally a partial LOF because it significantly differed from both wildtype MscS and vector only controls (Table S2). This is not surprising as I43 also interacts with the lipid bilayer in the closed state of the channel and has previously been shown to be functionally a partial LOF [2]. Additionally, all MscS mutants expressed at levels comparable to wildtype MscS (Figure 2B).
Figure 2.
Loss of function analysis and Western blots of mutated MscS channels. A) Results from osmotic downshock analysis of wildtype MscS and MscS harboring mutations of lipid lining residues mutated to either alanine or leucine. Error bars represent the standard error of the mean for 6 independent trials. The dark gray bar indicates the construct that is statistically different from wild-type MscS as determined using a Student’s T-test (* = p < 0.05). B) Western blot analysis for wildtype and mutated MscS proteins under conditions identical to those used for the osmotic downshock assay.
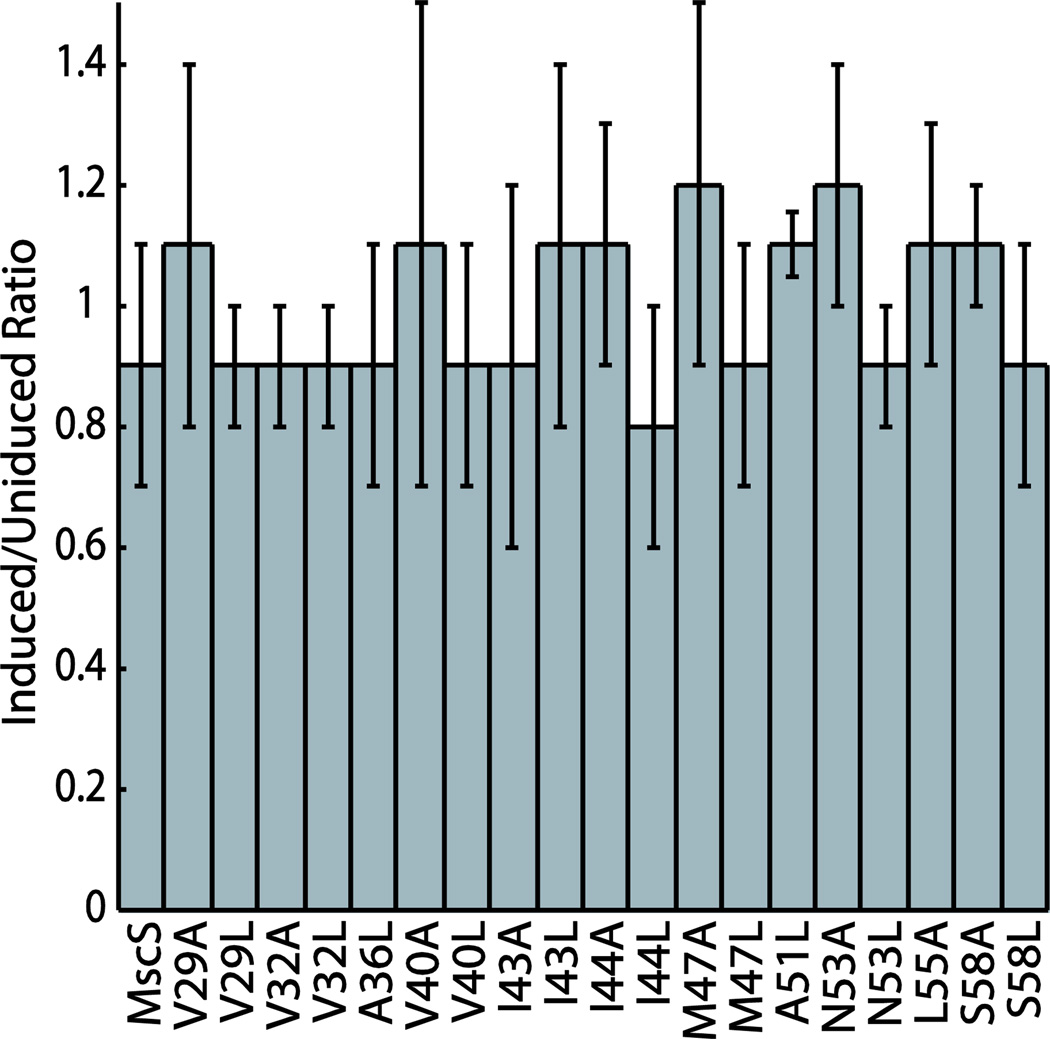
MscS mutations that produce “leaky” channels upon reintroduction of the gene on an inducible plasmid have been shown to reduce the bacterial growth rate and are known as functionally “gain of function” (GOF) mutations [25]. To determine if our mutations to MscS produced channels that were functionally GOF, two different growth based assays were carried out for each mutation: steady state optical density analysis [2, 41, 42] and a plate-based gain of function assay [39]. None of the data derived from the mutated MscS channels were found to be statistically different from wildtype MscS controls in the steady state optical density measurements (Figure S2). To further confirm that these mutated proteins were functionally wildtype, a plate based assay was conducted comparing bacteria grown on normal LB plates with bacteria grown on plates supplemented with IPTG. Serial dilutions of bacterial cultures were plated onto the different growth media and plates were scored by counting the number of dilutions that showed bacterial growth. The ratio of the induced and uninduced scores was determined for wildtype MscS and each mutant (Figure 3, Figure S2). All of the mutated proteins were found to be functionally indistinguishable from wildtype MscS using a Student’s T-test.
Figure 3.
Gain of function analysis of MscS mutations. Plates were scored by counting the number of dilutions with bacterial growth. The ratio of induced to uninduced was determined by dividing the induced score by the uninduced score. Representative plates for each mutated protein can be found in Figure S2. Error bars reflect the standard deviation of four independent trials.
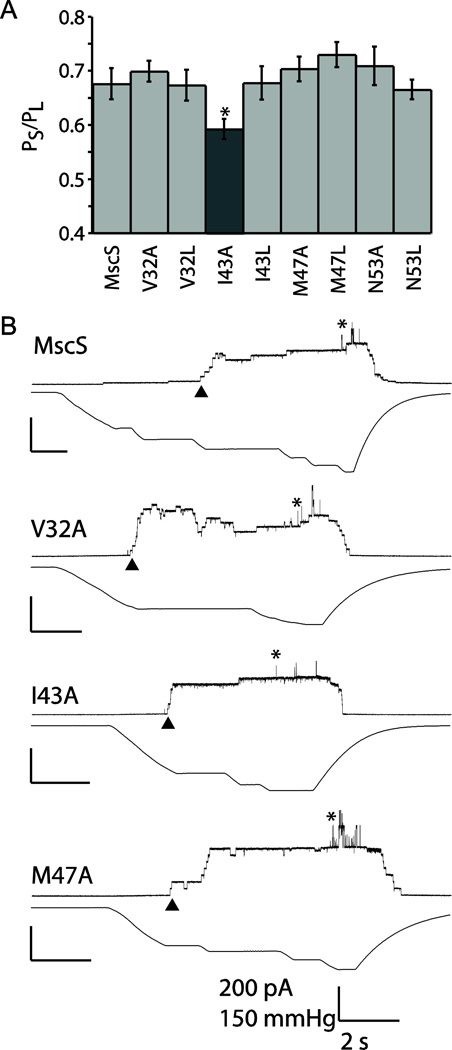
Patch clamp electrophysiology was carried out on a subset of the mutated channels to confirm that they displayed similar gating characteristics to wildtype MscS. Non-adjacent mutations that spanned the entire face of the lipid exposed helix were chosen to provide full coverage of the open state lipid-interacting residues. Gating thresholds relative to the native MscL were determined for each mutation using at least six patches from a minimum of two independent spheroplast preparations. The thresholds of V32A, V32L, I43L, M47A, M47L, N53A, and N53L were indistinguishable from wildtype MscS, and, as anticipated, an altered phenotype was observed for I43A (Figure 4). This mutation would be historically considered partial GOF based its electrophysiology, since it exhibits a reduced gating threshold relative to wildtype MscS. However, it exhibits partial LOF behavior in osmotic downshock assays and is functionally wildtype in growth assay. The apparent disconnect between the electrophysiology and the in vivo assay is unclear, but could be due to a number of factors including very subtle differences in channel activity that are difficult to quantify or differences in the membrane potential applied in the patch (-21mV) and in vivo (circa −165mv, which cannot be applied to the patch with the required gating tension without losing the patch integrity) [43–45].
Figure 4.
Patch clamp analysis of wildtype and mutated MscS channels at +20 mV. A) Pressure threshold values for a selection of mutants. Error bars represent the standard error of the mean for 6 patches from a minimum of two independent preps. The dark gray bar indicates the construct that is statistically different from wild-type MscS as determined using a Student’s T-test (* = p < 0.05). B) Representative traces are shown for wildtype MscS and three additional mutants, showing wildtype behavior. For simplicity in patch traces channel openings are shown as upward inflections, MscS openings are indicated by ▲ and MscL openings are indicated with *. Scale bars represent 2 seconds on the X-axis and 200 pA and 150 mmHg on the Y-axis.
To further classify the mutations electrophysiologically, single channel conductances were measured. None of the mutants had a single channel conductance or conductance ratio (MscL/MscS) that was outside of the published values for wildtype (Table 1) [6]. The similarity of the single channel conductances suggests that the pore shape and size of the mutant channels are not measurably different. The single channel electrophysiology confirms that with the exception of I43A, which is presented to the lipids in both the closed and open states, there is no difference from the wildtype channel function. No significant changes in channels kinetics were observed for any of the mutants.
Table 1.
Single channel conductance for MscS mutants and MscL/MscS conductance ratio. Unitary conductance was measured using single channel patch-clamp electrophysiology on spheroplasts at +20 mV under symmetric conditions, error is standard deviation.
| Conductance (pS) | MscL/MscS Conductance Ratio (pS) |
Number of Patches |
|
|---|---|---|---|
| MscS | 1.5 ± 0.3 | 2.8 ± 0.3 | 6 |
| V32A | 1.7 ± 0.2 | 3.0 ± 0.6 | 6 |
| V32L | 1.7 ± 0.2 | 2.7 ± 0.5 | 6 |
| I43A | 1.6 ± 0.5 | 2.7 ± 0.4 | 6 |
| I43L | 1.2 ± 0.1 | 3.3 ± 0.2 | 6 |
| M47A | 1.9 ± 0.3 | 3.1 ± 0.4 | 6 |
| M47L | 1.7 ± 0.2 | 3.1 ± 0.2 | 6 |
| N53A | 1.6 ± 0.2 | 3.1 ± 0.6 | 6 |
| N53L | 1.6 ± 0.3 | 3.2 ± 0.5 | 6 |
Taken together, the results of the in vivo analysis and the patch clamp electrophysiology indicate that residues that are lipid exposed only in the open state of MscS can be readily mutated without functional alteration. This suggests that these mutations do not differentially stabilize or destabilize MscS in the closed state, open state, any intermediate states, or any transition states, since no phenotypic alterations are observed. Therefore open state lipid-interactions are not critical for open state stabilization and, thus, this rules out a gating mechanism that is driven by an equilibrium of molecular interactions between the lipid-lining residues in the open and closed sates, and the lipid tail groups of the bilayer. Instead, gating in MscS appears to be driven by relief of intrinsic lipid bilayer pressure, which is a mechanism that does not require energetically significant lipid interactions in the open state of the channel.
3.3 The Jack-In-The-Box Model of MscS Gating
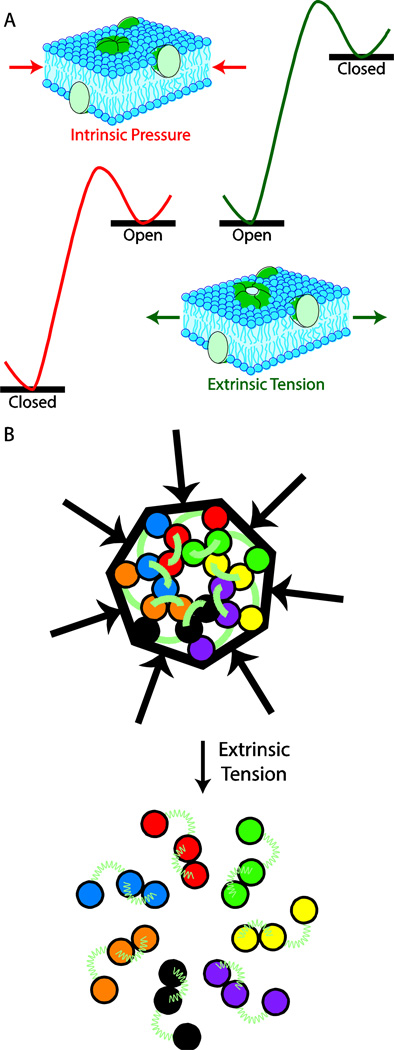
The data suggest a model in which intrinsic pressure from the lipid bilayer, stabilizes the closed state of MscS by pushing in on the channel. As a result, under normal conditions the closed state of MscS lies energetically below the open state of the channel. Upon application of extrinsic tension to the membrane, the intrinsic lipid bilayer pressure is relieved resulting in reversal of the energetics of the open and closed states (Figure 5A). The relief of intrinsic tension upon application of extrinsic tension is a result of the force vectors, relative to the channel, being in opposite direction for intrinsic and extrinsic tension. This leads to a Jack-In-The-Box type gating mechanism for MscS, where the intrinsic lipid bilayer pressure serves as the box that compresses the channel. Upon relief of this strain, the channel springs open allowing ions to pass (Figure 5B). A Jack-In-The-Box model of channel gating is consistent with quantitative mathematical models that have described protein free energy changes due to changes in bilayer properties [21–24].
Figure 5.
The Jack-In-A-Box model of MscS gating. A) Differences in the thermodynamic gating profile for MscS in the presence of intrinsic membrane pressure and extrinsic membrane tension. B) Schematic representation of the Jack-In-A-Box model of MscS gating with helix positions based on the experimentally derived closed and open state structures of MscS [2, 10].
This model is supported by observed structural differences in the open state and closed states of MscS. In the closed state of the channel, the transmembrane helices are tightly packed into a small footprint. However, upon transition to the open state, the helices expand and become further apart. Figure 5B shows the changes in helical packing of the transmembrane domains along the top face of the channel, which occur upon gating. The relative positions of the transmembrane helices were derived from the open state crystal structure and our closed state model [2, 10]. It should be noted, that the exact helix positions vary depending on which closed state model is used and some have argued that first and second transmembrane domains move as a unit [46]. While these domains do not appear to move as a unit in Figure 5, this lack of concerted motion is in part because of reorientations of the transmembrane helices in the open state structure relative to the membrane normal.
While crystal structures have been obtained for both the open and desensitized states of the channel, a closed state crystal structure has thus far been elusive. This may be because removal of MscS from the lipid bilayer into detergent micelles relieves the intrinsic lipid pressure resulting in stabilization of either the open state or desensitized state of the channel. The original MscS crystal structure is a snap shot of MscS in an inactive or desensitized state [14]. To obtain the initial open state structure an A106V mutant of MscS that exhibited a gating threshold greater than wildtype MscS was used [10]. The crystallization of a mutant that gates with a higher gating threshold than wildtype MscS into an open state structure, seems counterintuitive upon first glance. However, it is possible that this mutation simply reduced the probability of the channel to enter the desensitized state upon insertion of the channel into detergent micelles. If the mutation prevents desensitization, the “Jack-In-The-Box” gating model would predict that this mutation should give rise to an open state structure, which is the observed structure for this mutant. Our model is further supported by a recent report of wildtype MscS crystalizing in the open state under different crystallization conditions than employed for the initial structure [47]. Pliotas and co-workers have determined two unique crystal structures of wildtype MscS in detergent micelles that are consistent with the previously published open state structure of an MscS mutant.
When the gating mechanism of the MscL and MscS were compared significant differences were observed, which further suggest that the two channels may function by different mechanisms [48–50]. Recently Martinac and co-workers demonstrated that addition of cholesterol to lipid bilayers greatly alters the activation threshold for MscS, but does not affect MscL activation [51]. These data fit within the Jack-In-The-Box gating model because introduction of cholesterol significantly alters the intrinsic lipid bilayer pressure [52].
4. Conclusions
Our analyses indicate that open-state lipid interactions are not critical for MscS channel gating. Mutation of open state lipid lining residues to residues that would enhance or reduce these interactions gave functionally wildtype channels. The complete lack of phenotypic changes upon extensive mutagenesis is rare for bacterial mechanosensitive channels and similar mutations to lipid interacting residues in the closed state of MscS gave rise to partial loss of function phenotypes. This has led us to propose a “Jack-In-The-Box” model of gating for MscS, in which intrinsic lipid bilayer pressure holds the channel in the closed state. Upon relief of the intrinsic bilayer pressure by application of opposing extrinsic tension, MscS springs into the open state.
Supplementary Material
Highlights.
The open state of MscS is not stabilized by lipid interactions.
MscS opens in response to a relief of intrinsic lipid bilayer pressure.
MscS functions as a Jack-In-The-Box.
MscS and MscL exhibit contrasting gating mechanisms.
Acknowledgements
The authors thank Professor Donald E. Elmore (Wellesley College) for assistance in creating the bilayer embedded MscS open state structure and Professor Ian Booth (University of Aberdeen) for providing the MJF429 and MJF465 bacterial strains. This work was supported in part by Washington University in St. Louis (J.A.M.), Grant I-1420 of the Welch Foundation (P.B.), Grant RP100146 from the Cancer Prevention & Research Institute of Texas (CPRIT; http://www.cprit.state.tx.us/) (P.B.), and Grant GM061028 from the National Institutes of Health (P.B.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding organizations.
Abbreviations
- MscS
Mechanosensitive channel of small conductance
- MscL
Mechanosensitive channel of large conductance
- GOF
Gain of function
- LOF
Loss of function
- E. coli
Escherichia coli
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anishkin A, Akitake B, Sukharev S. Characterization of the resting MscS: modeling and analysis of the closed bacterial mechanosensitive channel of small conductance. Biophysical journal. 2008;94:1252–1266. doi: 10.1529/biophysj.107.110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malcolm HR, Heo YY, Elmore DE, Maurer JA. Defining the role of the tension sensor in the mechanosensitive channel of small conductance. Biophysical journal. 2011;101:345–352. doi: 10.1016/j.bpj.2011.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller S, Bartlett W, Chandrasekaran S, Simpson S, Edwards M, Booth IR. Domain organization of the MscS mechanosensitive channel of Escherichia coli. EMBO J. 2003;22:36–46. doi: 10.1093/emboj/cdg011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukharev S. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophysical journal. 2002;83:290–298. doi: 10.1016/S0006-3495(02)75169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasquez V, Sotomayor M, Cordero-Morales J, Schulten K, Perozo E. A structural mechanism for MscS gating in lipid bilayers. Science. 2008;321:1210–1214. doi: 10.1126/science.1159674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards MD, Bartlett W, Booth IR. Pore mutations of the Escherichia coli MscS channel affect desensitization but not ionic preference. Biophysical journal. 2008;94:3003–3013. doi: 10.1529/biophysj.107.123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koprowski P, Kubalski A. C termini of the Escherichia coli mechanosensitive ion channel (MscS) move apart upon the channel opening. J Biol Chem. 2003;278:11237–11245. doi: 10.1074/jbc.M212073200. [DOI] [PubMed] [Google Scholar]
- 9.Nomura T, Sokabe M, Yoshimura K. Interaction between the cytoplasmic and transmembrane domains of the mechanosensitive channel MscS. Biophysical journal. 2008;94:1638–1645. doi: 10.1529/biophysj.107.114785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Black SS, Edwards MD, Miller S, Morrison EL, Bartlett W, Dong C, Naismith JH, Booth IR. The structure of an open form of an E. coli mechanosensitive channel at 3.45 A resolution. Science. 2008;321:1179–1183. doi: 10.1126/science.1159262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards MD, Li Y, Kim S, Miller S, Bartlett W, Black S, Dennison S, Iscla I, Blount P, Bowie JU, Booth IR. Pivotal role of the glycine-rich TM3 helix in gating the MscS mechanosensitive channel. Nat Struct Mol Biol. 2005;12:113–119. doi: 10.1038/nsmb895. [DOI] [PubMed] [Google Scholar]
- 12.Vasquez V, Sotomayor M, Cortes DM, Roux B, Schulten K, Perozo E. Three-dimensional architecture of membrane-embedded MscS in the closed conformation. J Mol Biol. 2008;378:55–70. doi: 10.1016/j.jmb.2007.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinbacher S, Bass R, Strop P, Rees DC. Structures of the prokaryotic mechanosensitive channels MscL and MscS. Mechanosensitive Ion Channels, Part A. 2007;58:1–24. [Google Scholar]
- 14.Bass RB, Strop P, Barclay M, Rees DC. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298:1582–1587. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- 15.Koprowski P, Grajkowski W, Isacoff EY, Kubalski A. Genetic screen for potassium leaky small mechanosensitive channels (MscS) in Escherichia coli: recognition of cytoplasmic beta domain as a new gating element. The Journal of biological chemistry. 2011;286:877–888. doi: 10.1074/jbc.M110.176131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belyy V, Anishkin A, Kamaraju K, Liu N, Sukharev S. The tension-transmitting 'clutch' in the mechanosensitive channel MscS. Nat Struct Mol Biol. 2010;17:451–458. doi: 10.1038/nsmb.1775. [DOI] [PubMed] [Google Scholar]
- 17.Nomura T, Sokabe M, Yoshimura K. Lipid-protein interaction of the MscS mechanosensitive channel examined by scanning mutagenesis. Biophysical journal. 2006;91:2874–2881. doi: 10.1529/biophysj.106.084541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmore DE, Dougherty DA. Investigating lipid composition effects on the mechanosensitive channel of large conductance (MscL) using molecular dynamics simulations. Biophysical journal. 2003;85:1512–1524. doi: 10.1016/S0006-3495(03)74584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurer JA, Dougherty DA. Generation and evaluation of a large mutational library from the Escherichia coli mechanosensitive channel of large conductance, MscL: implications for channel gating and evolutionary design. The Journal of biological chemistry. 2003;278:21076–21082. doi: 10.1074/jbc.M302892200. [DOI] [PubMed] [Google Scholar]
- 20.Maurer JA, Elmore DE, Lester HA, Dougherty DA. Comparing and contrasting Escherichia coli and Mycobacterium tuberculosis mechanosensitive channels (MscL). New gain of function mutations in the loop region. The Journal of biological chemistry. 2000;275:22238–22244. doi: 10.1074/jbc.M003056200. [DOI] [PubMed] [Google Scholar]
- 21.Andersen OS, Koeppe RE., 2nd Bilayer thickness and membrane protein function: an energetic perspective. Annual review of biophysics and biomolecular structure. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 22.Jensen MO, Mouritsen OG. Lipids do influence protein function-the hydrophobic matching hypothesis revisited. Biochimica et biophysica acta. 2004;1666:205–226. doi: 10.1016/j.bbamem.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459:379–385. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolf TB, Roux B. Structure, energetics, and dynamics of lipid-protein interactions: A molecular dynamics study of the gramicidin A channel in a DMPC bilayer. Proteins. 1996;24:92–114. doi: 10.1002/(SICI)1097-0134(199601)24:1<92::AID-PROT7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Ou X, Blount P, Hoffman RJ, Kung C. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:11471–11475. doi: 10.1073/pnas.95.19.11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wild J, Altman E, Yura T, Gross CA. DnaK and DnaJ heat shock proteins participate in protein export in Escherichia coli. Genes Dev. 1992;6:1165–1172. doi: 10.1101/gad.6.7.1165. [DOI] [PubMed] [Google Scholar]
- 27.Blount P, Sukharev SI, Moe PC, Schroeder MJ, Guy HR, Kung C. Membrane topology and multimeric structure of a mechanosensitive channel protein of Escherichia coli. EMBO Journal. 1996;15:4798–4805. [PMC free article] [PubMed] [Google Scholar]
- 28.Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura K, Batiza A, Schroeder M, Blount P, Kung C. Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophysical journal. 1999;77:1960–1972. doi: 10.1016/S0006-3495(99)77037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booth IR, Edwards MD, Black S, Schumann U, Bartlett W, Rasmussen T, Rasmussen A, Miller S. Physiological analysis of bacterial mechanosensitive channels. Methods Enzymol. 2007;428:47–61. doi: 10.1016/S0076-6879(07)28003-6. [DOI] [PubMed] [Google Scholar]
- 31.Caldwell DB, Malcolm HR, Elmore DE, Maurer JA. Identification and experimental verification of a novel family of bacterial cyclic nucleotide-gated (bCNG) ion channels. Biochimica et biophysica acta. 2010;1798:1750–1756. doi: 10.1016/j.bbamem.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Malcolm HR, Heo YY, Caldwell DB, McConnell JK, Hawkins JF, Guayasamin RC, Elmore DE, Maurer JA. Ss-bCNGa: a unique member of the bacterial cyclic nucleotide gated (bCNG) channel family that gates in response to mechanical tension. European biophysics journal : EBJ. 2012;41:1003–1013. doi: 10.1007/s00249-012-0855-z. [DOI] [PubMed] [Google Scholar]
- 33.Blount P, Sukharev SI, Moe PC, Martinac B, Kung C. Mechanosensitive channels of bacteria. In: Conn PM, editor. Methods in Enzymology. Vol. 294. San Diego, CA: Academic Press; 1999. pp. 458–482. [DOI] [PubMed] [Google Scholar]
- 34.Blount P, Krause JE. Functional nonequivalence of structurally homologous domains of neurokinin-1 and neurokinin-2 type tachykinin receptors. Biol. J. Chem. 1993;268:16388–16395. [PubMed] [Google Scholar]
- 35.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blount P, Moe PC. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol. 1999;7:420–424. doi: 10.1016/s0966-842x(99)01594-2. [DOI] [PubMed] [Google Scholar]
- 37.Blount P, Sukharev SI, Schroeder MJ, Nagle SK, Kung C. Single residue substitutions that change the gating properties of a mechanosensitive channel in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11652–11657. doi: 10.1073/pnas.93.21.11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshimura K, Batiza A, Schroeder M, Blount P, Kung C. Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophysical Journal. 1999;77:1960–1972. doi: 10.1016/S0006-3495(99)77037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maurer J, Elmore D, Lester H, Dougherty D. Comparing and contrasting Escherichia coli and Mycobacterium tuberculosis mechanosensitive channels (MscL). New gain of function mutations in the loop region. Journal of Biological Chemistry. 2000;275:22238–22244. doi: 10.1074/jbc.M003056200. [DOI] [PubMed] [Google Scholar]
- 40.Moe PC, Levin G, Blount P. Correlating a protein structure with function of a bacterial mechanosensitive channel. Journal of Biological Chemistry. 2000;275:31121–31127. doi: 10.1074/jbc.M002971200. [DOI] [PubMed] [Google Scholar]
- 41.Iscla I, Wray R, Blount P. On the structure of the N-terminal domain of the MscL channel: helical bundle or membrane interface. Biophys J. 2008;95:2283–2291. doi: 10.1529/biophysj.107.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levin G, Blount P. Cysteine scanning of MscL transmembrane domains reveals residues critical for mechanosensitive channel gating. Biophysical journal. 2004;86:2862–2870. doi: 10.1016/S0006-3495(04)74338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kashket ER. Effects of K+ and Na+ on the proton motive force of respiring Escherichia coli at alkaline pH. Journal of bacteriology. 1985;163:423–429. doi: 10.1128/jb.163.2.423-429.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorence RM, Green GN, Gennis RB. Potentiometric analysis of the cytochromes of an Escherichia coli mutant strain lacking the cytochrome d terminal oxidase complex. Journal of bacteriology. 1984;157:115–121. doi: 10.1128/jb.157.1.115-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pittman MS, Robinson HC, Poole RK. A bacterial glutathione transporter (Escherichia coli CydDC) exports reductant to the periplasm. The Journal of biological chemistry. 2005;280:32254–32261. doi: 10.1074/jbc.M503075200. [DOI] [PubMed] [Google Scholar]
- 46.Naismith JH, Booth IR. Bacterial mechanosensitive channels--MscS: evolution's solution to creating sensitivity in function. Annual review of biophysics. 2012;41:157–177. doi: 10.1146/annurev-biophys-101211-113227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pliotas C, Ward R, Branigan E, Rasmussen A, Hagelueken G, Huang H, Black SS, Booth IR, Schiemann O, Naismith JH. Conformational state of the MscS mechanosensitive channel in solution revealed by pulsed electron-electron double resonance (PELDOR) spectroscopy. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2675–E2682. doi: 10.1073/pnas.1202286109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Booth IR, Rasmussen T, Edwards MD, Black S, Rasmussen A, Bartlett W, Miller S. Sensing bilayer tension: bacterial mechanosensitive channels and their gating mechanisms. Biochemical Society transactions. 2011;39:733–740. doi: 10.1042/BST0390733. [DOI] [PubMed] [Google Scholar]
- 49.Romantsov T, Battle AR, Hendel JL, Martinac B, Wood JM. Protein localization in Escherichia coli cells: comparison of the cytoplasmic membrane proteins ProP, LacY, ProW, AqpZ, MscS, and MscL. Journal of bacteriology. 2010;192:912–924. doi: 10.1128/JB.00967-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belyy V, Kamaraju K, Akitake B, Anishkin A, Sukharev S. Adaptive behavior of bacterial mechanosensitive channels is coupled to membrane mechanics. The Journal of general physiology. 2010;135:641–652. doi: 10.1085/jgp.200910371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nomura T, Cranfield CG, Deplazes E, Owen DM, Macmillan A, Battle AR, Constantine M, Sokabe M, Martinac B. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8770–8775. doi: 10.1073/pnas.1200051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Needham D, Nunn RS. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophysical journal. 1990;58:997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.