Abstract
Various DNA repair pathways protect the structural and chemical integrity of the human genome from environmental and endogenous threats. Polymorphisms of genes encoding the proteins involved in DNA repair have been found to be associated with cancer risk and chemotherapeutic response. In this study, we aim to establish the normative frequencies of DNA repair genes in South Indian healthy population and compare with HapMap populations. Genotyping was done on 128 healthy volunteers from South India, and the allele and genotype distributions were established. The minor allele frequency of Xeroderma pigmentosum group A (XPA) G23A, Excision repair cross-complementing 2 (ERCC2)/Xeroderma pigmentosum group D (XPD) Lys751Gln, Xeroderma pigmentosum group G (XPG) His46His, XPG Asp1104His, and X-ray repair cross-complementing group 1 (XRCC1) Arg399Gln polymorphisms were 49.2%, 36.3%, 48.0%, 23.0%, and 34.0% respectively. Ethnic variations were observed in the frequency distribution of these polymorphisms between the South Indians and other HapMap populations. The present work forms the groundwork for cancer association studies and biomarker identification for treatment response and prognosis.
Keywords: XPA, XPD, XPG, XRCC1, DNA repair
Introduction
The human genome is vulnerable to structural and chemical damage by various environmental and endogenous noxious stimuli. The integrity and stability of the genome are of paramount importance to its function as any alteration would result in malfunctioning of downstream pathways that could lead to disease or dysfunction. Alteration in the genome is a key step for cancer initiation and progression. Hence, it is not surprising that the human genome has more than 130 genes involved in recognizing and repairing DNA defects.1 These genes control various DNA repair pathways involved in maintaining the genomic integrity. Polymorphisms in the genes coding for DNA repair proteins result in decreased removal of DNA damaged products, thereby, increasing the risk of developing cancer and age-related disorders.2
The nucleotide excision repair (NER) pathway is a complex and versatile DNA repair mechanism that rectifies various DNA defects caused by ultraviolet (UV)-induced lesions and bulky chemical adducts, and drug-induced DNA damage.3 Various proteins are involved in the functioning of the NER pathway, and variants in the genes encoding for these proteins have been studied. Seven complementation groups (XPA–XPG) have been described, and they code for proteins involved in the NER pathway.4
The Xeroderma pigmentosum group A (XPA) gene codes for a protein that, along with the replication protein A (RPA) and Xeroderma pigmentosum group C (XPC), is involved in the initial sensing of the damage in the DNA strands,5 and, hence, is called the damage recognition factor. A polymorphism in the XPA, the G23A polymorphism, is located in the 5′ untranslated region and is four nucleotides upstream of the start codon. Mutations in this region are known to cause low levels of protein expression because of the differential binding of the 40S ribosome to the promoter region.6 The Xeroderma pigmentosum group D (XPD), also known as the Excision repair cross-complementing 2 (ERCC2), codes for a protein that forms three of the nine subunits that constitute the transcription factor IIH (TFIIH).7 TFIIH is involved in the transcription of the DNA during the DNA repair process. Mutations in the XPD can diminish the activity of these proteins and result in an abnormal DNA repair process. A total of 17 single nucleotide polymorphisms (SNPs) of XPD have been described, of which the Lys751Gln (rs13181) results in an amino acid change and, consequently, can affect protein function.8 The XPG gene codes for an endonuclease that helps in the nicking of damaged DNA fragments and plays a pivotal role in the NER pathway.9 Two important SNPs in the XPG gene are the XPG His46His (rs1047768) and XPG Asp1104His (rs17655). Mutations of the genes encoding proteins in the NER pathway have been studied and found to be associated with various cancers.
The X-ray repair cross-complementing group 1 (XRCC1) gene codes for a scaffolding protein that plays a role in the base excision repair (BER) pathway. DNA repair is brought about by its complex interactions with DNA polymerase-β, poly (ADP-ribose) polymerase, and DNA ligase III.10,11 XRCC1 is required for the efficient repair of single-strand breaks (SSBs).12 SSBs occur in the cell because of the oxidative damage produced by the reactive oxygen species (ROS), because of the anomalous activity of certain enzymes involved in DNA replication, or during repair of DNA by other repair pathways.13 Genetic alterations of the XRCC1 may result in a dysfunctional protein with impaired repair capacity, thus, increasing risk of cancer in an individual. A common genetic variant of XRCC1, Arg399Gln, has been implicated in various cancers.14
Apart from conferring increased susceptibility to cancer risk, mutations in the DNA repair pathway also alter the sensitivity to cancer chemotherapy.15 Platinum-based chemotherapeutic agents, which includes cisplatin, carboplatin, and oxaliplatin, are commonly used as first-line chemotherapeutic agents for many cancers. The platinum compounds form DNA adducts and cause cell death. Efficient NER of the DNA damage decreases the efficiency to cause tumor cell death and might result in resistance to these compounds, whereas a deficiency in the DNA repair will increase the efficiency.16
India is a country with a vast population derived from various ethnic groups. Hence, genetic differences are common among various groups within India, and the South Indian population represents a genetically distinct group.17 Such genetic differences within populations can confound genotype–phenotype association studies and are the major reasons for the conflicting results. In this study, we aim to establish the normative frequency of five SNPs of genes involved in DNA repair pathways, viz, XPA G23A (rs1800975), ERCC2/XPD Lys751Gln (rs13181), XPG His46His (rs1047768), XPG Asp1104His (rs17655), and XRCC1 Arg399Gln (rs25487) (details of the polymorphisms are shown in Table 1). We also aim to evaluate the similarity or dissimilarity between various HapMap populations such as CEU (Utah residents with Northern and Western European ancestry), HCB (Han Chinese in Beijing, China), JPT (Japanese in Tokyo, Japan), YRI (Yoruba in Ibadan, Nigeria), GIH (Gujarat Indians in Houston), and MEX (Mexican ancestry in Los Angeles, CA).
Table 1.
Characteristic features, rs IDs, and assay IDs of the DNA repair gene polymorphisms studied.
| GENE | SNP | BASE PAIR CHANGE | GENE LOCATION | SNP LOCATION | rs ID | ASSAY ID |
|---|---|---|---|---|---|---|
| XPA | G23A | G>A | 9q22.3 | 5′ UTR | rs1800975 | C____482935_1_ |
| ERCC2/XPD | Lys751Gln | A>C | 19q13.3 | Exon 23 | rs13181 | C___3145033_10 |
| XPG | His46His | T>C | 13q33.1 | Exon 2 | rs1047768 | C___1891769_20 |
| XPG | Asp110 4His | G>C | 13q33.1 | Exon 15 | rs17655 | C____1891743_10 |
| XRCC1 | Arg399Gln | G>A | 19q13.2 | Exon 10 | rs25487 | C____622564_10 |
Abbreviations: SNP, single nucleotide polymorphism; 5′ UTR, untranslated region; Lys, lysine; Gln, glutamine; His, histidine; Asp, aspartate; Arg, arginine.
Methodology
Study subjects
The study was carried out on 128 healthy individuals between 18 and 70 years of age. Unrelated individuals from the general population, residing in the Southern states of India (Tamil Nadu, Kerala, Karnataka, and Andhra Pradesh) for three consecutive generations and speaking a Dravidian language (Tamil, Malayalam, Kannada, and Telugu), were selected for the study. The study subjects comprised 73 males and 55 females, and the mean age (±SD) was 34.8 (±14.5) years. Ethical approval was obtained from the Institute Ethics Committee, JIPMER (Jawaharlal Institute of Postgraduate Medical Education and Research), Pondicherry, India. Written informed consent was obtained from all subjects participating in the study. The study was in compliance with the good clinical practice according to the principles of the Declaration of Helsinki.
DNA extraction
Approximately 5 mL of venous blood was collected from each study subject in tubes containing 100 μL of 10% ethylene diaminetetraacetic acid (EDTA). Genomic DNA was extracted using the standard phenol–chloroform extraction method.18 The isolated DNA was stored at −20°C until genotyping.
Genotyping
Five SNPs in DNA repair genes—XPA G23A (rs1800975), ERCC2/XPD Lys751Gln (rs13181), XPG His46His (rs1047768), XPG Asp1104His (rs17655), and XRCC1 Arg399Gln (rs25487)—were genotyped by RT-PCR (Real time polymerase chain reaction) (Applied Biosystems 7300) using TaqMan SNP genotyping assay kits (Table 1). Genomic DNA was diluted to 50 ng/μL, and 2.5 μL was used for RT-PCR. In all, 5 μL of TaqMan Universal PCR master mix and 0.25 μL of TaqMan genotyping assay were added to the diluted DNA, and deionized water was added to make up the final volume to 10 μL. The thermocycler was set at 50°C for two minutes and at 95°C for 10 minutes to activate polymerase AmpliTaq Gold. In all, 40 cycles of denaturation (92°C for 15 seconds) and annealing-extension (60°C for 1 minute) were used to amplify the DNA sequence. Allelic discrimination was done by 7300 sequence detection software (SDS), version 1.4. The allelic discrimination plots of the five SNPs are shown in Figures 1–5. For quality control, 10% of the samples were reanalyzed, and the results were found to be 100% concordant.
Figure 1.
Allelic discrimination plot of XPA G23a (rs1800975) polymorphism.
Figure 5.
Allelic discrimination plot of XRCC1 Arg399Gln (rs25487) polymorphism.
Statistical analysis
The genotype and allele frequencies were determined by direct gene count method. The genotype frequencies were tested for Hardy-Weinberg equilibrium using the chi-square (χ2) test by comparing the observed frequencies with the expected frequencies. Chi-square test was also used to assess the differences in allele frequencies between the study population and populations of different ethnicities. Statistical analysis was performed using GraphPad InStat 3.
Results
The observed allele frequencies of all the five SNPs were in Hardy–Weinberg equilibrium. Gender-wise stratification of the genotype and allele frequencies of the five SNPs was done and is shown in Table 2. The allele frequencies did not differ significantly between males and females.
Table 2.
Gender-wise genotype and allele frequency distribution of DNA repair gene polymorphisms in the study population.
| SNP | GENOTYPE FREQUENCY (%) | ALLELE FREQ UENCY (%) | P VALUE | |||
|---|---|---|---|---|---|---|
| rs13181 | AA | AC | CC | A | C | 0.1928 |
| Male (73) | 39.7 | 41.1 | 19.2 | 60.3 | 39.7 | |
| Female (55) | 47.3 | 41.8 | 10.9 | 68.2 | 31.8 | |
| rs25487 | GG | GA | AA | G | A | 0.8693 |
| Male (73) | 46.6 | 39.7 | 13.7 | 66.4 | 33.6 | |
| Female (55) | 47.3 | 36.4 | 16.4 | 65.5 | 34.5 | |
| rs1800975 | GG | GA | AA | G | A | 0.6387 |
| Male (73) | 30.1 | 43.8 | 26.0 | 52.1 | 47.9 | |
| Female (55) | 29.1 | 40.0 | 30.9 | 49.1 | 50.9 | |
| rs1047768 | TT | TC | CC | T | C | 0.6399 |
| Male (73) | 30.1 | 41.1 | 28.8 | 50.7 | 49.3 | |
| Female (55) | 27.3 | 52.7 | 20.0 | 53.6 | 46.4 | |
| rs17655 | GG | GC | CC | G | C | 0.2740 |
| Male (73) | 63.0 | 32.9 | 4.1 | 79.5 | 20.5 | |
| Female (55) | 54.5 | 38.2 | 7.3 | 73.6 | 26.4 | |
XPA G23A (rs1800975)
The XPA G23A had G allele frequency of 50.8% and A allele frequency of 49.2%. The heterozygous genotype GA was seen in 42.2% while the homozygous genotypes GG and AA were seen in 29.7% and 28.1%, respectively (Table 3). The allele frequencies were significantly different from those observed among the CEU, YRI, and MEX. They were similar to the HCB, JPT, and GIH.
Table 3.
Comparison of the genotype and allele frequencies of XPA G23a polymorphism with HapMap populations.
| POPULATION | N | GENOTYPE FREQUENCY (%) | ALLELE FREQUENCY (%) | P VALUE | |||
|---|---|---|---|---|---|---|---|
| GG | GA | AA | G | A | |||
| Present study (SI) | 128 | 29.7 | 42.2 | 28.1 | 50.8 | 49.2 | Ref. |
| CEU | 226 | 37.2 | 49.6 | 13.3 | 61.9 | 38.1 | 0.0038 |
| HCB | 86 | 20.9 | 60.5 | 18.6 | 51.2 | 48.8 | 0.9383 |
| JPT | 168 | 29.8 | 42.9 | 27.4 | 51.2 | 48.8 | 0.9214 |
| YRI | 226 | 56.6 | 40.7 | 2.7 | 77.0 | 23.0 | <0.0001 |
| GIH | 176 | 30.7 | 47.7 | 21.6 | 54.5 | 45.5 | 0.3585 |
| MEX | 100 | 40.0 | 42.0 | 18.0 | 61.0 | 39.0 | 0.0294 |
Abbreviations: N, number of subjects; SI, South Indian; CEU, Utah residents with Northern and Western European ancestry; HCB, Han Chinese in Beijing, China; JPT, Japanese in Tokyo, Japan; YRI, Yoruba in Ibadan, Nigeria; GIH, Gujarat Indians in Houston, TX; and MEX, Mexican ancestry in Los Angeles, CA.
ERCC2/XPD Lys751Gln A>C (rs13181)
The observed genotype and allele frequencies of ERCC2/XPD Lys751Gln are shown in Table 4. The genotype frequencies of AA, AC, and CC were 43.0%, 41.4%, and 15.6%, respectively. The minor allele C had a frequency of 36.3%, highest among the populations compared. The allele frequencies observed in South Indians were significantly different from HCB, JPT, YRI, and MEX populations, while being similar to CEU and GIH. The C allele was as low as 7.6% in the JPT.
Table 4.
Comparison of the allele and genotype frequencies of ERCC2/XPD Lys751Gln (A>C) polymorphism with HapMap populations.
| POPULATION | N | GENOTYPE FREQUENCY (%) | ALLELE FREQUENCY (%) | P VALUE | |||
|---|---|---|---|---|---|---|---|
| AA | AC | CC | A | C | |||
| Present study (SI) | 128 | 43.0 | 41.4 | 15.6 | 63.7 | 36.3 | Ref. |
| CEU | 226 | 40.7 | 52.2 | 7.1 | 66.8 | 33.2 | 0.3975 |
| HCB | 86 | 83.7 | 16.3 | 0.0 | 91.9 | 8.1 | <0.0001 |
| JPT | 172 | 86.0 | 12.8 | 1.2 | 92.4 | 7.6 | <0.0001 |
| YRI | 226 | 64.6 | 33.6 | 1.8 | 81.4 | 18.6 | <0.0001 |
| GIH | 176 | 39.8 | 48.9 | 11.4 | 64.2 | 35.8 | 0.8925 |
| MEX | 100 | 62.0 | 34.0 | 4.0 | 79.0 | 21.0 | 0.0004 |
Abbreviations: N, number of subjects; SI, South Indian; CEU, Utah residents with Northern and Western European ancestry; HCB, Han Chinese in Beijing, China; JPT, Japanese in Tokyo, Japan; YRI, Yoruba in Ibadan, Nigeria; GIH, Gujarat Indians in Houston, TX; and MEX, Mexican ancestry in Los Angeles, CA.
XPG His46His T>C (rs1047768)
The genotype and allele frequencies of XPG His46His are shown in Table 5. T allele and C allele had frequencies of 52.0% and 48.0%, respectively. The TT and CC genotypes were seen in 28.9% and 25.0%, respectively, while the heterozygous TC was present in 46.1%. The allele frequencies were found to be significantly different from all the populations compared.
Table 5.
Comparison of the genotype and allele frequencies of XPG His46His (T>C) polymorphism with HapMap populations.
| POPULATION | N | GENOTYPE FREQUENCY (%) | ALLELE FREQUENCY (%) | P VALUE | |||
|---|---|---|---|---|---|---|---|
| TT | TC | CC | T | C | |||
| Present study (SI) | 128 | 28.9 | 46.1 | 25.0 | 52.0 | 48.0 | Ref. |
| CEU | 226 | 17.7 | 49.6 | 32.7 | 42.5 | 57.5 | 0.0151 |
| HCB | 86 | 55.8 | 34.9 | 9.3 | 73.3 | 26.7 | <0.0001 |
| JPT | 172 | 68.6 | 29.1 | 2.3 | 83.1 | 16.9 | <0.0001 |
| YRI | 224 | 8.0 | 42.0 | 50.0 | 29.0 | 71.0 | <0.0001 |
| GIH | 176 | 38.6 | 45.5 | 15.9 | 61.4 | 38.6 | 0.0205 |
| MEX | 100 | 36.0 | 52.0 | 12.0 | 62.0 | 38.0 | 0.0318 |
Abbreviations: N, number of subjects; SI, South Indian; CEU, Utah residents with Northern and Western European ancestry; HCB, Han Chinese in Beijing, China; JPT, Japanese in Tokyo, Japan; YRI, Yoruba in Ibadan, Nigeria; GIH, Gujarat Indians in Houston, TX; and MEX, Mexican ancestry in Los Angeles, CA.
XPG Asp1104His G>C (rs17655)
The genotype and allele frequencies of XPG Asp1104His are shown in Table 6. The GG and GC genotypes were seen in 59.4% and 35.1%, respectively, while the homozygous mutant CC was observed in 5.5%. The allele frequencies of G and C alleles were 77.0% and 23.0%, respectively. The allele frequencies of the South Indian population studied differed significantly from all the populations compared.
Table 6.
Comparison of the genotype and allele frequencies of XPG Asp1104His (G>C) polymorphism with HapMap populations.
| POPULATION | N | GENOTYPE FREQUENCY (%) | ALLELE FREQUENCY (%) | P VALUE | |||
|---|---|---|---|---|---|---|---|
| GG | GC | CC | G | C | |||
| Present study (SI) | 128 | 59.4 | 35.1 | 5.5 | 77.0 | 23.0 | Ref. |
| CEU | 120 | 6.7 | 40.0 | 53.3 | 26.7 | 73.3 | <0.0001 |
| HCB | 90 | 15.6 | 57.8 | 26.7 | 44.4 | 55.6 | <0.0001 |
| JPT | 88 | 25.0 | 54.5 | 20.5 | 52.3 | 47.7 | <0.0001 |
| YRI | 120 | 28.3 | 51.7 | 20.0 | 54.2 | 45.8 | <0.0001 |
Abbreviations: N, number of subjects; SI, South Indian; CEU, Utah residents with Northern and Western European ancestry; HCB, Han Chinese in Beijing, China; JPT, Japanese in Tokyo, Japan; and YRI, Yoruba in Ibadan, Nigeria.
XRCC1 Arg399Gln G>A (rs25487)
The genotype frequencies of GG, GA, and AA of XRCC1 Arg399Gln were 46.9%, 38.3%, and 14.8%, respectively (Table 7). The G allele frequency was calculated to be 66.0%, and the A allele frequency was 34.0%. The allele frequencies were found to be statistically divergent from YRI. The minor allele frequency varied from 11.1% in YRI to 40.9% in GIH.
Table 7.
Comparison of the genotype and allele frequencies of XRCC1 Arg399Gln (G>A) polymorphism with HapMap populations.
| POPULATION | N | GENOTYPE FREQUENCY (%) | ALLELE FREQUENCY (%) | P VALUE | |||
|---|---|---|---|---|---|---|---|
| GG | GA | AA | G | A | |||
| Present study (SI) | 128 | 46.9 | 38.3 | 14.8 | 66.0 | 34.0 | Ref. |
| CEU | 224 | 38.4 | 50.0 | 11.6 | 63.4 | 36.6 | 0.4846 |
| HCB | 84 | 52.4 | 40.5 | 7.1 | 72.6 | 27.4 | 0.1518 |
| JPT | 172 | 52.3 | 40.7 | 7.0 | 72.7 | 27.3 | 0.0788 |
| YRI | 226 | 77.9 | 22.1 | 0 | 88.9 | 11.1 | <0.0001 |
| GIH | 176 | 34.1 | 50.0 | 15.9 | 59.1 | 40.9 | 0.0824 |
| MEX | 98 | 42.9 | 49.0 | 8.2 | 67.3 | 32.7 | 0.7662 |
Abbreviations: N, number of subjects; SI, South Indian; CEU, Utah residents with Northern and Western European ancestry; HCB, Han Chinese in Beijing, China; JPT, Japanese in Tokyo, Japan; YRI, Yoruba in Ibadan, Nigeria; GIH, Gujarat Indians in Houston, TX; and MEX, Mexican ancestry in Los Angeles, CA.
Discussion
The aim of the present study was to investigate the frequency of genetic polymorphisms in the XPA, XPD, XPG, and XRCC1 genes in a South Indian healthy population. We report the genotype and allele frequencies of five different polymorphisms in DNA repair genes.
In our study, the allele frequencies of XPA G23A showed a significant deviation from the CEU, YRI, and MEX. The presence of G allele has been shown to decrease the risk of lung cancer among non-Caucasians, while this effect was not seen in the Caucasians.19 Further, ethnic specific differences were observed in its association with head and neck and colorectal cancers, a protective effect of the G allele being greater in the Asian population.20 Moreover, XPA polymorphisms have a role as a predictive biomarker of response to platinum-based chemotherapy, especially in non-small-cell lung cancer.21,22
The ERCC2/XPD Lys751Gln polymorphism frequencies showed a significant deviation from the populations of HCB, JPT, YRI, and MEX. The minor allele frequency was found to be similar to a study on the Northern Indian population by Gangwar et al23 and on South Indians by Vettriselvi et al.24 This polymorphism has been associated with an increased risk of cancers of the lung,25 bladder,26 prostate,27 and brain.28 A recent study also revealed that the XPD polymorphism can be useful as a biomarker for tamoxifen response in breast cancer patients.29 Recently, we established the frequency distribution of another polymorphism ERCC2/XPD Asp312Asn and two important polymorphisms of the ERCC1 in South Indian population.30
We report, to the best of our knowledge, for the first time in South Indian population, the allele and genotype frequencies of XPG His46His and Asp1104His. We observe the allele frequencies of both the SNPs to be completely deviant in the rest of the populations from the HapMap such as the CEU, HCB, JPT, and YRI. Heterozygous genotype of the XPG His46His was observed in a larger group of subjects similar to other populations, unlike the HCB or JPT. The present study population also had almost equal frequencies of both the alleles. Interestingly, the GG genotype of the XPG Asp1104His was higher in our population, quite contrary to the rest of the populations compared with. CC was the major genotype in CEU, while the heterogeneous genotype GC was major genotype in HCB, JPT, and YRI. Very few studies evaluating XPG gene polymorphisms are available from India, and a North Indian study reported the heterogeneous genotype to be the commonest genotype in the healthy population.31 This probably suggests that inter-ethnic variations exist in the distribution of XPG gene polymorphisms. XPG His46His has been associated with colon32 and prostate cancer.31 It also has a role in predicting response to platinum-based chemotherapy.33,34 The Asp1104His, on the other hand, has been associated with an increased risk of cutaneous melanoma35 and breast cancer.36 Other studies show weak or no association with cancer.37,38
The XRCC1 Arg399Gln gene polymorphism frequency in South Indians was comparable to the populations such as CEU, HCB, JPT, GIH, and MEX. However, it showed a significant deviation from YRI. A study from the northern part of India of 150 healthy volunteers found the minor allele frequency to be 43%, higher than the present study population (34%).39 A recent study from Northern India, evaluating the role of Arg399Gln in colorectal cancer, found a higher percentage of homozygous mutants than our study.40 Arg399Gln has been found to be associated strongly with hepatocellular carcinoma in the Asian population and breast cancer in Indians.41 Various studies have shown its association with the risk of lung cancer,42,43 glioma,28,44 endometrial carcinoma,45 breast cancer,46 etc.
We did not find a difference in the distribution of these polymorphisms between the genders, and the differential risk that these genotypes confer on males and females might be because of the dietary habits and interaction with other environmental factors as explained by Wang et al.47
To the best of our knowledge, this is the first study to establish the frequency distribution of XPA and XPG gene polymorphisms in South Indian population. To conclude, we report the frequency distribution of five SNPs in DNA repair pathways, and the present work forms the groundwork for cancer association studies and biomarker identification for treatment response and prognosis.
Figure 2.
Allelic discrimination plot of ERCC2/XPD Lys751Gln (rs13181) polymorphism.
Figure 3.
Allelic discrimination plot of XPG His46His (rs1047768) polymorphism.
Figure 4.
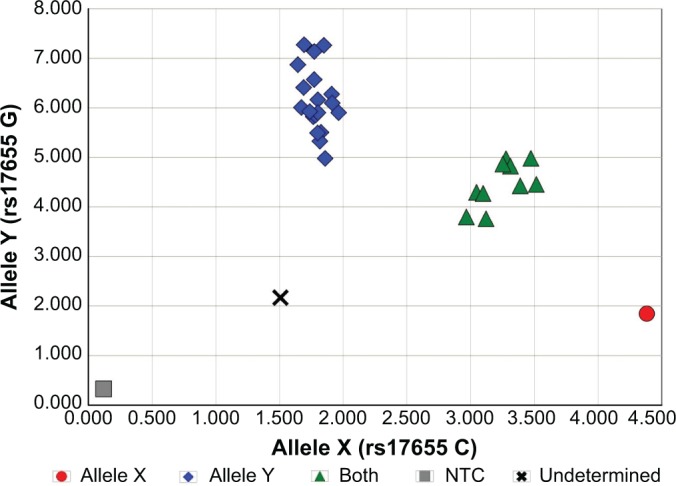
Allelic discrimination plot of XPG Asp1104His (rs17655) polymorphism.
Footnotes
Author Contributions
Conceived and designed the experiments: KSR, BD, KG, and SAD. Recruitment of healthy volunteers and DNA extraction: ASA and KSR. Genotyping: GU and KSR. Analyzed the data: KSR and AP. Wrote the manuscript: AP, KSR, and SAD. All authors reviewed and approved the final manuscript.
ACADEMIC EDITOR: Barbara Guinn, Editor in Chief
FUNDING: An intramural research fund was provided by JIPMER. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. all editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291:1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 2.Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1513–1530. [PubMed] [Google Scholar]
- 3.De Laat WL, Jaspers NG, Hoeijmakers JH. Molecular mechanism of nucleotide excision repair. Genes Dev. 1999;13:768–785. doi: 10.1101/gad.13.7.768. [DOI] [PubMed] [Google Scholar]
- 4.Sancar A, Tang M. Nucleotide excision repair. Photochem Photobiol. 1993;57:905–921. doi: 10.1111/j.1751-1097.1993.tb09233.x. [DOI] [PubMed] [Google Scholar]
- 5.Wakasugi M, Sancar A. Order of assembly of human DNA repair excision nuclease. J Biol Chem. 1999;274:18759–18768. doi: 10.1074/jbc.274.26.18759. [DOI] [PubMed] [Google Scholar]
- 6.Miller KL. XPA, haplotypes, and risk of basal and squamous cell carcinoma. Carcinogenesis. 2005;27:1670–1675. doi: 10.1093/carcin/bgi376. [DOI] [PubMed] [Google Scholar]
- 7.Coin F, Bergmann E, Tremeau-Bravard A, Egly JM. Mutations in XPB and XPD helicases found in xeroderma pigmentosum patients impair the transcription function of TFIIH. EMBO J. 1999;18:1357–1366. doi: 10.1093/emboj/18.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen MR, Jones IM, Mohrenweiser H. Nonconservative amino acid substitution variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res. 1998;58:604–608. [PubMed] [Google Scholar]
- 9.Clarkson SG. The XPG story. Biochimie. 2003;85:1113–1121. doi: 10.1016/j.biochi.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Caldecott KW, Aoufouchi S, Johnson P, Shall S. XRCC1 polypeptide interacts with DNA polymerase β and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular “nick-sensor” in vitro. Nucleic Acids Res. 1996;24:4387–4394. doi: 10.1093/nar/24.22.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldecott KW, McKeown CK, Tucker JD, Ljungquist S, Thompson LH. An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Mol Cell Biol. 1994;14:68–76. doi: 10.1128/mcb.14.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brem R. XRCC1 is required for DNA single-strand break repair in human cells. Nucleic Acids Res. 2005;33:2512–2520. doi: 10.1093/nar/gki543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 14.Hu Z, Ma H, Chen F, Wei Q, Shen H. XRCC1 polymorphisms and cancer risk: a meta-analysis of 38 case–control studies. Cancer Epidemiol Biomarkers Prev. 2005;14:1810–1818. doi: 10.1158/1055-9965.EPI-04-0793. [DOI] [PubMed] [Google Scholar]
- 15.Fojo T, Cancer DNA. repair mechanisms, and resistance to chemotherapy. J Natl Cancer Inst. 2001;93:1434–1436. doi: 10.1093/jnci/93.19.1434. [DOI] [PubMed] [Google Scholar]
- 16.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–1295. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 17.Tamang R, Singh L, Thangaraj K. Complex genetic origin of Indian populations and its implications. J Biosci. 2012;37:911–919. doi: 10.1007/s12038-012-9256-9. [DOI] [PubMed] [Google Scholar]
- 18.Blin N, Stafford DW. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976;3:2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou J-H, An L, Chen S, Ren LQ. XPA A23G polymorphism and lung cancer risk: a meta-analysis. Mol Biol Rep. 2012;39(2):1435–1440. doi: 10.1007/s11033-011-0878-z. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Zhang Z, Cao XL, et al. XPA A23G polymorphism and susceptibility to cancer: a meta-analysis. Mol Biol Rep. 2012;39:6791–6799. doi: 10.1007/s11033-012-1504-4. [DOI] [PubMed] [Google Scholar]
- 21.Cheng H, Qin Q, Sun X, et al. Predictive effect of XPA and XPD polymorphisms on survival of advanced NSCLC patients treated with platinum-based chemotherapy: a three-dimensional (3-D), polyacrylamide gel-based DNA microarray method. Technol Cancer Res Treat. 2013;12:473–482. doi: 10.7785/tcrt.2012.500337. [DOI] [PubMed] [Google Scholar]
- 22.Feng J, Sun X, Sun N, et al. XPA A23G polymorphism is associated with the elevated response to platinum-based chemotherapy in advanced non-small cell lung cancer. Acta Biochim Biophys Sin. 2009;41:429–435. doi: 10.1093/abbs/gmp027. [DOI] [PubMed] [Google Scholar]
- 23.Gangwar R, Manchanda PK, Mittal RD. Implications of XRCC1, XPD and APE1 gene polymorphism in North Indian population: a comparative approach in different ethnic groups worldwide. Genetica. 2009;136:163–169. doi: 10.1007/s10709-008-9329-8. [DOI] [PubMed] [Google Scholar]
- 24.Vettriselvi V, Vijayalakshmi K, Solomon PFD, Venkatachalam P. XRCC1 and XPD gene polymorphisms in a South Indian population. Asian Pac J Cancer Prev. 2007;8:283–286. [PubMed] [Google Scholar]
- 25.Wu H, Ding L. Comprehensive assessment of the association between XPD rs13181 polymorphism and lung cancer risk. Tumor Biol. 2014;35(8):8125–8132. doi: 10.1007/s13277-014-1948-3. [DOI] [PubMed] [Google Scholar]
- 26.Li SX, Dai QS, Chen SX, et al. Xeroderma pigmentosum complementation group D (XPD) gene polymorphisms contribute to bladder cancer risk: a meta-analysis. Tumor Biol. 2014;35:3905–3915. doi: 10.1007/s13277-013-1519-z. [DOI] [PubMed] [Google Scholar]
- 27.Yang B, Chen WH, Wen XF, Liu H, Liu F. Role of DNA repair-related gene polymorphisms in susceptibility to risk of prostate cancer. Asian Pac J Cancer Prev. 2013;14:5839–5842. doi: 10.7314/apjcp.2013.14.10.5839. [DOI] [PubMed] [Google Scholar]
- 28.Fahmideh MA, Schwartzbaum J, Frumento P, Feychting M. Association between DNA repair gene polymorphisms and risk of glioma: a systematic review and meta-analysis. Neuro-Oncol. 2014;16:807–814. doi: 10.1093/neuonc/nou003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tengström M, Mannermaa A, Kosma VM, Soini Y, Hirvonen A, Kataja V. MnSOD rs4880 and XPD rs13181 polymorphisms predict the survival of breast cancer patients treated with adjuvant tamoxifen. Acta Oncol. 2014;53:769–775. doi: 10.3109/0284186X.2014.892210. [DOI] [PubMed] [Google Scholar]
- 30.Rao KS, SureshKumar S, Umamaheswaran G, et al. Frequency distribution of DNA repair genes ERCC1 and ERCC2 polymorphisms in South Indian healthy population. Environ Toxicol Pharmacol. 2014;38:480–488. doi: 10.1016/j.etap.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Berhane N, Sobti RC, Mahdi SA. DNA repair genes polymorphism (XPG and XRCC1) and association of prostate cancer in a north Indian population. Mol Biol Rep. 2012;39:2471–2479. doi: 10.1007/s11033-011-0998-5. [DOI] [PubMed] [Google Scholar]
- 32.Negandhi AA, Hyde A, Dicks E, et al. MTHFR Glu429Ala and ERCC5 His46His polymorphisms are associated with prognosis in colorectal cancer patients: analysis of two independent cohorts from Newfoundland. PLoS One. 2013;8:e61469. doi: 10.1371/journal.pone.0061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T, Sun J, Lv M, et al. XPG is predictive gene of clinical outcome in advanced non-small-cell lung cancer with platinum drug therapy. Asian Pac J Cancer Prev. 2013;14:701–705. doi: 10.7314/apjcp.2013.14.2.701. [DOI] [PubMed] [Google Scholar]
- 34.Kweekel DM, Antonini NF, Nortier JWR, Punt CJA, Gelderblom H, Guchelaar HJ. Explorative study to identify novel candidate genes related to oxaliplatin efficacy and toxicity using a DNA repair array. Br J Cancer. 2009;101:357–362. doi: 10.1038/sj.bjc.6605134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schrama D, Scherer D, Schneider M, et al. ERCC5 p. Asp1104His and ERCC2 p. Lys751Gln polymorphisms are independent prognostic factors for the clinical course of melanoma. J Invest Dermatol. 2011;131:1280–1290. doi: 10.1038/jid.2011.35. [DOI] [PubMed] [Google Scholar]
- 36.Kumar R, Hoglund L, Zhao C, Forsti A, Snellman E, Hemminki K. Single nucleotide polymorphisms in the XPG gene: determination of role in DNA repair and breast cancer risk. Int J Cancer. 2003;103:671–675. doi: 10.1002/ijc.10870. [DOI] [PubMed] [Google Scholar]
- 37.He XF, Liu LR, Wei W, et al. Association between the XPG Asp1104His and XPF Arg415Gln polymorphisms and risk of cancer: a meta-analysis. PLoS One. 2014;9:e88490. doi: 10.1371/journal.pone.0088490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu ML, Wang M, Cao ZG, et al. Association between the ERCC5 Asp1104His polymorphism and cancer risk: a meta-analysis. PLoS One. 2012;7:e36293. doi: 10.1371/journal.pone.0036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiran M, Saxena R, Kaur J. Distribution of XRCC1 genotypes in north Indian population. Indian J Med Res. 2010;131:71–75. [PubMed] [Google Scholar]
- 40.Sameer A. Arg399Gln polymorphism of XRCC1 gene and risk of colorectal cancer in Kashmir: a case control study. Oncol Lett. 2013;5:959–963. doi: 10.3892/ol.2013.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi L, Xiao-feng H, Yun-tao L, Hao L, Ye S, Song-tao Q. Association between the XRCC1 Arg399Gln Polymorphism and Risk of Cancer: evidence from 297 case–control studies. PLoS One. 2013;8:e78071. doi: 10.1371/journal.pone.0078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Huang Y, Cao YS, et al. Assessment of the association between XRCC1 Arg399Gln polymorphism and lung cancer in Chinese. Tumour Biol. 2013;34:3681–3685. doi: 10.1007/s13277-013-0950-5. [DOI] [PubMed] [Google Scholar]
- 43.Natukula K, Jamil K, Pingali UR, Attili VSS, Madireddy URN. The codon 399 Arg/Gln XRCC1 polymorphism is associated with lung cancer in Indians. Asian Pac J Cancer Prev. 2013;14:5275–5279. doi: 10.7314/apjcp.2013.14.9.5275. [DOI] [PubMed] [Google Scholar]
- 44.Gao K, Mu SQ, Wu ZX. Investigation of the effects of single-nucleotide polymorphisms in DNA repair genes on the risk of glioma. Genet Mol Res. 2014;13:1203–1211. doi: 10.4238/2014.February.27.5. [DOI] [PubMed] [Google Scholar]
- 45.Hosono S, Matsuo K, Ito H, et al. Polymorphisms in base excision repair genes are associated with endometrial cancer risk among postmenopausal Japanese women. Int J Gynecol Cancer. 2013;23:1561–1568. doi: 10.1097/IGC.0b013e3182a80a7e. [DOI] [PubMed] [Google Scholar]
- 46.Bu T, Liu L, Sun Y, et al. XRCC1 Arg399Gln polymorphism confers risk of breast cancer in American population: a meta-analysis of 10846 cases and 11723 controls. PLoS One. 2014;9:e86086. doi: 10.1371/journal.pone.0086086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Zhao Y, Jiang J, et al. Polymorphisms in DNA repair genes XRCC1, XRCC3 and XPD, and colorectal cancer risk: a case–control study in an Indian population. J Cancer Res Clin Oncol. 2010;136:1517–1525. doi: 10.1007/s00432-010-0809-8. [DOI] [PubMed] [Google Scholar]


