Abstract
Motor behavior requires selecting between potential actions. The role of inhibition in response selection has frequently been examined in tasks in which participants are engaged in some advance preparation prior to the presentation of an imperative signal. Under such conditions, inhibition could be related to processes associated with response selection, or to more general inhibitory processes that are engaged in high states of anticipation. In Experiment 1, we manipulated the degree of anticipatory preparation. Participants performed a choice reaction time task that required choosing between a movement of the left or right index finger, and used transcranial magnetic stimulation (TMS) to elicit motor evoked potentials (MEPs) in the left hand agonist. In high anticipation blocks, a non-informative cue (e.g., fixation marker) preceded the imperative; in low anticipation blocks, there was no cue and participants were required to divide their attention between two tasks to further reduce anticipation. MEPs were substantially reduced before the imperative signal in high anticipation blocks. In contrast, in low anticipation blocks, MEPs remained unchanged before the imperative signal but showed a marked suppression right after the onset of the imperative. This effect occurred regardless of whether the imperative had signaled a left or right hand response. After this initial inhibition, left MEPs increased when the left hand was selected and remained suppressed when the right hand was selected. We obtained similar results in Experiment 2 except that the persistent left MEP suppression when the left hand was not selected was attenuated when the alternative response involved a non-homologous effector (right foot). These results indicate that, even in the absence of an anticipatory period, inhibitory mechanisms are engaged during response selection, possibly to prevent the occurrence of premature and inappropriate responses during a competitive selection process.
Keywords: inhibition, action selection, competition resolution, impulse control, transcranial magnetic stimulation, motor preparation, movement
Introduction
Most daily life situations require making decisions between several actions (Cisek, 2012; Oliveira, Diedrichsen, Verstynen, Duque, & Ivry, 2010). Computational and neurobiological approaches view decision making as a continuous process in which evidence simultaneously accumulates for different options, with selection occurring when the activity associated with a particular action reaches a threshold (Cisek, 2006; Cos, Duque, & Cisek, 2014; Domenech & Dreher, 2010; Kim & Basso, 2010; Klein-Flugge & Bestmann, 2012; Klein, Olivier, & Duque, 2012; Link & Heath, 1975; Mazurek, Roitman, Ditterich, & Shadlen, 2003; Tosoni, Galati, Romani, & Corbetta, 2008). Many variants of decision-making models assume that inhibitory mechanisms contribute to this accumulation process (Coles, Gratton, Bashore, Eriksen, & Donchin, 1985; Usher & McClelland, 2004) but see Brown and Heathcote (2008)). In general, these inhibitory processes are assumed to help ensure that non-optimal actions are prevented from reaching threshold, although the manner in which they contribute to response preparation and initiation remains the subject of considerable debate (Aron, 2007; Munakata, et al., 2011; Wiecki & Frank, 2013).
TMS applied over the primary motor cortex (M1) has been used to probe the dynamics of corticospinal (CS) excitability during response selection. When preparing a unimanual movement, CS excitability of selected hand muscles increases (Chen & Hallett, 1999). In contrast, nonselected hand muscles typically show a transient decrease in excitability (Duque, et al., 2005; Duque, et al., 2008; Leocani, Cohen, Wassermann, Ikoma, & Hallett, 2000), suggesting the existence of processes that not only promote activation of the selected action, but also inhibition of actions that have not been selected (Klein, Petitjean, Olivier, & Duque, 2014; Koch, et al., 2006). This inhibition, or what we have called “inhibition for competition resolution (CR)”, can sharpen response selection in a competitive process (Duque, Olivier, & Rushworth, 2013; van Campen, Keuken, van den Wildenberg, & Ridderinkhof, 2014). Moreover, when both hands are potential responders, this inhibitory process might be essential to negate the likelihood of mirror movements that could result from bilateral planning (Davare, Duque, Vandermeeren, Thonnard, & Olivier, 2007; Duque, et al., 2009; Swinnen, 2002).
However, it is also possible that CS suppression of a nonselected muscle reflects remnants of anticipatory inhibitory influences that are not directly related to selection processes (Duque & Ivry, 2009). That is, in most choice reaction time studies, the imperative signal is preceded by an alerting cue such as the onset of a fixation marker to initiate the trial. Such cues are included to allow participants to anticipate the task since the instructions generally encourage participants to respond as quickly as possible following the presentation of the imperative. Interestingly, this anticipation includes suppression of task-relevant muscles (Duque, Lew, Mazzocchio, Olivier, & Ivry, 2010; Fetz, Perlmutter, Prut, Seki, & Votaw, 2002; Hasbroucq, Kaneko, Akamatsu, & Possamai, 1999).
The aim of the present study was to re-examine the operation of inhibitory processes during response preparation, employing a design that can separate effects related to anticipation from those related to response selection. In all conditions, participants had to select between a left or right index finger movement. In a high anticipation condition, the imperative signal was preceded by two successive events, a fixation marker and an alerting cue. In a low anticipation condition, we eliminated these two events, precluding any explicit advance warning of the imperative signal. In the latter condition, we also included an unrelated secondary task, making it impossible for the participant to predict the task for the forthcoming trial. These manipulations were expected to greatly reduce the participants’ ability to anticipate the imperative for the choice reaction time (RT) task in the low anticipation condition. While we did not include a direct measure of anticipation (e.g., EEG-based measure such as readiness potential), the RTs in the low and high anticipation conditions provided a proxy: We assumed RTs would be faster in the high anticipation condition.
We measured MEPs elicited in the left hand following TMS of right M1. The TMS pulses were administered either before or after the imperative signal. We predicted that, before the imperative signal, left MEPs would be attenuated relative to baseline in the high anticipation condition, consistent with previous results (Davranche, et al., 2007; Duque & Ivry, 2009; Duque, Labruna, Verset, Olivier, & Ivry, 2012). In contrast, we predicted that MEPs would be unchanged in the low anticipation condition given that the participants could not anticipate the imperative.
Of greater interest was the dynamics of CS excitability changes following the imperative. In the high anticipation condition, left MEPs should remain inhibited when the imperative signals a right hand response, indicative of either anticipatory inhibition and/or the operation of inhibition related to response selection (CR). We considered three possible outcomes for the low anticipation condition. First, if the post-imperative inhibition reported in previous studies is related to selection processes, we would expect to observe a suppression of left MEPs after an imperative signalling a right hand response. Second, observing no post-imperative inhibition in the low anticipation condition would suggest that the inhibition observed in previous studies was due to anticipatory effects. Third, left MEP suppression following left and right hand cues would suggest the recruitment of a more generic inhibitory process during response selection and preparation. We conducted a second experiment in which we varied the relationship between the two response options to explore the generality of these preparatory dynamics.
Methods
Participants
A total of twenty-five right-handed healthy volunteers participated; 10 in Experiment 1 (5 women, 23 ± 1.7 years old) and 15 in Experiment 2 (6 women, 20 ± 0.6 years old). Participants were financially compensated and were naive to the purpose of the study. All participants gave written informed consent under a protocol approved by the Committee for the Protection of Human Subjects at UC, Berkeley.
Experiment 1
Experimental procedure
The participants sat in front of a computer screen with both hands resting on a pillow, palms down and the arms semi-flexed. Responses involved abductions of either the left or right index finger. We used a virtual soccer game task in which the required response was indicated by the position of the “ball” on the computer screen (Fig. 1A). The instructions emphasized that the participants should imagine shooting the ball with the index finger into the goal. In separate blocks of trials, the soccer game was performed under SINGLE or DUAL TASK conditions, designed to create conditions of high and low anticipation, respectively.
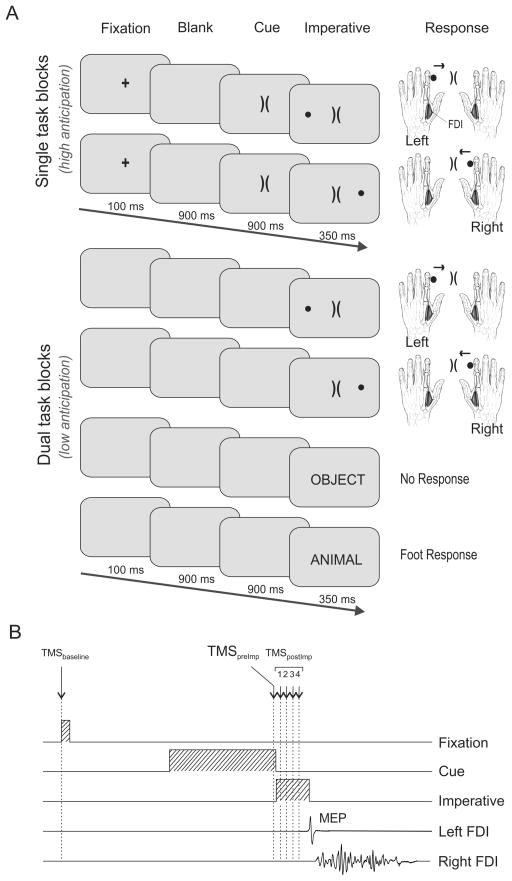
Figure 1.
A: Trial events in Experiment 1. In the SINGLE TASK blocks, a fixation marker (100 ms) signalled the start of the trial. 900 ms later, an uninformative preparatory cue was presented for 900 ms. The imperative signal was then added on the left or right side (350 ms), indicating a left or right index finger abduction movement. In the DUAL TASK blocks, the fixation marker and the preparatory cue were not shown. The soccer trials were intermingled with another task in which the imperative signal consisted of a word depicting an object or an animal. On these trials, participants responded with a left foot extension movement when the word was an animal. No response was required when the word depicted an object.
B: Sequence and timing of trial events. The example depicts a right hand SINGLE TASK trial. A TMS pulse was applied over the right M1 at one of six possible times, selected to assess left hand CS excitability during a baseline probe (TMSbaseline), a pre-imperative probe (TMSpreImp) and four different post-imperative probes (TMSpostImp, see methods for details). FDI = First Dorsal Interosseous (index finger abductor). MEP = Motor Evoked Potential.
Trials in the SINGLE TASK blocks were similar to those used in Duque et al. (2010). Each trial began with the brief presentation (100 ms) of a fixation marker at the centre of the screen. 900 ms later, an alerting cue appeared which consisted of two adjacent central brackets, the “goals”, oriented to the left and right. After 900 ms, an informative imperative signal was added to the display. The imperative was a filled circle, the “ball” and was positioned on the left or right side of the goals. The participant was instructed to perform the specified abduction movement as quickly as possible. To emphasize reaction time, the imperative only remained visible for 350 ms. Note that with this design, response selection was only possible after the appearance of the imperative signal. However, participants could anticipate the imperative given that the fixation marker and alerting cue occurred at fixed intervals in advance of the imperative. The duration of the inter-trial interval was randomly selected to be between 2700 and 3150 ms.
The DUAL TASK condition was designed to minimize the role of inhibitory processes that operate during delay periods between a cue and an imperative signal (Duque & Ivry, 2009). In the DUAL TASK blocks, the soccer trials were randomly intermixed with a secondary task (see Fig. 1A). For the latter, the imperative was a word that appeared at the centre of the screen. Participants responded with a left foot extension when the word denoted an animal and withheld responding when the word denoted an inanimate object. No fixation marker or alerting cue was provided during the DUAL TASK blocks. As a consequence, participants did not know which task (soccer or word) would be performed from trial to trial, nor were there any events to indicate the onset of the imperative. As such, we expected minimal anticipation and/or preparation of the manual responses. As in the SINGLE TASK blocks, the duration of the inter-trial interval was randomly selected to be between 2700 and 3150 ms.
The participants practiced the two block types for a few minutes to become familiar with the basic procedure. The main phase of the experiment consisted of six blocks, two SINGLE TASK blocks (high anticipation) and four DUAL TASK blocks (low anticipation). The blocks for a given condition were run successively, with the order of the two conditions counterbalanced. Each SINGLE TASK block consisted of 96 trials and each DUAL TASK block consisted of 84 trials (42 for each soccer or word task). Within each block, half of the soccer trials required a left hand response and half required a right hand response.
We used single-pulse TMS applied over right M1 to elicit MEPs in the left FDI. This procedure allowed us to assess net changes in CS excitability during response preparation and initiation, regardless of the locus (cortical or spinal) of inhibitory influences (Duque & Ivry, 2009; Duque, et al., 2012; Duque, et al., 2010). Only one TMS pulse was given on each trial, with six possible timings (see Fig. 1B). To establish a baseline, stimulation was applied at the onset of the fixation in SINGLE TASK blocks and at the corresponding time (but with a blank screen) in DUAL TASK blocks. We obtained 16 MEPs (TMSbaseline) in each SINGLE TASK block [= 32 total] and 12 MEPs in each DUAL TASK block [= 48 total]. In each DUAL TASK block, 10 of the TMSbaseline pulses were applied on the word trials and 2 on the soccer trials. We note that, while the TMS pulse could provide an alerting cue for the forthcoming trial, these baseline probes were relatively infrequent. Moreover, given that only one pulse was applied in each trial, any anticipatory effects in these trials should not impact the non-baseline measures.
For the second timing, TMS was applied 10 ms before the imperative signal in SINGLE TASK blocks and at the corresponding time in DUAL TASK blocks, again when the screen was blank (pre-imperative pulse: TMSpreImp). This timing corresponded to 890 ms after the onset of the alerting cue in the SINGLE TASK blocks. We assumed that, at this time, anticipatory inhibitory effects associated with potential index finger movements would be high in the SINGLE TASK blocks and relatively low in the DUAL TASK blocks. Eight MEPs were obtained at this time in each DUAL TASK block [=32 total] and 16 MEPs in each SINGLE TASK block [=32 total].
For the four remaining time points, the TMS pulse occurred 50, 100, 150, or 200 ms after the onset of the imperative signal in the SINGLE TASK blocks and 100, 150, 200, or 250 ms after the onset of the imperative signal in the DUAL TASK blocks (post-imperative period; TMSpostImp1–4). Within a block, 16 MEPs were obtained for each time point for the left and right hand soccer task cues. We used different TMSpostImp timings for the SINGLE and DUAL TASK blocks because pilot work indicated that RTs were slower in the latter, consistent with our assumption that anticipation would be lower in that condition. By using different pulse times, we sought to roughly equate the TMS timings between the two conditions with respect to movement onset.
For the word trials in the DUAL TASK blocks, TMS was applied at the baseline time, but not at the TMSpreImp or TMSpostImp times. As such, most word trials (n=30 in each block) occurred without TMS.
Transcranial magnetic stimulation
TMS was applied using a figure-of-eight magnetic coil (diameter of wings 70 mm) connected to a Magstim 200 magnetic stimulator (Magstim, Whitland, Dyfed, UK). The magnetic coil was placed tangentially on the scalp over right M1. The handle was oriented backward and laterally at a 45° angle away from the midline, approximately perpendicular to the central sulcus. After fitting the participant with a tight EEG cap, we first identified the optimal spot for eliciting MEPs in the left first dorsal interosseous (FDI), the index finger agonist for this task (abduction). This hotspot was marked on the EEG cap to provide a reference point for the experimental session. At this position, MEPs could also be elicited in the left Abductor Digiti Minimi (ADM), a pinkie abductor, in most subjects (n=9), providing a measure of CS excitability changes associated with a task-irrelevant muscle.
The resting motor threshold (rMT) was defined as the minimal TMS intensity required to evoke MEPs larger than 50 μV peak-to-peak in the relaxed FDI on 5 out of 10 consecutive trials. Across participants, the rMT corresponded to 46% [SE=2.1] of maximum stimulator output (MSO). The intensity of TMS was always set at 115% of rMT. Using this intensity, the mean peak-to-peak amplitude of left FDI MEPs during the baseline period was 1.27 mV [SE=0.18] and 1.12 mV [SE=0.15] for the SINGLE and DUAL TASK blocks, respectively (n=10). These values were not significantly different (p>0.2). In nine participants, MEPs were also consistently evoked in the left ADM. The amplitude of these MEPs at baseline were 0.48 mV [SE=0.09] and 0.50 mV [SE=0.09] for the SINGLE and DUAL TASK blocks, respectively. MEPs were larger in the FDI than in the ADM (main effect of MUSCLE in the SINGLE TASK, F=13.4, p=0.008, and DUAL TASK, F=14.2, p=0.007, conditions), consistent with the fact that the TMS location was chosen to obtain the best response in the FDI (and not in the ADM).
EMG Recording
EMG activity was recorded from surface electrodes placed over the left and right FDI muscles as well as over the left ADM. EMG activity was also recorded in the left gastrocnemius muscle to measure RT for the word trials in the DUAL TASK blocks. EMG data were collected for 2000 ms on each trial, starting a minimum of 200 ms before the TMS pulse. The EMG signals were amplified and bandpass filtered on-line (50–2000 Hz; Delsys Inc., Boston, USA), and stored on a personal computer for off-line analysis. This off-line analysis included the measurement of RTs and the peak-to-peak amplitude of the MEPs. RT was defined as the time interval between the onset of the imperative signal and a movement-related increase in the EMG activity of the agonist (FDI for soccer task; gastrocnemius for word task). In order to prevent contamination of MEP measurements by background EMG activity, trials with any EMG activity greater than 100μV in the 200 ms window preceding the TMS artefact were excluded from the analysis (Duque & Ivry, 2009; Duque, et al., 2007). All trials in which the subjects provided the wrong response were also not included in the analysis. Given that the task was very easy, this represented a minimal number of trials.
Data selection for MEPs elicited at TMSpostImp
Because the amplitude of MEPs changes rapidly just prior to EMG onset in an agonist muscle, we selected left hand MEP data with respect to RT onset, focusing on those trials in which the TMS pulse was applied late after the imperative signal (TMSpostImp_late), just before EMG onset. For this analysis, we identified trials in which the TMS pulse was applied from 100 ms to 20 ms before EMG onset. Within this epoch, TMS occurred on average 70 ± 1.6 ms and 74 ± 1.6 ms prior to EMG onset in the SINGLE and DUAL TASKS, respectively, thus equating the timing for the two conditions with respect to movement onset. Note that this window was applied regardless of whether the response was made with the left (selected) or right (nonselected) hand. On average, the MEPs included in this window were elicited 112 ± 4.9 ms and 110 ± 4.7 ms after the imperative onset in the selected (mean number of trials: 23 ± 3.5) and nonselected conditions (mean number of trials: 26 ± 2.3) of the SINGLE TASK blocks. The corresponding values in the DUAL TASK blocks were 190 ± 5.5 ms (selected; mean number of trials: 14 ± 2.8) and 194 ± 4.9 ms (nonselected; mean number of trials: 22 ± 1.7). Given the longer RTs in the DUAL TASK, we were also able to define a second (earlier) epoch that extended from 160 ms to 100 ms prior to EMG onset (TMSpostImp_early, mean time: 130 ± 1.2 ms prior to EMG onset). On average, the MEPs included in this earlier window were elicited 150 ± 10.9 ms (selected; mean number of trials: 20 ± 2.0) and 133 ± 9.0 ms (nonselected; mean number of trials: 17 ± 1.4) after the imperative onset. There were insufficient trials falling within this window in the SINGLE TASK condition. For each trial, we also recorded the MEP value for the left ADM to obtain a measure of CS excitability changes in a task-irrelevant muscle (again, in the selected or nonselected hand depending on whether the response was performed with the left or right hand).
Statistical analyses
To compare RTs in the two tasks, we restricted the data to trials in which TMS was applied before the imperative signal (TMSbaseline and TMSpreImp). The RT data from these trials were analysed with a two-way repeated-measure ANOVA (ANOVARM) with TASK (single, dual) and HAND (left, right) as factors. To assess the impact of TMS on RTs, we also performed separate ANOVAs for the two tasks with the factors HAND (left, right) and TMS-CONDITION (TMSbaseline&preImp, TMSpostImp_late and, for the DUAL TASK blocks, also TMSpostImp_early).
For the evaluation of CS excitability, we first performed an analysis of the MEP data for each task (SINGLE and DUAL) separately. A logarithmic transformation was applied to the raw MEP values and these normalized data were analyzed in a two-way ANOVARM with MUSCLE (FDI, ADM) and TMS-CONDITION (TMSbaseline, TMSpreImp, TMSpostImp_late-selected, TMSpostImp_late-nonselected and, for the DUAL Task blocks, also TMSpostImp_early-selected, TMSpostImp_early-nonselected) as factors. In a second analysis, we directly compared CS excitability changes in the two tasks. MEPs elicited at TMSpreImp and TMSpostImp_late were defined with respect to baseline (%), with these normalized values again log-transformed. To assess the impact of anticipatory preparation on MEPs elicited at TMSpreImp, we used a two-way ANOVARM with the factors TASK (single, dual) and MUSCLE (FDI, ADM). In addition, to compare the MEPs elicited at TMSpostImp_late in the SINGLE and DUAL TASKS, we used a three-way ANOVARM with TASK (single, dual), TMS-CONDITION (selected, nonselected) and MUSCLE (FDI, ADM) as factors. We did not include the TMSpostImp_early data given that we did not have these data for the SINGLE TASK. When appropriate, post hoc comparisons were conducted using the Fisher’s LSD procedure. All of the data are expressed as mean ± SE.
Experiment 2
We conducted a second experiment to examine how CS excitability changes after an imperative signal are influenced by the relationship between the response alternatives. In a recent study (Labruna, et al., 2014), CR inhibition during a delay period was found to be modulated by the relationship of the different response alternatives. CR was stronger in a task that required choosing between left and right finger responses (Finger-Finger choice) compared with a task that required choosing left finger responses against right foot responses (Finger-Foot choice), perhaps reflecting distances in cortical space or the degree to which the actions compete in natural behavior. We aimed at testing whether a similar effect of choice is observed when inhibitory changes are probed after the imperative signal.
Fifteen naïve participants were tested in a modified version of the DUAL TASK condition. Similar to Experiment 1, subjects performed word and soccer trials that were intermingled within the same blocks. However, the soccer trials in Experiment 2 involved Finger-Finger or Finger-Foot choices, with the pairs tested in separate blocks. MEPs were elicited in the left hand following TMS over right M1 for both pairings. Because the right foot was used as one of the two responses in the Finger-Foot blocks, participants were now required to make vocal responses for word trials, reading the word when it denoted an animal and making no response when the word denoted an inanimate object. MEPs were elicited at baseline (TMSbaseline) or at one of the four post-imperative timings (TMSpostImp1–4); we did not include a TMSpreImp timing as we were interested in focusing on MEP changes that occur after the imperative signal in the two choice conditions. All other aspects were identical to Experiment 1. The rMT of subjects was 43% [SE=1.0] of MSO and the intensity of TMS set at 115% of rMT.
For the RT analysis, we only used trials in which TMS was applied at TMSbaseline. These data were analysed using a two-way ANOVARM with CHOICE (Finger-Finger, Finger-Foot) and HAND (left, right) as factors. We did not evaluate RTs when the TMS was applied after the imperative since the results of Experiment 1 showed that the TMS pulse altered RTs in a response-specific manner (see below).
For the analysis of CS excitability, the left FDI MEPs were assessed as a function of whether the left index finger was selected or nonselected for the forthcoming response. In addition, we calculated the average MEP amplitudes at TMSpostImp_early (epoch extending from 160 ms to 100 ms prior to EMG onset) and TMSpostImp_late (epoch extending from 100 ms to 20 ms prior to EMG onset). MEPs at these two post-imperative epochs had to be analysed separately as we only had sufficient data sets (minimum of 8 MEPs falling withing this window) from 10 of the 15 participants for the TMSpostImp_late condition when the left hand was selected for the forthcoming response. On average, the MEPs in the TMSpostImp_early window were elicited 173 ± 8.4 ms and 153 ± 6.2 ms after the imperative onset in the selected (n=10, mean number of trials: 14 ± 1.4) and nonselected (n=15: mean number of trials: 15 ± 1.1) trials of the Finger-Finger choice condition. The comparable values for the Finger-Foot condition were 182 ± 6.0 ms (selected; n=10, mean number of trials: 12 ± 1.1) and 161 ± 7.0 ms (nonselected; n=15, mean number of trials: 14 ± 1.0). On average, the MEPs included in the TMSpostImp_late were elicited 199 ± 4.0 ms (selected; n=10, mean number of trials: 17 ± 1.8) and 192 ± 5.3 ms (nonselected; n=15, mean number of trials: 18 ± 1.9) after the imperative onset in the Finger-Finger condition. For the Finger-Foot condition, the values were 210 ± 3.8 ms (selected; n=10, mean number of trials: 11 ± 1.4) and 197 ± 4.8 ms (nonselected; n=15, mean number of trials: 11 ± 1.1).
For the analysis of MEPs at TMSpostImp_early (n=15; always log-transformed), we used a two-way ANOVARM with CHOICE (Finger-Finger, Finger-Foot) and TMS-CONDITION (TMSbaseline, TMSpostImp_early-selected, TMSpostImp_early-nonselected) as factors; one-tailed paired t-tests were used for the post hoc comparisons given our a-priori hypothesis based on Experiment 1 and a previous study (Labruna, et al., 2014). We also assessed CS excitability just before response initiation by comparing the amplitude of MEPs at TMSpostImp_late (log-transformed) to baseline using paired t-tests (always one-tailed). For this analysis, we had data for 10 participants in the selected condition and 15 participants in the nonselected condition. Finally, when appropriate, one-tailed paired t-tests were also used to compare MEPs elicited at TMSpostImp_late with those evoked at TMSpostImp_early. All of the data are expressed as mean ± SE, similar to Experiment 1.
Results
Experiment 1
Reaction times
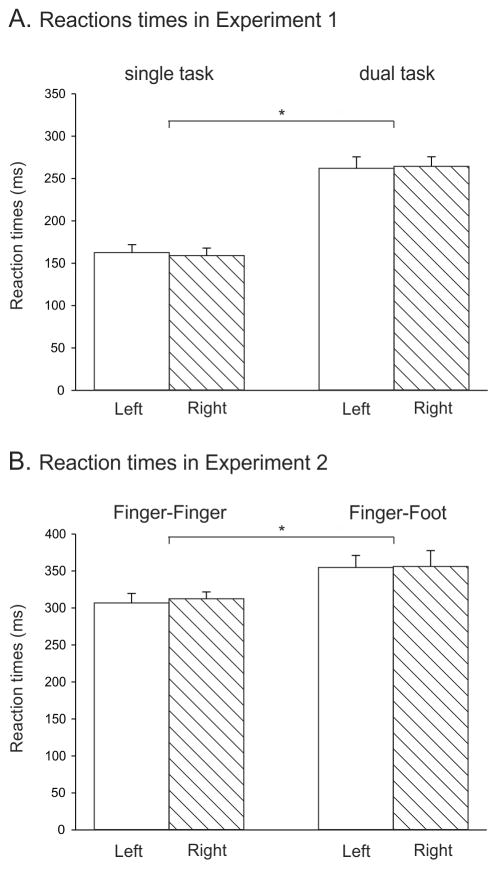
RTs in the SINGLE TASK blocks were considerably faster than in the DUAL TASK blocks (main effect of TASK: F=112.9, p<0.0001, see Fig. 2A). The mean RT of the left index finger was 163 ms [SE=8.7] and 262 ms [SE=14.2] in the SINGLE and DUAL TASK conditions, respectively. The corresponding means for the right index finger were 160 ms [SE=8.2] and 265 ms [SE=11.2]. There was no effect of HAND or HAND X BLOCK interaction. The 100 ms advantage in the SINGLE TASK blocks is consistent with our assumption that the fixation maker and alerting cue, as well as task certainty, allowed participants to anticipate the imperative signal in the SINGLE TASK blocks, even if the actual response remained unknown during the delay period. Note that the RTs on these trials (TMSbaseline and TMSpreImp) in the DUAL TASK may be faster than on no-TMS DUAL TASK trials (data not available) because the TMS pulse may have provided an alerting signal.
Figure 2.
A: Group means with SE bars of Experiment 1 reaction times (RT, ms) on trials in which TMS was applied before the imperative signal (TMSbaseline and TMSpreImp) for left (white fill) and right (dashed fill) index finger responses in SINGLE TASK BLOCKS (left side) and DUAL TASK BLOCKS (right side). B: RT data for DUAL TASK BLOCKS of Experiment 2 on trials in which TMS was applied at TMSbaseline. * = p < 0.05 for the comparisons indicated.
The HAND X TMS-CONDITION interaction was significant for both tasks (both F>5.3, t<0.02). Applying a TMS pulse just before movement onset (TMSpostImp_late) delayed RTs when the response had to be provided with the left hand. As such, RTs were longer for the left hand in the SINGLE TASK (193 ± 6.3 ms, p<0.0001 when compared to TMSbaseline&preImp) and DUAL TASK (275 ± 6.3 ms, p<0.03) blocks but not for right hand responses (167 ± 5.6 and 258 ± 5.7 ms, respectively, both p>0.1). In the DUAL TASK, RTs were also prolonged when TMS was applied relatively early in the RT interval (TMSpostImp_early) preceding left index finger (283 ± 11.4 ms, p<0.001) but not right index finger responses (263 ± 9.7 ms, p>0.7). Hence, TMS delayed RTs when the response required a contraction of the muscle (FDI) in which MEPs were elicited, but not when the response involved a contraction of the homologous muscle in the other hand.
CS excitability
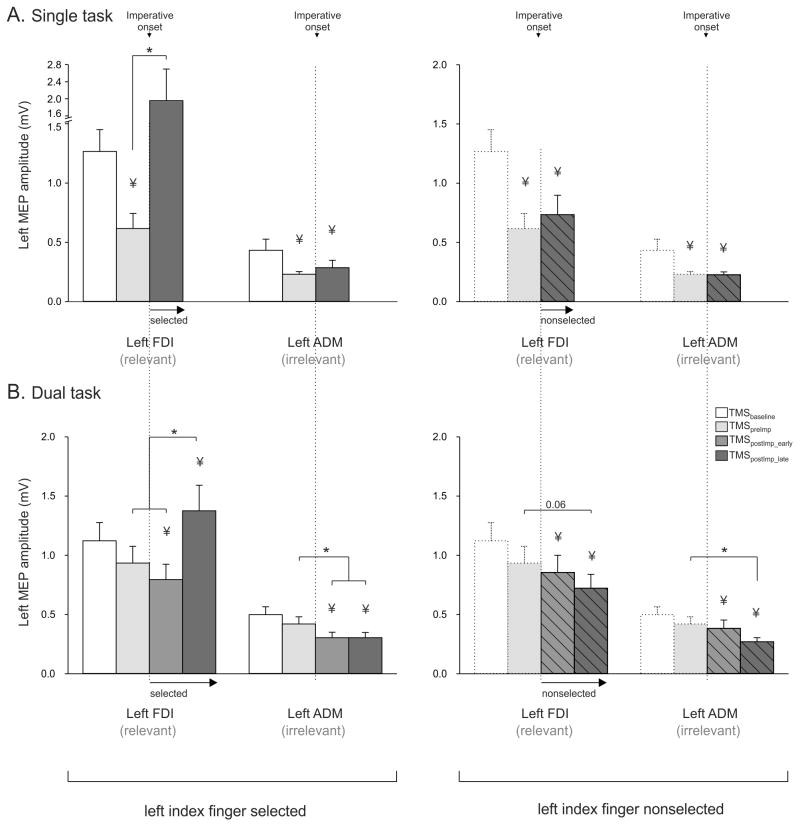
Consistent with previous reports (Duque, et al., 2012; Duque, et al., 2010), marked inhibition was observed in the SINGLE TASK blocks when participants were in a heightened state of anticipation for the imperative signal (TMSpreImp; p<0.0001 when compared to TMSbaseline, see Fig. 3A). This inhibition was observed despite the fact that the actual response was only specified by the imperative signal. When the imperative signalled a left hand response, this inhibition reversed such that the MEPs became strongly facilitated (TMSpostImp_late-selected relative to TMSpreImp; p<0.0001). In contrast, when the imperative signalled a right hand response, the inhibition persisted (TMSpostImp_late-nonselected relative to TMSpreImp; p=0.35). We also found a significant suppression of the task-irrelevant ADM muscle just prior to the imperative, relative to baseline (TMSpreImp: p<0.004, see Fig. 3A). This suppression of left ADM MEPs remained after the imperative signal, regardless of the response side (TMSpostImp_late relative to TMSpreImp; both selected and nonselected p>0.25). These observations were supported by a significant MUSCLE X TMS-CONDITION interaction (F=4.7, p<0.02).
Figure 3.
Group means and SE bars (Experiment 1, n=10) of MEP amplitudes (mV), recorded from the left first dorsal interosseous (FDI) and the left adbuctor digiti minimi (ADM) following TMS of right M1 in the SINGLE (A) and DUAL TASK (B) blocks. TMSbaseline are from MEPs elicited at the onset of the fixation cross. TMSpreImp are from MEPs elicited just prior to the onset of the imperative signal (vertical dashed line). TMSpostImp are from MEPs elicited after the imperative signal, with the MEPs divided into bins in which the pulse occurred 160 ms to 100 ms (TMSpostImp_early) or from 100 ms to 20 ms (TMSpostImp_late) before EMG onset for left index finger responses (left side, uniform fill) or right index finger responses (right side, dashed fill). MEPs are shown as raw data but were log-transformed for statistical analyses. * = p < 0.05 for the comparisons indicated. ұ = p < 0.05 when compared with MEPs elicited at TMSbaseline. FDI = First Dorsal Interosseous (task-relevant muscle: index finger abductor). ADM = Abductor Digiti Minimi (task-irrelevant muscle: pinkie abductor).
A different pattern of CS excitability changes was observed in the DUAL TASK condition (Fig. 3B). Left FDI MEPs showed a small reduction in amplitude prior to the onset of the imperative signal, although this effects was not reliable (TMSpreImp compared to TMSbaseline; p=0.10). When the full set of timings were considered, there was a significant MUSCLE X TMS-CONDITION interaction (F=7.1, p<0.0002). Surprisingly, there was a significant reduction in the MEPs when the TMS pulse was applied early in the reaction time interval. This suppression was observed on both left and right hand trials (TMSpostImp-early relative to TMSbaseline; selected and nonselected p<0.02). Hence, in a state of low anticipation, inhibitory processes are recruited during the initial stages of response selection and preparation. The response for the selected and nonselected hands diverge when excitability is probed just before the onset of the response (TMSpostImp-late), with the pattern similar to that observed in the SINGLE TASK blocks: Left FDI MEPs became facilitated on left hand trials (TMSpostImp_late-selected p<0.007) and remained suppressed on right hand trials (TMSpostImp_late-nonselected relative to TMSbaseline; p<0.001).
MEPs elicited in the ADM were not significantly suppressed at TMSpreImp (when compared to TMSbaseline). However, MEPs were inhibited after the onset of the imperative signal for this task irrelevant muscle. Moreover, this effect was observed on left and right hand trials (TMSpostImp_early relative to TMSbaseline; both p<0.01) and remained attenuated close to EMG onset (TMSpostImp_late relative to TMSbaseline; both p<0.007).
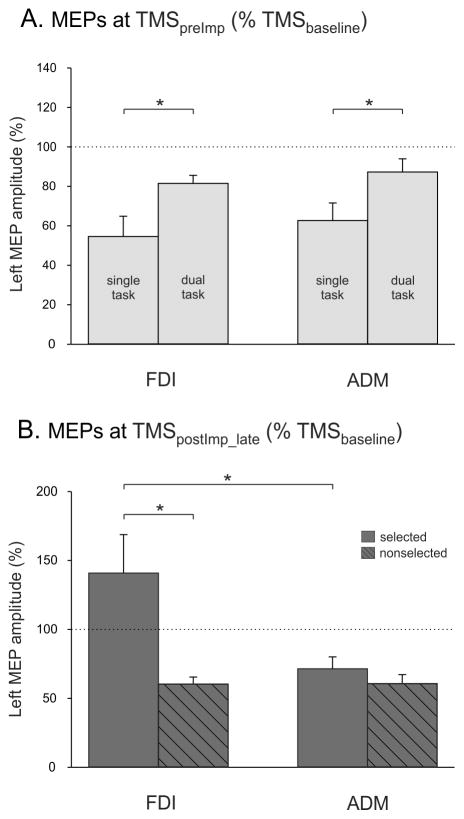
We performed a direct comparison of the SINGLE and DUAL TASKS, expressing the MEPs as a percentage of baseline (see Fig. 4AB). At TMSpreImp, the FDI and ADM MEPs were smaller in the SINGLE TASK condition compared to the DUAL TASK condition (F=8.76, p=0.018; Fig. 4A), consistent with an inhibitory process being invoked when anticipation is high. This effect occurred regardless of the muscle (TASK x MUSCLE F=1.70, p=0.23). Close to EMG onset (at TMSpostImp_late), there was a significant MUSCLE X TMS-CONDITION interaction (F=23.64, p=0.0018; Fig 4B). When averaged across the two tasks (the triple interaction was not significant: F=0.12, p=0.74), FDI and ADM MEPs remained suppressed when the imperative signalled a right hand response (nonselected condition, comparison of FDI and ADM p=0.12). In contrast, when the left index finger was selected, the left FDI MEPs became facilitated, whereas the left ADM MEPs remained suppressed (p<0.0001 when compared to the FDI MEPs).
Figure 4.
Group means and SE bars (Experiment 1, n=10) of left FDI and ADM MEP amplitudes, expressed with respect to baseline (horizontal dashed line), elicited (A) prior to the imperative signal (TMSpreImp) or (B) during the interval 100 ms to 20 ms (TMSpostImp_late) prior to the onset of the EMG for left index finger (selected) or the right index finger (nonselected) trials. MEPs are shown as raw data (% Baseline) but were log-transformed for statistical analyses. * = p < 0.05 for the comparisons indicated.
Experiment 2
Reaction times
The mean RT of the left index finger responses was 307 ms [SE=12.9] and 355 ms [SE=16.3] in the Finger-Finger and Finger-Foot choice conditions, respectively. The corresponding means for the right effectors (index finger or foot, respectively) were 312 ms [SE=9.3] and 356 ms [SE=21.4]. Participants were faster in making Finger-Finger than Finger-Foot choices (main effect of CHOICE F=12.5, p<0.004, see Fig. 2B). There was no effect of HAND or HAND X CHOICE interaction (all F<0.1, all p>0.75). We note that the mean RTs in the Finger-Finger condition are about 50 ms longer than in Experiment 1, an effect we assume reflects a random variation between the two samples.
CS excitability
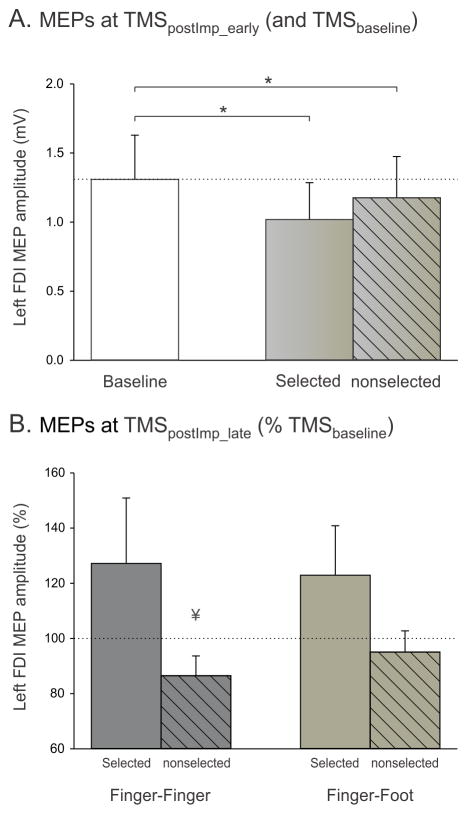
In this experiment, we compared the post-imperative amplitude of MEPs when the DUAL TASK required a Finger-Finger or a Finger-Foot choice. Our main goal was to investigate whether the post-imperative inhibitory changes observed in the DUAL TASK of Experiment 1 using a Finger-Finger choice would generalize (or not) to another type of choice, one that does not involve homologous muscles. The ANOVARM revealed that at TMSpostImp_early, the MEPs were significantly reduced with respect to MEPs elicited at TMSbaseline (factor TMS-CONDITION F=4.98, p<0.02; see Fig. 5A with data collapsed over Finger-Finger and Finger-Foot pairings); this suppression occurred regardless of whether the MEPs were elicited in a trial when the left index finger was selected or nonselected (both t>2.13, p<0.026) and regardless of the type of choice (TMS-CONDITION X CHOICE F=0.67, p=0.52; factor CHOICE F=0.10, p=0.76). Hence, at TMSpostImp_early, both selected and nonselected MEPs are suppressed and this effect is present during Finger-Finger and Finger-Foot choices.
Figure 5.
Group means and SE bars of left FDI MEP amplitudes in the DUAL TASK blocks of Experiment 2. A: Mean MEPs (mV) at TMSbaseline and TMSpostImp_early for the selected (uniform fill) and nonselected (dashed fill) conditions. Data here are collapsed over the Finger-Finger and Finger-Foot conditions. MEPs are shown as raw data but were log-transformed for statistical analyses. B: MEPs at TMSpostImp_late expressed relative to baseline for the Finger-Finger and Finger-Foot conditions. ұ = p < 0.05 when compared with MEPs elicited at TMSbaseline. * = p < 0.05 for the comparisons indicated.
Figure 5B depicts CS excitability changes, relative to baseline, just prior to EMG onset (TMSpostImp_late). The figure allows a visual comparison across the two choice conditions, although statistics were performed on raw (log-transformed) data. The left hand MEPs showed the expected increase when the left hand was selected for the forthcoming response in both the Finger-Finger and Finger-Foot conditions. As such, left hand MEPs at TMSpostImp_late were no longer suppressed with respect to baseline (both t<0.74, p>0.48) and were also significantly greater than those elicited at TMSpostImp_early (both t>1.98, p<0.04). When the left hand was not selected for the forthcoming response, the left hand MEPs elicited at TMSpostImp_late only remained suppressed in the Finger-Finger condition (t=2.076, p=0.03 when compared to baseline). This persistent CS suppression of a nonselected muscle was not found in the Finger-Foot choice condition (t=0.94, p=0.182), although a direct comparison of the nonselected MEPs in the Finger-Finger and Finger-Foot conditions was not significant (t=1.29, p=0.11). The amplitude of nonselected MEPs at TMSpostImp_late in the Finger-Foot condition was also significantly larger than that elicited at the early post-imperative TMS probe (t=2.083, p=0.03), an effect not observed in the Finger-Finger condition (t=0.05, p=0.48). These findings suggest a release of inhibition just before response onset when the left hand was nonselected in the Finger-Foot choice condition.
Discussion
TMS has proven to be a powerful tool to examine the dynamics of CS excitability changes in targeted muscles during movement preparation and initiation. In the early studies using this method, facilitation was observed for muscles associated with the forthcoming response and inhibition was observed for muscles associated with nonselected responses (Chen & Hallett, 1999; Duque, et al., 2005; Leocani, et al., 2000). These findings led to the hypothesis that inhibitory mechanisms are recruited during response selection to suppress activity in muscles associated with actions that are not selected (Koch, et al., 2006; Schel, et al., 2014; van den Wildenberg, et al., 2010), a process we have termed competition resolution (CR) (Duque, et al., 2010).
These previous studies used choice RT tasks in which an imperative stimulus both indicated the appropriate response and served as a signal for movement initiation. Moreover, this imperative was preceded by an alerting cue (e.g., a fixation marker), included to allow the participants to prepare for the task. Inhibition of CS excitability can be observed in task-relevant muscles following the presentation of such alerting cues (Hasbroucq, et al., 1999). This inhibition is evident with both non-informative and informative cues, and in the latter case, pronounced in both selected and nonselected muscles (Duque, et al., 2012; Duque, et al., 2010).
The presence of cue- or imperative-related inhibition is problematic. As such, it is possible, as generally assumed, that the post-imperative inhibition observed in nonselected muscles reflects the operation of a mechanism related to response selection (e.g. CR). Alternatively, it might also be the remnant of an inhibition associated with task anticipation. The aim of the present study was to disentangle these hypotheses. To do so, we devised a condition that minimized task anticipation by eliminating alerting cues and introducing task uncertainty (DUAL TASK blocks).
In Experiment 1, we compared CS excitability changes occurring during movement preparation in this low anticipation condition with those occurring when using a standard high anticipation procedure (SINGLE TASK blocks). Consistent with previous work, in the SINGLE TASK blocks, left FDI MEPs were strongly suppressed prior to the imperative signal (Davranche, et al., 2007; Hasbroucq, et al., 1999; Touge, Taylor, & Rothwell, 1998; van Elswijk, Kleine, Overeem, & Stegeman, 2007). Following the informative imperative, left FDI MEPs increased when a left hand response was selected. We did not observe any further strengthening of inhibition when the left hand was not selected, preceding a right hand response. Hence in this task, the post-imperative inhibition observed in a nonselected muscle might reflect the sustained effect of a process associated with anticipation per se, or the emergence of a CR inhibitory effect after the imperative has indicated the required response.
The DUAL TASK blocks help shed light on these alternatives. When anticipation was low, pre-imperative inhibition was markedly attenuated relative to baseline. Following the onset of the imperative, left FDI MEPs became significantly inhibited regardless of whether the trial required a left or right hand response (Fig. 3B). Then, the pattern diverged as a function of the forthcoming response. On left hand trials, the MEPs quickly became facilitated. In contrast, on right hand trials, the MEPs showed a trend of becoming more inhibited over time. Hence, when anticipatory processes are minimized before the imperative signal, an initial inhibitory effect was observed in all task-relevant respondents, followed by response specific changes that include inhibition of nonselected muscles. The presence of post-imperative inhibition, even in the absence of anticipation, provides the most compelling evidence of the operation of an inhibitory process associated with response selection.
Though, the early inhibition observed in left FDI following an imperative signal indicating a left hand response is puzzling. This effect is clearly seen in delay tasks where a cue indicates the forthcoming response in advance of an imperative. We have proposed that inhibition of the selected response under such conditions reflects a distinct mechanism, one designed to prevent premature responses, or what we have called impulse control (IC) (Duque, et al., 2012; Duque, et al., 2010). As such, several lines of evidence support the idea of two separate inhibitory mechanisms operating during action selection (e.g., IC and CR), including evidence that only inhibition of the selected effector is manifest at the spinal level (Duque, et al., 2010) and that TMS-induced disruption of lateral prefrontal cortex and dorsal premotor cortex has differential effects on signatures of these two processes (Duque, et al., 2012).
The initial post-imperative inhibition in the low anticipation condition of Experiment 1 might reflect either process. It may be that inhibition is targeted at both responses to prevent premature movements while the participant identifies the required response. Or it may be that the discrimination process engages inhibition related to CR, similar to that described in some accumulation models of decision making (Coles, et al., 1985; Usher & McClelland, 2004); but see also Brown and Heathcote (2008)). The fact that early inhibition was similar for selected and nonselected trials would favor the former hypothesis. That is, based on the latter hypothesis, one would expect greater CR inhibition in the nonselected condition given the assumption that the operation of discrimination processes would quickly favor the selected response, a pattern of change that we did not observe.
Regardless of the underlying mechanism, the current results indicate that inhibition of the selected response is not specific to delayed response tasks. Interestingly, previous studies have shown inhibitory changes in selected response representations following imperative signals in choice RT tasks but not in simple RT task (Chen & Hallett, 1999; Leocani, et al., 2000). Hence, it may be that this form of inhibition is automatically engaged during response preparation, keeping movements in check until a selection threshold is reached, an interesting question for future investigation.
To further explore post-imperative inhibition, we conducted a second experiment in which we varied the relationship between the two response options. In a recent study (Labruna, et al., 2014), we observed that only the magnitude of CR was modulated by the relationship between the response options, with greater inhibition of nonselected responses when the two alternatives were similar; IC was comparable for all pairings. In that study, we proposed that the efficacy of CR is a function of the cortical overlap of the effector representations (e.g., stronger for finger-hand pairings compared to finger-foot) or the degree to which a pair of effectors compete in natural behavior.
In Experiment 2, we asked if the post-imperative inhibition of nonselected responses would also be sensitive to the relationship of the response pair in our low anticipation condition, comparing here a condition in which the left index finger was either paired with a right index finger (Finger-Finger choice) or with a right foot response (Finger-Foot choice). As in Experiment 1, left FDI MEPs were suppressed right after the imperative, regardless of the response pairing or whether this finger was selected or not for the forthcoming response. This inhibition quickly turned to facilitation when the left hand was selected. Of critical interest is the finding that just prior to EMG onset, the left hand inhibition persisted when the selected response involved the right hand, but not when the selected response involved the right foot. This result provides additional evidence for the operation of inhibitory processes during response selection that cannot be attributed to anticipation. In addition, the fact that the relationship of the two competing alternatives influenced nonselected MEP suppression close to movement onset is consistent with a CR origin of the inhibitory effect observed at that timing (Labruna, et al., 2014).
One caveat is in order here. The direct comparison of left FDI MEPs in the nonselected condition for the Finger-Finger and Finger-Foot conditions was only marginally reliable. The dissociation of these pairings is based on the finding that left FDI remained inhibited at the late timing in the Finger-Finger condition and not in the Finger-Foot condition (Fig. 5B), as well as the finding that left FDI excitability increased from the early to late epochs in the Finger-Foot but not in the Finger-Finger condition. These within-pairing contrasts seem most appropriate here given the RT differences between the two pairings. As such, while the MEP bins are equated with respect to movement onset, they differ with respect to the onset of the imperative.
In Experiment 1, we concurrently obtained recordings of left FDI and left ADM, providing an opportunity to compare CS excitability changes occurring in a task-relevant and a task-irrelevant muscle. Overall, the results for left ADM were quite similar to those observed for left FDI when the left hand was not selected for the forthcoming response. In the SINGLE TASK blocks, MEPs elicited in left ADM were suppressed prior to the imperative and remained suppressed throughout the selection and initiation period of left and right finger responses. In the DUAL TASK blocks, ADM MEPs were only inhibited after the onset of the imperative, and remained so independent of whether left FDI was selected or not selected for the forthcoming response.
These results suggest that inhibition related to anticipation influences representation of both task-relevant and task-irrelevant muscles. We note that in an earlier study, we did not observe changes in CS excitability in a task-irrelevant muscle in the delayed response task (Duque, et al., 2010). One major difference between the two studies is that, for the prior study, the left hand was task-irrelevant (both potential responses were with the right hand), whereas here the left hand was task-relevant. It may be that the target of anticipatory inhibitory influences is not completely specific in that it extends to motor representations that “surround” task-relevant muscles (Touge, et al., 1998). Further evidence that anticipation-related inhibition has some degree of task specificity comes from a study showing that when an anticipated response does not involve the hands (e.g. reading), facilitation (rather than inhibition) is observed in hand MEPs (Seyal, Mull, Bhullar, Ahmad, & Gage, 1999).
Future studies are required to identify the neural mechanisms underlying post-imperative inhibitory effects observed when participants are in a low state of anticipation. As a starting point, reflex measurements could be obtained to determine if inhibition is manifest at the spinal level or restricted to supraspinal levels. Our previous results with this method using a delayed response task (Duque et al., 2010) suggest that attenuation of the H-reflex is associated with inhibition required to prevent premature responses rather than competition between response alternatives. It would be interesting to determine if inhibition occurring during the post-imperative period is also evident at the spinal level. One may also measure the duration of silent periods following single-pulse TMS (Davranche, et al., 2007) or intra-cortical inhibitory effects following paired-pulse TMS (Duque & Ivry, 2009) to evaluate specifically the cortical component of inhibitory effects during this period. Finally, the combination of rTMS and single-pulse TMS offers the opportunity to investigate the origins of post-imperative inhibition (Duque et al., 2012). Possible candidates are the pre-supplementary motor area, the lateral prefrontal cortex and the premotor cortex (Duque, et al., 2012; Duque, et al., 2013). Investigating the role of the basal ganglia would require the use of alternative methodologies (Jahfari, et al., 2012; Wiecki & Frank, 2013).
In conclusion, by using a DUAL TASK procedure, we were able to examine inhibitory effects observed during response preparation, evaluating the contribution of anticipatory and preparatory processes. Even in the absence of anticipation, CS excitability shows an initial attenuation after the presentation of an imperative signal. This inhibition may be similar to the inhibition of selected muscles in delayed response tasks, or what we have called IC; as stimulus discrimination and response selection unfolds, an inhibitory mechanism is recruited to prevent premature responding. Alternatively, it could reflect the reciprocal operation of a competitive process, with each possible response (and surrounding representations) being inhibited. As the selection process evolves, CS excitability for the selected and nonselected responses diverge with an intriguing degree of task specificity that depends on the relationship between the two response alternatives, consistent with the operation of a CR form of inhibition.
Highlights.
Used TMS to assess inhibition during movement preparation and initiation.
Manipulated task expectancy to unconfound anticipation and preparation.
Even in state of low anticipation, corticospinal excitability was inhibited.
These results support the view that inhibitory processes assist response selection.
Acknowledgments
This work was supported by the National Institute of Health (NS0085570, NS040813) and the National Institute of General Medical Sciences (T34GM092702). J.D. was supported by the Belgian National Funds for Scientific Research (FRS - FNRS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Brown SD, Heathcote A. The simplest complete model of choice response time: linear ballistic accumulation. Cogn Psychol. 2008;57:153–178. doi: 10.1016/j.cogpsych.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Chen R, Hallett M. The time course of changes in motor cortex excitability associated with voluntary movement. Can J Neurol Sci. 1999;26:163–169. doi: 10.1017/s0317167100000196. [DOI] [PubMed] [Google Scholar]
- Cisek P. Integrated neural processes for defining potential actions and deciding between them: a computational model. J Neurosci. 2006;26:9761–9770. doi: 10.1523/JNEUROSCI.5605-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Making decisions through a distributed consensus. Curr Opin Neurobiol. 2012;22:927–936. doi: 10.1016/j.conb.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Coles MG, Gratton G, Bashore TR, Eriksen CW, Donchin E. A psychophysiological investigation of the continuous flow model of human information processing. J Exp Psychol Hum Percept Perform. 1985;11:529–553. doi: 10.1037//0096-1523.11.5.529. [DOI] [PubMed] [Google Scholar]
- Cos I, Duque J, Cisek P. Rapid Prediction of Biomechanical Costs during Action Decisions. J Neurophysiol. 2014;112(6):1256–66. doi: 10.1152/jn.00147.2014. [DOI] [PubMed] [Google Scholar]
- Davare M, Duque J, Vandermeeren Y, Thonnard JL, Olivier E. Role of the ipsilateral primary motor cortex in controlling the timing of hand muscle recruitment. Cereb Cortex. 2007;17:353–362. doi: 10.1093/cercor/bhj152. [DOI] [PubMed] [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 2007;25:3766–3774. doi: 10.1111/j.1460-9568.2007.05588.x. [DOI] [PubMed] [Google Scholar]
- Domenech P, Dreher JC. Decision threshold modulation in the human brain. J Neurosci. 2010;30:14305–14317. doi: 10.1523/JNEUROSCI.2371-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Davare M, Delaunay L, Jacob B, Saur R, Hummel F, Hermoye L, Rossion B, Olivier E. Monitoring Coordination during Bimanual Movements: Where Is the Mastermind? J Cogn Neurosci. 2010 Mar;22(3):526–42. doi: 10.1162/jocn.2009.21213. [DOI] [PubMed] [Google Scholar]
- Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex. 2009;19:2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci. 2012;32(3):806–816. doi: 10.1523/JNEUROSCI.4299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci. 2010;30:3793–3802. doi: 10.1523/JNEUROSCI.5722-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically Specific Interhemispheric Inhibition Operating in the Process of Generation of a Voluntary Movement. Cereb Cortex. 2005;15:588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Stefan K, Hummel F, Olivier E, Cohen LG. Memory formation in the motor cortex ipsilateral to a training hand. Cereb Cortex. 2008;18:1395–1406. doi: 10.1093/cercor/bhm173. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual Differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Duque J, Olivier E, Rushworth M. Top-down inhibitory control exerted by the medial frontal cortex during action selection under conflict. J Cogn Neurosci. 2013;25:1634–1648. doi: 10.1162/jocn_a_00421. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y, Seki K, Votaw S. Roles of primate spinal interneurons in preparation and execution of voluntary hand movement. Brain Res Brain Res Rev. 2002;40:53–65. doi: 10.1016/s0165-0173(02)00188-1. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamai CA. The time-course of preparatory spinal and cortico-spinal inhibition: an H-reflex and transcranial magnetic stimulation study in man. Exp Brain Res. 1999;124:33–41. doi: 10.1007/s002210050597. [DOI] [PubMed] [Google Scholar]
- Jahfari S, Verbruggen F, Frank MJ, Waldorp LJ, Colzato L, Ridderinkhof KR, Forstmann BU. How preparation changes the need for top-down control of the basal ganglia when inhibiting premature actions. J Neurosci. 2012;32:10870–10878. doi: 10.1523/JNEUROSCI.0902-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. A probabilistic strategy for understanding action selection. J Neurosci. 2010;30:2340–2355. doi: 10.1523/JNEUROSCI.1730-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Flugge MC, Bestmann S. Time-dependent changes in human corticospinal excitability reveal value-based competition for action during decision processing. J Neurosci. 2012;32:8373–8382. doi: 10.1523/JNEUROSCI.0270-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PA, Olivier E, Duque J. Influence of Reward on Corticospinal Excitability during Movement Preparation. J Neurosci. 2012;32:18124–18136. doi: 10.1523/JNEUROSCI.1701-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PA, Petitjean C, Olivier E, Duque J. Top-down suppression of incompatible motor activations during response selection under conflict. Neuroimage. 2014;86:138–149. doi: 10.1016/j.neuroimage.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, Rothwell JC. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006;26:7452–7459. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna L, Lebon F, Duque J, Klein PA, Cazares C, Ivry RB. Generic inhibition of the selected movement and constrained inhibition of nonselected movements during response preparation. J Cogn Neurosci. 2014;26:269–278. doi: 10.1162/jocn_a_00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123(Pt 6):1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Link SW, Heath RA. A sequential theory of psychological discrimination. Psychometrika. 1975;40:77–105. [Google Scholar]
- Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. A role for neural integrators in perceptual decision making. Cereb Cortex. 2003;13:1257–1269. doi: 10.1093/cercor/bhg097. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC. A unified framework for inhibitory control. Trends Cogn Sci. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira FT, Diedrichsen J, Verstynen T, Duque J, Ivry RB. Transcranial magnetic stimulation of posterior parietal cortex affects decisions of hand choice. Proc Natl Acad Sci U S A. 2010;107:17751–17756. doi: 10.1073/pnas.1006223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schel MA, Kuhn S, Brass M, Haggard P, Ridderinkhof KR, Crone EA. Neural correlates of intentional and stimulus-driven inhibition: a comparison. Front Hum Neurosci. 2014;8:27. doi: 10.3389/fnhum.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyal M, Mull B, Bhullar N, Ahmad T, Gage B. Anticipation and execution of a simple reading task enhance corticospinal excitability. Clin Neurophysiol. 1999;110:424–429. doi: 10.1016/s1388-2457(98)00019-4. [DOI] [PubMed] [Google Scholar]
- Swinnen SP. Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci. 2002;3:348–359. doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- Tosoni A, Galati G, Romani GL, Corbetta M. Sensory-motor mechanisms in human parietal cortex underlie arbitrary visual decisions. Nat Neurosci. 2008;11:1446–1453. doi: 10.1038/nn.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touge T, Taylor JL, Rothwell JC. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol. 1998;109:489–495. doi: 10.1016/s0924-980x(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Usher M, McClelland JL. Loss aversion and inhibition in dynamical models of multialternative choice. Psychol Rev. 2004;111:757–769. doi: 10.1037/0033-295X.111.3.757. [DOI] [PubMed] [Google Scholar]
- van Campen AD, Keuken MC, van den Wildenberg WP, Ridderinkhof KR. TMS over M1 reveals expression and selective suppression of conflicting action impulses. J Cogn Neurosci. 2014;26:1–15. doi: 10.1162/jocn_a_00482. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, Wylie SA, Forstmann BU, Burle B, Hasbroucq T, Ridderinkhof KR. To head or to heed? Beyond the surface of selective action inhibition: a review. Front Hum Neurosci. 2010;4:222. doi: 10.3389/fnhum.2010.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elswijk G, Kleine BU, Overeem S, Stegeman DF. Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosci. 2007;19:121–131. doi: 10.1162/jocn.2007.19.1.121. [DOI] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol Rev. 2013;120:329–355. doi: 10.1037/a0031542. [DOI] [PubMed] [Google Scholar]