Abstract
Mottled-dappled (Mo-dp) is a mouse model of Menkes disease caused by a large, previously uncharacterized deletion in the 5' region of Atp7a, the mouse ortholog of ATP7A. Affected mutants die in utero at embryonic day 17, and show bending and thickening of the ribs and distortion of the pectoral and pelvic girdles and limbs. To characterize this allele, we designed a custom 4×180K microarray on the mouse X chromosome and performed comparative genomic hybridization using extracted DNA from normal and carrier Mo-dp females, and identified an approximately 9 kb deletion. We used PCR to fine-map the breakpoints and amplify a junction fragment of 630 bp. Sequencing of the junction fragment disclosed the exact breakpoint locations and that the Mo-dp deletion is precisely 8,990 bp, including approximately 2 kb in the promoter region of Atp7a. Western blot analysis of Mo-dp heterozygotes brains showed diminished amounts of Atp7a protein, consistent with reduced expression due to the promoter region deletion on one allele. In heterozygous females, brain copper levels tended to be lower compared to wild type whereas neurochemical analyses revealed higher dihydroxyphenylacetic acid: dihydroxyphenylglycol (DOPAC: DHPG) and dopamine: norepinephrine (DA:NE) ratios compared to normal (p=0.002 and 0.029, respectively), consistent with partial deficiency of dopamine-beta-hydroxylase, a copper-dependent enzyme. Heterozygous females showed no significant differences in body weight compared to wild type females. Our results delineate the molecular details of the Mo-dp mutation for the first time and define novel biochemical findings in heterozygous female carriers of this allele.
Keywords: Copper, Mottled-dappled, Menkes disease, Atp7a
1. Introduction
Menkes disease (MD) is an often lethal X-linked recessive trait primarily affecting human male infants and caused by mutations in the copper transporting ATPase, ATP7A [1]. The condition is associated with defective copper homeostasis leading to brain copper deficiency and neurodegeneration [2]. Copper is essential for numerous copper–dependent enzymes involved in important cellular functions including pigmentation, catecholamine biosynthesis, and connective tissue formation [1].
In cells cultured under normal copper conditions, ATP7A is localized in the trans-Golgi network, and trafficks to the plasma membrane to mediate copper export under conditions of high intracellular copper [3, 4]. The ATP7A transporter provides copper to cuproenzymes, such as tyrosinase, involved in skin pigmentation, lysyl-oxidase, needed for connective tissue formation, and dopamine beta-hydroxylase (DBH), a crucial enzyme in the catecholamine biosynthetic pathway [1]. Distinctively abnormal catecholamine levels in blood and cerebrospinal fluid provide useful markers for early diagnosis of Menkes patients [5].
The mottled series of mice are animal models of Menkes disease, so-named because carrier females manifest coat color variegation due to deficiency of tyrosinase, another copper-dependent enzyme [6]. The mottled alleles harbor various mutations in the Atp7a gene on the mouse X chromosome. Since human and murine ATP7A proteins share 89% homology, the mottled alleles represent valuable tools to study the molecular pathophysiology of Menkes disease [1]. The Atp7a gene in mice is around 100 kb, spanning 23 exons with the initiation codon in exon 2 as in the human homolog. Based on the Mouse July 2007 (NCBI37/mm9) assembly, the mouse genome project which we adopted in this study and was the most recent at the time of our experiments, the Atp7a gene is located on the mouse X chromosome from 103,222,563 to 103,323,499 bp.
Phenotypic severity and survival in affected male mottled mutants appear to vary depending on the type of Atp7a mutation. In the Mo-dp mouse, hemizygous males die around embryonic day 17 (E17) whereas mice carrying other alleles, e.g., Mottled-brindled (Mo-br), a six base pair in-frame deletion, survive until postnatal day 13 (P13) approximately [7]. We previously documented the safety and survival benefit of brain-directed gene addition in Mo-br C57Bl/6J mice in combination with copper chloride, both delivered by intracerebroventricular injection [7]. Although the Atp7aMo-br allele allows <15% copper transport in a yeast complementation assay compared to wild type (WT), experimental treatment of a null or knockout allele may also contribute information useful for clinical translation [7].
The Mo-dp mouse harbors one of the most severe Atp7a mutations and harbors a large deletion of at least 1.8 kb in the promoter region of Atp7a [8]. Heterozygous Mo-dp females show similar vibrissae to normal mottled females although the former some show curliness of whiskers (Figure 1A,B). Some also have clubbing of the forefeet at birth or, at weaning and a tendency to walk on the dorsal surface of the hind feet. With age, calcified periosteal lumps may appear in the thoracic and lumbar vertebrae [9, 10].
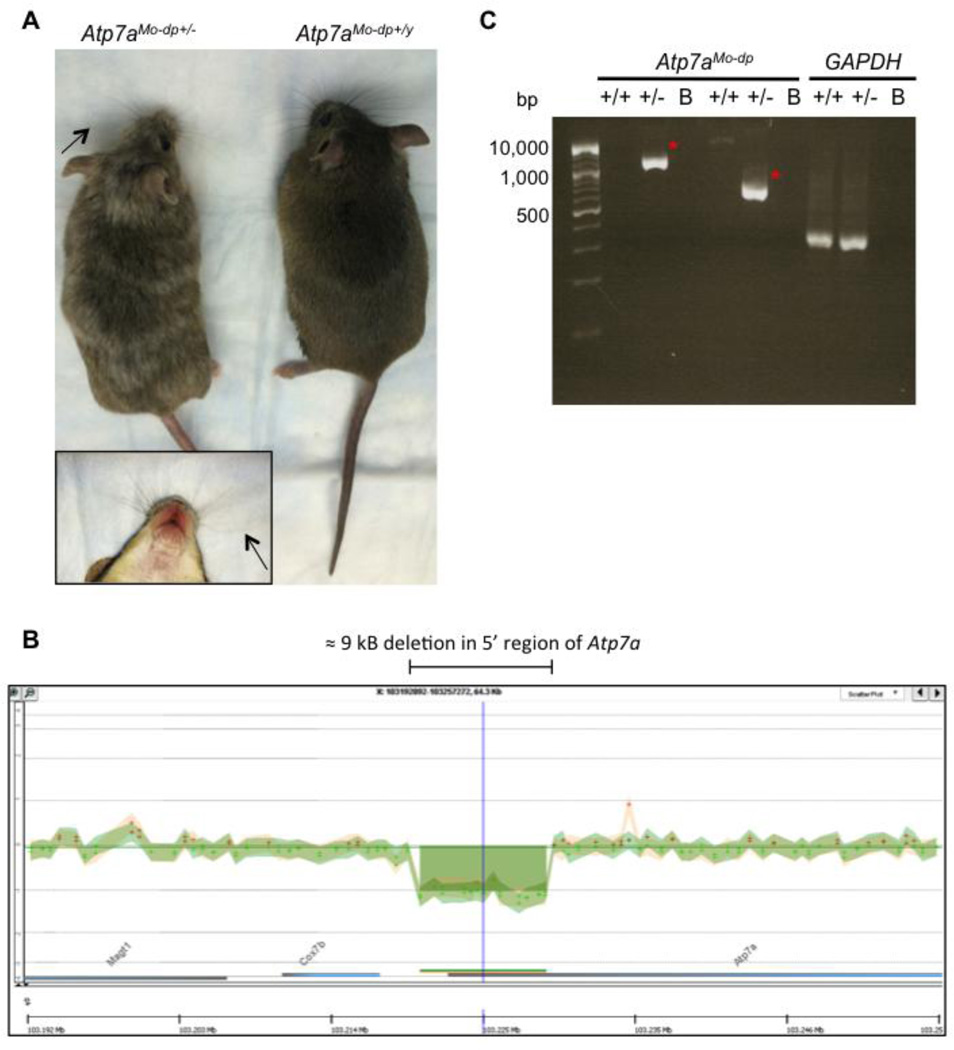
Figure 1.
Phenotype and molecular characterization of Mo-dp heterozygous females. A. Photograph of a Mo-dp breeding pair showing a heterozygous Mo-dp female (Atp7aModp+/−) on the left and a wild type male (Atp7aMo-dp+/y) on the right. Carrier females show a variegated coat color due to deficiency of tyrosinase, a copper-dependent enzyme and sometimes slightly curly vibrissae (arrows), enlarged in the boxed area. B. Ideogram of the Mo-dp mouse X chromosome in the vicinity of Atp7a gene. Custom X chromosome 4×180K CGH microarray revealed a ≈9kb deletion in the 5' untranslated region of the copper transporter Atp7a. C. Amplification of the junction fragment from heterozygous female genomic DNA by PCR using two different primer pairs produced 1,275 bp and 630 bp amplicons, respectively (red asterisks). +/+: Mo-dp +/+ normal C3H101H female; +/−: Mo-dp +/− heterozygous female; B: blank; bp: base pair.
Southern blot analysis of DNA from Mo-dp mutants revealed a deleted restriction fragment length variation (RFLV) in the upstream region of Atp7a upon digestion with the restriction enzymes EcoRI and HindIII. However, the Mo-dp defect has not been further characterized, nor fine mapping of the deletion reported. The mutation first appeared spontaneously in the germline of a F1 male born from a cross between a PT background female and an irradiated 3H1 mixed background (C3Hx101 F1 hybrid) male [8, 11, 12]. This male, on outcrossing to unrelated females, sired ten dappled females, 278 normal females and 290 normal males. The ten dappled females were all sired in the first four weeks of a total breeding life of 46 weeks [13]. Consequently, this large deletion in the 5’ untranslated region (5'UTR) of Atp7a, the Mo-dp allele represents a complete null allele and an interesting mouse model of MD for further study. However, a major obstacle in studying this model is prenatal lethality of Mo-dp male mutants.
In this study, we 1) characterized the Mottled-dappled deletion in C3H101H carrier females, adding much more precise molecular information when compared to the previous literature [8], 2) reported a genotyping assay that identifies the colony’s different alleles, and 3) assessed the copper-related biochemical phenotype in heterozygous female brains.
2. Materials and Methods
2.1. Animals
We obtained Mottled-dappled mouse (C3H101H-Atp7a<Mo-dp>/H) breeding pairs from MRC Harwell (London, UK) on a mixed genetic background, C3H101H (MGI:1856099). To expand the colony we mated C3H101H WT male (Atp7aMo-dp+/y) with heterozygous females from the same mixed genetic background (Atp7aMo-dp+/−). The NICHD Animal Care and Use Committee approved all animal experimental procedures. Heterozygous Mo-dp females (Figure 1A) show a variegated coat color and are similar in vibrissae color to other Mottled female carriers but show subtle curliness of the whiskers. Given the poor breeding efficiency of Mo-dp females, we used four heterozygous mice and three wild type mice for copper and neurochemical measurements, two mice per group for genomic DNA extraction and molecular analysis, and one mouse brain per genotype for Western blot analysis.
2.2. Dissection and genomic DNA extraction
Mouse brain dissections were carried out in WT ATP7AMo–dp+/+ and ATP7AMo–dp+/− C3H101H females. Each brain was bisected sagittally. Each half-brain was snap frozen on dry ice, stored at −80 °C, and used for brain copper measurements and brain neurochemical determinations (see below). Similarly, mouse livers were dissected and snap frozen, and genomic DNA isolated using DNeasy blood and tissue kit following the manufacturer's instructions (Qiagen, Limburg, Netherlands). The extracted DNA was used for MicroArray analysis and as template for polymerase chain reactions (PCR).
2.3. Array comparative genomic hybridization
We designed a custom 4×180K microarray on the mouse X chromosome and tiled 60-mer probes around the region of interest near the Atp7a locus. The average resolution in the vicinity of the deletion was 675 bp. Comparative genomic hybridization (CGH) was performed using the Sureprint G3 Mouse 4×180k plus Custom X microarrays (AMADID: 043258), printed by Agilent Technologies (Santa Clara, CA) (Biomedical Genomics Core, Nationwide Children’s Hospital, Columbus, OH). The features were selected from Agilent’s eArray (Agilent, https://earray.chem.agilent.com/earray) probe library. Agilent’s array-based CGH application uses a “two-color” process to measure DNA copy number changes in an experimental sample relative to a reference sample. DNA concentration was determined using a NanoDrop spectrophotometer (NanoDrop products, Wilmington, DE) and quality determined by SYBR Gold stained Flash Gel. For enzymatic labeling, 1.0 µg of starting genomic DNA was digested by Alu1/Rsa1 restriction enzymes and labeled by incorporation of cyanine-dUTP via Exo-Klenow and random primers. The labeled DNA was purified and free dye is removed using column purification. After column purification, the labeled target was mixed with Mouse Cot-1 DNA, blocking agent, buffer and hybridized for 24hr at 65C. Hybridized microarrays were washed, dried and scanned on the Agilent microarray scanner (G2505C). Feature Extraction software was used for image analysis and the resulting statistics analyzed via Agilent’s CytoGenomics Workbench 2.0.6 software (Figure 1B).
2.4. Polymerase chain reactions
To fine map the Mo-dp deletion, the genomic DNA from liver was PCR-amplified, based on microarray results, to identify junction fragments. We used the University of California, Santa Cruz (UCSC) genome browser on Mouse July 2007 (NCBI37/mm9) Assembly to design primers. Two primer pairs were utilized, forward primer: 5’-TTGAAATGAAGTTGAGTTTTGTCC-3’ (designed from the probe A_67_P21975404) and reverse primer: 5’-GAGCATGACTTTGAAGCCAATA-3’ (designed from the probe A_67_P21975466), and forward 2 primer: 5’-TGTCAGCCTTTCTGGGAAGT-3’ and reverse 2 primer: 5’ CAGCACTGGGGAGGTTAAGA-3’ in order to obtain a smaller junction fragment (Figure 1C). The respective PCR products were 1,275 and 630 bp in size. PCR was carried out using a platinum high-fidelity Taq polymerase (Invitrogen, Carlsbad, CA) in a 50µl final volume, according to the manufacturer’s instructions. Samples were amplified under the following conditions in a Perkin-Elmer 9600 thermocycler: 95°C for 5 min, 35 cycles consisting of denaturation at 95°C for 30 sec, annealing at 59°C for 30 sec, and extension at 72°C for 90 sec, followed by the final extension at 72°C for 10 min. Oligonucleotide primers for PCR amplification were designed from the published sequence of the mouse Atp7a gene, as it follows:
Mo-dp F3: 5’ TGTGAGTGACGGGTAAACCA 3’;
Mo-dp R3 N: 5’ GCCACTTGGGACACATTTTC 3’; and
Mo-dp R2 D: 5’ CAGCACTGGGGAGGTTAAGA 3’.
2.5. DNA sequencing
DNA sequencing was performed using an ABI prism 377 automated DNA sequencer (Applied Biosystems, Carlsbad, CA) at the National Institute of Neurological Disorders and Stroke (NINDS) intramural DNA Sequencing Facility.
2.6. Protein extraction and Western blotting
Total proteins were extracted from sagittally bisected mouse brains from WT females (ATP7AMo–dp+/+) or heterozygous females harboring the (ATP7AMo–dp+/−) deletion on one allele. Protein extraction was performed using RIPA buffer containing 50 mM HEPES buffer, 150 mM NaCl, 0.5% Triton X-100 and 10% glycerol. Supernatants from the lysed tissues were collected. The total proteins were denatured by adding 4X NuPage, LDS sample buffer (Invitrogen, Life Technologies, NY, USA) with 1% of β-mercaptoethanol, then heating at 95 °C for 5 min. Total proteins (70 µg) from each sample were electrophoresed through precast 4–12% NOVEX Tris–glycerin sodium dodecyl sulfate (SDS) polyacrylamide gels (Invitrogen) for 1 h at 170 V and transferred to polyvinylidine fluoride (PVDF) membranes for 2 h at 25 V (150 mA). The membranes were then incubated at 4 °C overnight in phosphate buffered saline blocking solution containing 0.1% Tween 20 (PBST) and 5% non-fat milk (Bio-Rad, Hercules, CA). Blots were washed with PBST, and incubated for 2h at room temperature (RT) with a 1:1000 dilution of a rabbit carboxyl-terminal anti-ATP7A antibody [5] or a 1:5000 dilution of anti-β-actin monoclonal antibody coupled with HRP (Abcam, Cambridge, UK). The membrane was washed 3 times with PBS 0.1% Tween20 and incubated with a 1:2000 dilution of a goat anti-rabbit IgG horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology, Dallas, TX) for 1h at RT. Membranes were then incubated in SuperSignal West Pico Luminol/Enhancer Solution (Thermo Fisher Scientific, Waltham, MA), exposed to a Kodak BioMax MR autoradiography film, and developed using a Kodak X-OMAT 2000A processor.
2.7. Brain copper measurements
Copper levels in fresh frozen brains were determined by graphite furnace atomic absorption and confirmed by inductively coupled plasma mass spectrometry, as previously described [14, 15].
2.8. Brain neurochemicals
Half of each brain was weighed and immediately homogenized in 5–10 volumes of 0.4N perchloric acid containing 0.1% ethylene diamine tetra-acetic acid. The homogenates were centrifuged and supernatant frozen at −80°C until assay. Concentrations of brain neurochemicals dihydroxyphenylalanine (DOPA), DOPAC, DA, NE, and DHPG were determined by high-performance liquid chromatography with electrochemical detection, as previously described [16, 17].
2.9. Statistics
Brain copper, brain neurochemical levels and their relative ratios were compared between groups of Mo-dp heterozygous and normal females and evaluated for statistically significant differences by unpaired, two-tailed Student t-tests.
3. Results
Based on previous studies, the Mo-dp mouse model of Menkes disease harbors a large deletion in the 5’ UTR of Atp7a. Our initial goal was to obtain a clear molecular definition of this deletion: for this purpose, we sought to fine-map the chromosomal rearrangement in the Mo-dp mouse model. Since mutant males die by E17, in this study we used heterozygous female carriers (Figure 1A) as a source of the mutant allele. We designed a custom X chromosome 4×180K CGH Agilent MicroArray and, using labeled genomic DNA, we hybridized heterozygous female (Atp7aMo-dp+/−) DNA against normal C3H101H female DNA (Atp7aMo-dp+/+). After analyzing the MicroArray data, which focused on the mouse X chromosome and, more specifically, the vicinity of the Atp7a gene, we found a large deletion in the 5' untranslated region (Figure 1B). In order to identify the deletion breakpoints and the exact location on the mouse X chromosome, we analyzed the results in silico using the Agilent website and the UCSC genome browser. We found that the upstream deleted probe was A_67_P09384713 and the downstream deleted probe was A_67_P09384734. In order to amplify a junction fragment upstream and downstream of the deletion, we designed PCR primers within the adjacent proximal (A_67_P21975404) and distal (A_67_P21975466) probes that were not deleted according to our microarray data. We designed two pairs of primers predicted to amplify 1,275 bp and 630 bp Mo-dp Atp7a segments, respectively. Each primer pair amplified the expected-sized product. Given the fact that the normal allele should yield an amplicon ≥ 9kb, no band or only a faint band was observed for DNA samples from WT animals (Figure 1C).
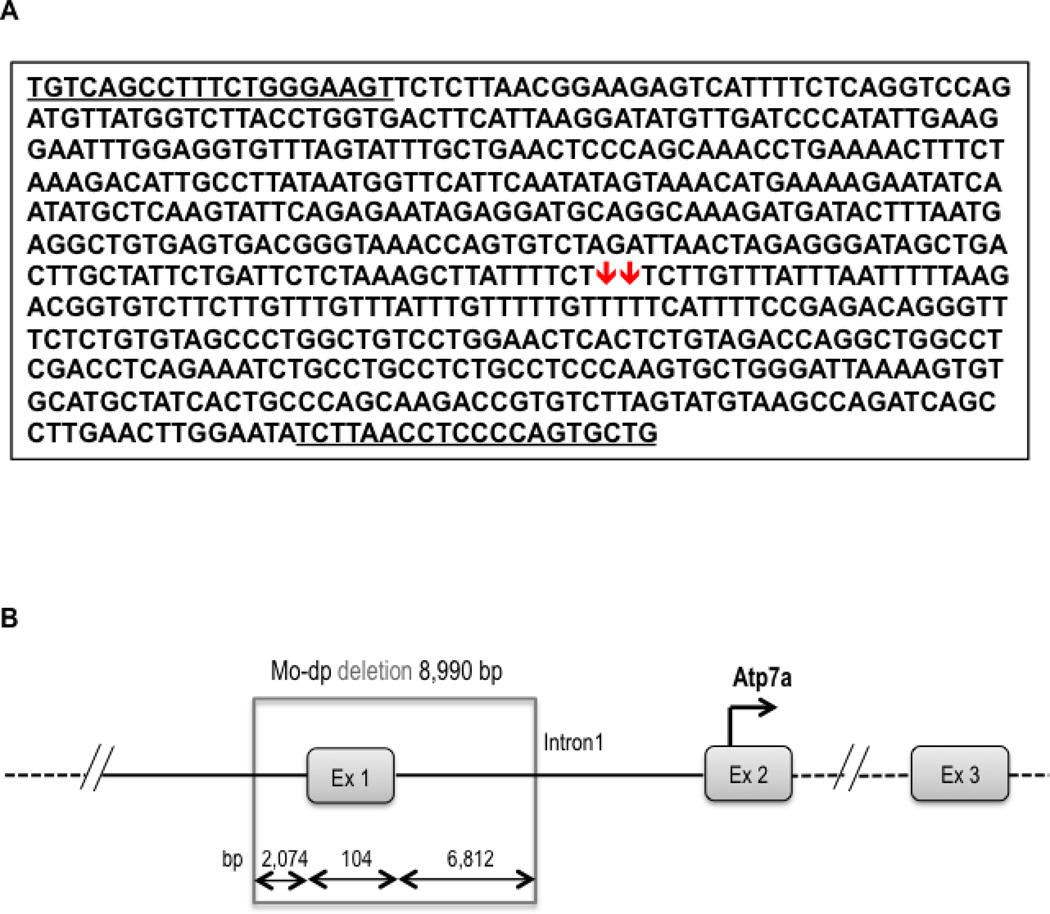
The amplified junction fragment was next sequenced and deletion breakpoints identified (Figure 2A), using the mouse genome assembly from July 2007 (NCBI37/mm9). Thus, the deletion location for the Mo-dp allele begins at 103,220,541 bp and ends at 103,229,530 bp on the mouse X chromosome. The exact size of the deletion is 8,990 bp, where 2,074 bp are in the promoter region, 104 bp in exon 1, and 6,812 bp in intron 1 of Atp7a (Figure 2B). This information enabled the design of PCR primers based on the DNA sequence from the region of the deletion (Mo-dp F3, Mo-dp R3N) and the junction fragment (Mo-dp R3D) for efficient detection of all possible genotypes for this allele (Figure 3A). In this genotyping assay, PCR fragments of 167 bp and 354 bp define female heterozygotes, whereas a single band of 167 bp indicates a WT male or female (Figure 3B). A single 354 bp would denote an affected male (−/y), however we did not detect this genotype, since the Mo-dp mutation is associated with early embryonic lethality and affected males do not survive to birth.
Figure 2.
Junction fragment sequence reveals Mo-dp deletion breakpoints. A. A 630 bp junction fragment was amplified using primers (underlined) from the 5’UTR (forward) and intron 1 (reverse) of Atp7a. The red arrows represent the location of the deleted fragment in the Mo-dp allele. B. Representation of the Atp7a genomic region containing the Mo-dp deletion’s breakpoints on the mouse X chromosome. The exact size of the deletion is 8,990 bp, where 2,074 bp are in the promoter region, 104 bp in exon 1, and 6,812 bp in intron 1 of Atp7a.
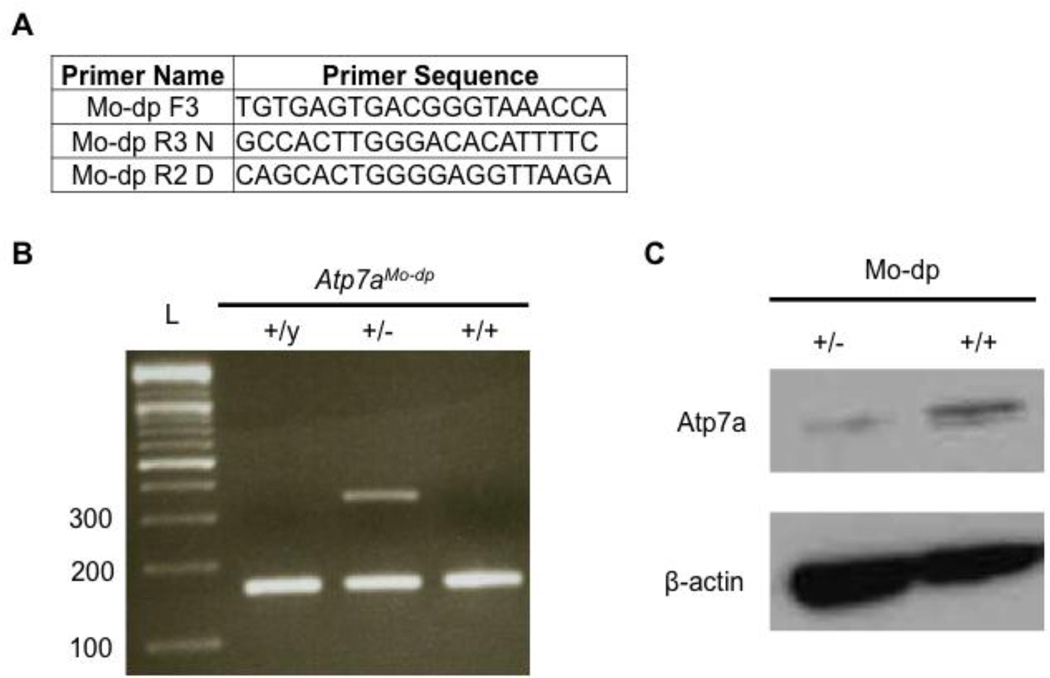
Figure 3.
Genotyping and Atp7a expression in Mo-dp+/− mouse brains. A. Primers used to amplify normal and dappled alleles. The normal allele was amplified by the primers Mo-dp F3 and Mo-dp R3 N, producing a 167 bp amplicon. The Mo-dp allele was amplified by the primers Mo-dp F3 and Mo-dp R2 D yielding to a 354 bp amplicon. B. Duplex PCR to genotype and identify normal (167 bp band) and mo-dappled (354 bp band) alleles. All three viable genotypes are represented in this figure. C. Western blot analysis of total proteins from mouse brains shows lower Atp7a levels in the heterozygous female (Atp7aMo-dp+/−) compared to a normal female (Atp7aMo-dp+/+).
To evaluate Atp7a expression, we performed Western blot analyses on total proteins extracted from the brains of carrier females (+/−), compared to WT males and females. Heterozygous females showed decreased Atp7a levels compared to normal females (Figure 3C) and normal males (data not shown), which led us to speculate that the carrier females may have biochemical abnormalities consistent with reduced Atp7a activity. To evaluate this hypothesis, we measured brain copper levels in the heterozygous and normal female brains (Table 1). Mean brain copper measurements in ATP7AMo–dp+/− females were approximately 25% lower compared to the normal ATP7AMo–dp+/+ females but the groups did not differ statistically based on the relatively small number of animals assessed (P = 0.1186).
Table 1.
Brain copper levels in wild type Atp7a controls and Mo-dp heterozygotes
| Mouse ID | Atp7aMo-dp genotype | Brain Cua |
|---|---|---|
| 117B | +/− | 13.9 |
| 180 | +/− | 15.9 |
| 181 | +/− | 19.2 |
| 203 | +/− | 22 |
| Mean/SD: 17.7 ± 3.58b | ||
| 177 | +/+ | 22.3 |
| 178 | +/+ | 22.5 |
| 182 | +/+ | 20.7 |
| Mean/SD: 21.8 ± 0.99 | ||
µg/g dry weight
P-value = 0.1186
SD: standard deviation
In order to evaluate the activity of the copper dependent enzyme DBH, we also measured brain neurochemical levels and calculated DOPAC:DHPG and DA:NE ratios. Interestingly, we observed significant reductions in NE and DHPG, the products of dopamine hydroxylation (Table 2). The DOPAC:DHPG ratios were approximately 3.5-fold increased in Mo-dp carrier females, reflecting DBH deficiency, a neurochemical pattern similar to the one observed in cerebrospinal fluid and plasma of infants with Menkes disease [5, 18]. The DA:NE ratios were approximately 2.5-fold increased in heterozygous females compared to WT (Table 2).
Table 2.
Brain catecholamine levels (pg/mg of wet weight) and ratios in Mo-dp heterozygotes compared to WT females
| Mouse ID |
Atp7a genotype |
DOPA | DOPAC | DA | NE* | DHPG* | DOPAC: DHPG* |
DA: NE* |
|---|---|---|---|---|---|---|---|---|
| 117B | +/− | 3.9 | 156.5 | 1131.4 | 127 | 6.7 | 23.4 | 8.9 |
| 180 | +/− | 9.2 | 178 | 1239.5 | 121.4 | 7.1 | 25.1 | 10.2 |
| 203 | +/− | 7.3 | 111.2 | 1191.6 | 187.6 | 7.7 | 14.4 | 6.4 |
| 318 | +/− | 10.4 | 146.4 | 834.7 | 213.3 | 8.5 | 17.2 | 3.9 |
| Mean | 7.7 | 148 | 1099.3 | 162.3 | 7.5 | 20.0 | 7.3 | |
| SEM | ±1.4 | ±13.9 | ±90.9 | ±22.7 | ±0.4 | ±2.5 | ±1.4 | |
| 117 | +/+ | 10.8 | 144.9 | 1217 | 339.7 | 19.6 | 7.4 | 3.6 |
| 178 | +/+ | 13.2 | 121.8 | 998.9 | 257.2 | 18.3 | 6.7 | 3.9 |
| 179 | +/+ | 6.1 | 117.9 | 1129.5 | 350 | 18.1 | 6.5 | 3.2 |
| 319 | +/+ | 8.6 | 100.1 | 757.5 | 376.9 | 18.4 | 5.4 | 2 |
| Mean | 9.7 | 121.2 | 1025.7 | 331 | 18.6 | 6.5 | 3.2 | |
| SEM | ±1.5 | ±9.2 | ±100 | ±25.8 | ±0.3 | ±0.4 | ±0.4 | |
| P-value | 0.378 | 0.159 | 0.606 | 0.003 | <0.0001 | 0.002 | 0.029 | |
Statistically significant
SEM: standard error of the mean
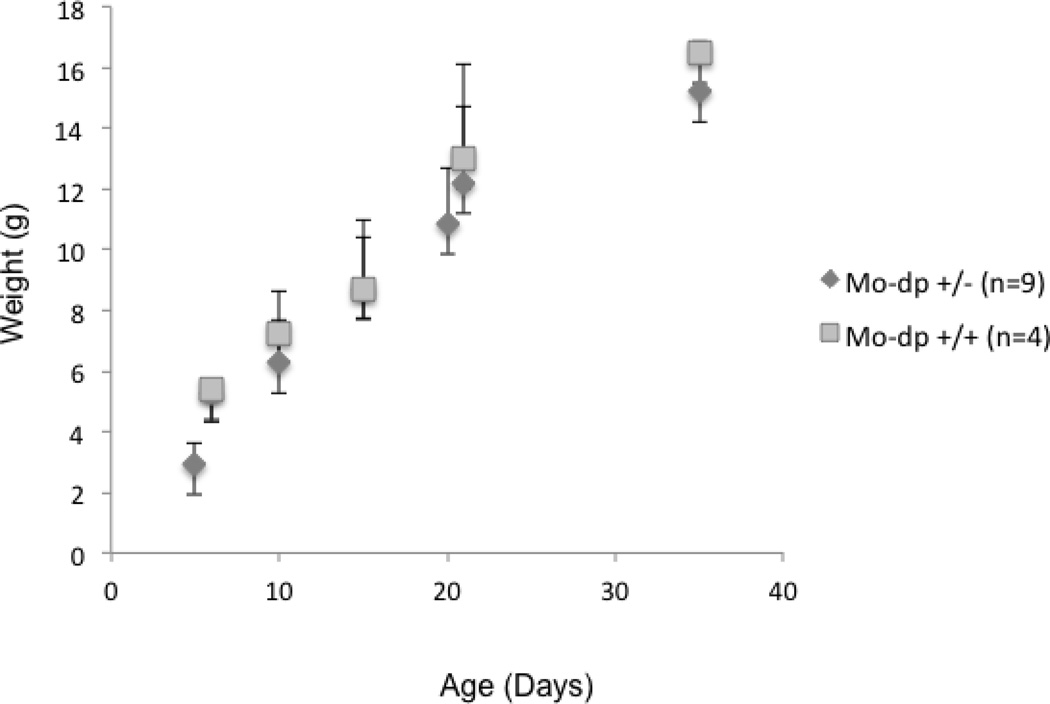
As an indicator of general health, we also compared mouse body weights between heterozygous and WT females during the first seven weeks of life. We found lower mean values in the former; however this difference was not statistically significant (Figure 4).
Figure 4.
Growth chart in heterozygous Mo-dp females. Body weights were measured in grams (g) from day 5 to day 35 of age. Carrier females show no statistically significant differences in body weights compared to age-matched normal females.
4. Discussion
The Mo-dp is a severe mouse model of MD caused by a large, previously uncharacterized deletion in the 5' region of Atp7a. It is one of the most severe mouse models of this disorder and harbors a complete loss-of-function mutation, thus representing a powerful model for experimental therapeutic studies in MD. However, prenatal lethality represents an important obstacle for the future study and evaluation of Mo-dp mutant males.
While the precise biochemical basis for embryonic lethality in mice lacking an intact Atp7a gene is unknown, the Atp7a protein is known to transport copper to at least three copper-dependent enzymes (DBH, lysyl oxidase, and peptidylglycine alpha-amidating monooxygenase) whose individual deletions cause lethality during gestation or in the perinatal period [19–21]. Wang et al. demonstrated the essential role of Atp7a in mouse embryonic development by generating a mouse model enabling conditional deletion of the Atp7a gene. Global deletion of Atp7a resulted in morphological and vascular defects in hemizygous male embryos and death in utero. Heterozygous deletion in females resulted in a 50% reduction in live births and a high postnatal lethality [21]. The Mottled mouse displays several distinct phenotypic severities: embryonic lethal (11H), perinatal lethal (brindled and macular) and a longer-lived viable (viable brindled) phenotypes. Previous evidence showed that the phenotypic severity of mottled mutations was associated with impairment of Atp7a-dependent copper transport as well as mislocalization of the Atp7a protein [22].
Array comparative genomic hybridization detects net copy differences between two samples of genomic DNA (e.g., insertions, deletions, etc.) and provides higher resolution than traditional FISH (Fluorescent In Situ Hybridization) and karyotype analysis [23]. In order to characterize the deletion on the Mo-dp allele, we designed a custom 4×180K microarray on the mouse X chromosome. Using CGH arrays followed by PCR and DNA sequencing, we defined, for the first time, the exact size and locations of the Mo-dp deletion on the mouse X chromosome, establishing also a genotyping assay to differentiate our colony’s genotypes. These findings add precise information and modify the previous statement of Levinson et al. who reported that the Mo-dp mice have a large deletion of 1.8 kb in the promoter region of Atp7a [8].
Related to our efforts to develop new therapies for Menkes disease, we have embarked on a project to rescue embryonic lethal Mo-dp mutant males by in utero viral gene therapy [24]. The molecular characterization of Mo-dp null mutation and establishment of a genotyping assay for the Mo-dp allele facilitate these efforts. We previously showed that prenatal copper replacement alone in a human fetus with a severe loss of function mutation in ATP7A gene failed to rescue the phenotype [14]; thus we speculate that neonatal ATP7A gene addition plus subcutaneous copper injections represents a reasonable approach in such circumstances. Given the complete loss-of-function mutation in Atp7a, rescue of affected male Mo-dp mice by viral gene therapy would provide further proof of concept for this therapeutic approach in human patients.
We also documented decreased Atp7a protein levels, somewhat lower brain copper levels, and abnormal brain neurochemical ratios in heterozygous Mo-dp females, presumably as a consequence of reduced copper transport. Subtle neurochemical abnormalities consistent with deficiency of DBH were previously reported in Mo-br heterozygous females [6, 25]. Similarly, human Menkes female carriers occasionally develop neurological symptoms such as epilepsy and learning disability, as reported by Moller et al [26]. In a cohort consisting of 517 families, nine neurologically affected carriers with normal karyotypes were identified. The clinical symptoms of affected females were generally milder than those of affected males in the families with the same mutations. X-inactivation analyses in affected female carriers suggested a correlation with the degree of mental retardation [26].
The relatively minor difference in brain copper levels we documented between Mo-dp heterozygotes and controls suggest compensatory mechanisms for copper delivery into the CNS. In contrast, the distinctive decline in catecholamine metabolites implies considerably less than normal ATP7A-dependent copper transport to DBH within the trans-Golgi compartment of dopaminergic neurons. In the Mo-dp mouse model, female carriers are mosaics of wild type and mutant cells due to the random X inactivation. Thus, brain biochemical abnormalities could be related to Atp7a haploinsufficiency in carrier females. Although Atp7a-mediated copper transport is likely not completely abolished at the blood-CSF barrier, the quantity of residual activity may be inadequate to transport copper to cuproenzymes such as DBH.
The present work represents the first description of copper-related brain neurochemical abnormalities in Mo-dp heterozygous females, and the pattern resembles what is observed in MD patients [28]. Future studies should dissect the spatiotemporal manifestations of these abnormalities and possible related behavioral deficits. These investigations may help further illuminate the role(s) of ATP7A expression during brain development.
5. Conclusions
The precise definition of the molecular basis for an embryonic-lethal Atp7aMo-dp mouse can provide a powerful tool for deciphering the physiological importance of Atp7a protein in the early phases of brain development. As there are currently no reliably effective treatments for MD and other ATP7A-related disorders [1, 4], correcting the phenotypic consequences of the complete null Atp7a mutation in Mo-dp may lead to new therapies for these conditions. Viral gene delivery to Mo-dp−/y hemizygous mutant embryos in utero represents one potential approach in this unique mouse model.
Highlights.
First detailed molecular characterization of mottled-dappled, a mouse model of Menkes disease
Reduced brain Atp7a expression was shown in the female carriers
First observation of abnormal brain copper and catecholamine in these female heterozygotes
Acknowledgements
We thank Daniel Abebe, Lynn Holtzclaw, and Matt Schech for animal care support and assistance and Diego Martinelli for helpful discussions and comments. We also wish to acknowledge the late David Newsom (Nationwide Children’s Hospital, Columbus, OH) for very helpful discussions and planning for array CGH. This work was supported by the Intramural Research Program of the National Institutes of Health.
Abbreviations
- DA
dopamine
- DHPG
dihydroxyphenylglycol
- DOPA
dihydroxyphenylalanine
- DOPAC
dihydroxyphenylacetic acid
- Mo-br
mottled-brindled
- Mo-dp
mottled-dappled
- NE
norepinephrine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marie Reine Haddad, Email: reina.haddad@nih.gov.
Keyur D. Patel, Email: keyur.patel19@gmail.com.
Patricia H. Sullivan, Email: psullivan1@ninds.nih.gov.
David S. Goldstein, Email: goldsteind@ninds.nih.gov.
Kevin M. Murphy, Email: kevin.m.murphy2@navy.mil.
Jose A. Centeno, Email: jose.centeno@afncr.af.mil.
Stephen G. Kaler, Email: kalers@mail.nih.gov.
References
- 1.Kaler SG. ATP7A-related copper transport diseases-emerging concepts and future trends. Nature reviews. Neurology. 2011;7:15–29. doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu PC, Chen YW, Centeno JA, Quezado M, Lem K, Kaler SG. Downregulation of myelination, energy, and translational genes in Menkes disease brain. Molecular genetics and metabolism. 2005;85:291–300. doi: 10.1016/j.ymgme.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. The EMBO journal. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- 4.Yi L, Donsante A, Kennerson ML, Mercer JF, Garbern JY, Kaler SG. Altered intracellular localization and valosin-containing protein (p97 VCP) interaction underlie ATP7A-related distal motor neuropathy. Human molecular genetics. 2012;21:1794–1807. doi: 10.1093/hmg/ddr612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaler SG, Holmes CS, Goldstein DS, Tang J, Godwin SC, Donsante A, Liew CJ, Sato S, Patronas N. Neonatal diagnosis and treatment of Menkes disease. The New England journal of medicine. 2008;358:605–614. doi: 10.1056/NEJMoa070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka S, Takeuchi T. Hair pigmentation in transgenic mice. Annals of the New York Academy of Sciences. 1991;642:407–418. doi: 10.1111/j.1749-6632.1991.tb24405.x. [DOI] [PubMed] [Google Scholar]
- 7.Donsante A, Yi L, Zerfas PM, Brinster LR, Sullivan P, Goldstein DS, Prohaska J, Centeno JA, Rushing E, Kaler SG. ATP7A gene addition to the choroid plexus results in long-term rescue of the lethal copper transport defect in a Menkes disease mouse model. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:2114–2123. doi: 10.1038/mt.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levinson B, Packman S, Gitschier J. Deletion of the promoter region in the Atp7a gene of the mottled dappled mouse. Nature genetics. 1997;16:224–225. doi: 10.1038/ng0797-224. [DOI] [PubMed] [Google Scholar]
- 9.Silvers WK. The Coat Colors of Mice A Model for Mammalian Gene Action and Interaction. New York, NY: Springer New York; 1979. SpringerLink (Online service) p. 1. online resource. [Google Scholar]
- 10.Lyon MF, Searle AG. Genetic variants and strains of the laboratory mouse. Oxford England; New York Stuttgart: Oxford University Press; G. Fischer Verlag; 1989. International Committee on Standardized Genetic Nomenclature for Mice. [Google Scholar]
- 11.Carter TC, Lyon MF, Phillips RJ. Genetic hazard of ionizing radiations. Nature. 1958;182:409. doi: 10.1038/182409a0. [DOI] [PubMed] [Google Scholar]
- 12.Reed V, Boyd Y. RFLVs in mottled dappled alleles. Nature genetics. 1994;8:11–12. doi: 10.1038/ng0994-11. [DOI] [PubMed] [Google Scholar]
- 13.Phillips RJ. Dappled, a New Allele at Mottled Locus in House. Mouse Genet Res. 1961;2:290-&. [Google Scholar]
- 14.Haddad MR, Macri CJ, Holmes CS, Goldstein DS, Jacobson BE, Centeno JA, Popek EJ, Gahl WA, Kaler SG. In utero copper treatment for Menkes disease associated with a severe ATP7A mutation. Molecular genetics and metabolism. 2012;107:222–228. doi: 10.1016/j.ymgme.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lem KE, Brinster LR, Tjurmina O, Lizak M, Lal S, Centeno JA, Liu PC, Godwin SC, Kaler SG. Safety of intracerebroventricular copper histidine in adult rats. Molecular genetics and metabolism. 2007;91:30–36. doi: 10.1016/j.ymgme.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donsante A, Sullivan P, Goldstein DS, Brinster LR, Kaler SG. L-threodihydroxyphenylserine corrects neurochemical abnormalities in a Menkes disease mouse model. Annals of neurology. 2013;73:259–265. doi: 10.1002/ana.23787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Basile MJ, Mash DC. Catechols in post-mortem brain of patients with Parkinson disease. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2011;18:703–710. doi: 10.1111/j.1468-1331.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein DS, Holmes CS, Kaler SG. Relative efficiencies of plasma catechol levels and ratios for neonatal diagnosis of menkes disease. Neurochemical research. 2009;34:1464–1468. doi: 10.1007/s11064-009-9933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czyzyk TA, Ning Y, Hsu MS, Peng B, Mains RE, Eipper BA, Pintar JE. Deletion of peptide amidation enzymatic activity leads to edema and embryonic lethality in the mouse. Developmental biology. 2005;287:301–313. doi: 10.1016/j.ydbio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Zhu S, Weisman GA, Gitlin JD, Petris MJ. Conditional knockout of the Menkes disease copper transporter demonstrates its critical role in embryogenesis. PloS one. 2012;7:e43039. doi: 10.1371/journal.pone.0043039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim BE, Petris MJ. Phenotypic diversity of Menkes disease in mottled mice is associated with defects in localisation and trafficking of the ATP7A protein. Journal of medical genetics. 2007;44:641–646. doi: 10.1136/jmg.2007.049627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ou Z, Kang SH, Shaw CA, Carmack CE, White LD, Patel A, Beaudet AL, Cheung SW, Chinault AC. Bacterial artificial chromosome-emulation oligonucleotide arrays for targeted clinical array-comparative genomic hybridization analyses. Genetics in medicine : official journal of the American College of Medical Genetics. 2008;10:278–289. doi: 10.1097/GIM.0b013e31816b4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddad MR, Donsante A, Zerfas P, Kaler SG. Fetal Brain-directed AAV Gene Therapy Results in Rapid, Robust, and Persistent Transduction of Mouse Choroid Plexus Epithelia Molecular therapy. Nucleic acids. 2013;2:e101. doi: 10.1038/mtna.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin PM, Irino M, Suzuki K, Lewis MH, Mailman RB. The female brindled mouse as a model of Menkes' disease: the relationship of fur pattern to behavioral and neurochemical abnormalities. Developmental neuroscience. 1991;13:121–129. doi: 10.1159/000112149. [DOI] [PubMed] [Google Scholar]
- 26.Moller LB, Lenartowicz M, Zabot MT, Josiane A, Burglen L, Bennett C, Riconda D, Fisher R, Janssens S, Mohammed S, Ausems M, Tumer Z, Horn N, Jensen TG. Clinical expression of Menkes disease in females with normal karyotype. Orphanet journal of rare diseases. 2012;7:6. doi: 10.1186/1750-1172-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiological reviews. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 28.Kaler SG, Goldstein DS, Holmes C, Salerno JA, Gahl WA. Plasma and cerebrospinal fluid neurochemical pattern in Menkes disease. Annals of neurology. 1993;33:171–175. doi: 10.1002/ana.410330206. [DOI] [PubMed] [Google Scholar]