Abstract
Hypoxia-inducible factors (HIFs) are stabilized during adverse inflammatory processes associated with disorders such as inflammatory bowel disease, pathogen infection and acute lung injury, as well as during ischaemia–reperfusion injury. HIF stabilization and hypoxia-induced changes in gene expression have a profound impact on the inflamed tissue microenvironment and on disease outcomes. Although the mechanism that initiates HIF stabilization may vary, the final molecular steps that control HIF stabilization converge on a set of oxygen-sensing prolyl hydroxylases (PHDs) that mark HIFs for proteasomal degradation. PHDs are therefore promising therapeutic targets. In this Review, we discuss the emerging potential and associated challenges of targeting the PHD–HIF pathway for the treatment of inflammatory and ischaemic diseases.
Hypoxia-inducible factors (HIFs) are ancient transcription factors that are stabilized when the availability of oxygen is limited (that is, during hypoxia), and drive a transcriptional programme that promotes hypoxia adaptation1. HIFs were initially discovered ~20 years ago by Gregg Semenza and colleagues; studies of erythropoietin (EPO) gene regulation showed that HIFs are transcriptional regulators of hypoxia- or anaemia-associated increases in EPO release and concomitant increases in erythropoiesis2,3. Subsequent studies then implicated HIFs in the regulation of the glycolytic pathway4,5.
Such findings have steered the field of hypoxia research into a completely new direction, with an exciting focus on understanding the molecular mechanisms that alter cellular responses to conditions of limited oxygen availability. Moreover, mounting evidence indicates that HIFs have important roles in a wide range of diseases, including conditions characterized by ischaemia and inflammation6–12. Consequently, pharmacological approaches to enhance or inhibit the stabilization of HIFs are considered promising11,12. Indeed, various ongoing clinical studies are examining orally bioavailable small-molecule activators of HIFs for the treatment of renal anaemia. Similarly, studies have identified digoxin and other cardiac glycosides as HIF inhibitors13. Given the important functional role of HIFs in ischaemic and inflammatory diseases, and the interdependent relationship between inflammation and hypoxia or ischaemia, strategies aimed at modulating hypoxia-signalling pathways for the treatment of ischaemic and inflammatory diseases are gaining considerable attention.
Cellular oxygen-sensing and signalling
HIFs are αβ-heterodimeric transcription factors that function as global oxygen homeostasis regulators and therefore have central roles in the transcriptional responses that regulate tissue metabolism, stress and adaptation to diminished availability of oxygen14,15. Both HIF1 and HIF2 are members of the Per-ARNT-Sim (PAS) basic helix–loop–helix family of transcription factors. HIF stabilization in hypoxic conditions occurs through the stabilization of the oxygen-dependent degradation domain (ODD) of the α-subunit, followed by the nuclear translocation and subsequent formation of a functional complex with the β-subunit (which is also known as the aryl hydrocarbon nuclear translocator (ARNT)) and other co−activators, such as the cofactor p300 (REFS 7,14) (FIG. 1). When adequate tissue levels of oxygen are available, iron- and oxygen-dependent hydroxylation of two proline residues (positions 402 and 564 on human HIF1α) within the ODD of HIF1α and HIF2α promotes the interaction with von Hippel–Lindau disease tumour suppressor protein (VHL), leading to the rapid degradation of HIFs via the E3 ubiquitin ligase proteasomal pathway16–19. An additional regulatory switch functions in the carboxy-terminal transactivation domain of HIF1α and HIF2α, whereby low oxygen levels block the hydroxylation of asparagine-803, thereby facilitating the recruitment of p300 (REF. 20).
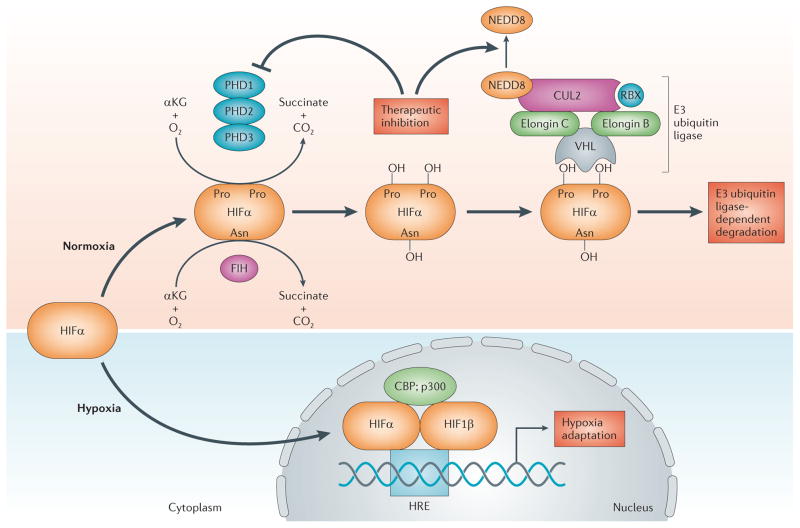
Figure 1. Molecular mechanism of oxygen sensing and signalling.
Depicted here is the biochemical pathway of hypoxia-inducible factor (HIF) hydroxylation through a combination of α-ketoglutarate (αKG), molecular oxygen (O2), one or more of the prolyl hydroxylase (PHD) isoenzymes and the asparaginyl hydroxylase factor inhibiting HIF (FIH) in normoxia. In normoxia, the von Hippel–Lindau disease tumour suppressor protein (VHL)-containing E3 ubiquitin ligase recognizes the α-subunit of HIFs and targets the subunit for polyubiquitylation and subsequent degradation. Regulation of the E3 ligase is maintained by the covalent modification of the ubiquitin-like protein NEDD8. The functional E3 ligase requires the COP9 signalosome to bind NEDD8 to CUL2. When oxygen becomes limited (hypoxia), the α-subunit is no longer hydroxylated and becomes stabilized. In the nucleus, the α-subunit binds to the HIF1β subunit and the complex becomes transcriptionally active upon binding to the hypoxia-response element (HRE) consensus sequence on DNA. This binding results in the transcriptional activation of genetically controlled survival pathways, which are central to the regulation of inflammatory outcomes. HIF activation can be elicited pharmacologically using PHD inhibitors, thereby promoting normoxic HIF stabilization and concomitant alterations in gene expression. Similarly, targeting of cullin 2 (CUL2) neddylation (for example, inhibition of NEDD8-activating enzyme) results in CUL2 deneddylation and a loss of E3 ubiquitin ligase activity with concomitant stabilization of HIFα. CBP, CREB-binding protein; RBX, RING-box protein.
HIF1α was the first HIF isoform identified; it was purified through oligonucleotide binding to the 3′ region of the EPO gene3. HIF2α was subsequently identified through homology searches2 and was discovered to be a heterodimeric binding partner of HIF1β21. Initially, HIF2α was thought to be predominantly expressed in endothelial cells (hence its alternative name endothelial PAS protein (EPAS))21. HIF3α is a more distantly related isoform that, when spliced appropriately, encodes a protein that antagonizes hypoxia response element (HRE)-dependent gene induction22–24.
For years, the mechanism through which low oxygen levels stabilize the expression of HIFs remained poorly understood. However, given the importance of prolyl hydroxylation of the ODD region of HIF1α and HIF2α to protein stability, attention turned to the potential role of enzyme-mediated hydroxylation. Because other mammalian prolyl hydroxylases (PHDs), such as pro-collagen PHDs, were dependent on the levels of 2-oxoglutarate (2-OG)25, it was predicted that HIF-modifying enzymes are also PHDs. Based on conserved structural features25, a candidate molecular approach was used to define HIF-modifying enzymes. This approach identified the HIF PHDs as the products of genes related to egl-9 in Caenorhabditis elegans (egl-9 was first described in the context of an egg-laying abnormal (EGL) phenotype)18. Subsequent studies found that three isoforms of PHDs (PHD1, PHD2 and PHD3) were expressed in mammalian cells18,19. In vitro studies confirmed that these PHDs can hydroxylate HIFα, thereby targeting HIF1α and HIF2α for degradation via the proteasomal pathway18,19 (FIG. 1)
The E3 SCF (SKP1–cullin-1–F-box) ubiquitin ligase specific to HIFα family members comprises elongin B, elongin C, RING-box protein (RBX), cullin 2 (CUL2) and the F-box domain of VHL, and is responsible for the polyubiquitylation of HIFα26 (FIG. 1). Regulation of the E3 SCF ligase is maintained by the covalent modification of the ubiquitin-like protein NEDD8. The functional E3 SCF ligase requires the COP9 signalosome to bind NEDD8 to CUL2, which can be deneddylated by the NEDD8-specific protease deneddylase 1 (DEN1; also known as SENP8). Defining specific aspects of NEDD8–cullin conjugation pathways in vivo has been hampered owing to the embryonic lethality of knocking out pathway components in mouse lines. Recently, a small molecule that disrupts NEDD8 conjugation to cullin proteins became commercially available. This compound, MLN4924, inhibits NEDD8-activating enzyme and results in the deneddylation of CUL1 and CUL2 (REFS 27,28). MLN4924 is an AMP analogue27,28 and, because adenosine can deneddylate cullin proteins29, MLN4924 could provide insights into the mechanism of cullin deneddylation and subsequent effects on HIF stability. Indeed, it was recently shown that MLN4924 is a potent HIF stabilizer in cultured endothelial cells30.
Interdependence of hypoxia and inflammation
Hypoxia and inflammation are intimately linked on many levels11,12 and have functional roles in many human diseases. Indeed, a wide range of clinical conditions are characterized by hypoxia- or ischaemia-driven inflammation or by inflammation-associated hypoxia (that is, inflammatory hypoxia). Accumulating evidence shows that inflammatory lesions are characterized by the occurrence of tissue hypoxia (inflammatory hypoxia), which is probably a result of increased metabolism and diminished oxygen supply. For example, this is the case during the intestinal inflammation observed in patients suffering from inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease11,12,31. Such inflammatory hypoxia is caused by dramatic shifts in metabolic supply and demand ratios32. On the one hand, inflammation leads to microenvironmental alterations in tissue metabolism that are characterized by profound increases in local metabolic demand. On the other hand, the metabolic supply from the blood-stream is decreased as a result of vascular occlusion and thrombosis33.
Experimental studies using histological staining approaches to map tissue hypoxia demonstrate that the intestinal mucosa and underlying tissues become profoundly hypoxic during experimentally induced intestinal inflammation34,35. This hypoxic effect was associated with the stabilization of HIFs and concomitant changes in protein expression. Moreover, pro-inflammatory molecules can directly stabilize HIFs; for example, lipopolysaccharide and signalling through Toll-like receptors (TLRs) result in HIF stabilization36 while, at the same time, HIFs can induce TLRs37. In addition, metabolic by-products (for example, citric acid cycle intermediates such as succinate) can also function as HIF activators during inflammation38. Similarly, during infections with different pathogens, HIFs are stabilized39–44 either owing to hypoxia of the infected tissues41 or through hypoxia-independent pathways40,42. For example, infections with Enterobacteriaceae such as Yersinia enterocolitica are characterized by the bacterial release of iron-binding siderophores that can function to stabilize HIFs via the formation of chelate complexes with Fe2+ ions, thereby inhibiting PHDs40. Other studies provide evidence that inflammatory pathways — such as the nuclear factor-κB (NF-κB) pathway — are intimately linked to hypoxia signalling (BOX 1). For example, experimental evidence indicates that NF-κB signalling controls the baseline mRNA levels of HIFs45. Taken together, these findings indicate that inflammatory diseases are characterized by the occurrence of tissue hypoxia, and that hypoxia-dependent and hypoxia-independent molecular pathways converge on the inflammatory stabilization of HIFs.
Box 1. The link between hypoxia and the NF-κB pathway.
Hypoxia-inducible factors (HIFs) and nuclear factor-κB (NF-κB) demonstrate an intimate interdependence at several mechanistic levels6. Indeed, oxygen-sensing hydroxylases control transcriptional adaptation to hypoxia through the regulation of both HIFs and NF-κB, which in turn regulate inflammatory responses179. Hypoxia can activate NF-κB and this seems, at least in part, to be dependent on prolyl hydroxylase (PHD)-mediated hydroxylation of IκB kinase-β (IKKβ)50,180. Indeed, within its activation loop, IKKβ contains an evolutionarily conserved consensus motif (LXXLAP, where X is any amino acid) for hydroxylation by PHDs50. In normoxia, IKKβ activity is suppressed through the putative hydroxylation of the LXXLAP motif by PHD1 and PHD2 (REF. 50). However, the hydroxylation of this peptide has not been definitively demonstrated (for example, this has yet to be shown by mass spectrometry).
Nonetheless, it is notable that conditional deletion of the NF-κB pathway in intestinal epithelial cells in mice led to a paradoxical increase in susceptibility to colitis181, similar to that of mice expressing mutant intestinal epithelial Hif1a34. This observation implicates epithelial NF-κB in a predominantly protective role in colitis, at least in part through the expression of epithelial anti-apoptotic genes and enhanced barrier function. Some studies have suggested that both the HIF pathway and the NF-κB pathway may also be influenced by mediators found within inflammatory sites, including microbial products, cytokines and even intact bacteria43.
NF-κB is an archetypal transcriptional regulator that is activated by a range of agonists, the activation of which drives a complex series of receptor-mediated signalling pathways. Recent studies indicate that transcription of HIF1α is activated by NF-κB-mediated signalling45. Inflammation-associated upregulation of HIF1A mRNA occurs in an NF-κB-dependent manner45. It also seems that increased NF-κB activity in hypoxia can be regulated by HIF1 (REF. 6), thereby creating a regulatory pathway that involves other transcriptional regulators with non-redundant PHD sensitivity, including Notch and activating transcription factor 4 (REFS 182,183), which both function as critical regulators of cell fate. Given that intestinal epithelial cells exist in an environment of constant exposure to potentially inflammatory stimuli (for example, lipopolysaccharide and pathogenic microorganisms), this cross-regulation of HIF and NF-κB may have profound implications for intestinal epithelial cell survival and function under both homeostatic and disease conditions.
Finally, a recent study indicates that, in addition to their direct effects on HIFs and NF-κB, hydroxylase inhibitors can mediate the anti-inflammatory functions of HIFs and NF-κB via hydroxylation of components of the interleukin-1B (IL-1B) pathway179. A combination of PHD1 and factor inhibiting HIF (FIH) hydroxylase isoforms regulates IL-1β-induced NF-κB at the level of (or downstream of) the tumour necrosis factor receptor-associated factor 6 complex. Indeed, multiple proteins of the distal IL-1β-signalling pathway are subject to hydroxylation and form complexes with either PHD1 or FIH179. These findings indicate that hydroxylase inhibition represents a unique approach to the inhibition of IL-1β-dependent inflammatory signalling179,184.
As we discuss later in this Review, there are many examples in which stabilization of HIFs dampens inflammatory responses33. However, it is important to note that hypoxia itself is an inflammatory stimulus because it breaks down tissue barriers, causes oedema and increases inflammatory cytokine levels. Studies in humans indicate that mountain climbers experience subclinical pulmonary oedema in the context of severe hypoxia46. Similarly, volunteers spending time at moderate altitude experience increased levels of circulating cytokines47. These findings in humans are mirrored in animal studies. For example, exposure of mice to severe hypoxia (8% oxygen) over 4 hours was associated with decreased barrier function of intestinal epithelial cells48 or increased vascular leakage into different organs49, indicating a prominent role of hypoxia on vascular barrier dysfunction. Mechanistic studies have also provided insights into hypoxia-driven inflammation. For example, hypoxic conditions were associated with increases in NF-κB activity (BOX 1), which is mediated through the negative regulation of IκB kinase-β by PHD1 (REF. 50). Collectively, these studies demonstrate that hypoxic conditions share many similarities with inflammatory diseases. However, emerging evidence indicates that concomitant inhibition of PHDs and subsequent stabilization of HIFs during tissue hypoxia could function as an endogenous adaptive response to counterbalance hypoxia-driven inflammation and to restore normal cellular functions51.
Pharmacological manipulation of PHDs and HIFs
So far, information regarding the potential effects of modulating the HIF hydroxylase system has mostly been acquired from genetic studies in animal models. A substantial number of pharmacological studies (generally using nonspecific 2-OG oxygenase inhibitors) have been conducted in animal models, and a few clinical studies have been performed. Indeed, several companies are involved in the discovery and development of PHD inhibitors for anaemia and other indications (see below), and the possibility of inhibiting HIF itself in different settings has also attracted interest.
Ischaemic and inflammatory disease states are areas in which PHD inhibitors are actively being pursued by many researchers as a novel therapeutic approach. However, there are many challenges that this field needs to overcome, including the question of how to obtain differential effects of HIF activation in the setting of a whole organism to regulate physiological outcomes. Other questions of interest include whether specific inhibitors of individual PHDs or activation of specific isoforms of HIFs are the best approach, and what the long-term consequences of HIF activation are. In addition, potential side effects of HIF activators and PHD inhibitors, as well as subgroups of patients in whom such therapeutic approaches would be beneficial, need to be identified. In the following sections we discuss examples of diseases that have been studied from the perspective of HIF activation or inhibition, and provide an update on the challenges that must be overcome to move therapeutic approaches that target the PHD–HIF pathway from bench to bedside.
PHDs and HIFs in inflammation and ischaemia
Inflammatory bowel disease
IBD, which includes ulcerative colitis and Crohn’s disease, has become a frequently used disease model to study HIFs and oxygen metabolism in the setting of ongoing inflammation. IBD is a chronic mucosal inflammatory disorder that results from a dysregulated immune response in genetically predisposed hosts52. Although the exact aetiology of Crohn’s disease and ulcerative colitis remains unclear, accumulating evidence suggests that dysfunction of the mucosal immune system has an important role in the pathogenesis of IBD. Strong evidence also implicates impaired innate immunity (involving granulocytes, macrophages and dendritic cells) in IBD, particularly in Crohn’s disease53.
IBD is a particularly relevant example of how oxygen metabolism contributes to inflammatory disease outcomes. Even under physiological conditions, the intestinal mucosa experiences profound fluctuations in blood flow and tissue oxygenation54. Using oxygen-sensitive compounds (for example, nitroimidazole-derived compounds), we and others have defined a steep oxygen gradient from the anaerobic lumen of the colon across the epithelium into a highly vascularized and metabolically active subepithelium54. From this perspective, it is perhaps not surprising that the epithelium has evolved several features to adapt to significant metabolic shifts during normal tissue function. For example, a comparison of barrier function responses between epithelial cells from different tissues revealed that intestinal epithelial cells seem to be uniquely resistant to low oxygen culture conditions34. Moreover, the normally low level of oxygenation within the healthy intestinal epithelium may be a regulatory adaptation mechanism to this steep oxygen gradient that can be observed morphologically34. Most recently, it was demonstrated that, during acute inflammatory disease, infiltrating neutrophils ‘mould’ the microenvironment in ways that significantly promote HIF-dependent transcriptional responses51. Together, these studies reveal that transmigrating neutrophils rapidly deplete the microenvironment of oxygen in an NADPH oxidase-dependent manner and transcriptionally imprint a molecular signature that significantly reflects HIF target genes. Importantly, this molecular signature promotes effective HIF-dependent inflammatory resolution. In this regard, a clinical corollary to these findings has indicated that patients who lack a functional NADPH oxidase (that is, those with chronic granulomatous disease) often present with an IBD-like syndrome55.
Many of the current studies examining HIFs in IBD have used mice with genetic alterations in the HIF pathway. For example, mice with deletions of Hif1a in intestinal epithelial cells are more susceptible to intestinal inflammation and have more severe disease in a wide range of murine models of intestinal inflammation33,34,54,56. By contrast, loss of Hif2a seems to be protective in similar models57. Tissue-specific deletion of Hif1a in intestinal epithelial cells was associated with increased weight loss, increased intestinal inflammation and more profound colonic shortening during 2,4,6-trinitrobenzenesulphonic acid (TNBS)-induced inflammation34. Similarly, mice with elevated HIF1α levels due to genetic deletion of the Vhl gene seemed to be protected during TNBS-induced colitis34. In addition, Phd1-null mice showed robust protection during intestinal inflammation, again implicating HIFs in gut protection during IBD58. However, it is important to note that Phd1 deletion could also be a driver of other transcriptional pathways, including NF-κB, and such pathways may also contribute to the gut protection observed in Phd1 deletion58. Interestingly, recent studies also implicate additional tissue-specific sources for HIF1α in gut protection during IBD. For example, tissue-specific deletion of Hif1a in regulatory T (TReg) cells attenuated their capability to repress gut inflammation in an immunological T cell transfer model of intestinal inflammation, thereby implicating T cell-dependent HIF1α in attenuating intestinal inflammation35.
Interestingly, a recent study highlighted a pro-inflammatory role for epithelial HIF2α in murine models of chemically induced and bacteria-induced colitis, which is in contrast to the barrier protective programme elicited by HIF1α57. However, epithelial HIF1-based signalling was also shown to promote inflammation in one study using dextran sodium sulphate-induced colitis59. Such contrasting findings probably reflect the context-dependent nature of these colitis models. Indeed, the temporal kinetics of HIF1α versus HIF2α in IBD seem to be different, with HIF1α stabilization occurring earlier in the disease course and a more prominent HIF2α signal occurring at later time points. Nonetheless, head-to-head comparisons have yet to fully elucidate the exact roles of HIF1α and HIF2α.
HIF-dependent target genes have been implicated in gut protection during intestinal inflammation. For example, the production and signalling effects of the anti-inflammatory signalling molecule adenosine mediate HIF-dependent attenuation of intestinal inflammation56,60–62 (BOX 2; FIG. 2). Other studies implicate HIF1α in the transcriptional induction of its target gene forkhead box P3 (FOXP3), and concomitant increases in TReg cell functions in attenuating mucosal inflammation35,63,64. In addition, the role of HIF-induced anti-inflammatory microRNAs must be considered in the mediation of HIF-dependent gut protection65.
Box 2. Adenine nucleotide metabolism and signalling.
Several studies have shown an important connection between hypoxia, hypoxia-inducible factors (HIFs) and the extracellular signalling molecule adenosine. Chemically, adenosine belongs to the group of purinergic signalling molecules that also include ATP and ADP11. Although the adenosine nucleotides ATP and ADP can promote inflammation32,185, nucleotide metabolism to adenosine and subsequent adenosine signalling events can also dampen inflammation11,186. Interestingly, hypoxia signalling through HIFs has been implicated in shifting the balance between ATP or ADP and adenosine towards promoting adenosine signalling77,187,188.
During inflammation or hypoxia, many cell types release extracellular ATP or ADP either from apoptotic or necrotic cells, or through molecularly controlled pathways189,190. Hypoxia is associated with the transcriptional induction of enzymes that control the extracellular conversion of ATP or ADP to adenosine191,192. For example, the pacemaker reaction for the extracellular generation of adenosine from precursor nucleotides is controlled by the ecto-5′-nucleotidase CD73 (REFS 48,193). As such, CD73 converts extracellular AMP to adenosine. Indeed, previous studies have shown that CD73 is a classic HIF target gene48 and that hypoxic tissue conditions stimulate its expression and function (FIG. 2).
Extracellular adenosine can signal through four distinct adenosine receptors: A1 (encoded by ADORA1), A2A (encoded by ADORA2A), A2B (encoded by ADORA2B) or A3 (encoded by ADORA3). In particular, the A2A and A2B receptors can dampen inflammation during conditions of hypoxia or ischaemia. Experimental studies provide evidence that ADORA2A is a target gene of HIF2α194, whereas ADORA2B is a HIF1α target gene195. In addition to a role for HIFs in promoting extracellular adenosine production from precursor nucleotides and adenosine signalling events, HIFs have also been implicated in attenuating the termination of adenosine signalling. As such, HIFs directly repress the expression of the equilibrative nucleoside transporters ENT1 and ENT2 (REFS 196–198), as well as their intracellular conversion of adenosine to AMP via the adenosine kinase to AMP199. Moreover, there are many instances in which the anti-inflammatory effects of HIF activators (for example, dimethyloxalylglycine) are abolished or attenuated in mouse models deficient in extracellular adenosine production (for example, Cd73−/− mice) or adenosine receptor signalling events (for example, Adora2b−/− mice)56,76. Taken together, these studies highlight a role for HIF activators in enhancing extracellular adenosine signalling events as a means for treating excessive inflammation or promoting ischaemia tolerance in different organs10,187.
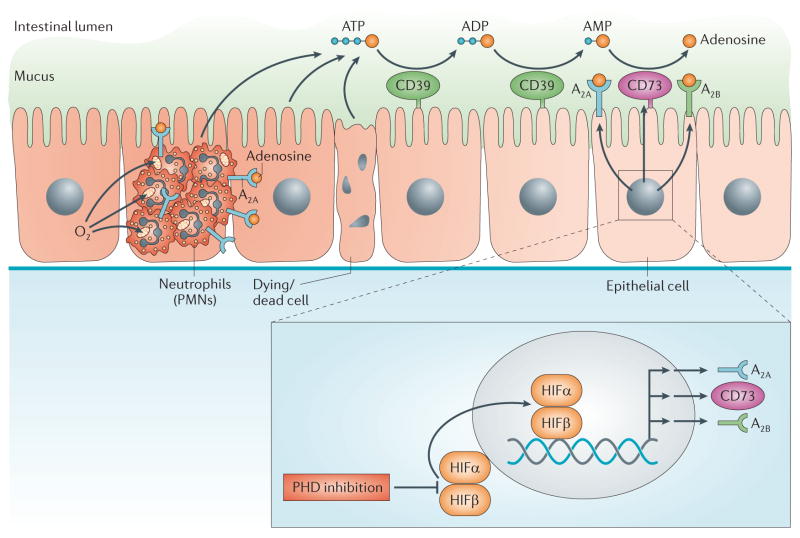
Figure 2. PHD inhibitor treatment of inflammatory bowel disease.
Inflammatory bowel disease is a result of intestinal inflammation, in which profound changes in metabolic supply and demand lead to an imbalance in oxygen supply. This imbalance causes severe hypoxia of the inflamed mucosa. Mucosal hypoxia during intestinal inflammation can be visualized using nitroimidazole compounds that stain hypoxic tissues33,34. Infiltrating polymorphonuclear neutrophils (PMNs) contribute to tissue hypoxia of the inflamed intestinal mucosa by localized oxygen depletion and concomitant stabilization of hypoxia-inducible factor (HIF), thereby contributing to the resolution of inflammation51. Inflammatory hypoxia causes the activation of hypoxia-elicited gene programmes. Coordinated gene programmes result in the increased production and signalling of extracellular adenosine. This gene programme is under the control of SP1-dependent induction of CD39, HIF-dependent induction of CD73 and the adenosine A2A and A2B receptors. These transcriptional changes lead to an increased turnover rate of the extracellular nucleotides ATP and ADP to AMP (via CD39) and subsequently via CD73 to adenosine. This pathway provides robust protection during intestinal inflammation. Importantly, orally available inhibitors of prolyl hydroxylases (PHDs) are available for the treatment of patients and are effective in increasing erythropoietin responses. Such compounds can promote intestinal protection from inflammation via enhancing extracellular adenosine production and signalling through adenosine receptors.
Several studies have demonstrated that pharmacological compounds that function to stabilize HIFs provided potent protection during intestinal inflammation. In most instances, the pharmacological approach to achieving HIF stabilization under normoxic conditions involved the inhibition of PHDs. Targeting the catalytic domain of PHDs can be achieved through the generation of molecules that interfere with critical cofactors such as 2-OG by structural mimicry, as is the case for the PHD inhibitor dimethyloxalylglycine (DMOG)66. Indeed, two studies simultaneously demonstrated a protective role for HIF activators in different models of intestinal inflammation. The first study used DMOG (a non-specific inhibitor of 2-OG oxygenases, including PHD) for the treatment of intestinal inflammation during chemically induced colitis67. A second study used the specific HIF activator FG-4497 during TNBS-induced intestinal inflammation. Similar to DMOG, FG-4497 blocks the active site of PHDs66,68. In both studies, HIF activator treatment was associated with profound improvements of multiple disease parameters, including weight loss, intestinal inflammation and histologically observed tissue injury67,68.
Indeed, the use of more HIF1-selective stabilizers, including AKB-4924 (REF. 69), holds promise in such intestinal inflammation models and suggests that IBD may be one of the more promising potential indications for PHD inhibitor treatment. The potential use of local oral delivery of an extended-release preparation to the inflamed mucosa could represent a novel therapeutic approach for IBD.
Myocardial ischaemia–reperfusion injury
Myocardial ischaemia–reperfusion injury is a pathological condition characterized by an initial restriction of blood supply to the myocardium followed by the restoration of perfusion and concomitant re-oxygenation. In its classic manifestation, occlusion of a coronary artery is caused by a coronary thrombus and results in a severe imbalance of metabolic supply and demand, causing tissue hypoxia10. In the second stage of the disease, blood flow is rapidly restored. Surprisingly, the restoration of blood flow together with re-oxygenation is, in many instances, associated with an exacerbation of tissue injury and a profound inflammatory response (that is, reperfusion injury)70.
Several studies implicate the PHD–HIF pathway in cardioprotection from ischaemia–reperfusion injury. For example, a critical link between cardioprotection by ischaemic preconditioning and HIF was shown. Ischaemic preconditioning is an experimental strategy whereby pre-exposure to short, non-lethal episodes of ischaemia results in attenuated myocardial tissue injury during subsequent ischaemia–reperfusion injury71. The cardioprotective effects of ischaemic preconditioning are profound; a >50% reduction in the size of infarct can be observed. Therefore, it is not surprising that numerous studies have attempted to identify the underlying molecular mechanism that mediates cardioprotection during ischaemic preconditioning, with the goal of identifying pharmacological approaches that can imitate this process72–75.
One study using an in situ model of murine ischaemic preconditioning74 pursued the hypothesis that HIFs can have a functional role in mediating cardioprotection by ischaemic preconditioning76. Indeed, preconditioning of the myocardium with four episodes of short, non-lethal myocardial ischaemia was associated with stabilization of HIF1 in the preconditioned myocardium. Moreover, in vivo small interfering RNA (siRNA) repression of cardiac Hif1a abolished the cardioprotective effects induced by ischaemic pre-conditioning. Pretreatment with the HIF activator DMOG elicited a similar extent of cardioprotection as that following ischaemic preconditioning itself. As an end point of HIF-dependent cardioprotection, the authors observed a functional role of HIF-dependent enhancement of purinergic signalling events76 (BOX 2). Taken together, these findings suggest that HIF1α has a functional role in mediating ischaemic preconditioning of the heart and implicate HIF activators such as DMOG in cardioprotection.
A recent study provided some interesting additional insights into the potential mechanisms of how HIF1α functions in mediating cardioprotection from ischaemia–reperfusion injury77,78. It has long been known that one of the critical functions of HIFs is the induction of glycolytic enzymes, which is considered an important mechanism involved in enhancing the capacity of hypoxic or ischaemic tissues to increase anaerobic glycolysis and concomitant ATP production during hypoxia or anoxia4,5. Interestingly, this function of HIF1α involves the interaction with the circadian rhythm protein period 2 (PER2). Indeed, HIF-dependent induction of the glycolytic programme during myocardial ischaemia was completely abolished in Per2−/− mice78. Additional studies in HIF or PER2 reporter mice provided further evidence for an interaction between HIF and PER in the heart. These studies indicated that, similar to the circadian alteration of PER2 levels over a 24-hour period, HIF1α also exhibits a circadian expression pattern77,78. Moreover, genetic ablation of Per2 in HIF reporter mice arrested the circadian expression pattern of HIF, indicating a functional interaction between HIF1α and PER2. Interestingly, light-induced stabilization of cardiac Per2 expression also promoted increases in the expression of glycolytic enzymes and concomitant cardioprotection, indicating that PER2 and HIF1α interact during myocardial ischaemia to increase the anaerobic glycolytic capacity of the ischaemic heart78.
An additional experimental strategy that is implicated in cardioprotection is referred to as remote ischaemic preconditioning79. Indeed, both preclinical and clinical studies suggest that brief cycles of ischaemia and reperfusion of an extremity (for example, the arm or the leg) can protect the heart from subsequent ischaemic myocardial injury79–82. For example, remote ischaemic preconditioning was associated with increased plasma interleukin-10 (IL-10) levels and concomitant decreased myocardial infarct size in wild- type mice but not in mice with gene-targeted deletion of Hif1a80. This effect could be pharmacologically elicited via injection of a recombinant adenovirus encoding a constitutively active form of HIF1α into the mouse hindlimb muscle80. Together, these studies provide strong experimental evidence that HIF1 activates IL10 transcription and is necessary for remote ischaemic preconditioning, again implicating HIF activators (such as PHD inhibitors) in cardioprotection from ischaemia.
Acute lung injury
Several studies provide strong evidence for an important role for HIF or HIF-specific target genes in dampening lung inflammation during acute lung injury (ALI)83–87. ALI is caused by injuries or acute infections to the lung88,89, which manifests itself as acute respiratory distress syndrome in patients90. Severe pulmonary oedema and uncontrolled lung inflammation are typical symptoms of ALI, causing difficulty in breathing and the need for mechanical ventilation. Survivors of acute respiratory distress syndrome experience long-term impairment of their physical and psychological quality of life, and increased costs and use of health care services91.
A landmark study that implicates HIFs in lung protection during ALI examined the consequences of supplemental use of high oxygen concentrations. Although oxygen therapy represents a life-saving measure, the discovery of HIF-dependent tissue protection92,93 raises the possibility that administration of high concentrations of oxygen to ALI patients with uncontrolled pulmonary inflammation may have dangerous side effects, such as preventing HIF stabilization94. In a mouse model of ALI induced by bacterial infections, mice exposed to 100% oxygen (which mimicked therapeutic oxygenation) had reduced survival rates compared with mice exposed to normal ambient levels of oxygen (21% oxygen). Indeed, five times as many mice died after receiving 100% oxygen than those breathing normal oxygen levels. These findings suggest that hypoxia and concomitant stabilization of HIFs protect against lung damage. Taken together, such studies indicate that high levels of inspired oxygen — as may be required to provide sufficient tissue oxygenation in patients suffering from ALI — may weaken the local tissue hypoxia-driven, HIF-mediated and adenosine-mediated (BOX 2) anti-inflammatory mechanisms, thereby further exacerbating lung injury92–94.
A subsequent study provided additional evidence for a direct role of HIF1α in lung protection during ALI95. It was reasoned that although ALI contributes significantly to critical illness, it spontaneously resolves in many instances. Because most patients experiencing ALI require mechanical ventilation, it was hypothesized that mechanical ventilation and concomitant stretch exposure of pulmonary epithelia might activate endogenous pathways important in lung protection. To address this hypothesis, pulmonary epithelia were exposed to cyclic mechanical stretch conditions — an in vitro model resembling mechanical ventilation. Surprisingly, a genome-wide screen revealed a transcriptional response in these mechanically stretched epithelia that was similar to hypoxia signalling, and subsequent studies confirmed stabilization of HIF1α during stretch conditions in vitro or during ventilator-induced ALI in vivo95. Extension of these findings identified a functional role for stretch-induced inhibition of succinate dehydrogenase (SDH) in mediating normoxic HIF1α stabilization and concomitant increases in glycolytic capacity95. Although the mechanism underlying these responses remains unclear, the tricarboxylic acid cycle flux was increased during ALI, which was abolished in mice with targeted deletion of Hif1a in alveolar epithelial cells95. Pharmacological studies with HIF activator or inhibitor treatment implicates HIF1α stabilization in attenuating pulmonary oedema and lung inflammation during ALI in vivo95. Systematic deletion of Hif1a in the lungs, endothelia, myeloid cells or pulmonary epithelia linked these findings to alveolar epithelial HIF1α95. In vivo analysis of 13C-glucose metabolites using liquid chromatography–tandem mass spectrometry demonstrated that increases in glycolytic capacity, improvement of mitochondrial respiration and concomitant attenuation of lung inflammation during ALI were specific for alveolar epithelial expressed Hif1a95. Collectively, these studies reveal a direct role for HIF1α in lung protection during ALI, in which normoxic HIF1α stabilization and HIF-dependent control of alveolar epithelial glucose metabolism function as an endogenous feedback loop to dampen lung inflammation95 (FIG. 3). Importantly, these findings support the idea of using HIF activators for ALI treatment. Indeed, in vivo treatment of mice with a PHD inhibitor was associated with dramatic increases in survival and attenuation of lung inflammation during ALI induced by mechanical ventilation95.
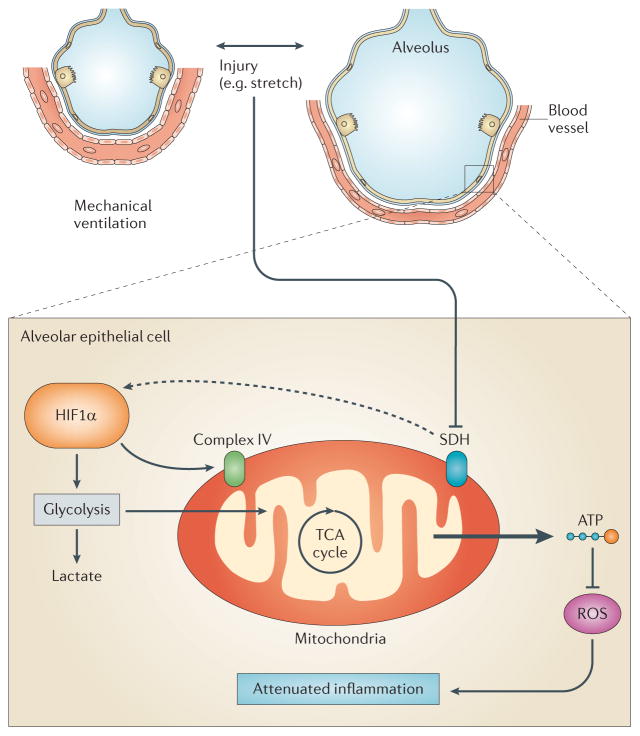
Figure 3. Mechanism of HIF1α stabilization during acute lung injury.
Patients with acute lung injury (ALI) frequently require mechanical ventilation of their lungs to maintain sufficient oxygen levels in their blood to oxygenate critical organs such as the brain, the kidneys or the heart. Cyclic mechanical stretch conditions during mechanical ventilation in vivo or during cyclic mechanical stretch exposure of the alveolar epithelial cells in vitro result in hypoxia-inducible factor 1, α-subunit (HIF1α) stabilization95. Stretch-induced HIF1α stabilization is mediated by the inhibition of succinate dehydrogenase (SDH), causing normoxic stabilization of alveolar epithelial HIF1α. Functional studies implicate alveolar epithelial HIF1α in optimizing carbohydrate metabolism of the injured lungs by increasing glycolytic capacity and tricarboxylic acid (TCA) cycle flux, and by optimizing mitochondrial respiration via induction of complex IV. HIF1α-dependent prevention of mitochondrial dysfunction during ALI is associated with increased alveolar epithelial capacity to produce ATP, while concomitantly preventing reactive oxygen species (ROS) accumulation and attenuating lung inflammation.
Infectious diseases and antimicrobial activity
The hypoxic microenvironment of the inflammatory lesion, specifically HIF1, has been implicated in the function of myeloid cells to clear infections. Not only is HIF1 essential to support glycolytic metabolism of phagocytes, but it also regulates key functions such as bacterial uptake, production of antimicrobial effector molecules (for example, cathelicidin-related antimicrobial peptide and serine proteases) and enhancing longevity of neutrophils12. In this regard, a fundamental difference between innate immunity and adaptive immunity is the means by which individual leukocyte populations obtain energy. Cells of myeloid lineages derive their energy almost exclusively from glycolysis, whereas lymphocytes predominantly use oxidative phosphorylation96.
Studies by Cramer97 and by Peyssonnaux44 revealed an essential role for HIF1 in innate immune function of myeloid phagocytes. These studies used conditional deletion of Hif1a in myeloid populations and showed a decreased bactericidal capacity of myeloid phagocytes that lacked functional HIF1α. Conversely, these same studies also revealed that genetic loss of myeloid VHL (that is, stabilization of HIF) resulted in enhanced acute inflammatory responses. Given that VHL has numerous substrates, it remains to be determined to what extent HIF contributes to this hyperinflammatory response. With regard to adaptive immunity, parallel studies using V(D)J recombination-activating protein 2 (RAG2)-deficient blastocyst complementation to bypass embryonic lethality revealed that HIF1α deficiency in T and B lymphocytes resulted in major defects in the development of B cells, significant autoimmunity reflected as immunoglobulin G (IgG) and IgM deposits in the kidney, and increased double-stranded DNA (dsDNA)-specific antibodies98.
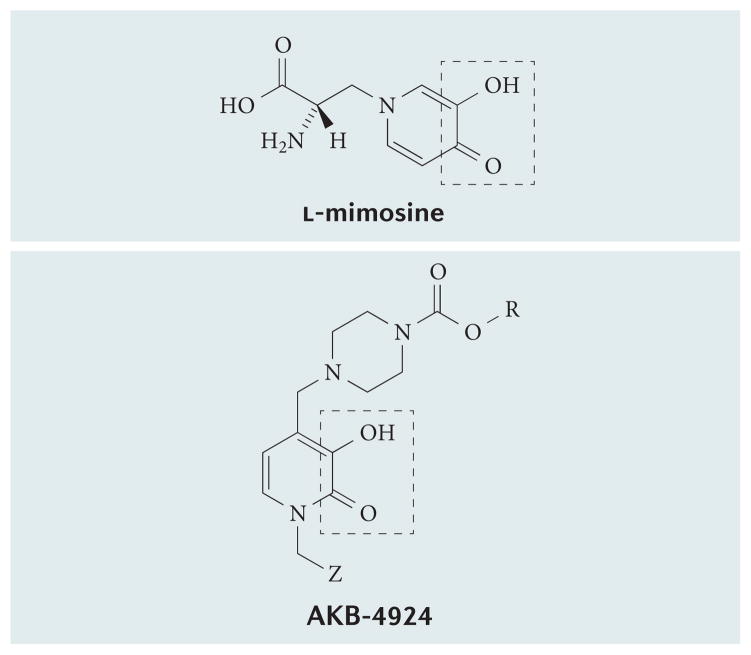
Recent extensions of these studies using the PHD inhibitor AKB-4924 (FIG. 4), which predominantly targets HIF1, revealed potent antimicrobial activity of this compound in stimulating the killing of the pathogens Pseudomonas aeruginosa and Acinetobacter baumannii99. Importantly, AKB-4924 was not directly bactericidal, and activity of this molecule mapped to the predominant induction of HIF1 but not HIF2. AKB-4924 contains an α-hydroxy carbonyl group similar to the iron-chelating inhibitor l-mimosine100 (FIG. 4), suggesting that AKB-4924 inhibits PHDs through a similar mechanism. Others have shown that pulmonary infections with P. aeruginosa are significantly attenuated with the PHD inhibitor DMOG in a HIF2- and Rho kinase-dependent manner101.
Figure 4. Structure of the prolyl hydroxylase inhibitor AKB-4924.
The AKB-4924 structure contains an iron-binding α-hydroxycarbonyl group similar to that of the iron chelator L-mimosine (outlined).
A crucial role for HIF-dependent signalling in the homeostatic regulation (that is, baseline expression) of epithelial antimicrobial peptide, particularly human β-defensin 1 (BD1), was recently recognized102. Antimicrobial peptides represent a substantial part of the mucosal barrier function, and β-defensins are the dominant class of antimicrobial peptide secreted by the epithelium. The four characterized human β-defensins (BD1, BD2, BD3 and BD4, encoded by DEFB1, DEFB4, DEFB103 and DEFB104, respectively) are small (30–47 amino acids), cationic, cysteine-rich peptides that have broad antimicrobial activity103,104. In the gut, BD1 has two characteristics that confer prominence. First, its anti-microbial activity is potentiated under reducing conditions that exist in the hypoxic gut lumen, whereas other antimicrobial peptides, such as BD3 are diminished in the reduced state105. Second, expression of BD1 is constitutive, whereas other defensins are expressed in response to microbial and inflammatory stimuli106–108. Given these properties, it is not surprising that defective expression of BD1 is associated with mucosal disease such as IBD109–111, candidia carriage112, periodontitis113 and dental carries114. It is likely that this homeostatic role of HIF in epithelial antimicrobial peptide expression complements other aspects of HIF-dependent mucosal barrier function33. Similarly, HIF1 is strongly expressed in the skin and regulates antimicrobial peptide production by keratinocytes (for example, cathelicidin). Keratinocyte-specific deletion of HIF1 enhanced susceptibility to group A streptococcal infection, whereas treatment with AKB-4924 enhanced keratinocyte bactericidal activity in vitro and in vivo99,115.
Wound healing
An important yet under-appreciated feature of wound repair is the availability of oxygen during the healing response116,117. Numerous factors, including localized vascular damage and increased tissue oxygen demand, significantly shift tissue metabolism during wound healing and can result in significant deprivation of molecular oxygen. HIF promotes mucosal barrier protection54,118,119 and is stabilized at the site of wounds during re-epithelialization120. Moreover, several HIF target genes (for example, vascular endothelial growth factor (VEGF) and inducible nitric oxide synthase) contribute to the wound healing process121,122. Although less is known about the role of HIF in tissue re-epithelialization (that is, epithelial restitution), Glover et al.123 recently showed an important role for HIF2-dependent expression of creatine kinase in intestinal epithelial cells. These studies identified several isoforms of creatine kinase as prominent HIF2 targets, and functional studies suggest that creatine metabolism provides an important source of high-energy phosphate that is necessary for epithelial restitution.
More recent studies have indicated that the induction and stabilization of HIF through the inhibition of PHDs not only ameliorates disease in murine models but also promotes wound re-epithelialization in vivo67,68. In vitro models of wound healing suggest that HIF stabilization increases fibroblast contraction within a collagen matrix, which is another important step in the wound healing process68. These results not only suggest an important role for HIFs in wound healing but also implicate HIF stabilization as a therapeutic target during active wound repair67,68.
Organ transplantation
Several studies suggest that HIF activators can prevent early graft failure during solid organ transplantation, such as heart, kidney, lung or liver transplantation. For example, early graft failure after liver transplantation is frequently caused by ischaemia–reperfusion injury and is associated with extremely high rates of morbidity and mortality. Owing to the shortage of available grafts for transplantation and a growing waiting list of recipients with an urgent need for liver transplantation, marginal livers are now being considered more frequently for transplantations. However, marginal grafts are more prone to ischaemia–reperfusion injury124.
Other studies indicate that ischaemia and reperfusion also have important immunological consequences in organ transplantation, such as affecting the severity of early liver rejection125 or the subsequent recurrence of viral hepatitis in liver transplantation recipients that require liver transplantation for hepatitis C126. Therefore, hepatic ischaemia and reperfusion are an area of intense investigation, and novel therapeutic strategies that dampen liver injury following ischaemia and reperfusion are urgently needed. Several studies suggest that HIF activators could treat acute graft failure during liver transplantation. For example, mice with genetic deletion of Phd1 and concomitant increases in HIFs are profoundly protected during hepatic ischaemia–reperfusion injury127. Additional evidence implicating HIF activators in the prevention of acute graft failure during liver transplantation is derived from studies that demonstrate a transcriptional HIF signature during ischaemia–reperfusion injury for purinergic HIF targets128,129 (BOX 2).
Other studies indirectly implicate HIF activators during β-cell transplantation, which is an important treatment option for diabetes mellitus. A study provided evidence that HIF1α is required for normal β-cell function and reserve, and that dysregulation may contribute to the pathogenesis of type 2 diabetes130. HIF1α is present at low levels in mouse and human normoxic β-cells and islets, and decreased levels of HIF1α impair glucose-stimulated ATP generation and β-cell function. Moreover, mice with β-cell-specific Hif1a deletion exhibit glucose intolerance and β-cell dysfunction, and develop severe glucose intolerance on a high-fat diet. By contrast, increasing HIF1α levels through PHD inhibitors improves insulin secretion and glucose tolerance, suggesting an important role for HIF1α in β-cell reserve and implicating HIFs as potential therapeutic targets for the β-cell dysfunction of type 2 diabetes130. Moreover, these findings indirectly suggest that HIF activators could also be used to improve β-cell function and outcomes during β-cell transplantation, which is one of the emerging therapeutic approaches for the treatment of type 2 diabetes131.
Autoimmune encephalitis
T helper type 17 (TH17) lymphocytes contribute to multiple sclerosis and to experimental autoimmune encephalomyelitis (a mouse model of multiple sclerosis)132. IL-17 is expressed on blood–brain barrier endothelial cells in multiple sclerosis lesions, and transmigrating TH17 lymphocytes promote central nervous system inflammation through CD4+ lymphocyte recruitment132. Recent studies implicate HIFs in the differentiation process of TH17 lymphocytes. Indeed, HIF1α enhances TH17 development through direct transcriptional activation of retinoid-related orphan receptor-γt (RORγt), as well as through tertiary complex formation with RORγt and p300 recruitment to the IL-17 promoter, thereby regulating TH17 signature genes133. Similarly, the HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 lymphocytes134. Upon antigen stimulation, the bioenergetic demands of T cells increased dramatically over the resting state and the glycolytic pathway, and expression of glycolytic enzymes were strongly upregulated during the differentiation of inflammatory TH17 cells. Indeed, blocking glycolysis inhibited TH17 development. Moreover, HIF1α was selectively expressed in TH17 cells, and the HIF1α-dependent transcriptional programme was important for mediating glycolytic activity, thereby contributing to differentiation of TH17 lymphocytes134.
Together, these studies provide indirect evidence that HIF1α inhibitors could be used for preventing TH17-dependent inflammation during autoimmune encephalitis. However, some studies suggest that the circumstances may be different in other T cell-driven inflammatory diseases. Indeed, a recent study showed protective functions for IL-17A in T cell-mediated intestinal inflammation in, for example, IBD135, suggesting the therapeutic potential of HIF activation. Using a T cell transfer model of intestinal inflammation, T cells deficient in the IL-17 receptor elicited an accelerated, aggressive wasting disease. Current studies indicate that HIF activators can promote the differentiation of TReg cells during intestinal inflammation via direct induction of the HIF target gene FOXP3 (REF. 136), providing additional evidence for HIF activator treatment of intestinal inflammation35.
Acute kidney injury
Acute kidney injury (AKI) is an acute disease with substantial impacts on morbidity and mortality of critical illness137. Indeed, several studies implicate AKI as one of the key initiating events in multiple-organ failure138. AKI is defined by an abrupt reduction in kidney function, observed clinically by a rise in serum creatinine values or experimentally by a rapid decrease in glomerular filtration rate. AKI is frequently caused by an obstruction of renal blood flow to the kidneys, which may occur during certain surgical procedures139. In addition, patients with sepsis often develop AKI, a combination that is associated with extremely high mortality rates140. Importantly, therapeutic approaches to prevent or treat AKI have been largely unsuccessful, and the majority of interventional clinical trials in AKI have failed141,142.
Several studies implicate HIF stabilization143 and the induction of HIF-dependent target genes in kidney protection during AKI144. For example, partial HIF1α deficiency substantially increased the severity of injury in mice subjected to renal ischaemia–reperfusion injury, whereas the HIF activator DMOG exerted robust kidney protection during ischaemic AKI143, suggesting that pharmacological activation of HIF may effectively protect the kidney from ischaemic injury. These findings are consistent with numerous studies suggesting that HIF target genes can provide potent kidney protection, including HIF-dependent enhancement of purinergic signalling pathways12. Moreover, a recent study has provided evidence for a kidney protective role of endothelial-expressed HIF2α during AKI145. Using a genetic approach, this study dissected the specific roles of endothelial HIF1 and HIF2 in murine models of hypoxic kidney injury induced by ischaemia and reperfusion or by ureteral obstruction. Interestingly, in both models, inactivation of endothelial HIF increased injury-associated renal inflammation and fibrosis. However, inactivation of endothelial HIF2α (but not endothelial HIF1α) was associated with increased expression of renal injury markers and inflammatory cell infiltration in the post-ischaemic kidney. Moreover, pharmacological or genetic activation of HIF via HIF PHD inhibition protected wild-type animals, but not mice lacking endothelial HIF2α, from ischaemic kidney injury and inflammation. Taken together, these findings implicate endothelial HIF2α in kidney protection during AKI145.
Pharmacological modulation of HIFs
The original description of HIF-selective PHDs as regulators of HIF expression has provided a template for the development of PHD-based molecular tools and therapies146,147. Pharmacological inactivation of the PHDs by 2-OG analogues is sufficient to stabilize HIF1α146, but this action is nonspecific with respect to individual PHD isoforms. In vitro studies do suggest significant differences in substrate specificity. For example, PHD3 prefers hydroxylation of proline-564 on HIF1α148, and comparisons of enzyme activity in vitro showed that the ODD sequence of HIF1α is hydroxylated most efficiently by PHD2 (REFS 7,25). These observations have generated considerable interest in identifying enzyme-modifying small-molecule inhibitors. Indeed, several PHD inhibitor classes have been described, including iron chelators, CUL2 deneddylators and 2-OG mimics (TABLE 1). The mechanism of action of these compounds is based on the observation that the binding of the co-substrate 2-OG to the catalytic domain, which harbours an essential Fe2+ ion, is crucial for enzymatic PHD and FIH (factor inhibiting HIF) activity. Therefore, chemical compounds that structurally mimic 2-OG, such as N-oxalylglycine or its precursor DMOG, inhibit PHDs and FIH by blocking the entry of the co-substrate66.
Table 1.
Classes and examples of HIF stabilizers and inhibitors
| Class | Examples | Refs |
|---|---|---|
| HIF stabilizers | ||
| 2-OG mimics | Dimethyloxalylglycine | 200 |
| N-oxalyl-d-phenylalanine | 201 | |
| Fe2+ chelators | Desferrioxamine | 202 |
| Hydralazine | 203 | |
| AKB-4924 | 99 | |
| FG-2229 | 204 | |
| TM-6008 | 205 | |
| L-mimosine | 206 | |
| PHD active-site blockers | Pyrazolopyridines | 207 |
| 8-hydroxyquinolines | 208 | |
| Compound A | 209 | |
| FG-4497 | 68 | |
| TM-6089 | 205 | |
| CUL2 deneddylators | MLN4924 | 30 |
| Fe2+ substitutes | Co2+, Ni2+ and Cu2+ | 202 |
| HIF inhibitors | ||
| PI3K and/or mTOR inhibitors | Wortmannin | 210 |
| LY294002 | 210 | |
| Temsirolimus | 211 | |
| DNA-binding inhibitors | Echinomycin | 149 |
| Histone deacetylase inhibitors | Romidepsin | 212 |
| LAQ842 | 213 | |
| Trichostatin A | 214 | |
| Heat-shock protein 90 inhibitors | Radicicol | 215 |
| Apigenin | 216 | |
| Geldanamycin | 217 | |
| 17-DMAG | 218 | |
| Cardiac glycosides | Digoxin | 13 |
| Transcription inhibitors | Bortezomib | 219 |
| Amphotericin B | 220 | |
| Chetomin | 221 | |
| Topoisomerase inhibitors | Camptothecin | 222 |
| Topotecan | 223 | |
2-OG, 2-oxoglutarate; 17-DMAG, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin; CUL2, cullin 2; HIF, hypoxia-inducible factor; PHD, prolyl hydroxylase; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinase.
The development of HIF inhibitors has been largely based on chemical compound screens (TABLE 1). Original studies by Kong et al.149 screened the National Cancer Institute small-molecule library and discovered that echinomycin (NSC-13502) potently inhibited HIF1 binding to its cognate HRE. Accordingly, echinomycin has been profiled among a number of direct and indirect HIF1 target drugs that show more or less promise as cancer therapeutics150 (TABLE 1). More recently, it was shown that cardiac glycosides inhibit HIF13. Indeed, Zhang et al.13,151 profiled the Hopkins Drug Library of 3,120 drugs that have been approved by the US Food and Drug Administration (FDA) or that have entered Phase II clinical trials, and identified 20 drugs that inhibited HIF1-dependent gene transcription. Interestingly, 11 of the most potent inhibitors are cardiac glycosides (for example, digoxin, oubain and proscillaridin A) and were revealed to be inhibitors of HIF1 protein synthesis. Other HIF inhibitors have been reviewed in detail elsewhere151 and include phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitors, heat-shock protein 90 inhibitors, histone deacetylase inhibitors and topoisomerase inhibitors (TABLE 1).
Emerging therapeutic agents
Targeting HIFs is a rapidly developing and highly dynamic field of drug discovery. Several ongoing clinical trials have examined HIF inhibitors in the context of cancer treatment; examples include the antisense oligonucleotide HIF inhibitor EZN-2968 (ClinicalTrials.gov identifier: NCT01120288) or pharmacological HIF inhibitors such as dutasteride152 (NCT00880672), topotecan153 (NCT00117013), PX-478 (REF. 154) (NCT00522652) or digoxin13 (NCT01763931). The usefulness of non-invasive PET imaging markers for detection of tumour hypoxia regions has also been tested (NCT01075399). However, one of the challenges regarding the use of digoxin as a clinical HIF inhibitor may be related to the relatively narrow therapeutic window of digoxin (for example, toxicity at the higher doses that could potentially be required for inhibiting HIF in patients). Beyond the cancer field, clinical studies are now evaluating HIFs as pharmacological targets in inflammatory and ischaemic diseases.
One area of study in which HIF activation was examined for the treatment of ischaemia is peripheral artery disease and intermittent claudication, a chronic vascular occlusion disease with extremely limited therapeutic approaches. Ischaemia normally induces the production of angiogenic cytokines and the homing of bone-marrow-derived angiogenic cells, but these adaptive responses become impaired with ageing because of reduced expression of HIFs155. As such, experimental studies analysed the effect of augmenting HIF1α levels in the ischaemic limb via intramuscular injection of AdCA5 (an adenovirus encoding a constitutively active form of HIF1α) and intravenous administration of bone-marrow-derived angiogenic cells that were cultured in the presence of the PHD inhibitor DMOG. The combined therapy increased perfusion, motor function and limb salvage in old mice subjected to femoral artery ligation155. Similarly, a different study provided strong evidence that mice with genetic deletion of Phd1 are protected during hindlimb ischaemia as a result of increased HIF levels156.
Based on these preclinical findings, a Phase I dose-escalation clinical trial was performed in patients with critical limb ischaemia. This study used an adenoviral delivery system for constitutively delivering the active form of HIF1α into the lower extremity of patients with critical limb ischaemia. Promising data showing that HIF1A gene therapy was well tolerated provided support for larger, randomized efficacy trials157. However, in a trial evaluating the efficacy of intramuscular administration of three different doses of Ad2/HIF1A/VP16 on walking time in patients with peripheral artery disease and intermittent claudication158, end points were not met. Indeed, no significant differences in claudication-onset time, ankle-brachial index or quality-of-life measurements between the placebo and each HIF1α dosage group were found, leading to the conclusion that gene therapy with intramuscular administration of Ad2/HIF1A/VP16 is not an effective treatment for patients with intermittent claudication158. Although the reasons underlying this failure remain unclear, concerns include the ability of intramuscular injection of HIF1A at multiple sites to establish contiguous collateral vessels, the duration of effect after a single treatment and the confounding effects of mechanisms other than blood supply that limit walking distance158. In particular, the efficacy of gene transfer in this study, including whether the gene transfer was increasing HIF to sufficient levels in the critical tissue compartment, remains unclear.
In contrast to the above studies that used gene-transfer approaches to increase HIF1α levels, a recent clinical trial in patients with renal anaemia examining the potential of an orally bioavailable PHD inhibitor to achieve HIF stabilization under normoxic conditions was successful159. Patients with renal anaemia have decreased red blood cell production, which leads to fatigue and other symptoms such as shortness of breath and palpitations160. In a small Phase I study, an orally active PHD inhibitor, FG-2216, was used to stabilize HIF independent of oxygen availability in 12 haemodialysis patients (6 of whom were anephric) and 6 healthy volunteers. FG-2216 increased plasma EPO levels by >30-fold in haemodialysis patients that were not anephric, >14-fold in anephric haemodialysis patients and 12.7-fold in healthy volunteers. These data demonstrate that pharmacological manipulation of the HIF system can stimulate endogenous EPO production and suggests that deranged oxygen sensing — but not a loss of EPO production capacity — causes renal anaemia159. However, owing to safety concerns with FG-2216 (fatal hepatic necrosis; see below), the compound was not developed further, and no further experimental activity has been performed on humans with FG-2216 (REF. 161). Nevertheless, a second-generation PHD inhibitor from FibroGen (FG-4592) is currently being examined in Phase III trials160 (TABLE 2).
Table 2.
Examples of ongoing clinical trials targeting prolyl hydroxylases
| Drug | Patient population | Purpose of study* | ClinicalTrials.gov number (status)‡ |
|---|---|---|---|
| FG-4592 (FibroGen, Astellas and AstraZeneca) | Non-dialysis-dependent subjects with anaemia associated chronic kidney diseases (Phase III) | Treat anaemia in patients with chronic kidney disease | NCT01887600 (recruiting) |
| Subjects with end-stage renal disease receiving maintenance haemodialysis (Phase II) | Evaluate the efficacy and safety of FG-4592 in maintaining and/or correcting haemoglobin given to subjects | NCT01147666 (completed) | |
| AKB-6548 (Akebia Therapeutics) | Subjects with chronic kidney disease and anaemia (Phase II) | Evaluate the safety, efficacy and pharmacokinetics of repeat doses of orally administered AKB-6548 in pre-dialysis subjects with anaemia | NCT01235936 (recruiting) |
| GSK1278863 (Glaxo-SmithKline) | Patients undergoing elective descending thoracic aorta/thoracic aortic aneurysm (DTA/TAAA) repair (Phase II) | Test the hypothesis that GSK1278863 will reduce neurologic, renal and/or cardiac ischaemia in subjects | NCT01920594 (recruiting) |
| Non-dialysis-dependent subjects with anaemia associated chronic kidney diseases (Phase II) | Evaluate the safety of GSK1278863 and its efficacy in elevating haemoglobin levels in subjects | NCT01977573 (recruiting) |
Potential limitations of HIF modulation
The PHD–HIF pathway is among the most ancient and conserved signal transduction pathways in humans1. Metazoan diversification occurred during a time when atmospheric oxygen levels fluctuated between 15% and 30%. As such, HIF is known to function as a primary regulator of the adaptive transcriptional response to hypoxia and changes in environmental oxygen concentrations. Although the HIF pathway is highly conserved, its complexity increased during periods when atmospheric oxygen concentrations were increasing1. Consequently, altering such an ancient adaptive pathway for hypoxia adaptation raises many concerns, particularly in a more chronic setting.
HIF activation and cancer progression
An important concern for using HIF activators in the treatment of inflammatory or ischaemic diseases are the implications on neoplastic diseases. Cancerous lesions express high levels of HIF1α and HIF2α, and the expression levels of HIFs predict cancer mortality162. Important in the context of HIF activator treatment for ischaemic or inflammatory diseases is that HIF stabilization can occur as a result of the loss of function of tumour suppressor genes, such as VHL. For example, patients with VHL disease have an increased risk of developing various tumours, including renal cell carcinoma12,163. However, there is experimental evidence to show that the roles for HIFs in cancer are highly context-dependent.
Hypoxia stimulates angiogenesis; therefore, activation of HIFs in hypoxic cancer or stromal cells is implicated in fuelling tumour vascularization164,165. However, other studies show that tumour cells can promote a non-productive angiogenic response that impairs — rather than improves — tumour oxygenation12. In this environment, cancer cells are primed to escape from the primary tumour via HIF-driven epithelial-to-mesenchymal transition and metastasize166. Notably, haplodeletion of endothelial PHD2 normalizes the endothelial layer and improves tumour oxygenation, thereby reducing tumour invasiveness, metastasis and malignancy in murine models12,167. As these studies demonstrate, the effect of HIF activators or inhibitors on cancer biology is highly complex, and this has been discussed in depth elsewhere12,162,168,169. Based on experimental studies, the question of whether HIF activators can potentially cause cancer or tumour progression in humans cannot be clearly answered. Although diligent attention has to be paid to this issue, it is reassuring that, in several clinical trials with gene-therapy-mediated HIF overexpression or with pharmacological HIF activators, no concerns regarding neoplastic diseases were reported.
HIF activation and fatal hepatic necrosis
In a Phase II clinical trial of the orally available PHD inhibitor FG-2216, a patient developed fatal hepatic necrosis, and this was related temporally to administration of the HIF stabilizer. As a result of this single death, as well as of other patients who developed abnormal liver enzyme test results, the FDA suspended this clinical trial, and development of this compound ceased (REF. 161)
A second-generation HIF stabilizer from FibroGen, FG-4592 (TABLE 2), is currently in Phase II and III clinical trials (in collaboration with Astellas Pharma and AstraZeneca) for the treatment of renal anaemia in patients with end-stage kidney disease and could become a first-in-class treatment option160. As FG-4592 has moved safely forward into Phase II and III clinical trials, it is possible that the hepatic necrosis associated with FG-2216 may have been an off-target effect.
HIF activation and sepsis
HIFs are the key transcriptional drivers of VEGF, and HIF-stimulated VEGF release may be associated with increased vascular leakage and a sepsis-like syndrome. In a study examining the role of HIF activator treatment during bronchopulmonary dysplasia — a chronic lung disease affecting preterm neonates — the authors tested whether HIF activators could enhance angiogenesis and improve lung growth and function in vivo in prematurely born baboon neonates170.
Treatment of the preterm baboons for 14 days with an intravenous PHD inhibitor, FG-4095, indicated that HIF stimulation via PHD inhibition ameliorated pathological and physiological consequences of bronchopulmonary dysplasia170. FG-4095 treatment was generally well tolerated and elicited some beneficial lung outcomes. However, when the early death subgroups were examined, several worrying findings were identified. All four early deaths included FG-4095-treated animals, which showed a skin rash that had never been observed by the authors in this model before. This strikingly erythematous, papular rash was usually detected between days 4 and 6 of treatment, was not exfoliative, involved primarily the face and scalp, and was occasionally generalized. Even though overall mortality did not differ between the treated and untreated animals, there was a significant (P = 0.002) association between death and rash in FG-4095-treated animals versus untreated controls170.
Fortunately, such findings have not been reported in clinical trials of HIF activator treatments and may therefore be specific for intravenous treatment with the compound FG-4095. Moreover, DMOG treatment was reported to be protective in models of sterile sepsis (lipopolysaccharide-induced) in which inflammation is the key driver of disease, whereas it exacerbated bacterial sepsis (cecal ligation model) possibly by suppressing inflammation and making the host susceptible to an overwhelming infection171.
HIF activation and erythropoiesis
HIF activators can promote EPO release and concomitant increases in erythrocyte production. Although this effect may be desirable for the treatment of renal anaemia160, there may be situations in which HIF-driven erythropoiesis may be harmful. Several studies indicate that increases in HIF-dependent EPO release are predominantly mediated through HIF2α. For example, a family with a gain-of-function mutation in HIF2A exhibited stabilization of HIF2 and erythrocytosis, which suggests that wild-type HIF2A regulates EPO production in humans172. Similarly, loss-of-function mutations in HIF2A are common among Tibetan highlanders and protect these individuals from the detrimental effects of living at high altitude, including hypoxia-driven erythrocytosis173. Although there may be some clinical indications that might take advantage of the HIF-activator-driven effects of increasing erythrocytosis160, there are probably many indications in which uncontrolled stimulation of erythropoiesis could be detrimental, particularly during chronic treatment with HIF2α activators.
Potential limitations of HIF inhibitors
HIF inhibitors are currently being investigated for the treatment of neoplastic diseases and could potentially be used for specific inflammatory indications such as autoimmune encephalitis or renal fibrosis174. Possible concerns with these compounds include the prevention of tissue-protective responses during acute ischaemic and anti-inflammatory diseases. For example, it is conceivable that treatment with HIF inhibitors could lead to the exacerbation of intestinal inflammation in patients with IBD. Similarly, patients chronically taking HIF inhibitors could experience more severe ischaemia–reperfusion injury during myocardial infarction, or more severe AKI or ALI.
At present, these concerns are based on experimental studies. For example, a recent study examined ALI in mice treated with the HIF inhibitor echinomycin, which prevents binding of HIF1α to DNA, thereby preventing functional HIF1α activation149. Indeed, echinomycin-treated mice experienced attenuated survival times and increased lung inflammation during ventilator-induced lung injury95. Although there is currently no evidence in humans on the functional roles of HIF inhibitors on inflammatory or ischaemic diseases, this remains an important concern for designing clinical trials.
Future challenges
Development of specific PHD inhibitors
Significant progress in the pharmacological development of PHD inhibitors has been made, including orally bioavailable PHD inhibitors that have been safely used in patients for the treatment of renal anaemia159–161. However, pharmacological inhibition of specific PHDs has not been reported. Isoform-specific PHD inhibitors might be desirable for several reasons. The expression and function of individual PHDs will probably differ between individual tissues; therefore, it may be desirable to selectively inhibit PHD1 in mucosal epithelial cells for the treatment of intestinal inflammation58. As such, a selective PHD1 inhibitor could be more efficient regarding on-target effects while simultaneously reducing the risk for off-target effects. An elegant means of achieving selective PHD deletion could be with the use of antisense oligonucleotides targeting specific PHDs175.
Development of specific HIF activators or inhibitors
The same rationale for the development of isoform-specific PHD inhibitors applies to the development of specific HIF1α or HIF2α activators or inhibitors. This is a highly dynamic field of hypoxia research. Indeed, many studies are currently performing head-to-head comparisons of the functional roles of HIF1α versus HIF2α using specific genetic models.
Interestingly, there are several examples in which HIF1α and HIF2α mediate highly divergent functions. For example, studies on the role of HIFs in regulating nitric oxide responses revealed that the two homologous transcription factors HIF1α and HIF2α can have physiologically antagonistic functions176. Moreover, HIF1α and HIF2α were previously suspected to promote tumour progression through largely overlapping functions; however, this relatively simple model has now been challenged in light of recent data revealing unique and sometimes opposing activities of these HIFα isoforms177. Similarly, studies in murine models of colitis indicate that HIF1α and HIF2α may have opposing roles (see above). These effects are mediated partly through the regulation of unique target genes, as well as through direct and indirect interactions with important oncoproteins and tumour suppressors177,178.
Although clear experimental evidence is still lacking, it is conceivable that inhibition of specific PHDs could be associated with a more pronounced HIF1α or HIF2α response. In addition, specifically targeting HIF1α or HIF2α will also require additional progress to be made towards identifying the tissue-specific and individual functions of HIF1α and HIF2α. Only with the knowledge of their individual functions can further progress be made towards targeting HIF1α or HIF2α for the treatment of inflammatory and ischaemic diseases.
Development of tissue-specific delivery mechanisms
To limit off-target effects of HIF activators, tissue-specific delivery is desirable. For example, a recent study implicated systemic HIF activators (such as DMOG) in the treatment of ALI by improving alveolar epithelial carbohydrate metabolism95. In this study, the authors performed a head-to-head comparison of different mice with tissue-specific deletions of Hif1a in different tissue compartments of the lungs, and provided compelling evidence that the predominant source of HIF-dependent lung protection stems from alveolar epithelial HIF signalling. As such, it is conceivable that treatment with inhaled PHD inhibitors could result in highly efficient HIF stabilization in alveolar epithelial cells while simultaneously avoiding systemic effects. Similar approaches have been taken for the inhaled treatment of asthma with glucocorticoids.
Similarly, there is strong experimental evidence to suggest that HIF-dependent gut protection during experimentally induced colitis involves HIF expressed within the mucosal epithelium33. Indeed, targeted deletion of Hif1a in intestinal epithelial cells is associated with exacerbated intestinal inflammation during experimental colitis34. As such, treatment of IBD in patients with colitis could be achieved by local delivery of PHD inhibitors to the colon through slow-release preparations of the active compound. Similarly, a non-absorbed PHD inhibitor or a HIF inhibitor that is almost entirely metabolized during first-pass through the liver could help to avoid systemic effects without altering HIF activation in the intestinal mucosa.
Conclusions
Data obtained from studies using cultured cell systems, animal models and patient-derived materials have consistently confirmed hypoxia and HIF as important components of the inflammatory microenvironment. Animal models, particularly conditional deletion mutants, have been particularly informative and have demonstrated an almost uniformly beneficial influence of HIF stabilization on disease outcomes during inflammation or ischaemia. HIF-stabilizing agents (especially PHD inhibitors) have provided insights into disease mechanisms, and the promise they have shown for clinical development in the near future has spurred an intense interest in the continued development of these agents. Ongoing studies to define the differences and similarities between the pharmacological targets will continue to teach us important lessons about the complexities and pathogenesis of inflammatory disease, and will probably yield novel targets as templates for the development of therapies.
Acknowledgments
The authors acknowledge S. A. Eltzschig for artwork during the preparation of this manuscript. This work is supported by US National Institutes of Health grants (DK097075, HL092188, DK083385, HL098294, HL114457, DK50189, HL60569, DK95491 and HL119837) and by grants from the Crohn’s and Colitis Foundation of America.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Taylor CT, McElwain JC. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology. 2010;25:272–279. doi: 10.1152/physiol.00029.2010. [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang G, Jiang B, Rue E, Semenza G. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc Natl Acad Sci USA. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. Landmark paper that identified binding of a hypoxia-inducible transcription factor, which binds to the erythropietin promoter and was subsequently named HIF. [PubMed] [Google Scholar]
- 6.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-κB in hypoxic inflammation. J Physiol. 2008;586:4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nature Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 10.Eltzschig HK, Eckle T. Ischemia and reperfusion — from mechanism to translation. Nature Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, et al. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology. 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell PH, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 17.Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1α by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein AC, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. Landmark paper that identified mammalian PHDs in their functional role of regulating the stability of HIF. [DOI] [PubMed] [Google Scholar]
- 19.Jaakkola P, et al. Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 20.Lando D, Peet DJ, Whelan DA, Gorman JJ, Murray LW. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 21.Ema M, et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third α-class hypoxia inducible factor subunit, HIF3α. Gene Expr. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- 23.Makino Y, Kanopka A, Wilson WJ, Tanaka H, Poellinger L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3α locus. J Biol Chem. 2002;277:32405–32408. doi: 10.1074/jbc.C200328200. [DOI] [PubMed] [Google Scholar]
- 24.Makino Y, et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414:550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 25.Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 26.Mikus P, Zundel W. COPing with hypoxia. Semin Cell Dev Biol. 2005;16:462–473. doi: 10.1016/j.semcdb.2005.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boh BK, Smith PG, Hagen T. Neddylation-induced conformational control regulates cullin RING ligase activity in vivo. J Mol Biol. 2011;409:136–145. doi: 10.1016/j.jmb.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Soucy TA, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 29.Khoury J, Ibla JC, Neish AS, Colgan SP. Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J Clin Invest. 2007;117:703–711. doi: 10.1172/JCI30049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrentraut SF, et al. Central role for endothelial human deneddylase-1/SENP8 in fine-tuning the vascular inflammatory response. J Immunol. 2013;190:392–400. doi: 10.4049/jimmunol.1202041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol. 2012;74:153–175. doi: 10.1146/annurev-physiol-020911-153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nature Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karhausen JO, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clambey ET, et al. Hypoxia-inducible factor-1α-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA. 2012;109:E2784–E2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peyssonnaux C, et al. Cutting edge: essential role of hypoxia inducible factor-1α in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 37.Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE. 2007;2:e1364. doi: 10.1371/journal.pone.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tannahill GM, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werth N, et al. Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS ONE. 2010;5:e11576. doi: 10.1371/journal.pone.0011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartmann H, et al. Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology. 2008;134:756–767. doi: 10.1053/j.gastro.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Kempf VA, et al. Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation. 2005;111:1054–1062. doi: 10.1161/01.CIR.0000155608.07691.B7. [DOI] [PubMed] [Google Scholar]
- 42.Haeberle HA, et al. Oxygen-independent stabilization of hypoxia inducible factor (HIF)-1 during RSV infection. PLoS ONE. 2008;3:e3352. doi: 10.1371/journal.pone.0003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nature Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peyssonnaux C, et al. HIF-1α expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rius J, et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grocott MP, et al. Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med. 2009;360:140–149. doi: 10.1056/NEJMoa0801581. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann G, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12:246–252. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- 48.Synnestvedt K, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmit MA, et al. Vasodilator phosphostimulated protein (VASP) protects endothelial barrier function during hypoxia. Inflammation. 2012;35:566–573. doi: 10.1007/s10753-011-9347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cummins EP, et al. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc Natl Acad Sci USA. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. An important study that identified a functional role of PHDs in the stabilization of nuclear factor κB under hypoxic conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell EL, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 53.Marks DJ, Rahman FZ, Sewell GW, Segal AW. Crohn’s disease: an immune deficiency state. Clin Rev Allergy Immunol. 2010;38:20–31. doi: 10.1007/s12016-009-8133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med. 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 55.Huang JS, et al. Chronic granulomatous disease caused by a deficiency in p47(phox) mimicking Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:690–695. doi: 10.1016/s1542-3565(04)00292-7. [DOI] [PubMed] [Google Scholar]
- 56.Hart ML, et al. Hypoxia-inducible factor-1α-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol. 2011;186:4367–4374. doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Xue X, et al. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology. 2013;145:831–841. doi: 10.1053/j.gastro.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tambuwala MM, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–2101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 59.Shah YM, et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036–2048. doi: 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aherne CM, et al. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2012;61:695–705. doi: 10.1136/gutjnl-2011-300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frick JS, et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eltzschig HK, Rivera-Nieves J, Colgan SP. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin Ther Targets. 2009;13:1267–1277. doi: 10.1517/14728220903241666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ehrentraut H, et al. CD73+ regulatory T cells contribute to adenosine-mediated resolution of acute lung injury. FASEB J. 2013;27:2207–2219. doi: 10.1096/fj.12-225201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ehrentraut H, Westrich JA, Eltzschig HK, Clambey ET. Adora2b adenosine receptor engagement enhances regulatory T cell abundance during endotoxin-induced pulmonary inflammation. PLoS ONE. 2012;7:e32416. doi: 10.1371/journal.pone.0032416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 66.Fraisl P, Aragones J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nature Rev Drug Discov. 2009;8:139–152. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- 67.Cummins EP, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Robinson A, et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keely S, et al. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 2013;22:114–123. doi: 10.1038/mi.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 71.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 72.Kohler D, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 73.Eckle T, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 74.Eckle T, et al. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291:H2533–H2540. doi: 10.1152/ajpheart.00472.2006. [DOI] [PubMed] [Google Scholar]
- 75.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eckle T, Kohler D, Lehmann R, El Kasmi KC, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 77.Eltzschig HK, Bonney SK, Eckle T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol Med. 2013;19:345–354. doi: 10.1016/j.molmed.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eckle T, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nature Med. 2012;18:774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Botker HE, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 80.Cai Z, Luo W, Zhan H, Semenza GL. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci USA. 2013;110:17462–17467. doi: 10.1073/pnas.1317158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374:1557–1565. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- 82.Ali ZA, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116 (11 Suppl):I98–I105. doi: 10.1161/circulationaha.106.679167. [DOI] [PubMed] [Google Scholar]
- 83.Eckle T, et al. Crosstalk between the equilibrative nucleoside transporter ENT2 and alveolar Adora2b adenosine receptors dampens acute lung injury. FASEB J. 2013;27:3078–3089. doi: 10.1096/fj.13-228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schingnitz U, et al. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184:5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology. 2009;24:298–306. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- 86.Eckle T, et al. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dengler V, Downey GP, Tuder RM, Eltzschig HK, Schmidt EP. Neutrophil intercellular communication in acute lung injury: emerging roles of microparticles and gap junctions. Am J Respir Cell Mol Biol. 2013;49:1–5. doi: 10.1165/rcmb.2012-0472TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 90.Ranieri VM, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 91.Herridge MS, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 92.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1α and adenosine receptors. Nature Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 93.Sitkovsky MV, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 94.Thiel M, et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;3:e174. doi: 10.1371/journal.pbio.0030174. An important study indicating that hyperoxic conditions — which may be necessary for the treatment of patients with ALI to maintain oxygenation of different tissues — can be detrimental via inhibition of anti-inflammatory and tissue-protective functions of HIFs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eckle T, et al. HIF1A reduces acute lung injury by optimizing carbohydrate metabolism in the alveolar epithelium. PLoS Biol. 2013;11:e1001665. doi: 10.1371/journal.pbio.1001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol. 2010;184:4062–4068. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cramer T, et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kojima H, et al. Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1α-deficient chimeric mice. Proc Natl Acad Sci USA. 2002;99:2170–2174. doi: 10.1073/pnas.052706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okumura CY, et al. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J Mol Med. 2012;28:1079–1089. doi: 10.1007/s00109-012-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Warnecke C, et al. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB J. 2003;17:1186–1188. doi: 10.1096/fj.02-1062fje. [DOI] [PubMed] [Google Scholar]
- 101.Schaible B, et al. Hypoxia modulates infection of epithelial cells by Pseudomonas aeruginosa. PLoS ONE. 2013;8:e56491. doi: 10.1371/journal.pone.0056491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kelly CJ, et al. Fundamental role for HIF-1α in constitutive expression of human βdefensin-1. Mucosal Immunol. 2013;6:6. doi: 10.1038/mi.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human β-defensins. Cell Mol Life Sci. 2006;63:1294–1313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nature Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 105.Schroeder BO, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature. 2011;469:419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 106.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 107.O’Neil DA, et al. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–6724. [PubMed] [Google Scholar]
- 108.Zhao C, Wang I, Lehrer RI. Widespread expression of β-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- 109.Peyrin-Biroulet L, et al. Peroxisome proliferator-activated receptor gamma activation is required for maintenance of innate antimicrobial immunity in the colon. Proc Natl Acad Sci USA. 2010;107:8772–8777. doi: 10.1073/pnas.0905745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kocsis AK, et al. Association of β-defensin 1 single nucleotide polymorphisms with Crohn’s disease. Scand J Gastroenterol. 2008;43:299–307. doi: 10.1080/00365520701682615. [DOI] [PubMed] [Google Scholar]
- 111.Wehkamp J, et al. Inducible and constitutive β-defensins are differentially expressed in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9:215–223. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 112.Jurevic RJ, Bai M, Chadwick RB, White TC, Dale BA. Single-nucleotide polymorphisms (SNPs) in human β-defensin 1: high-throughput SNP assays and association with Candida carriage in type I diabetics and nondiabetic controls. J Clin Microbiol. 2003;41:90–96. doi: 10.1128/JCM.41.1.90-96.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schaefer AS, et al. A 3′ UTR transition within DEFB1 is associated with chronic and aggressive periodontitis. Genes Immun. 2010;11:45–54. doi: 10.1038/gene.2009.75. [DOI] [PubMed] [Google Scholar]
- 114.Ozturk A, Famili P, Vieira AR. The antimicrobial peptide DEFB1 is associated with caries. J Dent Res. 2010;89:631–636. doi: 10.1177/0022034510364491. [DOI] [PubMed] [Google Scholar]
- 115.Peyssonnaux C, et al. Critical role of HIF-1α in keratinocyte defense against bacterial infection. J Invest Dermatol. 2008;128:1964–1968. doi: 10.1038/jid.2008.27. [DOI] [PubMed] [Google Scholar]
- 116.Steinbrech DS, et al. Fibroblast response to hypoxia: the relationship between angiogenesis and matrix regulation. J Surg Res. 1999;84:127–133. doi: 10.1006/jsre.1999.5627. [DOI] [PubMed] [Google Scholar]
- 117.Tandara AA, Mustoe TA. Oxygen in wound healing — more than a nutrient. World J Surg. 2004;28:294–300. doi: 10.1007/s00268-003-7400-2. [DOI] [PubMed] [Google Scholar]
- 118.Furuta GT, et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Louis NA, et al. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem. 2006;99:1616–1627. doi: 10.1002/jcb.20947. [DOI] [PubMed] [Google Scholar]
- 120.Elson DA, Ryan HE, Snow JW, Johnson R, Arbeit JM. Coordinate up-regulation of hypoxia inducible factor (HIF)-1α and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000;60:6189–6195. [PubMed] [Google Scholar]
- 121.Albina JE, et al. HIF-1 expression in healing wounds: HIF-1α induction in primary inflammatory cells by TNF-α. Am J Physiol Cell Physiol. 2001;281:C1971–1977. doi: 10.1152/ajpcell.2001.281.6.C1971. [DOI] [PubMed] [Google Scholar]
- 122.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 123.Glover LE, et al. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc Natl Acad Sci USA. 2013;110:19820–19825. doi: 10.1073/pnas.1302840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 125.Pirenne J, et al. Influence of ischemia-reperfusion injury on rejection after liver transplantation. Transplant Proc. 1997;29:366–367. doi: 10.1016/s0041-1345(96)00122-4. [DOI] [PubMed] [Google Scholar]
- 126.Watt KD, Lyden ER, Gulizia JM, McCashland TM. Recurrent hepatitis C posttransplant: early preservation injury may predict poor outcome. Liver Transpl. 2006;12:134–139. doi: 10.1002/lt.20583. [DOI] [PubMed] [Google Scholar]
- 127.Schneider M, et al. Loss or silencing of the PHD1 prolyl hydroxylase protects livers of mice against ischemia/reperfusion injury. Gastroenterology. 2010;138:1143–1154. doi: 10.1053/j.gastro.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 128.Chouker A, et al. In vivo hypoxic preconditioning protects from warm liver ischemia–reperfusion injury through the adenosine A2B receptor. Transplantation. 2012;94:894–902. doi: 10.1097/TP.0b013e31826a9a46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hart ML, et al. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 2008;135:1739.e3–1750.e3. doi: 10.1053/j.gastro.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 130.Cheng K, et al. Hypoxia-inducible factor-1α regulates β cell function in mouse and human islets. J Clin Invest. 2010;120:2171–2183. doi: 10.1172/JCI35846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kebir H, et al. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nature Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dang EV, et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shi LZ, et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and TReg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.O’Connor W, Jr, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nature Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1α. Eur J Immunol. 2008;38:2412–2418. doi: 10.1002/eji.200838318. [DOI] [PubMed] [Google Scholar]
- 137.Bartels K, Karhausen J, Clambey ET, Grenz A, Eltzschig HK. Perioperative organ injury. Anesthesiology. 2013;119:1474–1489. doi: 10.1097/ALN.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park SW, et al. Paneth cell-mediated multiorgan dysfunction after acute kidney injury. J Immunol. 2012;189:5421–5433. doi: 10.4049/jimmunol.1200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gelman S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology. 1995;82:1026–1060. doi: 10.1097/00000542-199504000-00027. [DOI] [PubMed] [Google Scholar]
- 140.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 141.Bove T, et al. Renoprotective action of fenoldopam in high-risk patients undergoing cardiac surgery: a prospective, double-blind, randomized clinical trial. Circulation. 2005;111:3230–3235. doi: 10.1161/CIRCULATIONAHA.104.509141. [DOI] [PubMed] [Google Scholar]
- 142.Kumar AB, Suneja M. Cardiopulmonary bypass-associated acute kidney injury. Anesthesiology. 2011;114:964–970. doi: 10.1097/ALN.0b013e318210f86a. [DOI] [PubMed] [Google Scholar]
- 143.Hill P, et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2008;19:39–46. doi: 10.1681/ASN.2006090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grenz A, et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kapitsinou PP, et al. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest. 2014;124:2396–2409. doi: 10.1172/JCI69073. An important study that, for the first time, implicates kidney protection by HIF2α expressed in vascular endothelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mole DR, et al. 2-oxoglutarate analogue inhibitors of HIF prolyl hydroxylase. Bioorg Med Chem Lett. 2003;13:2677–2680. doi: 10.1016/s0960-894x(03)00539-0. [DOI] [PubMed] [Google Scholar]
- 147.Masson N, Ratcliffe PJ. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O2 levels. J Cell Sci. 2003;116:3041–3049. doi: 10.1242/jcs.00655. [DOI] [PubMed] [Google Scholar]
- 148.Chan DA, Sutphin PD, Yen SE, Giaccia AJ. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1α. Mol Cell Biol. 2005;25:6415–6426. doi: 10.1128/MCB.25.15.6415-6426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kong D, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- 150.Wang R, Zhou S, Li S. Cancer therapeutic agents targeting hypoxia-inducible factor-1. Curr Med Chem. 2011;18:3168–3189. doi: 10.2174/092986711796391606. [DOI] [PubMed] [Google Scholar]
- 151.Xia Y, Choi HK, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem. 2012;49:24–40. doi: 10.1016/j.ejmech.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 152.Ku JH, et al. Effect of dutasteride on the expression of hypoxia-inducible factor-1α, vascular endothelial growth factor and microvessel density in rat and human prostate tissue. Scand J Urol Nephrol. 2009;43:445–453. doi: 10.3109/00365590903337896. [DOI] [PubMed] [Google Scholar]
- 153.Puppo M, et al. Topotecan inhibits vascular endothelial growth factor production and angiogenic activity induced by hypoxia in human neuroblastoma by targeting hypoxia-inducible factor-1α and -2α. Mol Cancer Ther. 2008;7:1974–1984. doi: 10.1158/1535-7163.MCT-07-2059. [DOI] [PubMed] [Google Scholar]
- 154.Palayoor ST, et al. PX-478, an inhibitor of hypoxia-inducible factor-1α, enhances radiosensitivity of prostate carcinoma cells. Int J Cancer. 2008;123:2430–2437. doi: 10.1002/ijc.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rey S, et al. Synergistic effect of HIF-1α gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia. Proc Natl Acad Sci USA. 2009;106:20399–20404. doi: 10.1073/pnas.0911921106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aragones J, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nature Genet. 2008;40:170–180. doi: 10.1038/ng.2007.62. A study providing critically important insights into mechanisms of PHD-dependent tissue protection during ischaemia. Indeed, the authors found that PHD1 deletion and concomittant elevation in HIFs is associated with improved metabolic responses, thereby promoting hypoxia or ischaemia tolerance. [DOI] [PubMed] [Google Scholar]
- 157.Rajagopalan S, et al. Use of a constitutively active hypoxia-inducible factor-1α transgene as a therapeutic strategy in no-option critical limb ischemia patients: Phase I dose-escalation experience. Circulation. 2007;115:1234–1243. doi: 10.1161/CIRCULATIONAHA.106.607994. [DOI] [PubMed] [Google Scholar]
- 158.Creager MA, et al. Effect of hypoxia-inducible factor-1α gene therapy on walking performance in patients with intermittent claudication. Circulation. 2011;124:1765–1773. doi: 10.1161/CIRCULATIONAHA.110.009407. [DOI] [PubMed] [Google Scholar]
- 159.Bernhardt WM, et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21:2151–2156. doi: 10.1681/ASN.2010010116. The first published clinical trial that successfully uses a PHD inhibitor in patients to achieve HIF stabilization and concomitant increases in erythropoitin production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Flight MH. Deal watch: AstraZeneca bets on FibroGen’s anaemia drug. Nature Rev Drug Discov. 2013;12:730. doi: 10.1038/nrd4135. [DOI] [PubMed] [Google Scholar]
- 161.Macdougall IC. New anemia therapies: translating novel strategies from bench to bedside. Am J Kidney Dis. 2012;59:444–451. doi: 10.1053/j.ajkd.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 162.Semenza GL. Targeting HIF-1 for cancer therapy. Nature Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 163.Kaelin WG. Von Hippel–Lindau disease. Annu Rev Pathol. 2007;2:145–173. doi: 10.1146/annurev.pathol.2.010506.092049. An important review paper that summarizes the discovery and functional role of the VHL gene in the post-translational regulation of HIF levels. [DOI] [PubMed] [Google Scholar]
- 164.Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev Cell. 2009;16:167–179. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 165.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 166.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 167.Mazzone M, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Galluzzi L, Kepp O, Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nature Rev Drug Discov. 2013;12:829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- 169.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2009;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Asikainen TM, et al. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. FASEB J. 2006;20:1698–1700. doi: 10.1096/fj.06-5887fje. [DOI] [PubMed] [Google Scholar]
- 171.Hams E, et al. The hydroxylase inhibitor dimethyloxallyl glycine attenuates endotoxic shock via alternative activation of macrophages and IL-10 production by B1 cells. Shock. 2011;36:295–302. doi: 10.1097/SHK.0b013e318225ad7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Percy MJ, et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358:162–168. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Simonson TS, et al. Genetic evidence for highaltitude adaptation in Tibet. Science. 2010;329:72–75. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- 174.Higgins DF, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelialto-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kastelein JJ, et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006;114:1729–1735. doi: 10.1161/CIRCULATIONAHA.105.606442. [DOI] [PubMed] [Google Scholar]
- 176.Takeda N, et al. Differential activation and antagonistic function of HIF-α isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nature Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Branco-Price C, et al. Endothelial cell HIF-1α and HIF-2α differentially regulate metastatic success. Cancer Cell. 2012;21:52–65. doi: 10.1016/j.ccr.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Scholz CC, et al. Regulation of IL-1β-induced NF-κB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc Natl Acad Sci USA. 2013;110:18490–18495. doi: 10.1073/pnas.1309718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cockman ME, et al. Posttranslational hydroxylation of ankyrin repeats in IκB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH) Proc Natl Acad Sci USA. 2006;103:14767–14772. doi: 10.1073/pnas.0606877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zaph C, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 182.Coleman ML, et al. Asparaginyl hydroxylation of the Notch ankyrin repeat domain by factor inhibiting hypoxia-inducible factor. J Biol Chem. 2007;282:24027–24038. doi: 10.1074/jbc.M704102200. [DOI] [PubMed] [Google Scholar]
- 183.Koditz J, et al. Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood. 2007;110:3610–3617. doi: 10.1182/blood-2007-06-094441. [DOI] [PubMed] [Google Scholar]
- 184.Bartels K, Grenz A, Eltzschig HK. Hypoxia and inflammation are two sides of the same coin. Proc Natl Acad Sci USA. 2013;110:18351–18352. doi: 10.1073/pnas.1318345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Riegel AK, et al. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood. 2011;117:2548–2555. doi: 10.1182/blood-2010-10-313957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Koeppen M, Eckle T, Eltzschig HK. Interplay of hypoxia and A2B adenosine receptors in tissue protection. Adv Pharmacol. 2011;61:145–186. doi: 10.1016/B978-0-12-385526-8.00006-0. [DOI] [PubMed] [Google Scholar]
- 188.Aherne CM, Kewley EM, Eltzschig HK. The resurgence of A2B adenosine receptor signaling. Biochim Biophys Acta. 2011;1808:1329–1339. doi: 10.1016/j.bbamem.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Eltzschig HK, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 191.Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltzschig HK. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Eltzschig HK, et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thompson LF, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ahmad A, et al. Adenosine A2A receptor is a unique angiogenic target of HIF-2α in pulmonary endothelial cells. Proc Natl Acad Sci USA. 2009;106:10684–10689. doi: 10.1073/pnas.0901326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 196.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136:607–618. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 197.Eltzschig HK, et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Casanello P, et al. Equilibrative nucleoside transporter 1 expression is downregulated by hypoxia in human umbilical vein endothelium. Circ Res. 2005;97:16–24. doi: 10.1161/01.RES.0000172568.49367.f8. [DOI] [PubMed] [Google Scholar]
- 199.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–5580. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 200.Chan DA, Sutphin PD, Denko NC, Giaccia AJ. Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1α. J Biol Chem. 2002;277:40112–40117. doi: 10.1074/jbc.M206922200. [DOI] [PubMed] [Google Scholar]
- 201.McDonough MA, et al. Selective inhibition of factor inhibiting hypoxia-inducible factor. J Am Chem Soc. 2005;127:7680–7681. doi: 10.1021/ja050841b. [DOI] [PubMed] [Google Scholar]
- 202.Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–3615. [PubMed] [Google Scholar]
- 203.Knowles HJ, Tian YM, Mole DR, Harris AL. Novel mechanism of action for hydralazine: induction of hypoxia-inducible factor-1α, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ Res. 2004;95:162–169. doi: 10.1161/01.RES.0000134924.89412.70. [DOI] [PubMed] [Google Scholar]
- 204.Ivan M, et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci USA. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nangaku M, et al. A novel class of prolyl hydroxylase inhibitors induces angiogenesis and exerts organ protection against ischemia. Arterioscler Thromb Vasc Biol. 2007;27:2548–2554. doi: 10.1161/ATVBAHA.107.148551. [DOI] [PubMed] [Google Scholar]
- 206.Zaman K, et al. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, 21(waf1/cip1), and erythropoietin. J Neurosci. 1999;19:9821–9830. doi: 10.1523/JNEUROSCI.19-22-09821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Warshakoon NC, et al. Design and synthesis of a series of novel pyrazolopyridines as HIF-1α prolyl hydroxylase inhibitors. Bioorg Med Chem Lett. 2006;16:5687–5690. doi: 10.1016/j.bmcl.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 208.Choi SM, et al. Clioquinol, a Cu(II)/Zn(II) chelator, inhibits both ubiquitination and asparagine hydroxylation of hypoxia-inducible factor-1α, leading to expression of vascular endothelial growth factor and erythropoietin in normoxic cells. J Biol Chem. 2006;281:34056–34063. doi: 10.1074/jbc.M603913200. [DOI] [PubMed] [Google Scholar]
- 209.Siddiq A, et al. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280:41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jiang BH, et al. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- 211.Majumder PK, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nature Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 212.Mie Lee Y, et al. Inhibition of hypoxia-induced angiogenesis by FK228, a specific histone deacetylase inhibitor, via suppression of HIF-1α activity. Biochem Biophys Res Commun. 2003;300:241–246. doi: 10.1016/s0006-291x(02)02787-0. [DOI] [PubMed] [Google Scholar]
- 213.Qian DZ, et al. Class II histone deacetylases are associated with VHL-independent regulation of hypoxiainducible factor 1α. Cancer Res. 2006;66:8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- 214.Kong X, et al. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1α. Mol Cell Biol. 2006;26:2019–2028. doi: 10.1128/MCB.26.6.2019-2028.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hur E, et al. Reduction of hypoxia-induced transcription through the repression of hypoxia-inducible factor-1α/aryl hydrocarbon receptor nuclear translocator DNA binding by the 90-kDa heat-shock protein inhibitor radicicol. Mol Pharmacol. 2002;62:975–982. doi: 10.1124/mol.62.5.975. [DOI] [PubMed] [Google Scholar]
- 216.Osada M, Imaoka S, Funae Y. Apigenin suppresses the expression of VEGF, an important factor for angiogenesis, in endothelial cells via degradation of HIF-1α protein. FEBS Lett. 2004;575:59–63. doi: 10.1016/j.febslet.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 217.Mabjeesh NJ, et al. Geldanamycin induces degradation of hypoxia-inducible factor 1α protein via the proteosome pathway in prostate cancer cells. Cancer Res. 2002;62:2478–2482. [PubMed] [Google Scholar]
- 218.Isaacs JS, et al. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1α-degradative pathway. J Biol Chem. 2002;277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 219.Kaluz S, Kaluzova M, Stanbridge EJ. Proteasomal inhibition attenuates transcriptional activity of hypoxia-inducible factor 1 (HIF-1) via specific effect on the HIF-1α C-terminal activation domain. Mol Cell Biol. 2006;26:5895–5907. doi: 10.1128/MCB.00552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yeo EJ, et al. Amphotericin B blunts erythropoietin response to hypoxia by reinforcing FIH-mediated repression of HIF-1. Blood. 2006;107:916–923. doi: 10.1182/blood-2005-06-2564. [DOI] [PubMed] [Google Scholar]
- 221.Kung AL, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 2004;6:33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 222.Verma RP, Hansch C. Camptothecins: a SAR/QSAR study. Chem Rev. 2009;109:213–235. doi: 10.1021/cr0780210. [DOI] [PubMed] [Google Scholar]
- 223.Rapisarda A, et al. Schedule-dependent inhibition of hypoxia-inducible factor-1α protein accumulation, angiogenesis, and tumor growth by topotecan in U251-HRE glioblastoma xenografts. Cancer Res. 2004;64:6845–6848. doi: 10.1158/0008-5472.CAN-04-2116. [DOI] [PubMed] [Google Scholar]