Abstract
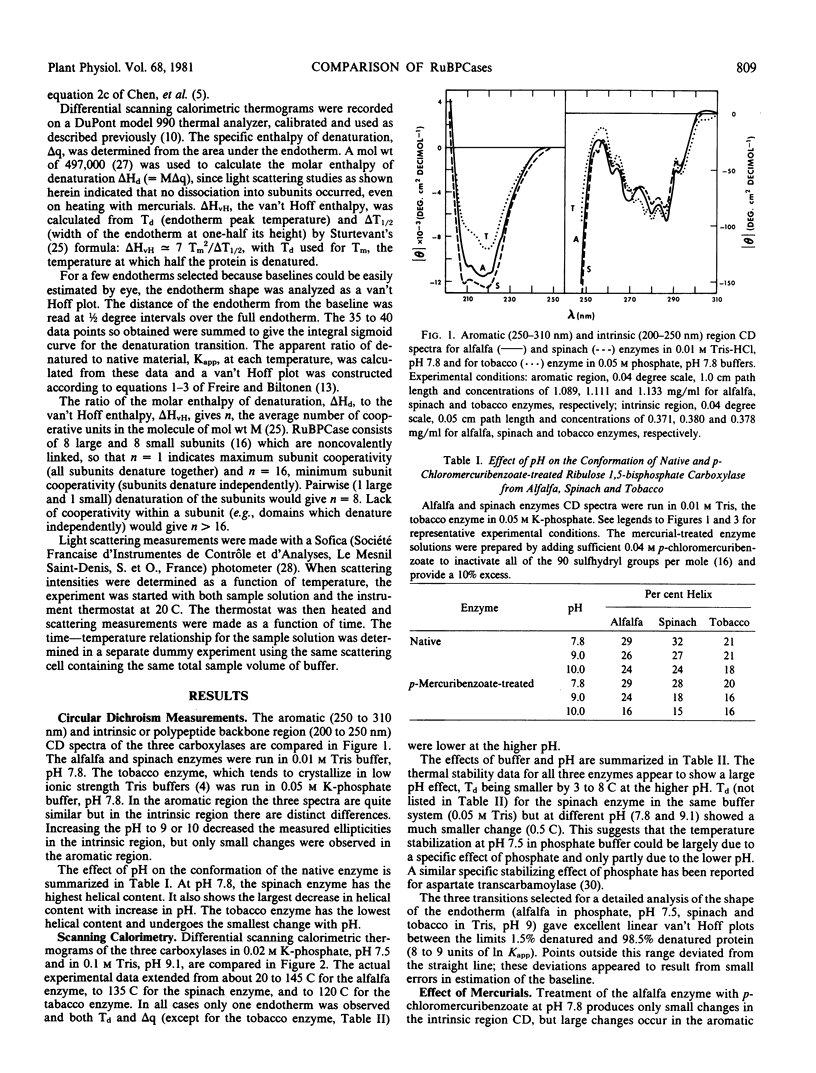
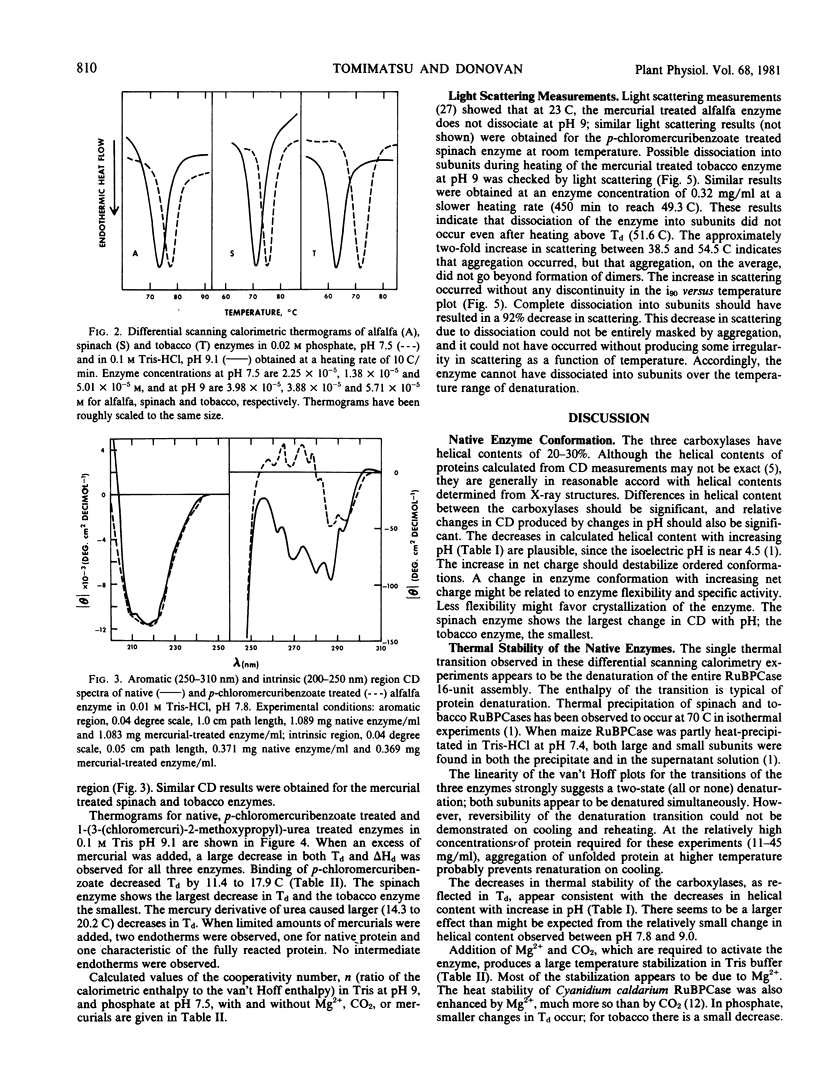
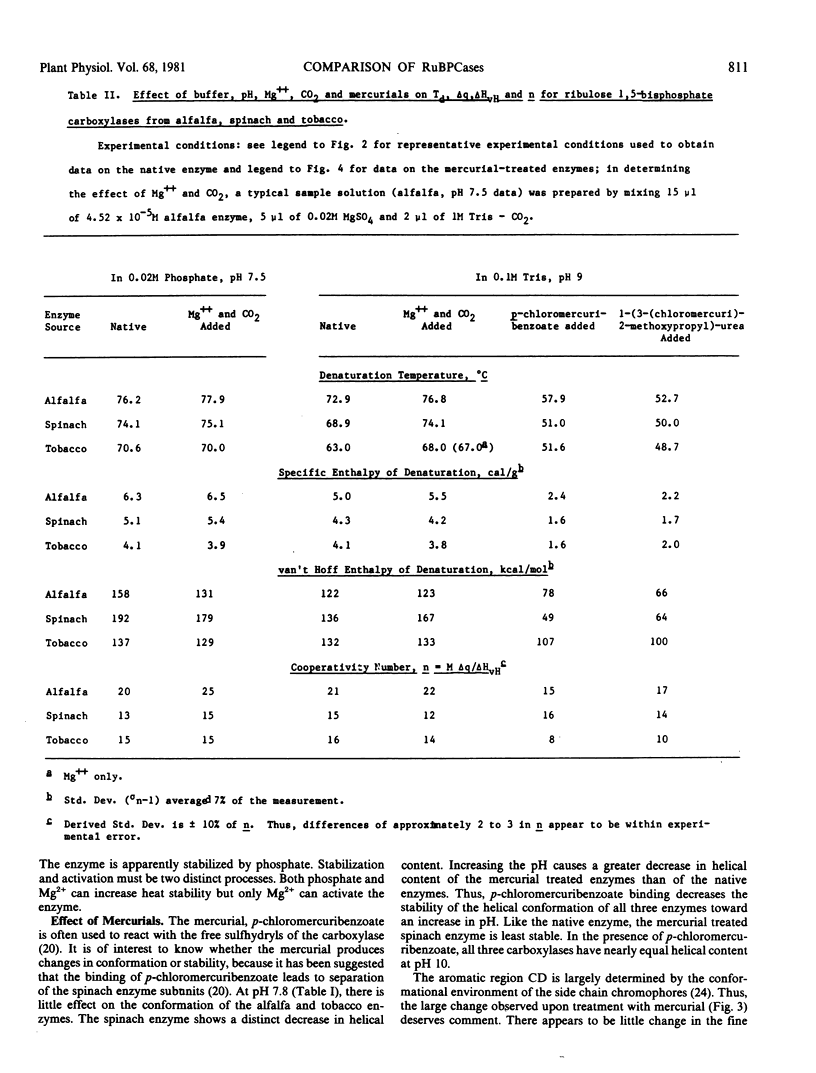
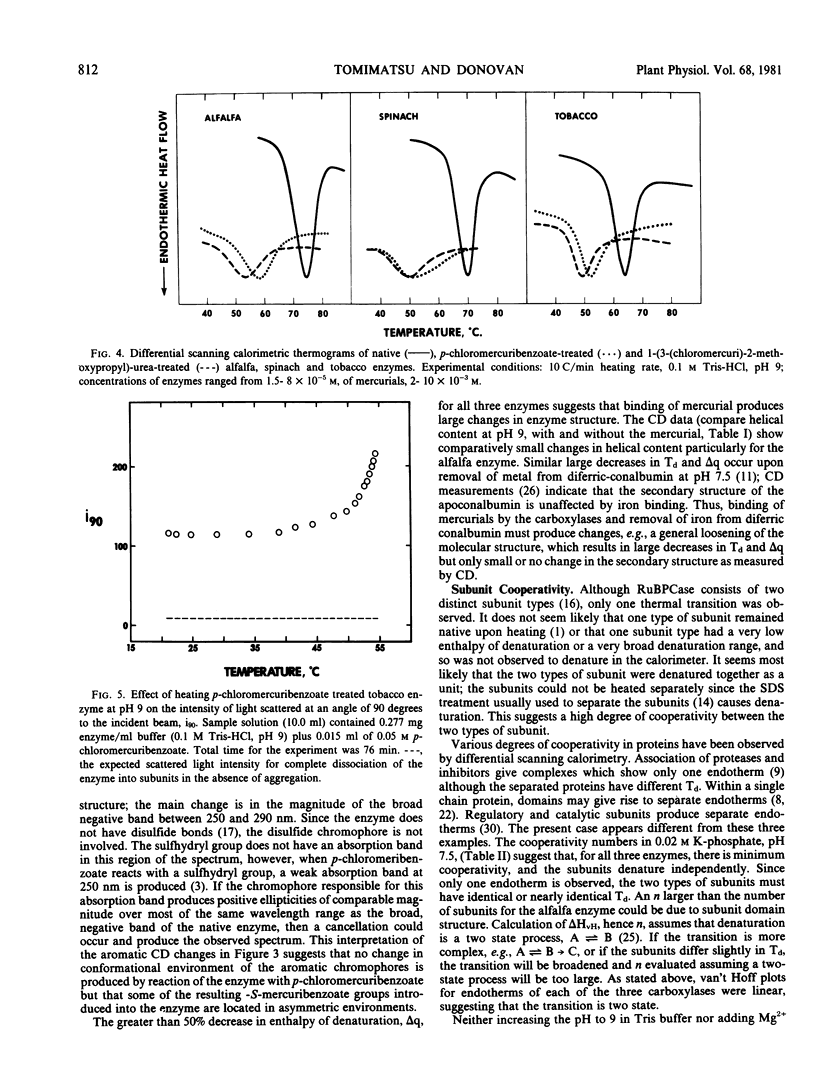
Circular dichroism, differential scanning calorimetry and light-scattering measurements of ribulose 1,5-bisphosphate carboxylase (E.C. 4.1.1.39) from alfalfa, spinach and tobacco show: a) The conformation and thermal stability of the native carboxylases are sensitive to changes in pH and to activation of the enzyme with Mg2+ and CO2. The helical content, denaturation temperature (Td) and specific enthalpy of denaturation (Δq) decreased with increase in pH. Addition of Mg2+ and CO2 at pH 9 increased Td by 4 to 5 C; at pH 7.5 the changes in Td were smaller. b) Addition of mercurials produced changes in conformation and thermal stability. The decrease in helical content of the enzymes with increase in pH was enhanced by the addition of p-chloromercuribenzoate. At pH 9, addition of p-chloromercuribenzoate or of 1-(3-(chloromercuri)-2-methoxypropyl)urea decreased Td by 11.4 to 20.2 C and Δq by 2.1 to 2.8 calories per gram. c) The spinach carboxylase undergoes the largest and the tobacco the smallest changes in conformation and thermal stability upon change in pH or treatment with mercurials. d) The calorimetric data suggest that the large and small subunits are heat denatured independently but at the same temperature. e) Light scattering measurements at pH 9 of p-chloromercuribenzoate treated tobacco enzyme showed that there is no dissociation into subunits upon heating to temperatures greater than Td. A `ball and string' model for the carboxylase molecule is proposed to reconcile independence of subunit denaturation with apparent strong interactions between subunits.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bourque D. P., Smith H. J. Solubility properties of fraction I proteins of maize, cotton, spinach, and tobacco. J Agric Food Chem. 1977 Jul-Aug;25(4):783–789. doi: 10.1021/jf60212a027. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Suh S. W., Eisenberg D. Structure of ribulose-1,5-bisphosphate carboxylase-oxygenase: Form III crystals. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1037–1041. doi: 10.1073/pnas.74.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. H., Sakano K., Singh S., Wildman S. G. Crystalline fraction I protein: preparation in large yield. Science. 1972 Jun 9;176(4039):1145–1146. doi: 10.1126/science.176.4039.1145. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- Chollet R., Anderson L. L., Hovsepian L. C. The absence of tightly bound copper, iron, and flavin nucleotide in crystalline ribulose 1,5-bisphosphate carboxylase-oxygenase from tobacco. Biochem Biophys Res Commun. 1975 May 5;64(1):97–107. doi: 10.1016/0006-291x(75)90224-7. [DOI] [PubMed] [Google Scholar]
- Chu D. K., Bassham J. A. Activation and inhibition of ribulose 1,5-diphosphate carboxylase by 6-phosphogluconate. Plant Physiol. 1973 Oct;52(4):373–379. doi: 10.1104/pp.52.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J. W., Beardslee R. A. Heat stabilization produced by protein-protein association. A differential scanning calorimetric study of the heat denaturation of the trypsin-soybean trypsin inhibitor and trypsin-ovomucoid complexes. J Biol Chem. 1975 Mar 25;250(6):1966–1971. [PubMed] [Google Scholar]
- Donovan J. W., Ross K. D. Increase in the stability of avidin produced by binding of biotin. A differential scanning calorimetric study of denaturation by heat. Biochemistry. 1973 Jan 30;12(3):512–517. doi: 10.1021/bi00727a024. [DOI] [PubMed] [Google Scholar]
- Donovan J. W., Ross K. D. Nonequivalence of the metal binding sites of conalbumin. Calorimetric and spectrophotometric studies of aluminum binding. J Biol Chem. 1975 Aug 10;250(15):6022–6025. [PubMed] [Google Scholar]
- Ford T. W. Ribulose 1,5-bisphosphate carboxylase from the thermophilic, acidophilic alga, Cyanidium caldarium (Geitler). Purification, characterisation and thermostability of the enzyme. Biochim Biophys Acta. 1979 Aug 15;569(2):239–248. doi: 10.1016/0005-2744(79)90059-7. [DOI] [PubMed] [Google Scholar]
- Johal S., Bourque D. P. Crystalline ribulose 1,5-bisphosphate carboxylase-oxygenase from spinach. Science. 1979 Apr 6;204(4388):75–77. doi: 10.1126/science.204.4388.75. [DOI] [PubMed] [Google Scholar]
- Kawashima N., Singh S., Wildman S. G. Reversible cold inactivation and heat reactivation of RuDP carboxylase activity of crystallized tobacco fraction I protein. Biochem Biophys Res Commun. 1971 Feb 19;42(4):664–668. doi: 10.1016/0006-291x(71)90539-0. [DOI] [PubMed] [Google Scholar]
- Kawashima N., Wildman S. G. Studies on fraction I protein. II. Comparison of physical, chemical, immunological and enzymatic properties between spinach and tobacco fraction I proteins. Biochim Biophys Acta. 1971 Mar 23;229(3):749–760. [PubMed] [Google Scholar]
- Nishimura M., Takabe T., Sugiyama T., Akazawa T. Structure and function of chloroplast proteins. XIX. Dissociation of spinach leaf ribulose-1,5-diphosphate carboxylase by p-mercuribenzoate. J Biochem. 1973 Nov;74(5):945–954. [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T., Sturtevant J. M., Ptashne M. The lambda repressor contains two domains. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1608–1612. doi: 10.1073/pnas.76.4.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Strickland E. H. Aromatic contributions to circular dichroism spectra of proteins. CRC Crit Rev Biochem. 1974 Jan;2(1):113–175. doi: 10.3109/10409237409105445. [DOI] [PubMed] [Google Scholar]
- Sturtevant J. M. Some applications of calorimetry in biochemistry and biology. Annu Rev Biophys Bioeng. 1974;3(0):35–51. doi: 10.1146/annurev.bb.03.060174.000343. [DOI] [PubMed] [Google Scholar]
- TROWN P. W. AN IMPROVED METHOD FOR THE ISOLATION OF CARBOXYDISMUTASE. PROBABLE IDENTITY WITH FRACTION I PROTEIN AND THE PROTEIN MOIETY OF PROTOCHLOROPHYLL HOLOCHROME. Biochemistry. 1965 May;4:908–918. doi: 10.1021/bi00881a018. [DOI] [PubMed] [Google Scholar]
- Tan A. T. Circular dichroism properties of conalbumin and its iron and copper complexes. Can J Biochem. 1971 Sep;49(9):1071–1075. doi: 10.1139/o71-156. [DOI] [PubMed] [Google Scholar]
- Tomimatsu Y. Macromolecular properties and subunit interactions of ribulose-1,5-bisphosphate carboxylase from alfalfa. Biochim Biophys Acta. 1980 Mar 26;622(1):85–93. doi: 10.1016/0005-2795(80)90160-9. [DOI] [PubMed] [Google Scholar]
- Vickers L. P., Donovan J. W., Schachman H. K. Differential scanning calorimetry of asparate transcarbamoylase and its isolate subunits. J Biol Chem. 1978 Dec 10;253(23):8493–8498. [PubMed] [Google Scholar]
- Wildman S. G., Kwanyuen P. Fraction I protein and other products from tobacco for food. Basic Life Sci. 1978;11:1–18. doi: 10.1007/978-1-4684-8106-8_1. [DOI] [PubMed] [Google Scholar]



