Abstract
Background:
The kidney is a target organ for injuries caused by advanced glycation end products (AGEs) in obesity. The receptor of AGEs (RAGE) is proinflammatory and appears to have a role in the pathogenesis of renal disease due to obesity.
Objective:
The aim was to verify the effect of obesity on renal damage and the effect of lycopene on these complications
Design and Methods:
Male Wistar rats were randomly assigned to receive a control diet (C, n=7) or a high-fat diet plus sucrose (HD+S, n=14) for 6 weeks. After this period, the HD+S animals were randomized into two groups: HD+S (n=7) and HD+S supplemented with lycopene (HD+S+L, n=7). The animals received maize oil (C and HD+S) or lycopene (HD+S+L) for a 6-week period.
Results:
The HD+S and HD+S+L animals demonstrated insulin resistance (OGTT glucose after 150 min; C: 117.6±3.9<HD+S: 138.1±5.1=HD+S+L: 137.8±5.2 mg dl−1; P=0.01); however, no changes were seen in fasting glucose, plasma lipids, blood pressure or renal function. Renal concentrations of RAGE and TNF-α increased in the HD+S group and lycopene supplementation restored these to control group values (RAGE: C: 3.1±0.3=DH+S+L: 3.1±0.3<DH+S: 3.6±0.4 μg g−1; P=0.014; TNF-α: C: 227.8±2.7=DH+S+L: 227.4±2.2<DH+S: 238.7±3.0 pg/ml; P=0.014).
Conclusions:
Lycopene may be beneficial in the prevention and treatment of oxidative stress and inflammation in the kidney due to obesity.
Introduction
Obesity is an excessive accumulation of body fat that impairs health. According to the World Health Organization,1 in 2011, 1.5 billion adults were overweight; of these, ~500 million were obese. On the basis of this information, obesity is now recognized as a public health problem and a risk factor for chronic diseases such as insulin resistance, type 2 diabetes mellitus and cardiovascular and renal diseases.2
Several factors can trigger kidney disease in obese individuals, including hyperglycemia, dyslipidemia, hypertension and oxidative stress.3 Under normal conditions, there is a balance between reactive oxygen and nitrogen species and antioxidants; in contrast, when the production of reactive species exceeds local antioxidant capacity, a situation of oxidative stress prevails.4 Excessive food intake leads to the oxidation of lipids (lipoxidation) and glucose (glycation), generating lipoxidation products (malondialdehyde, MDA; glyoxal; acrolein and 4-hydroxy-nonenal, 4-HNE) and glycation products (glyoxal and methyl glyoxal). These compounds are highly reactive and bind to amino acids, resulting in advanced glycation end products (AGEs) and lipoxidation products (ALEs).5
It has been described that AGEs are involved in common events in obesity, insulin resistance, diabetes mellitus 2 and inflammation,6 which may correspond with an important activator of renal injury in obesity.7 The AGEs remaining in the circulation are eliminated by the kidney8 and, depending on the intensity and duration of exposure, may be an important factor in kidney damage.9 AGEs contribute to tissue damage by binding to specific AGE receptors (RAGE) located on the cell membrane. RAGE is a member of the immunoglobulin superfamily, and is also expressed in the kidney.10 When binding occurs, AGE/RAGE triggers a cascade of events involving the transduction of kinases that culminate in the activation of nuclear transcription factor kappa β/nuclear factor kappa B, transcription factors that lead to the production of proinflammatory cytokines11 such as interleukin-6, tumor necrosis factor alpha (TNF-α) and monocyte chemoattractant factor (MCP-1/CCL2). Thus, AGE/RAGE binding is one of the interfaces between oxidative stress and inflammation.
Given the importance of oxidative stress in the pathogenesis of several diseases, several therapeutic strategies using antioxidants have been tested in order to mitigate the overproduction of reactive oxygen species and reactive nitrogen species.12
Lycopene is a potent antioxidant; it was recently demonstrated that lycopene supplementation may contribute to the attenuation of oxidative stress in metabolic diseases.13 Furthermore, it has been shown that lycopene is able to inhibit nuclear factor kappa B activation and the production of TNF-α, suggesting its utility as a therapy for inflammatory diseases.14 So far, we have found no studies that investigated the effect of supplementation with lycopene on the kidneys. Therefore, studies are needed to assess the effects of lycopene on the pro-oxidative state and renal inflammation that occur in obesity. Therefore, the aim of this study was to investigate the effect of obesity on renal damage and the possible effect of lycopene in ameliorating these complications.
Materials and Methods
Animals and experimental protocol
Male Wistar rats (10-week-old), from the Animal Center of Botucatu Medical School, Sao Paulo State University, UNESP (Botucatu, Brazil), were initially divided into groups receiving either a commercial chow diet (C, n=7; 12% energy from fat) or a high-fat diet (49.7% energy from fat) and 30% of sucrose in the drinking water (300 g/l) (HD+S, n=14), for 6 weeks.
The high-fat diet was designed in our laboratory to contain a powdered commercial chow diet—NUVILAB CR-1 (Nuvital; Sogorb Indústria e Comércio Ltda., Sao Paulo, Brazil), wafer biscuit, condensed milk, palm oil, vitamins and minerals. The ingredients were homogenized and pelletized. Diet-induced obesity was used to mimic obesity from occidental dietary habits. The nutritional composition of the diets is presented in Table 1. After 6 weeks under a high-fat diet plus sucrose, HD+S rats were randomly assigned into two groups: HD+S (n=7) and HD+S supplemented with lycopene (HD+S+L, n=7). Lycopene was mixed with corn oil equivalent to 10 mg lycopene per kg body weight and given orally every morning 5 days per week for a 6-week period. All groups received the same corn oil volume (~2 ml kg−1 body weight per day). Rats were housed in individual cages in Animal Experimental Facility (UNIPEX) at the Botucatu Medical School, UNESP, with a controlled room temperature (22–26 °C) and 12 h light/dark cycle. Dietary consumption was measured daily and body weight was assessed weekly. The 24-h urine samples were collected in metabolic cages 1 week before euthanasia. The animals were euthanized by decapitation under deep sodium pentobarbital anesthesia (50 mg kg−1, intraperitoneal injection). Plasma and kidneys were collected. The experiment was conducted in accordance with the Guidelines for the Care and Use of Experimental Animals and the diets followed the specifications on the Nutrient Requirements of the Laboratory Rat. The protocol was approved by the local Ethical Committee for Animal Research of Botucatu Medical School—UNESP (protocol no. 920-2012).
Table 1. Nutritional composition of the diets.
| Components |
Diet |
|
|---|---|---|
| Control | Hyperlipidic | |
| Protein (%) | 25 | 21 |
| Carbohydrate (%) | 58 | 45a |
| Fat (%) | 5 | 29 |
| % Energy from protein | 26.5 | 16.0 |
| % Energy from carbohydrate | 61.5 | 34.3a |
| % Energy from fat | 12.0 | 49.7 |
| % Energy from saturated fat | 2.1 | 24.7 |
| % Energy from unsaturated fat | 9.9 | 25.0 |
| Energy (kcal g−1) | 3.77 | 5.25a |
| Fatty acid composition (%) | ||
| Palmitic (16: 0) | 14.0 | 40.6 |
| Stearic (18: 0) | 2.7 | 6.2 |
| Oleic (18: 1n-9c) | 23.4 | 36.5 |
| Linoleic (18: 2n-6) | 53.1 | 11.3 |
| Others | 6.8 | 5.4 |
| Vitamin/mineral mixtureb | — | Added |
Abbreviations: C, control; HD+S, animals subjected to diet-induced obesity; HD+S+L, HD+S supplemented with lycopene.
Sucrose in the drinking water (300 g l−1) was not included.
Based on the vitamin/mineral amounts of the chow diet, for each kg of the high-fat diet, the following nutrients were added: Fe, 25.2 mg; K, 104·8 mg; Se, 73.1 mg; molybdenum sulphate, 150.0 mg; vitamin B12, 34.5 mg; vitamin B6, 6 mg; biotin, 0.12 mg; vitamin E, 32.6 mg; vitamin D, 61.2 mg; vitamin A, 4.6 mg.
Lycopene preparation
Lycopene (Lyc-O-Mato 6% dewaxed; LycoRed Natural Products Industries, Beer-Sheva, Israel) was mixed with corn oil and stored at 4 °C in the dark until use. The tomato oleoresin–corn oil mixture was stirred for 20 min in a water bath at 37 °C before being fed to the animals.
Total body fat
The adiposity index (%) was measured as the sum of the mass of the epididymal, retroperitoneal and visceral fat pads, divided by the body weight and multiplied by 100.
Glomerular filtration rate
Rats were housed in metabolic cages for 24 h and urine was collected. To calculate the glomerular filtration rate, creatinine concentrations in plasma and urine (glomerular filtration rate: urine creatinine (mg dl−1) × urinary flow (ml min−1)/plasma creatinine (mg dl−1)) were assessed.
Plasma and kidney lycopene analysis
A 400 μl aliquot of plasma or 100 μl of kidney homogenate (100 mg of tissue per 1 ml of phosphate buffered saline, PBS) was used for lycopene analysis, as described previously.15 Briefly, samples were extracted with 3 ml of chloroform–methanol (2:1) followed by 3 ml of hexane. The samples were dried under N2 and resuspended in 100 μl of ethanol, of which 25 μl were injected into the high-performance liquid chromatography (HPLC) apparatus.
The reverse phase HPLC system consisted of a Waters 2695 Alliance system (Waters Co., Milford, MA), a C30 semibore carotenoid column (3 μm, 150 × 3.0 mm, YMC, Wilmington, NC, USA) and a Waters 2996 photodiode array detector set at 450 nm. The HPLC mobile phase solvent A was MeOH/MBTE/water (85:12:3, v/v/v, with 1.5% ammonium acetate in the water), and the mobile phase solvent B was MeOH/MBTE/water (8:90:2, v/v/v, with 1% ammonium acetate in the water). The gradient was set up for 0.4 ml min−1 beginning with 100% solvent A followed by 45% solvent A over a 21 min linear gradient and held at 45% A for 1 min. This was followed by an 11 min linear gradient to 5% solvent A and 4 min hold at 5% solvent A, then a 2 min linear gradient back to 100% solvent A. The system was held at 100% solvent A for 8 min for equilibration back to the initial conditions. Lycopene was quantified by determining the peak areas in the HPLC chromatograms calibrated against known amounts of standards. The results were adjusted using an internal standard containing echinenone. The recovery of the added internal standard was consistently >90%.
Biochemical parameters
Plasma concentrations of glucose and total cholesterol were analyzed by an enzymatic colorimetric method using kits (Bioclin, Belo Horizonte, Brazil) and automated apparatus (Mindray BS-200, Shenzhen, China).
Oral glucose tolerance test
For the oral glucose tolerance test, the glucose solution was administered by gavage (3 g kg−1) to animals after 6 h of fasting. Capillary blood was collected from the tail immediately before, 30, 60, 90 and 150 min after glucose intake. The concentration of glucose was measured in whole blood (Accu-Chek Go glucometer kit, Roche, Sao Paulo, Brazil) and the area under the curve was estimated by the trapezoidal method.
Systolic blood pressure
Systolic blood pressure was measured by tail plethysmography, using a sphygmomanometer (electro-sphymomanometer, Narco Bio-System, Model 709-0610, International Biomedical, Inc., Tucson, AZ, USA). To trigger the vasodilatation of the caudal artery, rats were previously warmed at 40 °C for 5 min. After warming, the sensor was attached around the animal's tail. The cuff was inflated to a pressure of 200 mm Hg and subsequently deflated. The arterial pulse was registered on a polygraph (Gould RS 3200, Gould Instruments, Valley View, OH, USA).
Inflammatory and oxidative stress markers
TNF-α was analyzed in plasma and kidney homogenates (130 mg tissue per ml PBS) by a commercial ELISA kit (R&D Systems Inc., Minneapolis, MN, USA).
The concentration of the receptor for advanced glycation end products (RAGE) was quantified in plasma and kidney homogenates (50 mg tissue per ml PBS, diluted 1:200 in PBS) using a commercial ELISA kit (R&D Systems).
The AGEs in urine were quantified by a commercial ELISA kit (Cell Biolabs Inc., San Diego, CA, USA).
The 8-hydroxydeoxyguanosine, an indicator of oxidative DNA damage, was measured in urine by a commercial ELISA kit (Cell Biolabs).
All analyses were performed using a microplate reader (Spectra Max 190; Molecular Devices, Sunnyvale, CA, USA).
RAGE gene expression
Total RNA was extracted from the kidney using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The SuperScript II First-Strand Synthesis System for real-time PCR (Invitrogen) kit was utilized for the synthesis of 20 μl of complementary DNA from 1000 ng of total RNA. The mRNA levels of RAGE (Applied Biosystems, Thermo Fisher Scientific Inc., Foster City, CA, USA) were determined by real-time PCR. Quantitative measurements were made using a commercial kit (TaqMan qPCR; Applied Biosystems) in a detection system (StepOne Plus; Applied Biosystems). Cycling conditions were as follows: enzyme activation at 50 °C for 2 min, denaturation at 95 °C for 10 min, complementary DNA products amplification for 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. Gene expression was quantified in relation to the values of the control group after normalization to an internal control (cyclophilin; Applied Biosystems).
Malondialdehyde (MDA) analysis
A 100 μl aliquot of urine was used for MDA analysis. Briefly, we add 700 μl of 1% orthophosphoric acid and 200 μl of thiobarbituric acid (42 mm) to the sample and then heated it for 60 min in a water bath at 100 °C; the sample was cooled on ice immediately after. Two hundred microliters were transferred to a 2 ml tube containing 200 μl of sodium hydroxide–methanol (1:12 v/v). The sample was vortex-mixed for 10 s and centrifuged for 3 min at 13 000 g. We transferred 200 μl of the supernatant to a 300 μl glass vial, and injected 50 μl onto the column. The HPLC system used was a Shimadzu LC-10AD system (Shimadzu Corporation, Kyoto, Japan), with a C18 Luna column (5 μm, 150 × 4.60 mm, Phenomenex Inc., Torrance, CA, USA), a Shimadzu RF-535 fluorescence detector (excitation: 525 nm, emission 551 nm), and 0.5 ml min−1 flow of phosphate buffer (KH2PO4 1 mm, pH 6.8). MDA was quantified by area determination of the peaks in the chromatograms relative to a standard curve of known concentrations.
Total antioxidant performance
The total antioxidant performance in plasma and kidney was quantified by comparing the AUC on the oxidation kinetics of BODIPY581/591 (Invitrogen). The total antioxidant performance method was applied to measure the antioxidant status (lipophilic and hydrophilic compartments) using phosphatidylcholine as a reference. Aliquots (100 μl) of plasma or kidney homogenate (100 mg ml−1) were mixed with 300 μl of PBS and 100 μl of BODIPY581/591 (2 μm) and the samples were incubated in a water bath at 37 °C for 10 min. After incubation, 485 μl of ice-cold PBS was added. Fifteen microliters of the radical initiator MeO-AMVN (2 mm) was added and the mixture vortexed using small magnetic sphere. Then, 200 μl aliquots of each sample were transferred to a microplate. The oxidizability of the sample was monitored by the oxidation product of BODIPY581/591, indicated by green fluorescence (excitation: 500 nm, emission: 520 nm) using a counter (Wallac Victor 2, Perkin-Elmer, Boston, MA, USA).
Statistical analysis
The Kolmogorov–Smirnov test was used to verify the normality of the data. Results are expressed as mean and standard error (s.e.), and significance was calculated by one-way analysis of variance followed by post-hoc tests. For the correlation between variables, the Pearson correlation was used. The software used was SigmaStat version 3.5 for Windows (Systat Software, Inc., San Jose, CA, USA). Differences were considered significant at P<0.05.
Results
Body weight, food intake and adiposity index are shown in Table 2. The adiposity index was higher in the HD+S and HD+S+L groups (P<0.001). There was no difference between the groups regarding weight and energy intake. Chow intake was higher in the C group (P<0.001) and water consumption was lower in the HD+S+L group compared with the HD+S group (P=0.036).
Table 2. Adiposity index, body weight and chow intake (n=21).
| Variables |
Groups |
Pa | |||||
|---|---|---|---|---|---|---|---|
|
C |
HD+S |
HD+S+L |
|||||
| Mean | s.e. | Mean | s.e. | Mean | s.e. | ||
| Adiposity index (%) | 5.8a | 0.7 | 9.4b | 0.6 | 9.7b | 0.6 | <0.001 |
| Inicial body weight (g) | 344.0 | 17.3 | 345.0 | 15.7 | 347.5 | 11.3 | 0.986 |
| Final body weight (g) | 507.5 | 31.1 | 568.2 | 26.8 | 564.2 | 22.7 | 0.235 |
| Chow intake (g day−1) | 27.3a | 1.8 | 11.8b | 0.6 | 12.6b | 0.7 | <0.001 |
| Water intake (ml day−1) | 36.3a,b | 2.6 | 38.6a | 2.0 | 30.2b | 1.7 | 0.036 |
| Energy intake (kcal day−1)b | 105.8 | 6.8 | 109.6 | 3.8 | 103.6 | 3.9 | 0.695 |
Abbreviations: C, control; HD, animals subjected to diet-induced obesity; HD+L, HD supplemented with lycopene.
Mean values with unlike superscript letters were significantly different (P<0.05).
One-way analysis of variance with Holm–Sidak post-hoc test.
Energy intake includes energy from sugar in the drinking water.
Lycopene was detected in the plasma and kidney only in the HD+S+L group (plasma lycopene: mean 28.7, s.e. 6.3 nm; kidney lycopene: mean 1.49, s.e. 0.27 nm g−1 of protein).
Table 3 shows the biochemical parameters, blood pressure and glomerular filtration rate of animals. No significant differences were observed in these parameters.
Table 3. Biochemical, hemodynamic and glomerular filtration rate parameters (n=21).
| Variables |
Groups |
Pa | |||||
|---|---|---|---|---|---|---|---|
|
C |
HD+S |
HD+S+L |
|||||
| Mean | s.e. | Mean | s.e. | Mean | s.e. | ||
| Total cholesterol (mmol l−1) | 0.88 | 0.08 | 0.64 | 0.11 | 0.86 | 0.03 | 0.090 |
| Fasting glucose (mmol l−1) | 6.25 | 0.11 | 6.92 | 0.22 | 6.49 | 0.22 | 0.062 |
| Systolic blood pressure (mm Hg) | 125.2 | 4.1 | 131.5 | 6.2 | 127.1 | 3.2 | 0.628 |
| Glomerular filtration rate (ml min−1) | 1.27 | 0.24 | 1.02 | 0.15 | 1.28 | 0.14 | 0.561 |
Abbreviations: C, control; HD, animals subjected to diet-induced obesity; HD+L, HD supplemented with lycopene.
One-way analysis of variance.
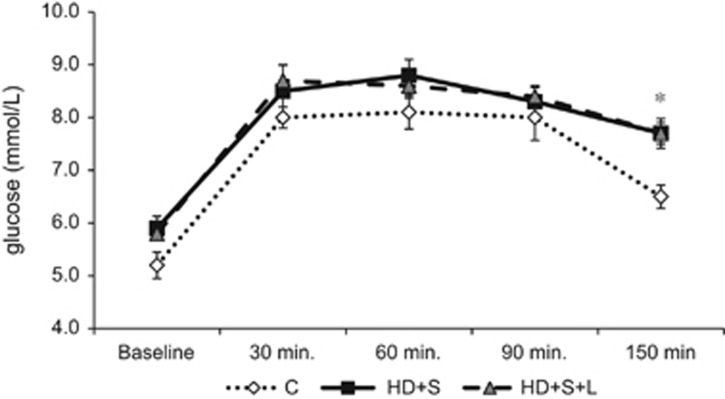
As an indicator of insulin resistance, the oral glucose tolerance test showed no differences between groups regarding the area under the curve. However, the groups HD+S and HD+S+L showed higher glucose levels (P<0.05) than the C group 150 min after glucose administration (Figure 1).
Figure 1.
Oral glucose tolerance test (OGTT, n=21). C, control; HD, animals subjected to diet-induced obesity; HD+L, HD supplemented with lycopene. *Comparison of HD+S and HD+S+L groups with the C group, One-way analysis of variance (ANOVA) with Holm–Sidak post-hoc test, P=0,010. Area under curve: C: 11714.9±341.2; DH+S: 12812.4±221.5; DH+S+L: 12902.4±493.6 mg dl−1 per 150 min. (One-way ANOVA, P=0.064).
Table 4 shows oxidative stress markers in kidney, plasma and urine. There was no difference between groups when the antioxidant performance in plasma and kidney was compared. In urine, the levels of MDA increased in HD+S animals (P=0.002) and HD+S+L animals showed similar values to the C animals (P=0.093). The levels of AGEs (P=0.005) and 8-OHdG (P<0.001) were increased in the HD+S and HD+S+L groups.
Table 4. Oxidative stress markers in kidney, plasma and urine (n=7) (n=21).
| Variables |
Groups |
Pa | |||||
|---|---|---|---|---|---|---|---|
|
C |
HD+S |
HD+S+L |
|||||
| Mean | s.e. | Mean | s.e. | Mean | s.e. | ||
| Kidney | |||||||
| TAP (% g−1 protein) | 54.6 | 3.8 | 59.3 | 2.4 | 59.4 | 3.4 | 0.243 |
| Plasma | |||||||
| TAP (%) | 40.4 | 3.1 | 42.6 | 4.4 | 49.3 | 3.5 | 0.498 |
| Urine | |||||||
| MDA (μM) | 2.17a | 0.67 | 5.6b | 0.55 | 4.08a,b | 0.60 | 0.003 |
| AGEs (μg ml−1) | 0.21a | 0.004 | 0.24b | 0.006 | 0.24b | 0.006 | 0.005 |
| 8-OHdG (ng ml−1) | 7.91a | 0.54 | 12.24b | 0.58 | 12.92b | 0.44 | <0.001 |
Abbreviations: AGE, advanced glycation end products; C, control; HD+S, animals subjected to diet-induced obesity; HD+S+L, HD+S supplemented with lycopene; MDA, malondialdehyde; 8-OHdG, 8-hydroxy-deoxy-guanosine; TAP, total antioxidant performance.
Mean values with unlike superscript letters were significantly different (P<0.05).
One-way analysis of variance with Holm–Sidak post-hoc test.
We found positive correlations between the index of adiposity and oxidative DNA damage (AI and 8-OHdG; r=0.60, P=0.005) and between the adiposity index and lipid peroxidation (AI and MDA; r=0.59, P=0.006).
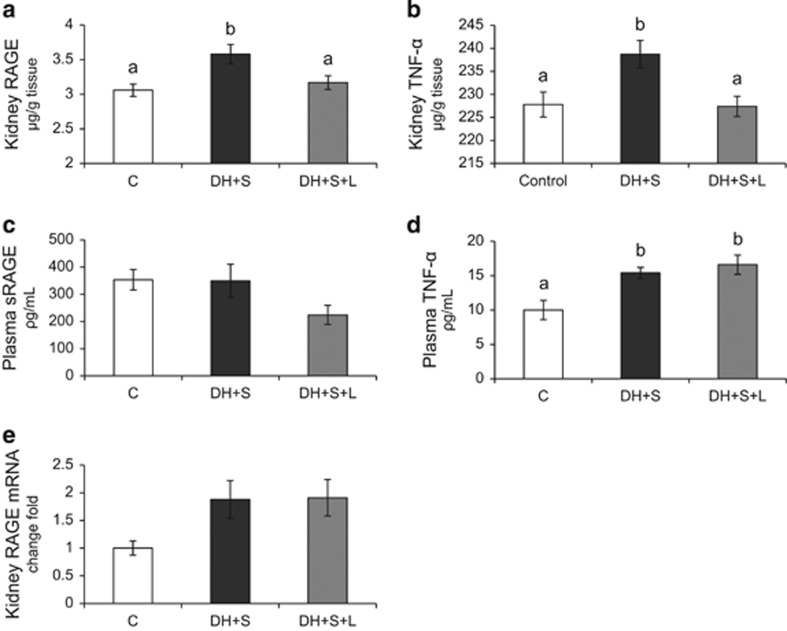
Kidney RAGE was increased in HD+S rats (P=0.005) and decreased in HD+S+L rats (HD+S+L vs HD+S, P=0.023; HD+S+L vs C, P=0.523) and returned to control levels (Figure 2a). Figure 2b shows the same pattern for TNF-α in the kidney. The concentration of soluble RAGE in plasma was not significantly different between groups (Figure 2c). Plasma TNF-α levels increased (P=0.003) in the HD+S and HD+S+L groups compared to the C group (Figure 2d). The analysis of the gene expression of RAGE in kidney showed no differences (P=0.063) between the groups (Figure 2e).
Figure 2.
Renal and plasma concentrations of RAGE and TNF-α (n=21). (a) Kidney RAGE; (b) kidney TNF-α (c) plasma soluble RAGE receptor; (d) plasma TNF-α (e) kidney RAGE mRNA. C, control; HD+S, animals subjected to diet-induced obesity; HD+S+L, HD+S supplemented with lycopene. a,b Mean values with unlike superscript letters were significantly different (P<0.05); one-way analysis of variance with Holm–Sidak post-hoc test.
Discussion
In the present study, obesity was induced in Wistar rats by high-fat diet plus sugar in drinking water aiming to characterize a Western diet. One of the first change with obesity is increase in fat mass, which brings metabolic disorders, inflammation and oxidative stress. These alterations reflect tissue damage that affects various organs, among them the kidney.16 Many studies have induce obesity by dietary changes, but there is still no uniformity in the period of manifestation of metabolic parameters or the increased adiposity index due to the diversity of composition and exposure to these diets.17
Our results showed that HD+S rats had increased fat mass, but this degree of obesity was not enough to cause changes in glucose, cholesterol, blood pressure and renal function; only insulin resistance increased with obesity, which was not reversed by lycopene supplementation. These data are consistent with the literature showing that the Western diet, rich in fat and sugar, is considered to increase visceral fat and the contribute to the development of insulin resistance;18 lycopene has no influence on these parameters.19
Oxidative stress involves reactive molecules that are composed of oxygen or hydrogen.20 In our study, the nutritional overload had no effect on total antioxidant performance in both the plasma and kidney, which can be justified by the absence of comorbidities. Venturini et al.21 demonstrated in clinical work that individuals with lower antioxidant performance suffered from metabolic syndrome, that is, they presented, in addition to obesity, increased blood sugar, triglycerides and blood pressure. Another clinical study reported that changes in antioxidant capacity occur when obesity is accompanied with metabolic complications.22 However, we observed a significant increase in the oxidative indicator of DNA damage, 8-OHdG, and MDA in the urine of HF+S rats. Hofer et al.,23 in humans, indicated that the body mass index is directly associated with increased risk of DNA damage due to oxidative stress. Regarding MDA, it has also been reported that this is related to increased body weight and a hyper-caloric diet.17, 24 These studies are in agreement with our data, as our animals (HD+S) had no comorbidities, so we can attribute the increase of these components to increased fat mass. Given the positive correlation found in our study, 8-OHdG and MDA are directly related to increased fat mass, and are thus particular indicators of oxidative stress in obesity.
The mechanisms by which obesity predisposes to renal damage are still being investigated.25 AGE/RAGE binding may contribute to the pathogenesis of obesity-related renal impairment.26 AGE/RAGE binding can also be promoted by the pro-oxidant state by triggering inflammation through the activation of nuclear factor kappa B and the production of proinflammatory cytokines.27
As noted above, the AGE/RAGE binding is associated with oxidative and inflammatory phenomena that result in increased cytokine expression.28 In this study, we observed that the high-fat diet plus sucrose promoted an increase in TNF-α and RAGE in renal tissue. This result suggests that this model of obesity induction resulted in the activation of inflammatory and oxidative pathways through AGE/RAGE binding.
Our study also shows that supplementation with lycopene can decrease the concentration of RAGE in the renal tissue of HF+S+L rats, which was also observed for urinary MDA. Regarding AGE, also urinary, with carotenoid supplementation did not interfere its concentration. It must be emphasized that this metabolite was formed since the initiation of obesity,29 which occurred in the sixth week. As the feed stimulus continued concomitantly with lycopene supplementation, these species could not be inactivated by antioxidants. Unlike MDA, which is a product that can be neutralized by antioxidants, AGEs are irreversible due to the irreversibility of carbonylation.30
These results suggest that lycopene had a direct action on RAGE which causes a decrease in inflammation as well as in the products of lipid peroxidation. Thus, we observed an anti-inflammatory effect of lycopene in the kidney via oxidative stress, which can be confirmed by the presence of lycopene in the plasma and kidneys of the supplemented rats.
The soluble RAGE receptor is found in the circulation, and a high concentration of soluble RAGE receptor has been associated with chronic inflammatory diseases, including atherosclerosis and diabetes.31 Our data show no change in the soluble receptor in induced obesity. This can be explained due to the time period of the study (12 weeks) and the early stage of obesity, which was not enough to cause changes in glucose levels. Brix et al.32 suggest that an increase in soluble RAGE is more common in diabetics and in more severe cases of obesity.
With regards to systemic inflammation, we confirmed increased plasma TNF-α levels in obese rats; these levels did not decrease with lycopene treatment. This may be due to the time of administration of the carotenoid (6 weeks). In a study on diabetic rats with similar timing to ours, 4 weeks of supplementation with lycopene was not able to reduce the levels of TNF-α.33 Moreover, the literature reports that diets rich in carotenoids result in a rapid increase of these components in plasma, but their effect on inflammation is slower, suggesting slow intracellular accumulation of carotenoids.34 Other researchers also found no decrease in plasma levels of TNF-α with administration of lycopene in obese individuals.35 This can also be explained by the fact that circulating levels of TNF-α are derived from other organs36, 37, 38 that are also affected by obesity, which might not respond as well to lycopene treatment as the kidney, since this organ is not the only one affected in this condition.39
In summary, it was observed that the high-fat diet plus sucrose led to changes in body composition, triggering obesity and insulin resistance, but without biochemical, hemodynamic or renal function changes. However, the diet promoted a systemic increase in lipid peroxidation, inflammation and oxidative damage to DNA, along with inflammation and oxidative stress in the kidney. Lycopene was able to decrease MDA, RAGE and TNF-α levels in the kidney. Thus, this carotenoid can be beneficial for the prevention and treatment of toxicity induced by oxidative stress in the kidney due to obesity.
Acknowledgments
We thank LycoRed Natural Products Industries (Beer-Sheva, Israel) for supplying the lycopene. We also thank FAPESP (2010/06100-9 and 2011/14132-0) and CAPES for financial support (022/11 and BEX: 9847/11-1). The material is original research, has not been previously published and has not been submitted for publication elsewhere.
The authors declare no conflict of interest.
References
- World Health Organization Obesity and overweight [internet] WHO, Geneva, Switzerland; 2011. Available at http://www.who.int//mediacentre/factsheets/fs311/en ( accessed 9 April 2013). [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamentos Familiares 2008-2009 [internet] IBGE, Brasília; 2009. Available at http://www.ibge.gov.br/home/estatistica/populacao/condicaodevida/pof/2008_2009/ . Accessed 9 April 2011. [Google Scholar]
- Guarnieri G, Zanetti M, Vinci P, Cattin MR, Pirulli A, Barazzoni R. Metabolic syndrome and chronic kidney disease. J Ren Nutr. 2010;20:S19–S23. doi: 10.1053/j.jrn.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- Aldini G, Dalle-Donne I, Facino RM, Milzani A, Carini M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med Res Rev. 2007;27:817–868. doi: 10.1002/med.20073. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, Baliga S, et al. Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. Am J Kidney Dis. 2003;42:532–538. doi: 10.1016/s0272-6386(03)00779-0. [DOI] [PubMed] [Google Scholar]
- Tuohy KM, Hinton DJ, Davies SJ, Crabbe MJ, Gibson GR, Ames JM. Metabolism of Maillard reaction products by the human gut microbiota—implications for health. Mol Nutr Food Res. 2006;50:847–857. doi: 10.1002/mnfr.200500126. [DOI] [PubMed] [Google Scholar]
- Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes. 2005;54:2314–2319. doi: 10.2337/diabetes.54.8.2314. [DOI] [PubMed] [Google Scholar]
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- Bierhaus A, Chevion S, Chevion M, Hofmann M, Quehenberger P, Illmer T, et al. Advanced glycation end product-induced activation of NF-kappaB is suppressed by alpha-lipoic acid in cultured endothelial cells. Diabetes. 1997;46:1481–1490. doi: 10.2337/diab.46.9.1481. [DOI] [PubMed] [Google Scholar]
- Ford ES, Ajani UA, Mokdad AH. Brief communication: the prevalence of high intake of vitamin E from the use of supplements among U.S. adults. Ann Intern Med. 2005;143:116–120. doi: 10.7326/0003-4819-143-2-200507190-00010. [DOI] [PubMed] [Google Scholar]
- Ali MM, Agha FG. Amelioration of streptozotocin-induced diabetes mellitus, oxidative stress and dyslipidemia in rats by tomato extract lycopene. Scand J Clin Lab Invest. 2009;69:371–379. doi: 10.1080/00365510802658473. [DOI] [PubMed] [Google Scholar]
- Bae JW, Bae JS. Barrier protective effects of lycopene in human endothelial cells. Inflamm Res. 2011;60:751–758. doi: 10.1007/s00011-011-0330-9. [DOI] [PubMed] [Google Scholar]
- Ferreira ALA, Russell RM, Rocha N, Ladeira MSP, Salvadori DMF, MCMO Nascimento, et al. Effect of lycopene on doxorubicin-induced cardiotoxicity: an echocardiographic, histological and morphometrical assessment. Basic Clin Pharmacol Toxicol. 2007;101:16–24. doi: 10.1111/j.1742-7843.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Woo MN, Bok SH, Lee MK, Kim HJ, Jeon SM, Do GM, et al. Anti-obesity and hypolipidemic effects of a proprietary herb and fiber combination (S&S PWH) in rats fed high-fat diets. J Med Food. 2008;11:169–178. doi: 10.1089/jmf.2007.082. [DOI] [PubMed] [Google Scholar]
- Noeman SA, Hamooda HE, Baalash AA. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr. 2011;3:17. doi: 10.1186/1758-5996-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida K, Fujimoto E, Higuchi M, Terada S. Effects of alternate-day fasting on high-fat diet-induced insulin resistance in rat skeletal muscle. Life Sci. 2013;93:208–213. doi: 10.1016/j.lfs.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Bahcecioglu IH, Kuzu N, Metin K, Ozercan IH, Ustundag B, Sahin K, et al. Lycopene prevents development of steatohepatitis in experimental nonalcoholic steatohepatitis model induced by high-fat diet. Veterinary medicine international. 2010;2010. [DOI] [PMC free article] [PubMed]
- Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- Venturini D, Simao AN, Scripes NA, Bahls LD, Melo PA, Belinetti FM, et al. Evaluation of oxidative stress in overweight subjects with or without metabolic syndrome. Obesity. 2012;20:2361–2366. doi: 10.1038/oby.2012.130. [DOI] [PubMed] [Google Scholar]
- Karamouzis I, Pervanidou P, Berardelli R, Iliadis S, Papassotiriou I, Karamouzis M, et al. Enhanced oxidative stress and platelet activation combined with reduced antioxidant capacity in obese prepubertal and adolescent girls with full or partial metabolic syndrome. Horm Metab Res. 2011;43:607–613. doi: 10.1055/s-0031-1284355. [DOI] [PubMed] [Google Scholar]
- Hofer T, Karlsson HL, Moller L. DNA oxidative damage and strand breaks in young healthy individuals: a gender difference and the role of life style factors. Free Radic Res. 2006;40:707–714. doi: 10.1080/10715760500525807. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang F, Li Y, Sun C. [Oxidative stress and the susceptibility to obesity in rats] Wei Sheng Yan Jiu. 2011;40:420–422. [PubMed] [Google Scholar]
- Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- Tomino Y, Hagiwara S, Gohda T. AGE-RAGE interaction and oxidative stress in obesity-related renal dysfunction. Kidney Int. 2011;80:133–135. doi: 10.1038/ki.2011.86. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, et al. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Yamamoto H. RAGE-mediated inflammation, type 2 diabetes, and diabetic vascular complication. Front Endocrinol. 2013;4:105. doi: 10.3389/fendo.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebekova K, Faist V, Hofmann T, Schinzel R, Heidland A. Effects of a diet rich in advanced glycation end products in the rat remnant kidney model. Am J Kidney Dis. 2003;41:S48–S51. doi: 10.1053/ajkd.2003.50084. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med. 2006;10:389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey JB, de Lemos JA, Cipollone F, Ayers CR, Rohatgi A, Morrow DA, et al. Association between circulating soluble receptor for advanced glycation end products and atherosclerosis: observations from the Dallas Heart Study. Diabetes Care. 2009;32:1218–1220. doi: 10.2337/dc09-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix JM, Hollerl F, Kopp HP, Schernthaner GH, Schernthaner G. The soluble form of the receptor of advanced glycation endproducts increases after bariatric surgery in morbid obesity. Int J Obes. 2012;36:1412–1417. doi: 10.1038/ijo.2012.107. [DOI] [PubMed] [Google Scholar]
- Yegin SC, Yur F, Ceylan E. Effect of lycopene application in rats with experimental diabetes using lipoprotein, paraoxonase and cytokines. J Membr Biol. 2013;246:621–626. doi: 10.1007/s00232-013-9575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzl B, Bub A, Briviba K, Rechkemmer G. Supplementation of a low-carotenoid diet with tomato or carrot juice modulates immune functions in healthy men. Ann Nutr Metab. 2003;47:255–261. doi: 10.1159/000072397. [DOI] [PubMed] [Google Scholar]
- Markovits N, Ben Amotz A, Levy Y. The effect of tomato-derived lycopene on low carotenoids and enhanced systemic inflammation and oxidation in severe obesity. Isr Med Assoc J. 2009;11:598–601. [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Vitseva OI, Tanriverdi K, Tchkonia TT, Kirkland JL, McDonnell ME, Apovian CM, et al. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity. 2008;16:932–937. doi: 10.1038/oby.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto T, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Orlicky DJ, Cicerchi C, et al. High fat and high sucrose (western) diet induce steatohepatitis that is dependent on fructokinase. Hepatology. 2013;58:1632–1643. doi: 10.1002/hep.26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro R, Azevedo I.Chronic inflammation in obesity and the metabolic syndrome Mediators Inflamm 2010e-pub ahead of print 14 July 2010; doi: 10.1155/2010/289645 [DOI] [PMC free article] [PubMed] [Google Scholar]