Abstract
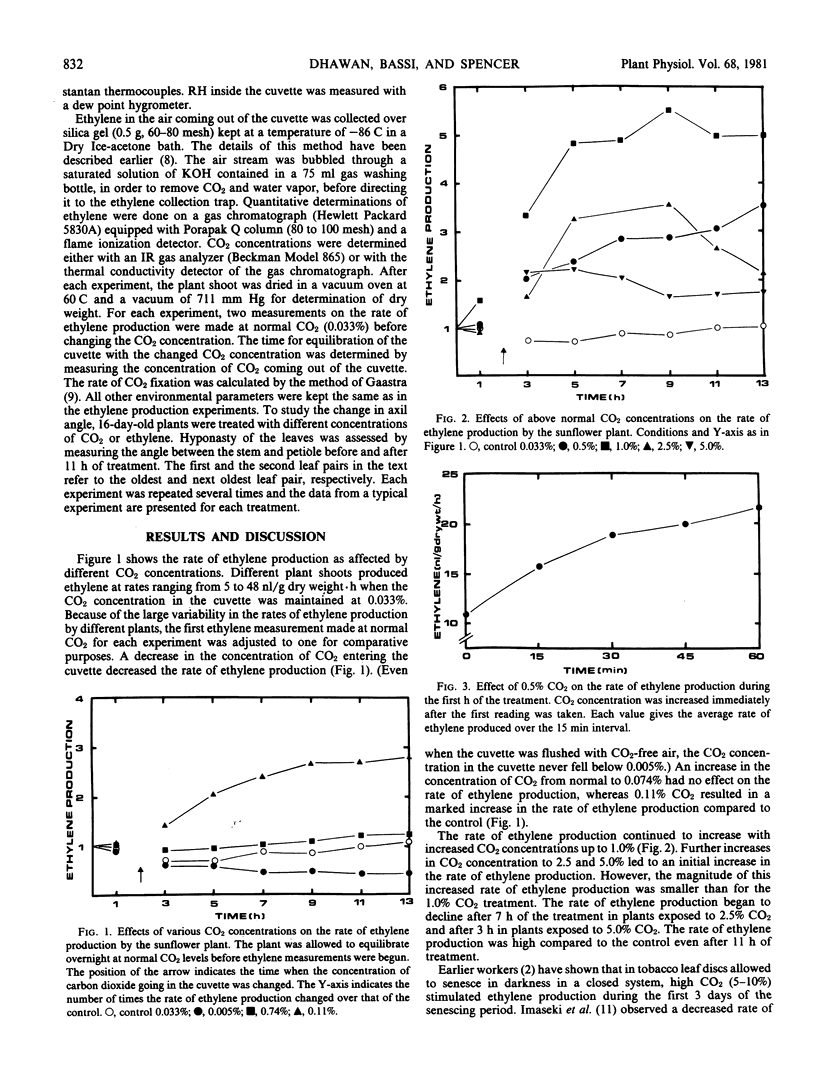
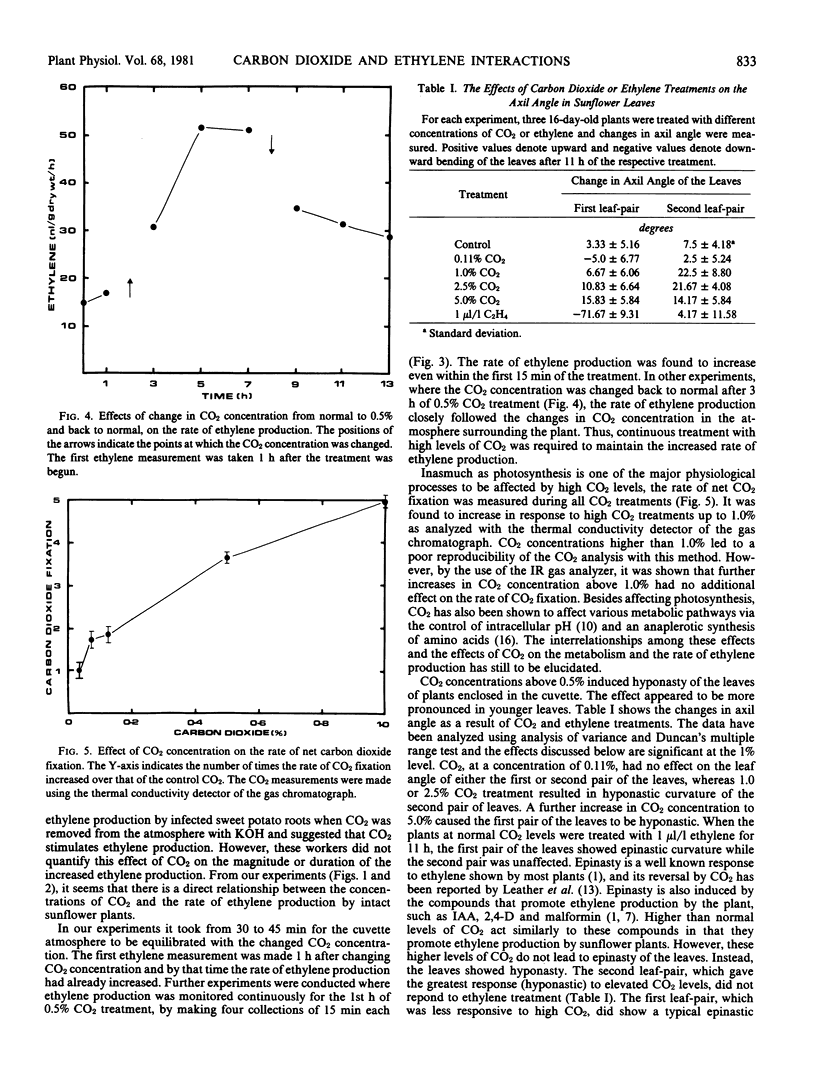
A continuous flow system was used to study the interactions between carbon dioxide and ethylene in intact sunflower (Helianthus annuus L.) plants. An increase in the concentration of carbon dioxide above the ambient level (0.033%) in the atmosphere surrounding the plants increased the rate of ethylene production, and a decrease in carbon dioxide concentration resulted in a decrease in the rate of ethylene production. The change in the rate of ethylene production was evident within the first 15 minutes of the carbon dioxide treatment. Continuous treatment with carbon dioxide was required to maintain increased rate of ethylene production. The rate of carbon dioxide fixation increased in response to high carbon dioxide treatment up to 1.0%. Further increases in carbon dioxide concentration had no additional effect on carbon dioxide fixation. Carbon dioxide concentrations higher than 0.11% induced hyponasty of the leaves whereas treatment with 1 microliter per liter ethylene induced epinasty of the leaves.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N., Lieberman M. Ethylene as a regulator of senescence in tobacco leaf discs. Plant Physiol. 1979 Nov;64(5):801–804. doi: 10.1104/pp.64.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi P. K., Spencer M. S. A Cuvette Design for Measurement of Ethylene Production and Carbon Dioxide Exchange by Intact Shoots under Controlled Environmental Conditions. Plant Physiol. 1979 Sep;64(3):488–490. doi: 10.1104/pp.64.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracker L. E., Abeles F. B. Abscission: role of abscisic Acid. Plant Physiol. 1969 Aug;44(8):1144–1149. doi: 10.1104/pp.44.8.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R. W. Mediation of a plant response to malformin by ethylene. Plant Physiol. 1968 Jan;43(1):76–80. doi: 10.1104/pp.43.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwell K. C., Bassi P. K., Spencer M. E. Comparison and evaluation methods for the removal of ethylene and other hydrocarbons from air for biological studies. Plant Physiol. 1978 Nov;62(5):723–726. doi: 10.1104/pp.62.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschke H. P., Lüttge U. Stoichiometric Correlation of Malate Accumulation with Auxin-dependent K-H Exchange and Growth in Avena Coleoptile Segments. Plant Physiol. 1975 Nov;56(5):696–698. doi: 10.1104/pp.56.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leather G. R., Forrence L. E. Increased Ethylene Production during Clinostat Experiments May Cause Leaf Epinasty. Plant Physiol. 1972 Feb;49(2):183–186. doi: 10.1104/pp.49.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splittstoesser W. E. Dark CO(2) Fixation and its Role in the Growth of Plant Tissue. Plant Physiol. 1966 May;41(5):755–759. doi: 10.1104/pp.41.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole V. K., Bailey W. K., Toole E. H. Factors Influencing Dormancy of Peanut Seeds. Plant Physiol. 1964 Sep;39(5):822–832. doi: 10.1104/pp.39.5.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. E., Romani R. J., Biale J. B. Carbon Dioxide Effects on Fruit Respiration . II. Response of Avocados, Bananas, & Lemons. Plant Physiol. 1962 May;37(3):416–422. doi: 10.1104/pp.37.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]


