Abstract
Fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) is not indicated or recommended in the initial staging of early breast cancer. Although it is valuable for detecting distant metastasis, providing prognostic information, identifying recurrence and evaluating response to chemotherapy, the role of FDG PET/CT in evaluating locoregional nodal status for initial staging of breast cancer has not yet been well-defined in clinical practice. FDG PET/CT has high specificity but compromised sensitivity for identifying axillary nodal disease in breast cancer. Positive axillary FDG PET/CT is a good predictor of axillary disease and correlates well with sentinel lymph node biopsy (SLNB). FDG PET/CT may help to identify patients with high axillary lymph node burden who could then move directly to axillary lymph node dissection (ALND) and would not require the additional step of SLNB. However, FDG PET/CT cannot replace SLNB or ALND due to unsatisfactory sensitivity. The spatial resolution of PET instruments precludes the detection of small nodal metastases. Although there is still disagreement regarding the management of internal mammary node (IMN) disease in breast cancer, it is known that IMN involvement is of prognostic significance, and IMN metastasis has been associated with higher rates of distant metastasis and lower overall survival rates. Limited clinical observations suggested that FDG PET/CT has advantages over conventional modalities in detecting and uncovering occult extra-axillary especially IMN lesions with upstaging the disease and an impact on the adjuvant management.
Keywords: Breast cancer, Fluorodeoxyglucose positron emission tomography/computed tomography, Locoregional nodal disease, Axillary lymph node, Internal mammary lymph node, Axillary lymph node dissection, Sentinel lymph node biopsy
Core tip: The presence and extent of locoregional nodal metastasis at diagnosis is the single most important prognostic factor in breast cancer. The predominant lymphatic drainage pathway from the breast cancer is toward the axilla. Fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) is a good predictor of axillary disease and correlates well with sentinel lymph node biopsy (SLNB). FDG PET/CT may help to identify patients with high axillary lymph node burden who could then move directly to axillary lymph node dissection (ALND) and would not require the additional step of SLNB. However, FDG PET/CT cannot replace SLNB or ALND due to unsatisfactory sensitivity secondary to the limitation of its spatial resolution. The internal mammary node (IMN) involvement is of prognostic significance in breast cancer, and IMN metastasis has been associated with higher rate of distant metastasis and lower overall survival rates. Limited preliminary data indicated that FDG PET/CT plays a role in identification of positive IMN, and it is superior to conventional imaging modalities.
INTRODUCTION
Breast cancer is the most common solid malignancy in women in the Western world, and a woman’s lifetime risk of developing breast cancer exceeds 10%[1]. The treatment and prognosis of breast cancer depend on the size of the lesion, pathologic grade, estrogen receptor status, locoregional nodal involvement and metastatic disease. Of these, the presence and extent of locoregional nodal metastasis at diagnosis is the single most important prognostic factor in breast cancer, which is reflected by 5-year survival rates of 99% in the absence of nodal metastasis, and 84% and 23% respectively for locoregional and distant metastatic disease[2,3]. An increase in the number of tumor-positive axillary nodes is related to a worsened prognosis irrespective of primary tumor size. Approximately 30% breast cancer with local or locoregional-confined disease eventually relapse[4,5]. Therefore, accurate evaluation of locoregional nodal status in breast cancer remains essential because of its implications for treatment and prognosis.
Imaging plays a critical role in the initial evaluation of breast cancer and serves as an important adjunct to surgical, pathologic and clinical staging. Several imaging modalities are used in the diagnosis and staging of breast cancer, including mammography, ultrasonography, computed tomography (CT), magnetic resonance imaging and positron emission tomography/computed tomography (PET/CT). For initial assessment of primary breast cancer, mammography is still the most used modality usually complemented with ultrasonography for the evaluation of axillary nodal involvement. Today metabolic imaging PET/CT with F18-fluoro-2-deoxy-D-glucose (FDG) has gained widespread clinical applications in oncology, and is accepted as a standard care in many malignancies. FDG is an analog of glucose and is used as a tracer of glycolysis. Malignant tissue and cells often demonstrate increased rate of glycolysis for rapid proliferation, due to increased numbers of glucose transporter protein and increased intracellular hexokinase and phosphofructokinase levels[6]. The intensity of FDG is proportional to the rate of glycolysis, therefore is potentially semi-quantitative with standardized uptake value (SUV). It is well known that FDG PET/CT is valuable for detecting distant metastasis, providing prognostic information, identifying recurrence, and evaluating response to chemotherapy[7]. However, knowledge of the value of FDG PET-CT for locoregional nodal staging is limited and somehow controversial. This review aims to present the role of FDG PET/CT in evaluating locoregional nodal status of breast cancer.
FDG PET/CT FOR AXILLARY LYMPH NODES
The most important prognostic factor in breast cancer is the axillary lymph node status. However, management of the axilla in patients with operable breast cancer is still one of most controversial areas in clinical oncology. The best procedure to examine the axillary lymph nodes is still axillary lymph node dissection (ALND) or sentinel lymph node biopsy (SLNB). SLNB remains the gold standard for axillary nodal evaluation. Lymphoscinitigraphy is a radionuclide imaging method to localize sentinel node in the axilla for biopsy. However, SLNB and ALNB carry significant surgical complications[8].
The accuracy of FDG PET-CT for detecting axillary nodal lesion has been studied primarily in early-stage disease. Veronesi et al[9] compared SLNB and FDG PET/CT in detecting occult axillary metastases in 236 consecutive patients. All patients with pathological confirmed cancer and negative axillae on clinical examinations underwent FDG PET/CT and SLNB. PET/CT identified positive axillary nodes in 43 patients (18.2%), 38 of these were confirmed true-positive on ALND (34) and SLNB (4). The results indicated a high positive predictive value of FDG PET/CT, and those with FDG PET positive axillae preoperatively should proceed directly to ALND without the need for SLNB. But SLNB identified 65 axillary positive cases, which was greater than the number of positive cases identified by FDG PET/CT. FDG PET/CT was unable to detect occult axillary metastases in one third of cases.
In a prospective multicenter trial, FDG PET-CT was performed in 360 women with newly diagnosed invasive breast cancer, and the PET results were compared to pathologic findings[10]. Overall, FDG PET was 61% sensitivity and 80% specificity for axillary metastases, with a positive predictive value of 62% and a negative predictive value of 79%. Patients with false negative PET had significantly smaller and fewer tumor-positive lymph nodes than true-positive cases. The results indicated the limitation of FDG PET in detection of micrometastases and small, tumor-infiltrated axillary lymph nodes.
Another prospective study was obtained by Ueda et al[11], which evaluated the utility of FDG PET/CT in combination with ultrasound for axillary staging in breast cancer. 183 patients underwent both FDG PET/CT and ultrasound within 5 wk of surgery. All patients had SLNB and/or ALND. FDG PET/CT was positive in the axillary lymph nodes in 40 (22%) patients, 34 (85%) of those were true-positive on correlation with surgical pathology. Of 143 negative PET/CTs, 25 (17%) were false-negative. The sensitivity and specificity of FDG PET/CT were 58% and 95%, respectively. The authors concluded that FDG PET/CT added incremental diagnostic confidence to axillary ultrasonography.
Kim et al[12] tried to determine whether pre-surgical FDG PET/CT could be used as a guide for ALND or SLNB. 137 patients with biopsy-proven breast cancer underwent preoperative FDG PET/CTs, those who were positive in the axillae went to ALND directly, and those who were negative in the axillae proceeded to SLNB. There were 8 false negative and no false positive scans. The overall sensitivity and specificity of FDG PET/CT in predicting axillary metastases were 77% and 100%. 27 patients were spared unnecessary SLNB by using FDG PET/CT.
A study by Choi et al[13] suggested that FDG PET/CT has similar sensitivity and specificity to breast ultrasonography in detecting axillary nodal lesions. One hundred and fifty-four consecutive biopsy-proven invasive breast cancer patients were enrolled in this study. All patients had preoperative FDG PET/CT and conventional image studies. Postoperative histopathologic results were used as the final standard. The sensitivity and specificity of FDG PET/CT to detect metastatic axilla were 37.3% and 95.8%, respectively; whereas the corresponding estimates of breast ultrasonography were 41.2% and 93.7%, respectively.
A meta-analysis by Cooper et al[14] examined 7 studies with a combined 862 patients who underwent PET-CT for breast cancer staging. The mean sensitivity for detecting nodal metastasis was 56%. However, although the overall sensitivity of nodal evaluation in early-stage breast cancer is low, PET-CT still outperformed traditional imaging modalities.
The sensitivity of FDG PET-CT for axillary nodal metastasis depends on both axillary tumor burden and FDG avidity of primary tumor[15,16]. A significantly higher proportion of metastases are detected in patients with aggressive histologic features.
In a study of 61 consecutive breast cancer patients by Heusner et al[17], FDG PET/CT was obtained and compared to either SLNB or ALND, or both. Of 24 histologically positive axillae, FDG PET/CT predicted lymph node involvement in 17. In 14 true positive PET/CT cases, the mean diameter of the nodal lesions was 9 mm (range 4-22 mm). In 10 false negative PET/CT cases, the mean diameter of the axillary nodes was 3 mm (range 0.8-6 mm). The overall sensitivity was 58%, and specificity was 92%.
In a more recently published Ontario Clinical Oncology Group Study with 325 patient enrollments and ALND or SLNB as the gold standard following FDG PET/CT, the sensitivity, specificity, positive predictive value and negative predictive value were 23.7%, 99.6%, 95.8% and 75.4%[18]. Using logistic regression, primary tumor size was predictive for prevalence of the nodal lesion and for PET sensitivity.
Table 1 outlines the major studies about the role of FDG PET/CT in evaluation of axillary nodal disease in breast cancer.
Table 1.
The studies evaluating the role of fluorodeoxyglucose positron emission tomography/computed tomography in axillary lymph nodes in breast cancer
| Author | Year | Patient No. | Ref. | PET/CT Sensitivity (%) | PET/CT Specificity (%) | PET/CT PPV (%) | PET/CT NPV (%) | Conclusions |
| Wahl et al[10] | 2004 | 360 | ALND | 61 | 80 | 62 | 79 | FDG PET was limited in detection of micrometastasis |
| Veronesi et al[9] | 2007 | 236 | SLNB | 37 | 96 | 88 | 66 | High specificity of FDG PET/CT indicated that patients with positive PET should have ALND directly |
| Ueda et al[11] | 2008 | 183 | SLNB and/or ALND | 58 | 95 | 85 | 83 | Diagnostic accuracy of PET/CT was nearly equal to ultrasound |
| Kim et al[12] | 2009 | 137 | ALND or SLNB | 77 | 100 | 100 | 94 | FDG PET/CT could help to select patients for either ALND or SLNB |
| Heusner et al[17] | 2009 | 61 | SLNB | 58 | 92 | 82 | 77 | FDG PET/CT could not replace invasive approaches for axillary staging |
| Choi et al[13] | 2012 | 154 | Biopsy or additional imaging and follow-ups | 37 | 96 | 83 | 74 | FDG PET/CT could not be recommended as a primary diagnostic procedure |
| Groheux et al[16] | 2011 | 70 | SLNB or US-FNA | 63 | 91 | 63 | 91 | FDG PET/CT might impact cancer management in small portions of patients |
| Koolen et al[3] | 2012 | 290 | SLNB or US-FNA | 82 | 92 | 98 | 53 | FDG PET/CT could be recommended as a standard staging procedure |
| Pritchard et al[18] | 2012 | 325 | SLNB or ALND | 24 | 100 | 96 | 75 | FDG PET/CT was not sufficiently sensitive to detect positive axillary nodes |
FDG PET/CT: Fluorodeoxyglucose positron emission tomography/computed tomography; PPV: Positive predictive value; NPV: Negative predictive value; ALND: Axillary lymph node dissection; SLNB: Sentinel lymph node biopsy.
In conclusion, the data above indicate that positive axillary FDG PET/CT is a good predictor of axillary disease and correlates well with SLNB. FDG PET/CT may help to identify patients with high axillary lymph node burden who could then move directly to ALND and would not require the additional step of SLNB[1]. However, FDG PET/CT cannot replace SLNB or ALND due to unsatisfactory sensitivity. False-negative FDG PET/CT in the evaluation of the axillary disease is mainly secondary to small size of the lymph nodes. A negative scan cannot exclude micrometastasis in the axilla. The spatial resolution (approximately 5-6 mm) of PET instruments precludes the detection of small nodal metastases.
FDG PET/CT FOR INTERNAL MAMMARY LYMPH NODES
Although the predominant lymphatic drainage pathway from the breast cancer is toward the axilla, nodal metastases outside the axilla may be present in up to 56% of breast cancer[1]. The sites of extra-axillary nodes include the internal mammary chain, interpectoral space, supraclavicular/infraclavicular region[19,20]. In which, breast cancer drainage to internal mammary node (IMN) happens in as many as 30% of patients[21,22]. Most of IMN metastases have concomitant axillary metastases, but 8%-10% patients with breast cancer may have IMN metastases only[23].
The presence of IMN metastasis is classified as stage N3. IMN involvement is of prognostic significance in breast cancer, and IMN metastasis has been associated with higher rate of distant metastasis and lower overall survival rates[24-28]. Yao et al[24] examined the relationship between lymphoscintigraphic evidence of IMN drainage and survival in early stage breast cancer patients. The results show a near 3-fold increase mortality in IMN positive patients. Prognosis of patients with both axillary and IMN metastases is poor when compared with axillary nodal metastasis only[29]. IMN involvement might predict treatment failure, early recurrence or distant metastasis in breast cancer[30]. Multiple studies have consistently found that medial breast cancers carry a worse prognosis compared with lateral cancer, even after adjusting for other known prognostic factors. Because there is no plausible evidence that medial tumors are more biologically aggressive, it is likely that the worse outcome is a result of the un-treatment of IMN metastases, which is more common in medial cancer[31,32].
The assessment of IMN remains a challenge. IMN metastasis is often clinically occult. Visualization of IMN drainage by lymphoscintigraphy depends on the use of peritumoral injection[24], and drainage to IMN varies by locations of tumors[24]. The peritumoral, or intratumoral injection of radiotracer was a useful method for identifying a significant portion of tumors that have primary IMN drainage because anatomic studies have shown that IMN is supplied primarily by retro-mammary lymphatic[33,34]. With peritumoral or intratumoral injection technique, up to 25% IMN drainage could be identified by lymphoscintigraphy[21,34]. However, subareolar injection, which improves axillary lymph node detection, rarely shows IMN uptake due to the superficial lymphatic channels. Although the gold standard for establishing the status of IMN metastasis is surgical, most surgeons do not routinely perform IMN sampling or dissection due to the relative inaccessibility and lack of convincing data for established survival benefit[26,33]. In studies where biopsy was performed for hot internal mammary sentinel node on lymphoscintigraphy, tumor was detected pathologically in 8% to 27% of patients[33]. Usually, if there is drainage to IMN, there is concomitant drainage to axillary node. In such case, general practice is surgical excision of only axillary sentinel node[22]. Systemic treatment strategy is rarely influenced by IMN metastasis, due to concurrent axillary nodal metastasis and unfavorable primary tumor characteristics. Most of these patients would need adjuvant therapy such as chemotherapy and/or radiation and/or hormonal therapy. However, two recent European studies showed that IMN dissection improved accuracy of breast cancer staging and survival[35,36]. The patients with IMN metastases had a better 5-year survival after IMN radiotherapy and chemotherapy[34]. Heuts et al[36] also suggested that tailored adjuvant systemic therapy and additional parasternal radiotherapy have a beneficial effect on the prognosis of these patients. However, the value of IMN radiation is uncertain and subject of debate partially due to a concern about the risk of cardiac toxicity associated with IMN radiotherapy[36].
Although data are limited regarding the role of FDG PET/CT for IMN metastasis in breast cancer, some studies had demonstrated that FDG PET is superior to conventional diagnostic techniques in the detection of extra-axillary nodal metastases, particular to the IMN. Eubank et al[37] compared the detection rates of CT and FDG PET/CT in 73 patients with recurrent or metastatic breast cancer. All patients had CT and PET/CT within 30 d of each other. The prevalence of abnormal FDG uptake in the IMN or mediastinum doubles that of abnormal CT findings in the extra-axillary nodal regions. FDG PET/CT could uncover disease in these nodal regions not detected by conventional staging methods. In another prospective study with 154 preoperative patients with breast cancer by Choi et al[13], 7 extra-axillary nodal lesions were detected by FDG PET/CT only although all patients had additional conventional imaging studies. Aukema et al[38] found that in 60 breast cancer patients with tumor size greater than 3 cm and/or proven axillary nodal metastasis, pre-chemotherapy FDG PET/CT detected abnormal IMN in 8 patients. In 4 of the 8 patients, treatment planning was changed and radiotherapy was added. Koolen et al[3] reported that in 310 patients with breast cancer and scheduled for neoadjuvant chemotherapy, FDG PET-CT detected 26 (8%) abnormal IMN. PET/CT findings helped selecting patients for postoperative internal mammary chain radiotherapy. Bernsdorf et al[39] analyzed FDG PET/CT data of 103 consecutive patients with newly diagnosed operable breast cancer and tumors greater than 2 cm. Extra-axillary lymph node involvement was detected in the internal mammary chain by PET/CT in 10 patients, 5 of them had adjuvant treatment modifications. Recently, Wang et al[40] reported the role of FDG PET/CT in the detection of IMN metastases with pathologic correlation in a large series of patients. One hundred and ten of 1259 patient had FDG avid IMNs on PET/CT. Twenty-five patients underwent ultrasound-guided fine needle aspiration of suspicious IMN based on PET/CT, and 20 IMNs (80%) were cytologically proven metastases form the primary breast malignancies. The results indicated a very high likelihood of malignant involvement of FDG avid IMNs. In another recently reported study by Koolen et al[41], pre-chemotherapy FDG PET/CT scans identified IMN nodal lesions in 17 of 278 patients with breast cancer. The results show that FDG PET/CT contributes to pretreatment staging and therapy planning, upstaging substantial proportion of patients to the high risk group, thereby potentially changing prognosis and possibly implicating postoperative irradiation.
In conclusion, there is a significant disagreement in the oncologic community regarding the management of IMNs in breast cancer, most likely due to the difficulties in assessment of IMN and conflicting evidence of significance of IMN treatment. Lymphoscintigraphy may help to identify the IMN drainage, but the IMN sampling or dissection is much more difficult than for axillary nodes, and only a small portion of hot sentinel IMNs are positive for metastases on pathological studies. As a non-invasive imaging modality, FDG PET/CT plays a role in identification of positive IMN, which may renew the oncologists’ interest in IMN management. But to date, the data regarding FDG PET/CT for IMN in breast cancer are very limited, and more clinical studies are warranted.
CASE EXAMPLES
Figure 1 illustrate the role of FDG PET/CT in locoregional nodal staging of breast cancer. In the case 1 and 2 (Figure 1A and B), FDG PET/CT was positive in the axillae. The patients avoided SLNB and directly had ALND. Pathology confirmed true-positive PET/CT findings.
Figure 1.
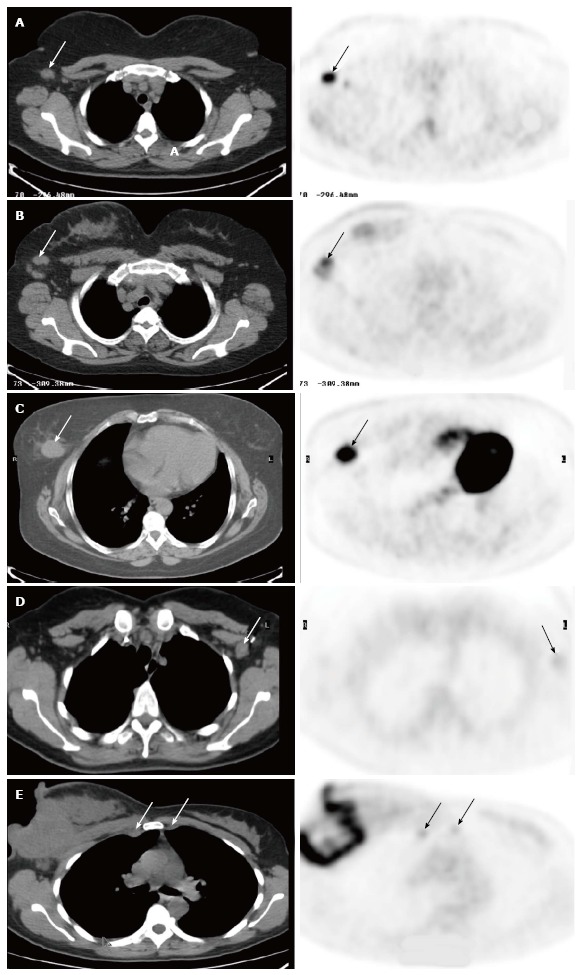
Fluorodeoxyglucose positron emission tomography/computed tomography. A: In a 61-year-old woman with newly diagnosed breast cancer. There was a single palpable axillary lymph node on physical examination. The images demonstrated a 1.5 cm axillary node with intense uptake (SUV 10, arrows), consistent with metastasis. There were also additional smaller axillary nodes with abnormal uptake. The patient underwent axillary lymph node dissection (ALND), and surgical pathology revealed that two axillary nodes were positive; B: In a 51-year-old woman with newly diagnosed right breast cancer. There were palpable right axillary lymph nodes on physical examination. The images demonstrated a few enlarged right axillary nodes with intense uptake (SUV 6.5, arrows). The patient had ALND, which showed three metastatic lymph nodes; C: In a 54-year-old woman with newly diagnosed right breast cancer. The images showed a 3.0 cm fluorodeoxyglucose (FDG) avid tumor in the right breast (arrows), but were negative in the right axilla. The patient had lymphscintigraphy for sentinel lymph node biopsy, which was negative for axillary metastasis; D: In a 40-year-old woman with history of left breast cancer and post lumpectomy. On physical examination, there was a palpable lymph node in the left axilla. Positron emission tomography/computed tomography (PET/CT) showed a 1.5 cm left axillary lymph node with very mild uptake (SUV 1.4, arrows), consistent with a benign etiology. Subsequent SLNB confirmed lymphadenitis; E: FDG PET/CT in a 41-year-old woman with newly diagnosed inflammatory carcinoma of the right breast. In addition to large right breast necrotic mass and axillary nodal lesions, there are a 1.2 cm right internal mammary node (IMN) and a 1.0 cm left IMN, and both are mildly FDG avid (SUV 3.2) and suspicious for IMN metastases (arrows). The patient was excluded as a surgical candidate based on FDG PET/CT findings. She received chemotherapy and radiation. SUV: Standardized uptake value.
FDG PET/CT was negative in the axilla in the case 3 (Figure 1C), and suggested a benign axillary lymph node in the case 4 (Figure 1D). In spite of negative PET/CT results, both patients had SLNB due to large primary tumor (the case 3) or palpable axillary lymph node (the case 4). SLNB demonstrated negative axillary disease consistent with PET/CT findings.
In the Case 5 (Figure 1E), FDG PET/CT identified positive IMNs, which updated staging, and subsequently changed the patients’ managements.
CONCLUSION
Although FDG PET/CT is valuable for detecting distant metastasis, identifying recurrence and evaluating response to chemotherapy, the role of FDG PET/CT in evaluating locoregional nodal status for initial staging of breast cancer has not yet been well-defined in clinical practice. FDG PET/CT is not recommended as a routine imaging modality for initial staging of early breast cancer[42], although the direct scientific evidence to support this recommendation is limited. FDG PET/CT has high specificity but compromised sensitivity for identifying axillary nodal disease in breast cancer. Positive axillary FDG PET/CT is a good predictor of axillary disease and correlates well with SLNB. FDG PET/CT may help to identify patients with high axillary lymph node burden who could then move directly to ALND and would not require the additional step of SLNB. However, FDG PET/CT cannot replace SLNB or ALND due to unsatisfactory sensitivity. The spatial resolution of PET instruments precludes the detection of small nodal metastases.
There are substantial disparities in regard to the significance of IMN in breast cancer, and evaluation of IMN status is difficult in practice. Limited Data suggested that FDG PET/CT has advantages over conventional modalities in detecting and uncovering occult extra-axillary especially IMN lesions with upstaging the disease and an impact on the adjuvant management.
Positive FDG PET/CT is highly predictive for locoregional nodal disease in locally advanced breast cancer. Although the indications and role in initial staging of breast cancer remain to be validated, FDG PET/CT can be used in concert with other imaging modalities especially for patients with high risk.
Footnotes
P- Reviewer: Hernanz F, Jun Y, Mentes O, Streckfus CF S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
References
- 1.Robertson IJ, Hand F, Kell MR. FDG-PET/CT in the staging of local/regional metastases in breast cancer. Breast. 2011;20:491–494. doi: 10.1016/j.breast.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Riegger C, Herrmann J, Nagarajah J, Hecktor J, Kuemmel S, Otterbach F, Hahn S, Bockisch A, Lauenstein T, Antoch G, et al. Whole-body FDG PET/CT is more accurate than conventional imaging for staging primary breast cancer patients. Eur J Nucl Med Mol Imaging. 2012;39:852–863. doi: 10.1007/s00259-012-2077-0. [DOI] [PubMed] [Google Scholar]
- 3.Koolen BB, Valdés Olmos RA, Elkhuizen PH, Vogel WV, Vrancken Peeters MJ, Rodenhuis S, Rutgers EJ. Locoregional lymph node involvement on 18F-FDG PET/CT in breast cancer patients scheduled for neoadjuvant chemotherapy. Breast Cancer Res Treat. 2012;135:231–240. doi: 10.1007/s10549-012-2179-1. [DOI] [PubMed] [Google Scholar]
- 4.Evangelista L, Panunzio A, Cervino AR, Vinante L, Al-Nahhas A, Rubello D, Muzzio PC, Polverosi R. Indeterminate pulmonary nodules on CT images in breast cancer patient: the additional value of 18F-FDG PET/CT. J Med Imaging Radiat Oncol. 2012;56:417–424. doi: 10.1111/j.1754-9485.2012.02408.x. [DOI] [PubMed] [Google Scholar]
- 5.Saez RA, McGuire WL, Clark GM. Prognostic factors in breast cancer. Semin Surg Oncol. 1989;5:102–110. doi: 10.1002/ssu.2980050206. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Ghesani NV, Zuckier LS. Physiology and pathophysiology of incidental findings detected on FDG-PET scintigraphy. Semin Nucl Med. 2010;40:294–315. doi: 10.1053/j.semnuclmed.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Groheux D, Espié M, Giacchetti S, Hindié E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266:388–405. doi: 10.1148/radiol.12110853. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH. Radionuclide methods for breast cancer staging. Semin Nucl Med. 2013;43:294–298. doi: 10.1053/j.semnuclmed.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U, De Cicco C, Galimberti VE, Fernandez JR, Rotmensz N, Viale G, Spano G, Luini A, Intra M, Veronesi P, et al. A comparative study on the value of FDG-PET and sentinel node biopsy to identify occult axillary metastases. Ann Oncol. 2007;18:473–478. doi: 10.1093/annonc/mdl425. [DOI] [PubMed] [Google Scholar]
- 10.Wahl RL, Siegel BA, Coleman RE, Gatsonis CG. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging breast cancer with PET Study Group. J Clin Oncol. 2004;22:277–285. doi: 10.1200/JCO.2004.04.148. [DOI] [PubMed] [Google Scholar]
- 11.Ueda S, Tsuda H, Asakawa H, Omata J, Fukatsu K, Kondo N, Kondo T, Hama Y, Tamura K, Ishida J, et al. Utility of 18F-fluoro-deoxyglucose emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in combination with ultrasonography for axillary staging in primary breast cancer. BMC Cancer. 2008;8:165. doi: 10.1186/1471-2407-8-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Lee J, Chang E, Kim S, Suh K, Sul J, Song I, Kim Y, Lee C. Selective Sentinel Node Plus Additional Non-Sentinel Node Biopsy Based on an FDG-PET/CT Scan in Early Breast Cancer Patients: Single Institutional Experience. World J Surg. 2009;33:943–949. doi: 10.1007/s00268-009-9955-z. [DOI] [PubMed] [Google Scholar]
- 13.Choi YJ, Shin YD, Kang YH, Lee MS, Lee MK, Cho BS, Kang YJ, Park JS. The Effects of Preoperative (18)F-FDG PET/CT in Breast Cancer Patients in Comparison to the Conventional Imaging Study. J Breast Cancer. 2012;15:441–448. doi: 10.4048/jbc.2012.15.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper KL, Meng Y, Harnan S, Ward SE, Fitzgerald P, Papaioannou D, Wyld L, Ingram C, Wilkinson ID, Lorenz E. Positron emission tomography (PET) and magnetic resonance imaging (MRI) for the assessment of axillary lymph node metastases in early breast cancer: systematic review and economic evaluation. Health Technol Assess. 2011;15:iii–iv, 1-134. doi: 10.3310/hta15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourgeois AC, Warren LA, Chang TT, Embry S, Hudson K, Bradley YC. Role of positron emission tomography/computed tomography in breast cancer. Radiol Clin North Am. 2013;51:781–798. doi: 10.1016/j.rcl.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Groheux D, Giacchetti S, Moretti JL, Porcher R, Espié M, Lehmann-Che J, de Roquancourt A, Hamy AS, Cuvier C, Vercellino L, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–435. doi: 10.1007/s00259-010-1640-9. [DOI] [PubMed] [Google Scholar]
- 17.Heusner TA, Kuemmel S, Hahn S, Koeninger A, Otterbach F, Hamami ME, Kimmig KR, Forsting M, Bockisch A, Antoch G, et al. Diagnostic value of full-dose FDG PET/CT for axillary lymph node staging in breast cancer patients. Eur J Nucl Med Mol Imaging. 2009;36:1543–1550. doi: 10.1007/s00259-009-1145-6. [DOI] [PubMed] [Google Scholar]
- 18.Pritchard KI, Julian JA, Holloway CM, McCready D, Gulenchyn KY, George R, Hodgson N, Lovrics P, Perera F, Elavathil L, et al. Prospective study of 2-[¹⁸F]fluorodeoxyglucose positron emission tomography in the assessment of regional nodal spread of disease in patients with breast cancer: an Ontario clinical oncology group study. J Clin Oncol. 2012;30:1274–1279. doi: 10.1200/JCO.2011.38.1103. [DOI] [PubMed] [Google Scholar]
- 19.Uren RF, Howman-Giles R, Renwick SB, Gillett D. Lymphatic mapping of the breast: locating the sentinel lymph nodes. World J Surg. 2001;25:789–793. doi: 10.1007/s00268-001-0006-7. [DOI] [PubMed] [Google Scholar]
- 20.Singletary SE, Greene FL. Revision of breast cancer staging: the 6th edition of the TNM Classification. Semin Surg Oncol. 2003;21:53–59. doi: 10.1002/ssu.10021. [DOI] [PubMed] [Google Scholar]
- 21.Hindié E, Groheux D, Brenot-Rossi I, Rubello D, Moretti JL, Espié M. The sentinel node procedure in breast cancer: nuclear medicine as the starting point. J Nucl Med. 2011;52:405–414. doi: 10.2967/jnumed.110.081711. [DOI] [PubMed] [Google Scholar]
- 22.Moncayo VM, Aarsvold JN, Grant SF, Bartley SC, Alazraki NP. Status of sentinel lymph node for breast cancer. Semin Nucl Med. 2013;43:281–293. doi: 10.1053/j.semnuclmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Huang O, Wang L, Shen K, Lin H, Hu Z, Liu G, Wu J, Lu J, Shao Z, Han Q, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008;107:379–387. doi: 10.1007/s10549-007-9561-4. [DOI] [PubMed] [Google Scholar]
- 24.Yao MS, Kurland BF, Smith AH, Schubert EK, Dunnwald LK, Byrd DR, Mankoff DA. Internal mammary nodal chain drainage is a prognostic indicator in axillary node-positive breast cancer. Ann Surg Oncol. 2007;14:2985–2993. doi: 10.1245/s10434-007-9473-x. [DOI] [PubMed] [Google Scholar]
- 25.Byrd DR, Dunnwald LK, Mankoff DA. Internal mammary lymph node drainage patterns in patients with breast cancer documented by breast lymphoscintigraphy. Ann Surg Oncol. 2001;8:234–240. doi: 10.1007/s10434-001-0234-y. [DOI] [PubMed] [Google Scholar]
- 26.Sugg SL, Ferguson DJ, Posner MC, Heimann R. Should internal mammary nodes be sampled in the sentinel lymph node era? Ann Surg Oncol. 2000;7:188–192. doi: 10.1007/BF02523652. [DOI] [PubMed] [Google Scholar]
- 27.Brito RA, Valero V, Buzdar AU, Booser DJ, Ames F, Strom E, Ross M, Theriault RL, Frye D, Kau SW, et al. Long-term results of combined-modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: The University of Texas M.D. Anderson Cancer Center experience. J Clin Oncol. 2001;19:628–633. doi: 10.1200/JCO.2001.19.3.628. [DOI] [PubMed] [Google Scholar]
- 28.Newman LA, Kuerer HM, Fornage B, Mirza N, Hunt KK, Ross MI. Adverse prognostic significance of infraclavicular lymph nodes detected by ultrasonography in patients with locally advanced breast cancer. Am J Surg. 2001;181:313–318. doi: 10.1016/s0002-9610(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 29.Madsen E, Gobardhan P, Bongers V, Albregts M, Burgmans J, De Hooge P, Van Gorp J, van Dalen T. The impact on post-surgical treatment of sentinel lymph node biopsy of internal mammary lymph nodes in patients with breast cancer. Ann Surg Oncol. 2007;14:1486–1492. doi: 10.1245/s10434-006-9230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellon JR, Livingston RB, Eubank WB, Gralow JR, Ellis GK, Dunnwalk LK. Evaluation of the internal mammary lymph nodes by FDG-PET in locally advanced breast cancer (LABC) Am J Clin Oncol. 2004;27:407–410. doi: 10.1097/01.coc.0000128869.19357.9b. [DOI] [PubMed] [Google Scholar]
- 31.Kroman N, Wohlfahrt J, Mouridsen HT, Melbye M. Influence of tumor location on breast cancer prognosis. Int J Cancer. 2003;105:542–545. doi: 10.1002/ijc.11116. [DOI] [PubMed] [Google Scholar]
- 32.Colleoni M, Zahrieh D, Gelber RD, Holmberg SB, Mattsson JE, Rudenstam CM, Lindtner J, Erzen D, Snyder R, Collins J, et al. Site of primary tumor has a prognostic role in operable breast cancer: the international breast cancer study group experience. J Clin Oncol. 2005;23:1390–1400. doi: 10.1200/JCO.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 33.Chen RC, Lin NU, Golshan M, Harris JR, Bellon JR. Internal mammary nodes in breast cancer: diagnosis and implications for patient management -- a systematic review. J Clin Oncol. 2008;26:4981–4989. doi: 10.1200/JCO.2008.17.4862. [DOI] [PubMed] [Google Scholar]
- 34.Estourgie SH, Nieweg OE, Olmos RA, Rutgers EJ, Kroon BB. Lymphatic drainage patterns from the breast. Ann Surg. 2004;239:232–237. doi: 10.1097/01.sla.0000109156.26378.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veronesi U, Arnone P, Veronesi P, Galimberti V, Luini A, Rotmensz N, Botteri E, Ivaldi GB, Leonardi MC, Viale G, et al. The value of radiotherapy on metastatic internal mammary nodes in breast cancer. Results on a large series. Ann Oncol. 2008;19:1553–1560. doi: 10.1093/annonc/mdn183. [DOI] [PubMed] [Google Scholar]
- 36.Heuts EM, van der Ent FW, von Meyenfeldt MF, Voogd AC. Internal mammary lymph drainage and sentinel node biopsy in breast cancer - A study on 1008 patients. Eur J Surg Oncol. 2009;35:252–257. doi: 10.1016/j.ejso.2008.06.1493. [DOI] [PubMed] [Google Scholar]
- 37.Eubank WB, Mankoff DA, Takasugi J, Vesselle H, Eary JF, Shanley TJ, Gralow JR, Charlop A, Ellis GK, Lindsley KL, et al. 18fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001;19:3516–3523. doi: 10.1200/JCO.2001.19.15.3516. [DOI] [PubMed] [Google Scholar]
- 38.Aukema TS, Straver ME, Peeters MJ, Russell NS, Gilhuijs KG, Vogel WV, Rutgers EJ, Olmos RA. Detection of extra-axillary lymph node involvement with FDG PET/CT in patients with stage II-III breast cancer. Eur J Cancer. 2010;46:3205–3210. doi: 10.1016/j.ejca.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 39.Bernsdorf M, Berthelsen AK, Wielenga VT, Kroman N, Teilum D, Binderup T, Tange UB, Andersson M, Kjær A, Loft A, et al. Preoperative PET/CT in early-stage breast cancer. Ann Oncol. 2012;23:2277–2282. doi: 10.1093/annonc/mds002. [DOI] [PubMed] [Google Scholar]
- 40.Wang CL, Eissa MJ, Rogers JV, Aravkin AY, Porter BA, Beatty JD. (18)F-FDG PET/CT-positive internal mammary lymph nodes: pathologic correlation by ultrasound-guided fine-needle aspiration and assessment of associated risk factors. AJR Am J Roentgenol. 2013;200:1138–1144. doi: 10.2214/AJR.12.8754. [DOI] [PubMed] [Google Scholar]
- 41.Koolen BB, Valdés Olmos RA, Vogel WV, Vrancken Peeters MJ, Rodenhuis S, Rutgers EJ, Elkhuizen PH. Pre-chemotherapy 18F-FDG PET/CT upstages nodal stage in stage II-III breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2013;141:249–254. doi: 10.1007/s10549-013-2678-8. [DOI] [PubMed] [Google Scholar]
- 42.Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, Coleman RE, Wahl R, Paschold JC, Avril N, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]


