Abstract
Respiratory chain deficiencies exhibit a wide variety of clinical phenotypes resulting from defective mitochondrial energy production through oxidative phosphorylation. These defects can be caused by either mutations in the mtDNA or mutations in nuclear genes coding for mitochondrial proteins. The underlying pathomechanisms can affect numerous pathways involved in mitochondrial physiology. By whole-exome and candidate gene sequencing, we identified 11 individuals from 9 families carrying compound heterozygous or homozygous mutations in GTPBP3, encoding the mitochondrial GTP-binding protein 3. Affected individuals from eight out of nine families presented with combined respiratory chain complex deficiencies in skeletal muscle. Mutations in GTPBP3 are associated with a severe mitochondrial translation defect, consistent with the predicted function of the protein in catalyzing the formation of 5-taurinomethyluridine (τm5U) in the anticodon wobble position of five mitochondrial tRNAs. All case subjects presented with lactic acidosis and nine developed hypertrophic cardiomyopathy. In contrast to individuals with mutations in MTO1, the protein product of which is predicted to participate in the generation of the same modification, most individuals with GTPBP3 mutations developed neurological symptoms and MRI involvement of thalamus, putamen, and brainstem resembling Leigh syndrome. Our study of a mitochondrial translation disorder points toward the importance of posttranscriptional modification of mitochondrial tRNAs for proper mitochondrial function.
Main Text
Defects of the mitochondrial respiratory chain underlie a diverse group of human disorders characterized by impaired oxidative phosphorylation (OXPHOS). The generation of a functional respiratory chain requires the coordinated expression of both the nuclear genome and mitochondrial DNA (mtDNA). Defective translation of mtDNA-encoded proteins, caused by mutations in either the mitochondrial or nuclear genomes, represents a rapidly expanding group of human disorders, which often manifest as severe infantile combined OXPHOS deficiencies.1 The mitochondrial genome contains a total of 37 genes, 13 of which encode protein subunits of the respiratory chain complexes and the ATP synthase. Translation of these genes is achieved by the organelle’s own protein synthesis machinery, of which only the RNA components (rRNAs and tRNAs) are encoded by mtDNA. All protein factors required for mitochondrial translation are encoded in the nucleus and must be imported after their synthesis in the cytoplasm. Mitochondrial (mt-) tRNAs require extensive posttranscriptional modifications before achieving translation competency. Modifications to tRNAs might contribute to their proper folding, stability, or decoding capacity. In mitochondria a minimal set of 22 different tRNAs is used to translate all codons.2 Modifications to the wobble position of the anticodon loop of mt-tRNAs play an important role in ensuring correct mRNA-tRNA interactions. In ten mt-tRNA species, all of which correspond to two codon sets, four different types of modified nucleotides have been identified at the wobble position.3,4 One of these modifications is 5-taurinomethyluridine (τm5U), found at position 34 (U34) of mt-tRNAsLeuUUR, Trp, Gln, Lys, and Glu, which has been suggested to be synthesized cooperatively by GTPBP3 and MTO1.5 In addition to τm5U, mt-tRNAs Gln, Lys, and Glu also contain a 2-thiouridine modification at U34 (s2U), introduced by TRMU (also known as MTU1). This results in a 5-taurinomethyl-2-thiouridine (τm5s2U) modification in these mt-tRNA molecules. Modifications of U34 have been proposed to modulate either the accuracy or the efficiency of translation.6,7 Three types of mutations affecting U34 have been associated with human mitochondrial disease: (1) mutations in the mt-tRNAs;8 (2) mutations in TRMU (MIM 610230) affecting U34 2-thiouridylation and leading to acute infantile liver failure resulting from combined OXPHOS deficiency;9 and (3) more recently, mutations in MTO1 (MIM 614667) found to underlie cases of hypertrophic cardiomyopathy and lactic acidosis, associated with impaired mitochondrial translation rate and reduced respiratory chain activities.10,11
Whole-exome sequencing (WES) of 790 individuals with suspected mitochondriopathy in five centers identified eight index case subjects (plus two affected siblings) with homozygous or two heterozygous rare variants (minor allele frequency < 0.1%) in GTPBP3 (MIM 608536), with no such case being found in 11,295 control subjects. This presents a genome-wide significant enrichment in GTPBP3 (RefSeq accession number NM_032620.3) mutation load in samples from individuals with the clinical diagnosis “mitochondrial disease” (p < 3.2 × 10−10, Fisher exact test) in comparison to nonmitochondrial disorder samples. In addition, when filtering for genes coding for mitochondrial proteins,12 in several individuals GTPBP3 was the only gene with two mutations. Further evidence for the pathogenic role of GTPBP3 mutations was derived from follow-up candidate gene sequencing of 18 individuals with similar phenotypes, which identified two more index cases. Collectively, mutations in GTPBP3 were detected in 12 individuals from 10 families. However, segregation analysis of a single affected individual (#66654) revealed that the two identified heterozygous mutations in GTPBP3 affected the same allele, leaving genetic evidence about 11 individuals from 9 families (Figure 1).
Figure 1.
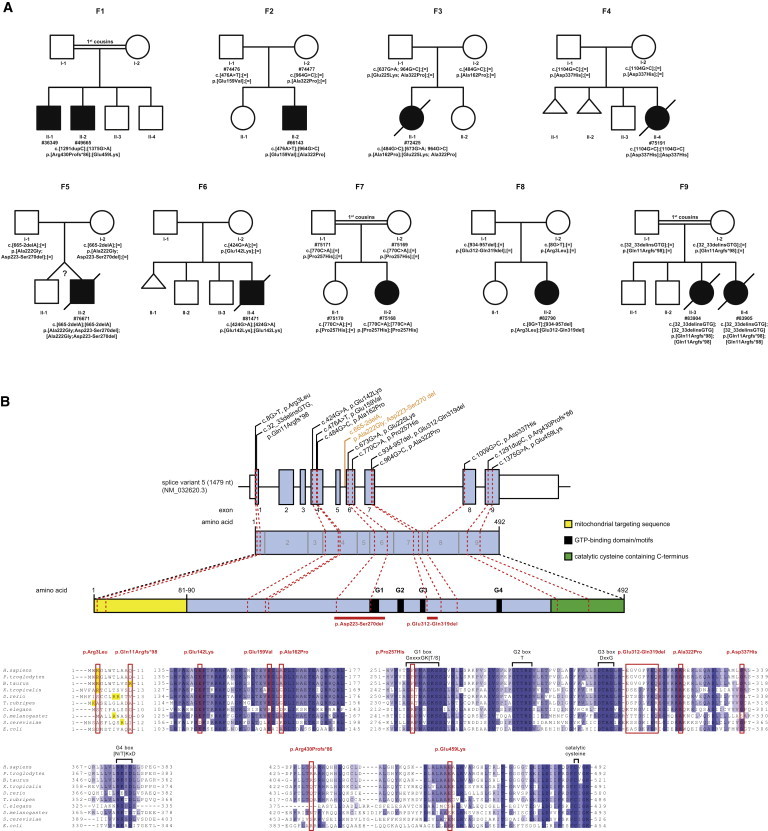
GTPBP3 Mutation Status and Gene Structure
(A) Pedigrees of nine families with mutations in GTPBP3.
(B) Gene structure of GTPBP3 with known protein domains of the gene product and localization and conservation of amino acid residues affected by mutations. Black and orange text indicate exonic and intronic variants. Intronic regions are not drawn to scale. Coloring in the sequence alignment represents the identity of amino acid residues.
Written informed consent was obtained from all individuals investigated or their guardians, and the ethics committee of the Technische Universität München approved the study.
Individual #49665 (family F1, Figure 1A) is a boy born to consanguineous parents from the UAE. He presented at the age of 10 years with mild intellectual disability, fatigability, mild hypertrophic cardiomyopathy, and visual impairment. At presentation he measured 134 cm with a body weight of 25 kg. Clinical examination revealed slight dyspnea when climbing stairs and mild intellectual disability. Plasma lactate was consistently elevated (3.0 to 7.2 mmol/l, reference < 2.1 mmol/l). Electroencephalogram, hearing test, and visual-evoked potentials showed no abnormalities. Electrocardiography (ECG) revealed signs of left ventricular hypertrophy confirmed by echocardiography. There was no obstruction of the left ventricular outflow tract. He had a pale optic disc on both sides but visual acuity and visual field could not be examined. Brain MRI was normal, but MR spectroscopy revealed lactate peaks in the parietal and precentral cortex. Respiratory chain (RC) measurement in muscle revealed a significant reduction of complex I and IV activities. He was substituted with CoQ10 (200 mg/day), riboflavin (400 mg/day), carnitine (1 g/day), and a fat-rich diet (60% of daily caloric intake). A follow-up examination 1 year after the initial presentation showed no significant changes of his clinical signs/symptoms.
His 17-year-old elder brother, individual #36349 (family F1, Figure 1A), had a very similar clinical picture.
Individual #66143 (family F2, Figure 1A), a boy, is the second child of healthy unrelated parents of Arab-Moslem origin from Israel. He presented at the age of 2 years with sudden respiratory failure. Heart ultrasonography indicated a hypertrophic cardiomyopathy and congestive heart failure. His cardiac symptoms improved on treatment with furosemide, spironolactone, carvedilole, and digoxin. In addition, a high-dose vitamin treatment (100 mg/day riboflavin, 100 mg/day vitamin B1, and 60 mg/day CoQ10) was initiated. RC enzyme measurement in muscle revealed a significant reduction of complex I and IV activities. On follow-up examinations (over 3 years), the child’s psychomotor development is normal and his parents reported that he is active like his peers. Digoxin and spironolacton treatment was stopped and his recent echocardiography revealed a stable condition of the heart including normal global function of left ventricle with no further hypertrophy of interventricular septum and no pulmonary hypertension.
Individual #72425 (family F3, Figure 1A) was a girl born to unrelated parents. At 3 months of age, she had feeding difficulties and failure to thrive. At the age of 7 months, she developed recurrent cough and fever and was admitted to the emergency room with severe fatigue, pallor, and progressive malaise. Blood exams showed leukocytosis, and 2 days later her general condition worsened, showing cyanosis and hyporeactivity. Echocardiography showed severe dilated cardiomyopathy with an ejection fraction of 20% that was unresponsive to therapy. She had severe refractory hyperlactatemia (23.3 mmol/l, reference range 0.5–2.3 mmol/l). Histochemical and spectrophotometric analysis of the muscle biopsy showed a severe complex IV deficiency. She died 10 days after admission from cardiac failure.
Individual #75191 (family F4, Figure 1A), a girl, was born to nonconsanguineous parents after an uneventful pregnancy of 40 weeks. The mother had had two miscarriages at 6 and 8 weeks and had a healthy son aged 16 months. In the first hours after birth, individual #75191 developed mild stridor and dyspnea which rapidly worsened. She fed poorly and became less responsive, and a Kussmaul breathing pattern was seen. She was transferred to a specialist center and was found to be severely hypotonic, moving very little, either spontaneously or after stimulation. She had hyperlactatemia (23 mmol/l), hypoglycemia (18 mg/dl), hyperammonemia (135 μmol/l, control value 11–48 μmol/l), and hyperlactaturia. She progressively developed respiratory insufficiency and bradycardia. Cardiac ultrasound showed apical right ventricular hypertrophy and an open duct of Botalli with minor shunting. Fractional shortening was 28% (mildly decreased). Cerebral ultrasound showed a minimal grade I bleeding, and the cerebral matter appeared mildly hyperechogenic. She died of asystolia at day 1. A muscle biopsy performed immediately after death showed decreased activities of RC complexes I and IV.
Individual #76671 (family F5, Figure 1A) was the second boy of nonconsanguineous parents. The infant was born at 41 weeks of gestation from a twin pregnancy. Generalized hypotonia and difficulty in suction was noted since birth and he rapidly developed failure to thrive. He acquired head control at the age of 7 months but parents reported normal cognitive skills. At the age of 9 months he was admitted to the intensive care unit for acute aspiration pneumonia that required intubation. Laboratory test revealed a metabolic acidosis with hyperlactatemia (5.2 mmol/l) and brain MRI showed bilateral thalamic T2-weighted hyperintense abnormalities with low diffusion. Analysis of a muscle biopsy revaled a clear reduction in histochemical cytochrome c oxidase activity and decreased complex I and IV enzyme activities. The cardiological examination disclosed hypertrophic cardiomyopathy and a Wolff-Parkinson-White pre-excitation syndrome (MIM 194200). The baby died after 15 days of hospitalization with clinical signs of heart failure.
Individual #81471 (family F6, Figure 1A) was a boy born to nonconsanguineous Romanian parents at 34 weeks gestation (birth weight 2.18 kg). His mother had premature and prolonged (85 hr) rupture of membranes before delivery, and the baby was treated with i.v. antibiotics before being discharged home on day 7. He was readmitted to hospital on day 25 with weight loss (2.23 kg). He was hypothermic and jaundiced and initial blood analysis showed profound metabolic acidosis. He was treated with i.v. antibiotics for presumed sepsis. The acidosis did not resolve, and serum lactate was elevated (11.0 mmol/l). ECG was abnormal and echocardiography showed concentric left ventricular hypertrophy. CSF lactate was 12.4 mmol/l (normal range 0.9–2.4 mmol/l) prompting bicarbonate treatment. Brain MRI showed abnormal diffusion of the subthalamic nuclei extending down to the brain stem. There was abnormal T2 signal in the midbrain and basal ganglia bilaterally. On examination he was thin but not dysmorphic. He was mildly jaundiced and had puffy feet. There was little spontaneous movement but normal muscle bulk and he was distinctly hypotonic. Feeding through a nasogastric tube was established but he did not become responsive despite high caloric intake. He developed recurrent apnea and died aged 5 weeks. Biochemical analysis performed in muscle revealed a significant decrease of RC complexes I and IV.
Individual #75168 (family F7, Figure 1A) is the second girl of first-cousin parents from India. She was first seen at the age of 2 years with development delay. She was able to walk but she couldn’t speak. She received occupational and speech therapy. During a febrile illness when she was 3 years old, she had an acute metabolic failure with hyperlactatemia and hyperlactatorachia. She recovered but had epileptic seizures and more severe intellectual disability. Brain MRI showed pronounced bilateral hyperintensities affecting the whole thalamus and extending to the mesencephalon. Hyperlactatemia (>10 mmol/l) and hyperlactatorachia (6 mmol/l) were noticed. RC activity in muscle was normal as well as PDH complex tested by immunoblot. The girl was treated with qa carnitine 3 × 350 mg/day, CoQ10 3 × 50 mg/day, vitamins B1 3 × 50 mg/day and B6 3 × 50 mg/day, and bicarbonate 4 × 1 g. Epilepsy was in good control with levetiracetame 40 mg/kg/day and a high-fat diet. The girl is in a special school for children with developmental delay. Her general condition is good. She is always in a good temper. Development is delayed about 1.5 years. She has continual hyperlactatemia (8–10 mmol/l).
Individual #82790 (family F8, Figure 1A) is a girl born at 40 weeks of gestation with normal birth weight to nonconsanguineous Japanese parents. At the age of 1 year, she developed frequent epileptic seizures, and she was medicated with phenobarbital. Severe developmental delay was noted and at the age of 15 months she was admitted to children’s hospital. Her weight gain (9.25 kg, −0.06 SD) is within the normal range, but she developed severe muscle hypotonia. There is no cardiac involvement by ECG and echocardiogram. Hyperlactatemia was noted (5.72–6.49 mmol/l) whereas metabolic profiling of amino acids, urinary organic acids, and acylcarnitine was normal. RC analysis in muscle showed a significant decrease in complexes I and IV activities. Brain MRI showed bilateral hyperintensities in the putamen and weakly also in the anterior thalamus. A lactate peak was detected on [H+]-MR spectroscopy. She is now 2 years of age and still presents with a severe global developmental delay.
Individual #83904 (family F9, Figure 1A) was the second child of consanguineous, healthy parents of Turkish origin. She was born at 39 weeks of gestational age (birth weight 2,740 g, length 49 cm, head circumference 32 cm). Shortly after birth, she presented with Wolff-Parkinson-White syndrome. Cardiac ultrasound was normal. Treatment was started with amiodarone and she remained stable, without cardiac symptoms or arrhythmia. At 7 months of age, she had cardiogenic shock and metabolic acidosis. Heart ultrasound detected dilated cardiomyopathy and decreased contractility (ejection fraction 35%). She presented hyperlactatemia (20 mmol/l), hyperalaninemia (1,175 μmol/l; normal range, 190–450 μmol/l), and an increased lactate-to-pyruvate ratio (47; normal range, 10–20). Her disorder progressed despite intensive medication for heart failure. She died at the age of 9 months of cardiac insufficiency with arrhythmia.
Her younger sister, individual #83905 (family F9, Figure 1A), had a very similar clinical picture. She died at 6 months of age of cardiac insufficiency unresponsive to resuscitation procedures.
Genetic, biochemical, and clinical findings are summarized in Table 1. Pedigrees of the families we studied are shown in Figure 1A. The location of the identified mutations within the gene and the conservation of the affected amino acid (aa) residues are shown in Figure 1B. Individual #49665 (F1: II-2) was found to carry a frame shift and one missense variant. The next generation sequencing (NGS) data demonstrated a compound heterozygous status of the two variants (Figure S1 available online). Individual #76671 (F5: II-2) was homozygous for an intronic single base pair deletion, c.665−2delA, which is predicted to cause the loss of a splice acceptor site. Analysis of cDNA from fibroblasts revealed a shorter transcript, and sequencing found that in more than 95% of transcripts, the downstream acceptor of exon 7 was used for splicing, resulting in the skipping of exon 6 including the conserved G1-box guanine nucleotide-binding signature motif (Figure S2). Individual #82790 (F8: II-2) was found to be compound heterozygous for a missense mutation c.8G>T (p.Arg3Leu) and a 24 bp deletion c.934_957del (p.Gly312_Val319del). The 24 bp deletion is predicted to cause the deletion of 8 amino acids containing conserved residues. The p.Arg3Leu substitution at the very N terminus of the protein is scored as a predicted polymorphism but causes a loss of a positively charged residue, which is predicted to interfere with mitochondrial targeting (Predotar, PsortII). The two missense variants found in individual #66654, c.[673G>A; 964G>C], p.[Glu225Lys; Ala322Pro], were identical to the variants found on the paternal allele of individual #72425 (F3: II-1). Analysis of parental DNA revealed that both variants were also located on the same allele in individual #66654, meaning that only one allele is affected. Because of this observation, combined with the absence of a heart phenotype and because this individual is the only one exhibiting an isolated complex I defect, we consider the mutations found in GTPBP3 not to be causative in subject #66654.
Table 1.
Genetic and Clinical Findings in Individuals with GTPBP3 Mutations
| ID | Sex |
GTPBP3 Mutations |
OXPHOS Activities in Skeletal Muscle |
Clinical Features |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| cDNA (NM_032620.3) and Protein (NP_116009.2) | RCC | % of Lower Control Range | Absolute Values | Reference Range | AO | Course | HCM | Histochemical COX Defect | Other Features | ||
| #49665a,b | male | c.[1291dupC; 1375G>A], p.[Pro430Argfs∗86; Glu459Lys] | I | 15% | 0.025 | 0.17–0.56 | 10 years | alive 14 years | yes | ND | consanguineous parents (1st cousins), mild intellectual disability, fatigability, limited vision, lactic acidosis |
| II | ND | ND | ND | ||||||||
| II+III | normal | 0.201 | 0.08–0.48 | ||||||||
| IV | 24% | 0.267 | 1.1–5.0 | ||||||||
| #36349b | male | c.[1291dupC; 1375G>A], p.[Pro430Argfs∗86; Glu459Lys] | I | no data | no data | no data | no data | alive 17 years | no data | no data | sibling of #49665 with similar clinical symptoms |
| II | |||||||||||
| II+III | |||||||||||
| IV | |||||||||||
| #66143a | male | c.[476A>T; 964G>C], p.[Glu159Val; Ala322Pro] | I | 7% | 0.01 | 0.19–0.48 | 2 years | alive 5 y ears | yes | ND | unrelated parents, sudden respiratory failure, lactic acidosis |
| II | normal | 0.10 | 0.07–0.12 | ||||||||
| II+III | normal | 0.12 | 0.09–0.22 | ||||||||
| IV | 28% | 0.12 | 0.44–0.92 | ||||||||
| #72425a | female | c.[484G>C; 673G>A; 964G>C], p.[Ala162Pro; Glu225Lys; Ala322Pro] | I | 14% | 0.015 | 0.11–0.30 | 3.5 months | died 8 months | DCM | yes | unrelated parents, cyanosis, hyporeactivity, DCM with residual ejection fraction of 20%, lactic acidosis |
| II | normal | 0.21 | 0.12–0.25 | ||||||||
| II+III | normal | 0.06 | 0.006–0.14 | ||||||||
| IV | 45% | 0.76 | 1.7–4.0 | ||||||||
| #75191a | female | c.[1009G>C; 1009G>C], p.[Asp337His; Asp337His] | I | 31% | 0.03 | 0.10–0.25 | birth | died 1 day | yes | yes | unrelated parents, Kussmaul breathing, stridor, hypotonic, hyporeactivity, RVH, lactid acidosis |
| II | normal | 0.16 | 0.14–0.25 | ||||||||
| II+III | normal | 0.12 | 0.13–0.25 | ||||||||
| IV | 15% | 0.09 | 0.60–1.48 | ||||||||
| #76671 | male | c.[665−2delA; 665−2delA], p.[Ala222Gly; Asp223_Ser270del; Ala222Gly; Asp223_Ser270del] | I | 45% | 0.05 | 0.11–0.30 | birth | died 10 months | yes | yes | unrelated parents, hypotonia from birth, RVH, WPW, lactic acidosis |
| II | normal | 0.16 | 0.12–0.25 | ||||||||
| II+III | ND | ND | 0.06–0.14 | ||||||||
| IV | 17% | 0.29 | 1.7–4.0 | ||||||||
| #81471a | male | c.[424G>A; 424G>A], p.[Glu142Lys; Glu142Lys] | I | 12% | 0.012 | 0.104 ± 0.036 | 4 weeks | died 5 weeks | yes | yes | consanguineous parents, two healthy siblings, one miscarriage, FTT, poor weight gain and feeding, concentric LVH, lactic acidosis |
| II | normal | 0.098 | 0.145 ± 0.047 | ||||||||
| II+III | normal | 0.850 | 0.544 ± 0.345 | ||||||||
| IV | 17% | 0.127 | 1.124 ± 0.511 | ||||||||
| #75168a | female | c.[770C>A; 770C>A], p.[Pro257His; Pro257His] | I | normal | no data | no data | 2 years | alive 5 years | no | ND | consanguineous parents (1st cousins), developmental delay, epileptic seizures, intellectual disability, MRI hyperintense lesions of basal ganglia typical to Leigh syndrome, lactic acidosis |
| II | normal | ||||||||||
| II+III | normal | ||||||||||
| IV | normal | ||||||||||
| #82790a | female | c.[8G>T; 934_957del], p.[Arg3Leu; Gly312_Val319del] | I | 36% | 0.107 | 0.301 ± 0.05 | 1 year | alive 2 years | no | ND | unrelated parents, seizures, severe hypotonia, developmental delay, lactic acidosis |
| II | normal | 0.424 | 0.272 ± 0.05 | ||||||||
| II+III | normal | 0.21 | 0.25 ± 0.093 | ||||||||
| IV | 21% | 0.008 | 0.035 ± 0.011 | ||||||||
| #83904a,c | female | c.[32_33delinsGTG; 32_33delinsGTG], p.[Gln11Argfs∗98; Gln11Argfs∗98] | I | 64% | 4.2 | 6.5–17 | 1 week | died 9 months | yes | ND | consanguineous parents (1st cousins), lactic acidosis, WPW |
| II | normal | 16.1 | 13.6–45.7 | ||||||||
| II+III | normal | 5.8 | 4.3–13.2 | ||||||||
| IV | 25% | 9.9 | 74–294 | ||||||||
| #83905a,c | female | c.[32_33delinsGTG; 32_33delinsGTG], p.[Gln11Argfs∗98; Gln11Argfs∗98] | I | no data | no data | no data | birth | died 10 days | yes | ND | consanguineous parents (1st cousins), lactic acidosis, WPW |
| II | |||||||||||
| II+III | |||||||||||
| IV | |||||||||||
| #66654a | female | c.[673G>A; 964G>A]; [=] p.[Glu255Lys; Ala322Pro]; [=] | I | 64% | 0.09 | 0.14–0.35 | 1.5 months | alive | no | ND | intrauterine growth retardation, lactic acidosis, leukodystrophy, generalized hypotonia |
| II | normal | 0.19 | 0.18–0.41 | ||||||||
| II+III | 90% | 0.27 | 0.30–0.67 | ||||||||
| IV | normal | 1.42 | 0.42–1.26 | ||||||||
Abbreviations are as follows: AO, age of onset; HCM, hypertrophic cardiomyopathy; DCM, dilated cardiomyopathy; FTT, failure to thrive; LVH/RVH, left/right ventricular hypertrophy; ND, not determined; WPW, Wolff-Parkinson-White syndrome.
Mitochondrial respiratory chain complexes (RCC) in muscle: I, NADH:CoQ-oxidoreductase; II, succinate:CoQ-oxidoreductase; II+III, succinate:cytochrome c reductase; IV, cytochrome c oxidase (COX).
Enzyme activities were determined in muscle biopsies and normalized to citrate synthase (CS). Absolute values and reference ranges are given in [mU / mU CS].
Investigated by exome sequencing.
These individuals are siblings.
In summary, the identification of 13 different alleles in 11 individuals with suspected mitochondrial disease from 9 families provides strong evidence for the pathological role of mutant GTPBP3 in the investigated families. It links GTPBP3 mutations to combined respiratory chain complex deficiency (9/11), cardiomyopathy (9/11), lactic acidosis (11/11), and encephalopathy (4/11).
Brain MRI was performed in three individuals (Figure 2). It showed bilateral T2 hyperintensities in the thalamus, ranging from weak (#82790) or small (#72425) changes in the anterior thalamus to very pronounced hyperintensities affecting the whole thalamus in individual #75168. In addition, T2 hyperintensities affected the putamen bilaterally in individual #82790 and extended markedly to the mesencephalon in individual #75168. Taken together, the MRI involvement of basal ganglia and brainstem resembles the (MRI) findings in Leigh syndrome (which is, however, an ill-defined entity).
Figure 2.
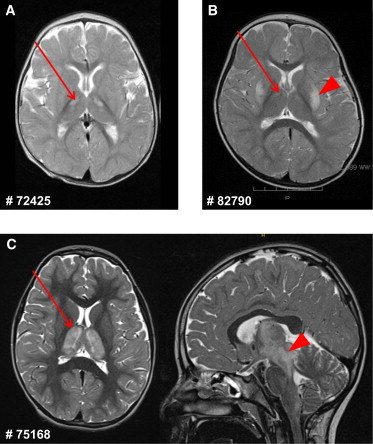
Brain MRI of Affected Individuals #72425, #82790, and #75168
(A) MRI of individual #72425 shows small T2 hyperintensities in the anterior thalamus bilaterally (arrow).
(B) In individual #82790, T2-weighted MRI shows bilateral hyperintensities in the putamen (arrowhead) and weakly also in the anterior thalamus (arrow).
(C) T2-weighted MRI of individual #75168 shows pronounced bilateral hyperintensities affecting the whole thalamus (arrow, axial view at the left) and extending to the mesencephalon (arrowhead, sagittal view at the right).
Skin fibroblast cell lines were available from seven individuals for functional studies. We first analyzed the cellular oxygen consumption rate (OCR13) by microscale respirometry with the XF96 extracellular flux analyzer (Seahorse Bioscience). When cells of individuals from families F1 to F5 were cultured in glucose-containing medium, only cell lines from individuals #75191 (F4: II-4) and #76671 (F5: II-2) showed a decreased OCR (of 59% and 58%, respectively) indicating defective oxidative phosphorylation (Figure 3A). When cells were cultured with galactose as the primary carbon source, rather than glucose, cells are forced to rely on oxidative phosphorylation rather than glycolysis in order to meet the energy demand.14,15 Accordingly, in control cells an increase in OCR of approximately 2-fold was observed when galactose was substituted as the primary carbon source. This increase in OCR was impaired in fibroblasts from affected individuals #49665 (F1: II-2), #66143 (F2: II-2), and #72425 (F3: II-1), which showed OCR increases of only 11%, 35%, and 22%, respectively (Figure 3B). In order to confirm that defects in GTPBP3 are the cause of this defect, we transduced three cell lines with a wild-type copy of GTPBP3 cDNA (RefSeq NM_32620.3) by using a lentiviral vector (pLenti 6.3/V5 TOPO, Life Technologies) as previously described.16,17 Fibroblasts from individuals #49665 and #66143 were used for the rescue experiment, with fibroblasts from #66654 (subject with only one affected allele) being included as a control (C6). Unfortunately, we were unable to recover any viable cells from subject #49665 after the transduction procedure. Although the transduction had no noticeable effect on the control cell line (C6-T), transduced fibroblasts from #66143 (#66143-T) displayed a significant improvement of OCR in galactose-containing medium (Figure 3B). Furthermore, we detected an isolated respiratory chain complex (RCC) I deficiency in a fibroblast cell line from family 9. Cotransfection of individual #83904 fibroblasts with two putative GTPBP3 isoforms amplified by RT-PCR, RefSeq NM_32620.3 and NM_0128855.2 (missing 63 base pairs of exon 8), significantly improved enzyme activities of RCC I (pIRES2-EGFP, Clontech) (Figure 3C). Analysis of the protein levels of GTPBP3 in five fibroblast cell lines demonstrated reduced or undetectable amounts in individuals #49665, #75191, #66143, #83904, and #83905, although they showed a clear increase after transduction or transfection (Figures S4 and 3D). In conclusion, our data demonstrate a causal role for GTPBP3 mutations in the oxidative metabolism deficiency in these individuals.
Figure 3.
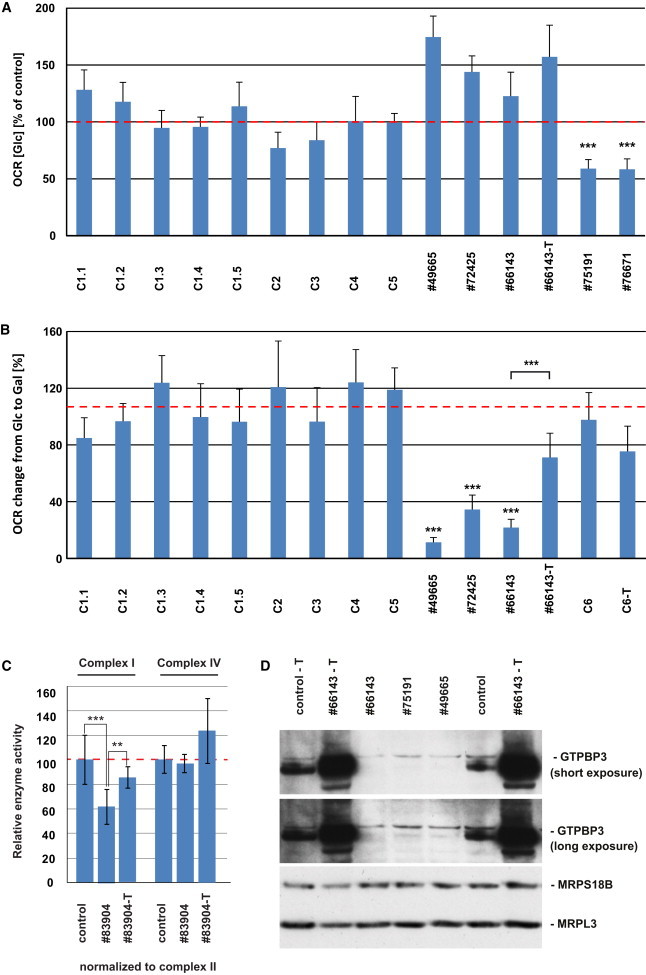
Analysis of Respiration Defects and GTPBP3 Protein Levels in Fibroblast Cell Lines
(A) Oxygen consumption rate (OCR) of fibroblast cell lines from five affected individuals and five control subjects cultured in high-glucose (Glc) medium. Each analysis was performed in more than 15 replicates. Control one (C1) was measured five time at different passage numbers (C1.1–1.5, NHDFneo, Lonza). OCR was expressed as percentage relative to the average of all controls. Cells from individuals #75191 and #76671 showed a significant reduction of oxygen consumption whereas cells from individuals #49665, #72425, and #66143 presented no defective respiration. Error bar indicates 1 SD; ∗∗∗p < 0.001.
(B) Oxygen consumption rate of fibroblast cells cultured in galactose (Gal) growth medium. The average increase of OCR from five control cells cultured in galactose-containing medium compared to glucose-containing medium was 107%. Cell lines from individuals #49665, #72425, and #66143 show significant lower increase in OCR. Lentiviral expression of wtGTPBP3 in cells from individual #66143 significantly increases the change in OCR although it has only little effect in control cells (C6-T). Error bar indicates 1 SD; ∗∗∗p < 0.001.
(C) Activities of respiratory chain complexes I and IV (expressed as ratio to CII activity) are decreased in individual #83904 cells transfected by electroporation with empty vector (pIRES2-EGFP) according to the manufacturer’s protocol (LONZA) but are improved upon expression of GTPBP3 cDNAs from the same plasmid. Measurements were performed as previously described.29,30 Error bar indicates 1 SD. Activity in controls was set as 100%. ∗∗p < 0.01, ∗∗p < 0.001.
(D) Levels of GTPBP3 were reduced in cells from individuals #49665, #75191, and #66143 and elevated after transduction with wtGTPBP3 cDNA. MRPS18B and MRPL3 served as mitochondrial loading controls.
Given that homologs of GTPBP3 in other systems have been implicated in protein synthesis, we next concentrated on the analysis of GTPBP3 in mitochondrial translation. The synthesis of mtDNA-encoded polypeptides, investigated by pulse-labeling of mitochondrial translation products via [35S]methionine in fibroblasts of affected individuals (for methods see Haack et al.18) was severely and uniformly decreased to 20%–30% of control levels in individuals #49665, #66143, and #75191 (Figures 4A and 4B). There was no detectable defect in fibroblasts from individual #72425, which might be explained by the relatively low conservation of the mutated residue in this individual (Figure 1B). In order to exclude possible defects of mitochondrial transcription or precursor RNA processing, we analyzed all mitochondrially encoded rRNAs and mRNAs in fibroblasts of individuals #49665, #66143, #72425, and #75191 by RNA blotting and by RNA-seq in fibroblasts of individual #49665. We found no differences in the expression levels of the mt-RNAs between case and control subjects. On average, the mt-RNA expression levels were only 6% lower in individual #49665 as compared to control individuals (data not shown). We did not observe any appreciable reduction in steady-state levels of mature RNAs, nor was there any accumulation of precursor RNAs (Figure S3A). Next, we analyzed the steady-state levels of mt-tRNAs, including those five species for which the τm5U modification has been reported in mammals (Gln, Glu, Lys, LeuUUR, and Trp).4 We again observed no appreciable changes in their steady-state levels (Figure S3B). In order to further corroborate a direct role of GTPBP3 in mitochondrial translation, we downregulated its expression via RNA interference in HeLa cells (Figure 4C). Reduction of GTPBP3 protein levels upon RNAi treatment of HeLa cells was comparable to the reduction of its level in GTPBP3 mutant fibroblasts (Figure 4D). Downregulation of GTPBP3 expression resulted in a general mitochondrial translation defect, similarly to the reduction observed in subject fibroblasts (Figure 4D). In conclusion, the reduced translation efficiency observed in three out of four GTPBP3 mutant cell lines, as well as in human cells treated with GTPBP3 RNAi, confirmed an important function for GTPBP3 in efficient mitochondrial protein synthesis.
Figure 4.
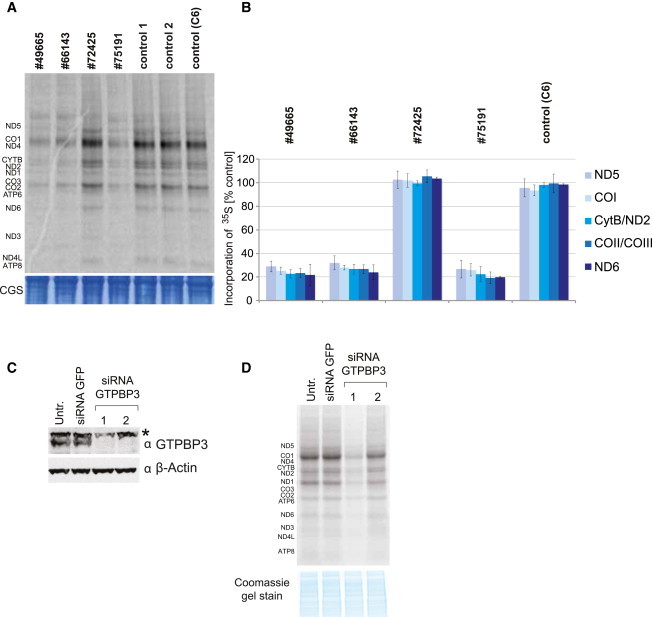
Analysis of Mitochondrial Protein Synthesis in Primary Fibroblasts and in HeLa Cells Treated with RNAi against GTPBP3
(A) [35S]methionine metabolic labeling of mitochondrial proteins in fibroblasts. Products of mitochondrial translation were labeled with [35S]methionine for 30 min, separated by a 4%–12% gradient SDS-PAGE, and visualized by autoradiography. To validate equal protein loading, a small section of the gel was stained with Coomassie (CGS). Fibroblasts from individuals #49665, #66143, and #75191 demonstrate significant inhibition of mitochondrial protein synthesis although translation in cells from individual #72425 is not affected.
(B) Quantification of radiolabelled products of mitochondrial translation. Incorporation of [35S] as in (A) was quantified by ImageQuant software after exposure to a PhosphorImager screen from three independent experiments. Error bar indicates 1 SD.
(C) Downregulation of GTPBP3 in HeLa cells via RNA interference. Immunoblot analysis of total HeLa cell lysate transfected with two different siRNA to GTPBP3 show decreased level of GTPBP3 upon RNAi treatment for 6 days. siRNA to GFP was used as transfection control. Asterisk indicates nonspecific band recognized by anti-GTPBP3 antibody in HeLa cells. β-actin serves as a loading control. Two different siRNA duplexes targeting GTPBP3 were used, 1 and 2.
(D) Mitochondrial translation in HeLa cells upon GTPBP3 downregulation. HeLa cells were transfected for 6 days with siRNA against GTPBP3 and subjected to [35S]methionine metabolic labeling. Inactivation of GTPBP3 leads to the reduced efficiency of mitochondrial translation. Two different siRNA duplexes targeting GTPBP3 were used, 1 and 2.
In order to test the consequences of this reduced translation rate upon the protein levels of OXPHOS complexes in mutant fibroblast cell lines, we analyzed the steady-state levels of several nuclear-encoded subunits of the OXPHOS system by immunoblotting. In fibroblasts from individuals #72425, #75191, and #76671 (F3: II-1, F4: II-4, and F5: II-2), we observed strongly reduced amounts of RCC IV. Fibroblasts from subjects #72425, #75191, and #49665 also showed reduced levels of RCC I, whereas the levels of RCC II and V remained normal in all cell lines (Figure 5). The diminished steady-state levels of respiratory chain complexes I and IV in fibroblast cell lines are in agreement with the impaired mitochondrial de novo translation rates in these cells and match the enzymatic defects identified in muscle biopsies of the same individuals.
Figure 5.
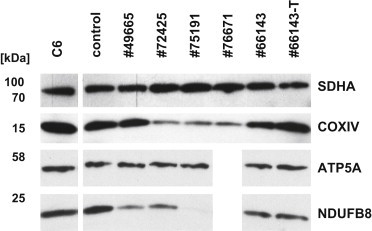
Immunoblot Analysis of OXPHOS Proteins in Fibroblasts
10 μg of detergent-solubilized total cell extract was subjected to immunoblot analysis of OXPHOS components. Amounts of SDHA (complex II) and ATP5A (ATPase) were unchanged in all individuals. In cells from individuals #72425, #75191, and #76671, a reduction of COXIV (complex IV) was observed. Cells from individuals #49665, #72425, and #75191 showed decreased levels of NDUFB8 (complex I). Antibodies used: mouse antibodies against SDHA (ab14715), NDUFB8 (ab110242), ATP5A (ab14748), and rabbit antibodies against COXIV (ab16056) from Abcam and rabbit anti GTPBP3 (HPA042158) from SIGMA Aldrich.
Within an international cooperation between European (Germany, UK, Italy, France, and Belgium), Israeli, and Japanese Centers for mitochondrial disorders, we provide statistically convincing evidence for GTPBP3 mutations leading to mitochondrial disease. To further support collaborative studies, the global mitochondrial disease community has established a Mitochondrial Disease Sequence Data Resource (MSeqDR) for common genomic data deposition and mining.
The genotype-driven analysis performed here was independent from the clinical presentation. Nevertheless, we identified common clinical features of the affected individuals that include lactic acidosis (11/11), cardiomyopathy (9/11), and neurological symptoms (6/11). The latter comprised symptoms such as development delay, intellectual disability, feeding difficulties, muscle hypotonia, fatigue, visual impairment, and epileptic seizures. Severity of the disease ranged from neonatal onset and death to late-infantile onset and survival into the second decade of life. Most affected individuals, however, manifested clinical symptoms before their first birthday. This is consistent with the normal cellular respiration, in organello translation, and normal levels of respiratory chain complexes reported in individuals less severely affected and the significantly reduced mitochondrial translation, respiration, and low levels of complex I and IV in those severely affected.
Modifications of the tRNA “wobble-base” in the anticodon loop are required for accurate and efficient codon recognition. The modification of position 5 (xm5) of the U34 wobble-base of certain tRNAs is evolutionarily well conserved, although different modified side chains have been identified in different species. In mammals, mitochondria 5-taurinomethyluridine (τm5U34) is found at the wobble-base position.19 Based upon studies in bacteria and yeast mitochondria, GTPBP3 and MTO1 have been proposed to generate this modification in mammalian mitochondria. Although this prediction awaits direct biochemical validation, the proposed functional conservation of GTPBP3 and MTO1 has been supported by the mitochondrial localization of these proteins in human cells and by complementation of the respiratory-deficient phenotype in yeast by their mammalian homolog cDNAs.20,21 Functional deficiency of homologs of GTPBP3 and MTO1 in bacteria and yeast mitochondria has been associated with abnormal U34 modification and consequently a reduced efficiency of translation.21–23 Our data support an analogous activity of GTPBP3 in human mitochondria since we identified a reduced efficiency of translation in three cell lines with GTPBP3 mutations and in cells with RNAi-mediated downregulation of GTPBP3 expression. Other groups have also reported impaired protein synthesis and reduced mitochondrial function in GTPBP3-depleted cells.24 The defect in mitochondrial translation was a likely cause of the combined respiratory chain complex deficiency detected in muscle tissues of all but one affected individual.
Like GTPBP3 mutations, MTO1 mutations are also associated with hypertrophic cardiomyopathy (HCM), lactic acidosis, and combined respiratory chain deficiency. An association of MTO1 mutations with impaired mitochondrial translation has yet to be shown for human mitochondria, but the common clinical presentation provides support for a common pathomechanism in the U34 modification for both diseases. So far, all individuals with MTO1 mutations presented a HCM. However, nearly all of them have been specifically screened for MTO1 mutations based on the clinical presentation of a HCM. Clinical and MRI signs of brain involvement are found for both GTPBP3 and MTO1 cases. The genotype-driven investigation presented here identified individuals with lactic acidosis, developmental delay, and MRI involvement of thalamus, putamen, and brainstem but without HCM. It can be expected that the clinical spectrum associated with MTO1 deficiency will also broaden, with more subjects being genome-wide investigated. In a very recent study, Taylor et al. indeed reported a case subject with MTO1 mutations and central neurological features who did not have a cardiomyopathy.25
Our study highlights that defects in mitochondrial translation, probably owing to incorrect posttranscriptional modification of mt-tRNAs, are an important contributory factor to the spectrum of human mitochondrial disease. Recent data have suggested that more than 7% of all mt-tRNA residues undergo posttranscriptional modification, with close to 30 different modifications so far described.4 Therefore, it is expected that future WES analyses of individuals clinically diagnosed with mitochondriopathy will reveal further mutations within genes coding for mt-tRNA modifiers. Indeed, in addition to the aforementioned mutations in MTO1 and TRMU, mutations in PUS1 (MIM 608109) (which introduces pseudouridine [Ψ] at base positions 27, 28, and 29 in several mt-tRNAs) have been reported in subjects affected with mitochondrial myopathy and sideroblastic anemia (MLASA)26 (MIM 600462) and very recent studies have identified mutations in TRIT1 (which is responsible for i6A37 modification of a subset of mt-tRNAs) in individuals with severe combined mitochondrial respiratory chain defects.27 Furthermore, mtDNA mutations in mt-tRNA genes, which are a very frequent cause of human respiratory chain deficiencies (MITOMAP), might also affect mt-tRNA modification. Related to the present study, it has been reported that τm5U34 is not present in mt-tRNALeuUUR harboring the m.3243A>G mutation (or other pathological mutations) responsible for mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) (MIM 540000). The absence of τm5U34 has been suggested to be responsible for the mitochondrial translation defect in these subjects.28 These results imply that deficiency of mt-tRNA modification plays a critical role in the molecular pathogenesis of human respiratory chain disease. Further studies of these pathways, such as analysis of tissue-specific regulation of mt-tRNA-modifying enzymes, might help to explain the clinical heterogeneity observed for mitochondrial diseases caused by mutations in mt-tRNA genes.
In conclusion, this study shows a mitochondrial translation disorder with a broad spectrum of clinical presentations, which emphasizes the importance of posttranscriptional modification of mitochondrial tRNAs for proper mitochondrial function.
Acknowledgments
We thank C. Terrile, M. Borzes, and C. Fischer for technical support and F. Miyake and T. Wada for referral of sample materials. This work was supported by the Deutsche Forschungsgemeinschaft within the framework of the Munich Cluster for Systems Neurology (EXC 1010 SyNergy), the German Bundesministerium für Bildung und Forschung (BMBF) through funding of the E-Rare project GENOMIT (01GM1207 for T.M. and H.P., 2011-RARE-005-03 for A.R. and M.D.M., J41J11000420001 for M.Z., and FWF I 920-B13 for J.A.M.), German Network for Mitochondrial Disorders (mitoNET 01GM1113C for T.M., H.P., and P.F. and 01GM1113A for T.K.), the German Center for Heart Research (Z76010017300 and Z56010015300 for T.M.), European Commission 7th Framework Program (Project N261123 GEUVADIS), Medical Research Council, UK (MC_U105697135 for T.J.N., J.R., S.F.P., C.A.P., and M.M.), Wellcome Trust Strategic Award (096919/Z/11/Z for R.W.T. and P.F.C.), MRC Centre for Neuromuscular Diseases (G0601943), UK NHS Highly Specialised “Rare Mitochondrial Disorders of Adults and Children” Service for R.W.T. and P.F.C., Fund for Scientific Research Belgium (FWO, contract number G.0200.10 for A.V., J.S., and R.V.C.), Fondazione Telethon (GGP11011 and GPP10005), Italian Ministry of Health (GR2010-2316392), CARIPLO (2011/0526), Pierfranco and Luisa Mariani Foundation, and Italian Association of Mitochondrial Disease Patients and Families (Mitocon) to D.G., F.I., E.L., and M.Z., Research Program of Innovative Cell Biology by Innovative Technology (Cell Innovation), Grant-in-Aid for the Development of New Technology from The Promotion and Mutual Aid Corporation for Private Schools of Japan from MEXT for Y.O., Grants-in-Aid for the Research on Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan for A.O., Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics for K. Murayama, Association Française contre les Myopathies (AFM) for A.R. and M.D.M., and Fellowship from the AFM (16615 for M.D.M.).
Contributor Information
Michal Minczuk, Email: michal.minczuk@mrc-mbu.cam.ac.uk.
Holger Prokisch, Email: prokisch@helmholtz-muenchen.de.
Supplemental Data
Web Resources
The URLs for data presented herein are as follows:
MITOMAP, http://www.mitomap.org/MITOMAP
MSeqDR, https://mseqdr.org/
Online Mendelian Inheritance in Man (OMIM), http://www.omim.org/
Predotar, https://urgi.versailles.inra.fr/predotar/predotar.html
PSORTII Prediction, http://psort.hgc.jp/form2.html
References
- 1.Boczonadi V., Horvath R. Mitochondria: impaired mitochondrial translation in human disease. Int. J. Biochem. Cell Biol. 2014;48:77–84. doi: 10.1016/j.biocel.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osawa S. ASM Press; Washington, DC: 1995. Evolution of the Genetic Code. [Google Scholar]
- 3.Suzuki T. Biosynthesis and function of tRNA wobble modifications. Top. Curr. Genet. 2005;12:23–69. [Google Scholar]
- 4.Suzuki T., Suzuki T. A complete landscape of post-transcriptional modifications in mammalian mitochondrial tRNAs. Nucleic Acids Res. 2014;42:7346–7357. doi: 10.1093/nar/gku390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colby G., Wu M., Tzagoloff A. MTO1 codes for a mitochondrial protein required for respiration in paromomycin-resistant mutants of Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:27945–27952. doi: 10.1074/jbc.273.43.27945. [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama S., Watanabe T., Murao K., Ishikura H., Yamaizumi Z., Nishimura S., Miyazawa T. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl. Acad. Sci. USA. 1985;82:4905–4909. doi: 10.1073/pnas.82.15.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Björk G.R., Huang B., Persson O.P., Byström A.S. A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA. 2007;13:1245–1255. doi: 10.1261/rna.558707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarham J.W., Elson J.L., Blakely E.L., McFarland R., Taylor R.W. Mitochondrial tRNA mutations and disease. Wiley Interdiscip. Rev. RNA. 2010;1:304–324. doi: 10.1002/wrna.27. [DOI] [PubMed] [Google Scholar]
- 9.Zeharia A., Shaag A., Pappo O., Mager-Heckel A.-M., Saada A., Beinat M., Karicheva O., Mandel H., Ofek N., Segel R. Acute infantile liver failure due to mutations in the TRMU gene. Am. J. Hum. Genet. 2009;85:401–407. doi: 10.1016/j.ajhg.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghezzi D., Baruffini E., Haack T.B., Invernizzi F., Melchionda L., Dallabona C., Strom T.M., Parini R., Burlina A.B., Meitinger T. Mutations of the mitochondrial-tRNA modifier MTO1 cause hypertrophic cardiomyopathy and lactic acidosis. Am. J. Hum. Genet. 2012;90:1079–1087. doi: 10.1016/j.ajhg.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baruffini E., Dallabona C., Invernizzi F., Yarham J.W., Melchionda L., Blakely E.L., Lamantea E., Donnini C., Santra S., Vijayaraghavan S. MTO1 mutations are associated with hypertrophic cardiomyopathy and lactic acidosis and cause respiratory chain deficiency in humans and yeast. Hum. Mutat. 2013;34:1501–1509. doi: 10.1002/humu.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elstner M., Andreoli C., Klopstock T., Meitinger T., Prokisch H. The mitochondrial proteome database: MitoP2. Methods Enzymol. 2009;457:3–20. doi: 10.1016/S0076-6879(09)05001-0. [DOI] [PubMed] [Google Scholar]
- 13.Invernizzi F., D’Amato I., Jensen P.B., Ravaglia S., Zeviani M., Tiranti V. Microscale oxygraphy reveals OXPHOS impairment in MRC mutant cells. Mitochondrion. 2012;12:328–335. doi: 10.1016/j.mito.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrova-Benedict R., Buncic J.R., Wallace D.C., Robinson B.H. Selective killing of cells with oxidative defects in galactose medium: a screening test for affected patient fibroblasts. J. Inherit. Metab. Dis. 1992;15:943–944. doi: 10.1007/BF01800243. [DOI] [PubMed] [Google Scholar]
- 15.Robinson B.H., Petrova-Benedict R., Buncic J.R., Wallace D.C. Nonviability of cells with oxidative defects in galactose medium: a screening test for affected patient fibroblasts. Biochem. Med. Metab. Biol. 1992;48:122–126. doi: 10.1016/0885-4505(92)90056-5. [DOI] [PubMed] [Google Scholar]
- 16.Danhauser K., Iuso A., Haack T.B., Freisinger P., Brockmann K., Mayr J.A., Meitinger T., Prokisch H. Cellular rescue-assay aids verification of causative DNA-variants in mitochondrial complex I deficiency. Mol. Genet. Metab. 2011;103:161–166. doi: 10.1016/j.ymgme.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Kornblum C., Nicholls T.J., Haack T.B., Schöler S., Peeva V., Danhauser K., Hallmann K., Zsurka G., Rorbach J., Iuso A. Loss-of-function mutations in MGME1 impair mtDNA replication and cause multisystemic mitochondrial disease. Nat. Genet. 2013;45:214–219. doi: 10.1038/ng.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haack T.B., Kopajtich R., Freisinger P., Wieland T., Rorbach J., Nicholls T.J., Baruffini E., Walther A., Danhauser K., Zimmermann F.A. ELAC2 mutations cause a mitochondrial RNA processing defect associated with hypertrophic cardiomyopathy. Am. J. Hum. Genet. 2013;93:211–223. doi: 10.1016/j.ajhg.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki T., Suzuki T., Wada T., Saigo K., Watanabe K. Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrial diseases. EMBO J. 2002;21:6581–6589. doi: 10.1093/emboj/cdf656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Guan M.-X. A human mitochondrial GTP binding protein related to tRNA modification may modulate phenotypic expression of the deafness-associated mitochondrial 12S rRNA mutation. Mol. Cell. Biol. 2002;22:7701–7711. doi: 10.1128/MCB.22.21.7701-7711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Li R., Lin X., Guan M.-X. Isolation and characterization of the putative nuclear modifier gene MTO1 involved in the pathogenesis of deafness-associated mitochondrial 12 S rRNA A1555G mutation. J. Biol. Chem. 2002;277:27256–27264. doi: 10.1074/jbc.M203267200. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Yan Q., Guan M.-X. Combination of the loss of cmnm5U34 with the lack of s2U34 modifications of tRNALys, tRNAGlu, and tRNAGln altered mitochondrial biogenesis and respiration. J. Mol. Biol. 2010;395:1038–1048. doi: 10.1016/j.jmb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy F.V., 4th, Ramakrishnan V., Malkiewicz A., Agris P.F. The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat. Struct. Mol. Biol. 2004;11:1186–1191. doi: 10.1038/nsmb861. [DOI] [PubMed] [Google Scholar]
- 24.Villarroya M., Prado S., Esteve J.M., Soriano M.A., Aguado C., Pérez-Martínez D., Martínez-Ferrandis J.I., Yim L., Victor V.M., Cebolla E. Characterization of human GTPBP3, a GTP-binding protein involved in mitochondrial tRNA modification. Mol. Cell. Biol. 2008;28:7514–7531. doi: 10.1128/MCB.00946-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor R.W., Pyle A., Griffin H., Blakely E.L., Duff J., He L., Smertenko T., Alston C.L., Neeve V.C., Best A. Use of whole-exome sequencing to determine the genetic basis of multiple mitochondrial respiratory chain complex deficiencies. JAMA. 2014;312:68–77. doi: 10.1001/jama.2014.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bykhovskaya Y., Casas K., Mengesha E., Inbal A., Fischel-Ghodsian N. Missense mutation in pseudouridine synthase 1 (PUS1) causes mitochondrial myopathy and sideroblastic anemia (MLASA) Am. J. Hum. Genet. 2004;74:1303–1308. doi: 10.1086/421530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yarham J.W., Lamichhane T.N., Pyle A., Mattijssen S., Baruffini E., Bruni F., Donnini C., Vassilev A., He L., Blakely E.L. Defective i6A37 modification of mitochondrial and cytosolic tRNAs results from pathogenic mutations in TRIT1 and its substrate tRNA. PLoS Genet. 2014;10:e1004424. doi: 10.1371/journal.pgen.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasukawa T., Suzuki T., Ueda T., Ohta S., Watanabe K. Modification defect at anticodon wobble nucleotide of mitochondrial tRNAs(Leu)(UUR) with pathogenic mutations of mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes. J. Biol. Chem. 2000;275:4251–4257. doi: 10.1074/jbc.275.6.4251. [DOI] [PubMed] [Google Scholar]
- 29.Rustin P., Chretien D., Bourgeron T., Gérard B., Rötig A., Saudubray J.M., Munnich A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta. 1994;228:35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 30.Rustin P., Chretien D., Bourgeron T., Wucher A., Saudubray J.M., Rotig A., Munnich A. Assessment of the mitochondrial respiratory chain. Lancet. 1991;338:60. doi: 10.1016/0140-6736(91)90057-v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


