Abstract
Delirium is a common cause and complication of hospitalization in older people, being associated with higher risk of future dementia and progression of existing dementia. However relatively little data are available on which biochemical pathways are dysregulated in the brain during delirium episodes, whether there are protein expression changes common among delirium subjects and whether there are any changes which correlate with the severity of delirium. We now present the first proteomic analysis of delirium cerebrospinal fluid (CSF), and one of few studies exploring protein expression changes in delirium. More than 270 proteins were identified in two delirium cohorts, 16 of which were dysregulated in at least 8 of 17 delirium subjects compared with a mild Alzheimer's disease neurological control group, and 31 proteins were significantly correlated with cognitive scores (mini-mental state exam and acute physiology and chronic health evaluation III). Bioinformatics analyses revealed expression changes in several protein family groups, including apolipoproteins, secretogranins/chromogranins, clotting/fibrinolysis factors, serine protease inhibitors and acute-phase response elements. These data not only provide confirmatory evidence that the inflammatory response is a component of delirium, but also reveal dysregulation of protein expression in a number of novel and unexpected clusters of proteins, in particular the granins. Another surprising outcome of this work is the level of similarity of CSF protein profiles in delirium patients, given the diversity of causes of this syndrome. These data provide additional elements for consideration in the pathophysiology of delirium as well as potential biomarker candidates for delirium diagnosis.
Introduction
Delirium is a common cause and complication of hospitalization, particularly prevalent in the elderly and within intensive care units. Although it is an acute condition from which most patients recover, it is also associated with multiple adverse outcomes including higher risk of future dementia as well as progression of existing dementia and higher mortality.1, 2, 3 The aetiology of delirium is thought to involve neurotransmitter changes arising from diverse sources including hypoxaemia, metabolic derangements, disturbance of the sleep–wake cycle, drug effects or systemic inflammation.4, 5 However there is a paucity of data relating to biochemical changes in delirium cerebrospinal fluid (CSF).6 There is much speculation; however, little evidence is available on which biochemical pathways are dysregulated in the brain during delirium episodes, whether there are protein expression changes common among delirium subjects and whether there are protein changes which correlate with delirium severity. Further, there is no available biochemical test which could facilitate delirium detection. Quantitative proteomics using iTRAQ tags is a well-established discovery-based tool, which allows unbiased evaluation of dysregulated protein expression in complex biological samples such as cellular and tissue extracts and body fluids. It has wide application in the biological and biomedical sciences and is increasingly applied to the study of neurodegenerative diseases such as Alzheimer's disease (AD).7, 8, 9 However, to date, few studies of delirium have utilized proteomics approaches.10, 11 None have utilized CSF which is the body fluid most likely to reflect biochemical changes in conditions which disrupt central nervous system function, and only one proteomics study has been reported on delirium subjects.10 This study utilized urine from post-cardiac surgery intensive care unit subjects, and applied MALDI-TOF mass spectrometric profiling to the intact proteins, which provided some quantitative data but precluded sequence identification. Furthermore few proteins were quantified, none of which were delirium specific. Another proteomics study of delirium plasma is in the planning phase,11 and will utilize SELDI-TOF, which is typically used for proteomics profiling, but will preclude protein sequence identification. In the current study, we used CSF samples from delirium subjects and neurological controls (Sydney study group),12 to identify proteins with altered expression levels relative to a mild dementia control group. We then validated the observed changes using a second study group based in Edinburgh (Edinburgh study group). Dysregulated proteins were analysed using bioinformatics tools DAVID and STRING to determine if functional relationships would emerge. This approach provides both protein sequence identification and quantification of relative protein expression changes, facilitating quantification of potential biomarkers and a better understanding of the pathology at a molecular level.
Materials and methods
Subjects
Sydney study
Patients admitted to the Geriatric Medicine Unit at the Prince of Wales Hospital were screened for delirium in the Emergency Department,12, 13 where they were admitted for a variety of medical diagnoses triggering the delirium, including infections, metabolic problems and adverse drug reactions, but on average were suffering from at least two identifiable causes of delirium, and on the Geriatric Medicine ward after admission from the Emergency Department, which is the source of 99% of Geriatric Medicine ward patients in our hospital. As part of the standard clinical care in this unit, patients suffering from delirium, not resolving after more than 5 days of treatment, are considered for additional investigations, including lumbar puncture. This procedure is discussed with their ‘person responsible' or substitute decision maker, and the patient where possible, who gave written consent. For the purposes of this study, a portion of the CSF obtained from hospitalized patients with current delirium for clinical purposes, was stored. Patients with delirium had mild-to-moderate levels of disorder on the basis of the delirium index score (DIS;14 Table 1 and Supplementary Table 2). Delirium severity was based on scores on the delirium index,14 and this was the severity scale measure used for comparison with proteomics data. Baseline assessment of the patients with delirium in the Sydney cohort also included; CAM (confusion assessment method),15 IQCODE (informant questionnaire on cognitive decline in the elderly); Barthel Index,16 modified instrumental activities of daily living index,17 acute physiology and chronic health evaluation III (APACHE III)18 and the Charlson comorbidity index.19 CSF was also obtained from neurological control subjects (AD with no delirium) where the patient and their ‘person responsible' consented to enter the study. Patients in the control group were from our outpatient clinic without any evidence of current delirium. They were diagnosed with dementia on the basis of the Diagnostic and Statistical Manual, 4th edition20 and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria.21 AD subjects had mild-to-moderate levels of disorder on the basis of the mini-mental state exam (MMSE).22
Table 1. Baseline characteristics of Sydney and Edinburgh Delirium Study groups.
| Sydney Delirium Study | Delirium, mean+s.d. | Dementia, mean+s.d. | P-valuea |
|---|---|---|---|
| Age | 80.3±9.0 | 83.1±5.8 | 0.364 |
| Sex (F:M) | 4:8 | 9:6 | 0.161 |
| Mini-mental state exam (/30) | 15.6±4.4 | 21.5±4.6 | 0.008 |
| Informant questionnaire on cognitive decline (/5) | 4.0±0.6 | 3.7±0.4 | 0.078 |
| Confusion assessment method | 6.1±1.3 | 0.0±0.0 | <0.001 |
| Delirium index | 15.6±4.8 | 2.9±1.4 | <0.001 |
| APACHE III (acute physiology and chronic health evaluation III) index | 47.6±23.2 | 30.6±3.6 | 0.028 |
| Geriatric Depression Scale (/15) | 5.6±1.4 | 6.7±2.5 | 0.248 |
| Barthel score (/20) | 17.4±3.8 | 19.1±1.8 | 0.186 |
| Instrumental activities of daily living (/12) | 6.1±4.1 | 8.5±2.7 | 0.100 |
| Charlson comorbidity index | 6.25±2.5 | 6.4±2.9 | 0.920 |
| Edinburgh Delirium Study | Delirium, mean+s.d. | Normal controls, mean+s.d. | P-value |
|---|---|---|---|
| Age | 83.6±10.1 | 81.1±10.84 | 0.685 |
| Sex (F:M) | 3:2 | 7:1 | 0.640 |
| Mini-mental state exam (/30)b | 19.6±10.5 | 26.9±1.8 | 0.198 |
| Informant questionnaire on cognitive decline (/5) | 3.3±0.4 | 3.1±0.1 | 0.289 |
| Postoperative DRS-R98b | 19.6±9.1 | 5.3±1.9 | 0.025 |
| APACHE II (acute physiology and chronic health evaluation II) index6 | 9.4±2.3 | 7.3±1.2 | 0.11 |
| Lawton instrumental activities of daily living | 5.2±2.3 | 6.9±1.1 | 0.19 |
All demographics data were compared using a two-tailed t-test, except for sex distribution, which was tested using the Fisher's exact test variant of the χ2.
Postoperative day 1 MMSE and DRS-R98 are shown here for the Edinburgh delirium study.
Edinburgh study
To ascertain whether dysregulated protein expression would show similar trends across studies, an independent study group based in Edinburgh was used. Community-dwelling patients over the age of 60 with acute hip fracture were recruited on the Orthopaedic Trauma Unit of the New Royal Infirmary of Edinburgh. They were assessed for delirium pre-operatively and daily postoperatively until day 4, and further on day 7 and between days 10–14. Precipitants of delirium included trauma, pain, medication, in particular, opiate analgesia and anaesthetic agents, and infection. CSF was obtained at the onset of spinal anaesthetic for the operation to repair their hip fracture.23, 24 Baseline assessment of the Edinburgh cohort included MMSE, pre- and postoperative Delirium Rating Scale—revised—98 (DRS-R98)25, 26 APACHE II index,27 IQCODE28 and Lawton instrumental activities of daily living.17 Delirium severity was based on scores on the Delirium Rating Scale—revised—98 (DRS-R98),25, 26 and this was the severity scale measure used for comparison with proteomics data. Demographics for the study groups as well as test scores are shown in Supplementary Tables 1 and 2.
Ethics approval was obtained from the Human Research Ethics Committee of the South Eastern Sydney Health Area (eastern section) and the University of New South Wales Ethics Committee. The proposal was also reviewed by the NSW Guardianship Tribunal that has to approve interventional studies involving participants who are unable to give consent, but not observational studies, and no objections were raised. The Edinburgh study was approved by the Scotland A Research Ethics Committee.
CSF collection and total protein assay
CSF samples were collected from the Sydney study group by lumbar puncture on the ward or under X-ray guidance, using local anaesthetic but no sedation or restraining devices and from the Edinburgh study group by lumbar puncture administered to induce spinal anaesthesia for the repair of their neck of femur fracture. CSF samples were transported from Edinburgh to Sydney on dry ice and all laboratory-based experiments using both cohorts, including proteomics, enzyme-linked immunosorbent assay (ELISA) and total protein assay, were carried out in the Bioanalytical Mass Spectrometry Facility, School of Medical Sciences, University of New South Wales. The CSF was centrifuged, aliquoted and stored at −80 °C until required. The total protein level of CSF samples was assayed using the bicinchoninic acid assay purchased from Pierce (Sydney, NSW, Australia) and following the manufacturer's instructions exactly. Bovine serum albumin was used to construct the standard curve (0.025–2 mg ml−1 concentration range, triplicate standards), and the absorbance read at a wavelength of 595 nm. A third-order polynomial curve was fitted, with an r2 typically >0.99 and the equation used to calculate protein concentrations in CSF.
iTRAQ, two-dimensional liquid chromatography-tandem mass spectrometry and data analysis
Isobaric labelling using iTRAQ reagents, two-dimensional liquid chromatography-tandem mass spectrometry and data analysis were performed according to published approaches.7, 29, 30, 31 An 8-plex iTRAQ design was used, which represents the maximum number of iTRAQ isobaric tag reagents available for any single experiment. With 17 delirium subjects and additional controls, a minimum of three iTRAQ experiments were therefore required (experiments 1 and 2 using Sydney study subjects and experiment 3 using Edinburgh study subjects). The experimental design is outlined in Supplementary Table 2, showing which iTRAQ tag was used for each subject. The 119 iTRAQ tag in each experiment was the mild AD control, which was used as the denominator for all iTRAQ ratios in both the Sydney and Edinburgh groups to facilitate comparison across groups. A detailed description of sample preparation and liquid chromatography-tandem mass spectrometry analysis is provided in the Supplementary Section.
ELISA assay of α-1-acid glycoprotein 1
To partially validate the iTRAQ proteomics data using an independent method, an ELISA was carried out using additional delirium18 and AD12 subjects from the Sydney cohort. An Assaypro AGP ELISA kit specific for human α-1-acid glycoprotein (Saint Charles, MO, USA) was used, following the manufacturer's instructions. This assay has a detection limit of ~0.06 μg ml−1 and intra- and inter-assay CV% of 4.7 and 7.3, respectively. CSF samples were diluted (1:100) using the kit mix diluent before assay. All data used for statistical analysis fell within the range of the standard curve. Log data were used for both concentration and absorbance values, and a third-order polynomial curve was fitted to the standards (r2=0.997), with a concentration range of 0.063–4 μg ml−1.
Statistics and bioinformatics; DAVID and STRING analyses
SPSS 20 was used to perform the Levine's test and t-test analyses of the alpha-1-acidic glycoprotein ELISA data, and to compare basal characteristics of the delirium vs dementia or delirium vs no delirium subjects in the Sydney and Edinburgh study groups, respectively. The Fisher's exact test variant of the χ2 was used to compare ratios of male to female subjects. The list of proteins deregulated in delirium relative to mild dementia which were identified by ProteinPilot analysis were further evaluated using WEB-based bioinformatics tools DAVID v6.7refs 32,33 and STRING v9.05.34 DAVID software determines whether statistically significant enrichment of specific protein functional clusters occurs within a target group of proteins relative to a control group (we used the full human genome as control). The STRING clustering tool was used to explore currently known associations between proteins, indicated in the scientific literature. However in the absence of literature-based evidence for specific connections, some proteins may miss inclusion in a cluster. Therefore in addition to using the clustering function, the STRING tool was used to explore enrichment of biological processes, molecular functions, cellular components and KEGG pathways. Significance of enrichment was based on a P-value of at least 0.05 using FDR correction. The regression analysis tool in the Microsoft XL data analysis package was used to perform regression analysis on iTRAQ scores vs MMSE, APACHE III and DIS values.
Results
Group demographics
The subject demographics are shown in Table 1. There were 17 delirium, 17 AD and 8 normal control subjects used for iTRAQ experiments. Psychometric performance of the Sydney cohort of delirium and dementia subjects significantly differed on four instruments (Table 1 and Supplementary Table 2); MMSE (in which dementia subject scores were significantly higher than those of delirium subjects), CAM, DIS and APACHE III Index, in all three of which delirium subjects had higher scores than dementia subjects (Supplementary Table 2). In the Edinburgh study, the delirium subjects had a lower mean MMSE score and higher postoperative DRS-R98 and APACHE II scores relative to controls, however, only the postoperative DRS-R98 scores were significantly different (P=0.025).
Summary of iTRAQ experiment outcomes
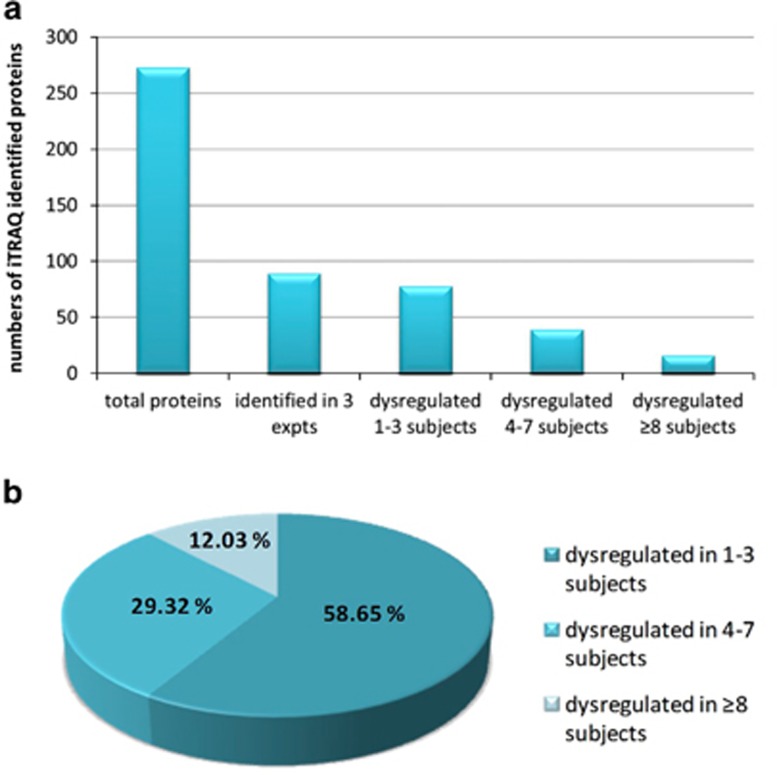
A total of 273 proteins were identified in three separate iTRAQ experiments (Supplementary Table 4), of which 89 were common to all three experiments. Approximately 12% had dysregulated protein expression in a minimum of eight subjects, however, many had dysregulated expression in just a few delirium patients (Figure 1). In two iTRAQ experiments, comprising subjects from the Sydney group, 26 proteins had dysregulated protein expression in at least 50% of delirium subjects relative to the mild AD neurological control group (Supplementary Table 3). When all three iTRAQ experiments were compared, comprising both the Sydney and Edinburgh groups, 16 proteins had dysregulated protein expression in eight or more subjects with delirium, across all three iTRAQ experiments and across both study groups (Table 2). Direction of expression change was the same for a few proteins, but most often a mixture of responses was observed (Table 2). Proteins with altered expression in at least 4 out of 17 delirium subjects were processed using the DAVIDv6.7 and STRING v9.05 bioinformatics tools to identify enrichment in specific functions (Supplementary Tables 1 and 5). Statistically significant enrichment in a variety of biological functions was identified, some of which were sample related, such as secreted and plasma proteins, but all the remaining clusters reflected specific functions, such as responses to wounding, inorganic substances and external stimulus, regeneration, protease inhibition, acute-phase response and high-density lipoprotein particle.
Figure 1.
Proteins identified in the three iTRAQ experiments from two studies (Sydney Delirium Study and Edinburgh Delirium Study), and a total of 17 subjects with either mild or moderate-to-severe delirium are shown. All proteins were identified with a confidence of 95% or greater (unused score of ⩾1.3 in ProteinPilot v3.0), were based on 4–7 iTRAQ technical replicates and significantly dysregulated proteins were identified at the P⩾0.05 level. (a) Total numbers of unique proteins identified in any of the three experiments, proteins identified in all three experiments and numbers of dysregulated proteins identified in 1–3, 4–7 or ⩾8 subjects are shown. (b) Percentage of proteins identified in all three experiments and dysregulated in 1–3, 4–7 or ⩾8 subjects. The list of proteins dysregulated in eight or more delirium subjects, and which are identified in all three iTRAQ experiments are shown in Table 2.
Table 2. Proteins identified in three iTRAQ experiments from two independent studies (Sydney Delirium Study and Edinburgh Delirium Study), with a total of 17 subjects with either mild or moderate-to-severe delirium.
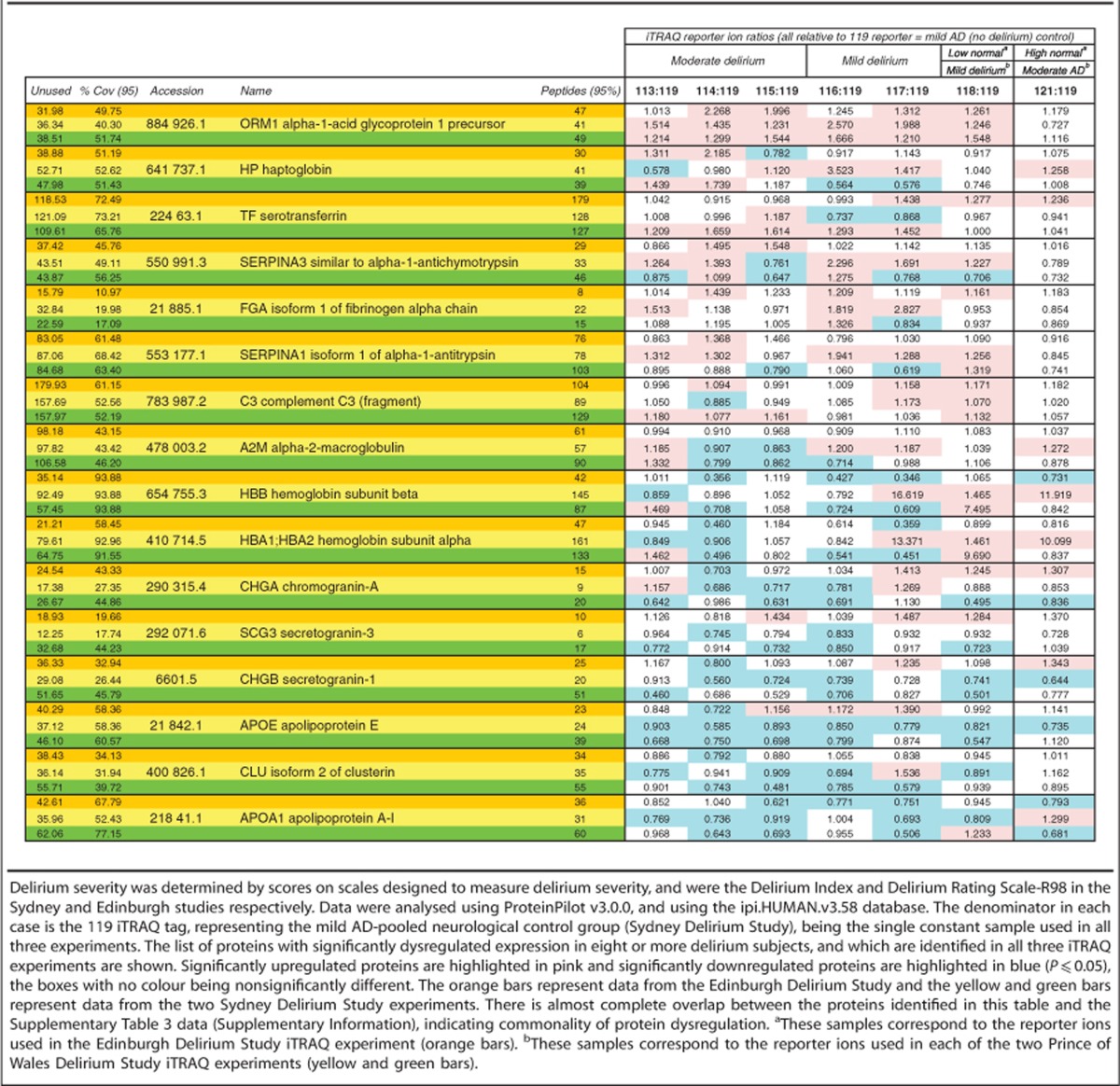
Expression changes of specific proteins
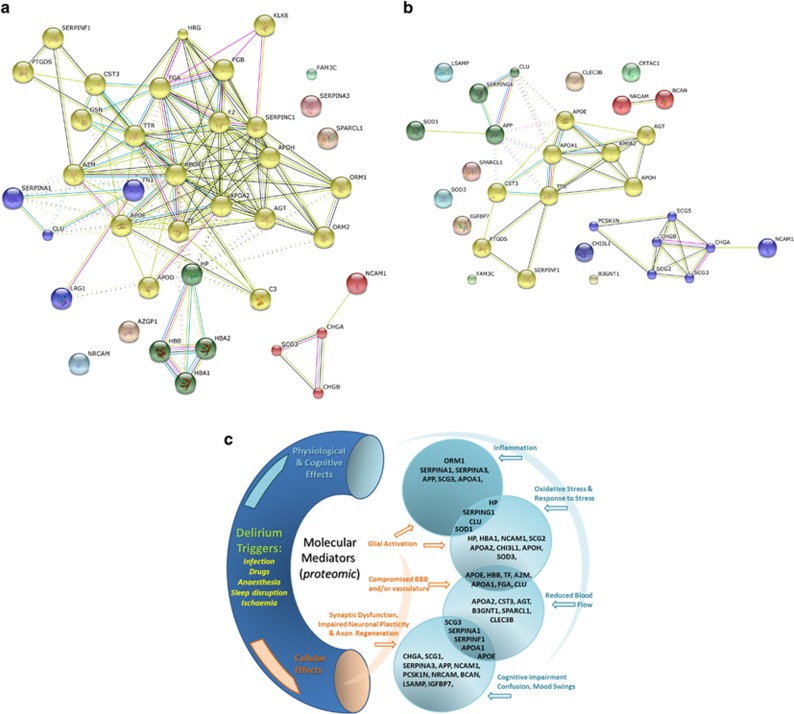
Proteins with altered expression levels in the majority of delirium subjects (Table 2) included alpha-1-antitrypsin (SERPINA3) and alpha-1-acid glycoprotein 1 (ORM1), both of which were upregulated in the majority of delirium subjects. Several proteins were significantly downregulated in the majority of subjects, including secretogranin-1 (CHGB), apolipoprotein E (APOE), clusterin (CLU)—also known as apolipoprotein J and apolipoprotein A1 (APOA1). Subsequent bioinformatics analyses using STRING and DAVID software only included proteins with significantly altered expression level in at least 4 out of 17 of the delirium subjects. The MCL clustering function of the STRING v9.05 bioinformatics analysis tool identified four clusters, broadly representing; granins, haptoglobin/haemoglobin complex, serpins and apolipoproteins (Figure 2a). Not all significantly deregulated proteins formed part of a STRING cluster, because literature evidence for an association is currently lacking. However sometimes non-clustered proteins are structurally or functionally linked to proteins which have formed part of a cluster. For example, in Figure 2a, SERPINS A1, C1 and F1 are clustered, but SERPIN A3 is not. Similarly NCAM1 is clustered but NRCAM is not. It is very likely that these related proteins may be involved in similar processes, and our data set provides a good rationale for further investigation. Other proteins such as FAM3C and SPARCL1 do not cluster with any of the other proteins in the set, and for these it remains to be seen whether connections will emerge or if their links to delirium will be through other proteins and pathways, as yet not identified here. The clustering function has not identified direct connections for all the proteins; however, the STRING enrichment tool has identified biological process, molecular function, cellular component and/or KEGG pathway enrichment for all of the proteins (Supplementary Tables 5 and 6). This offers insight into the roles that particular protein groups may have, some of which include platelet degranulation/activation, response to wounding, inflammation, coagulation, enzyme activity, biological regulation, cholesterol and lipoprotein processing, neuronal regeneration and extracellular vesicle components (Supplementary Tables 5 and 6). Many proteins appear across multiple groups, reflecting the multifunctional nature of most proteins.
Figure 2.
(a) Pathway analysis (STRING v9.05) of 39 proteins significantly dysregulated in the CSF of subjects with mild or moderate delirium relative to the mild AD control with no delirium. Subjects from both the Sydney and Edinburgh Delirium Studies were used. These are a subset of proteins listed in Supplementary Table 4, which are dysregulated in at least 4 out of 17 delirium patients. The STRING MCL clustering tool35 was used to identify up to four clusters in this data set (clustered proteins are marked in same colour circles), and include; chromogranin and secretogranin, apolipoproteins, clotting factors and regulatory proteins. The protein acronyms were as provided by the ProteinPilot software, which uses the gene symbol nomenclature based on the Guidelines for Human Gene Nomenclature36 and http://www.genenames.org/guidelines.html. (b) Pathway analysis (STRING v9.05) of proteins whose iTRAQ ratio is significantly correlated with MMSE score (regression analysis P-values are shown in Table 3). Delirium and normal control subjects from both the Sydney Delirium and Edinburgh Studies were used for the regression analysis. The STRING MCL clustering tool was used to identify up to four clusters in this data set. (c) Theoretical model of the relationship between proteomic changes and delirium pathophysiology. The proteins shown on this schematic are the significantly dysregulated proteins listed in Tables 2 and 3. CSF, cerebrospinal fluid.
ELISA of alpha-1-acid glycoprotein
ELISA assay of alpha-1-acid glycoprotein confirmed significant upregulation of expression in delirium CSF relative to AD subjects (P=0.026 using a two-tailed t-test, assuming unequal variances). Mean values for delirium and AD subjects were 277.85 μg ml−1 (s.e.m. 19.51, n=12) and 207.44 μg ml−1 (s.e.m. 22.81, n=18), respectively.
Regression analyses; iTRAQ ratios vs cognition or delirium severity scores
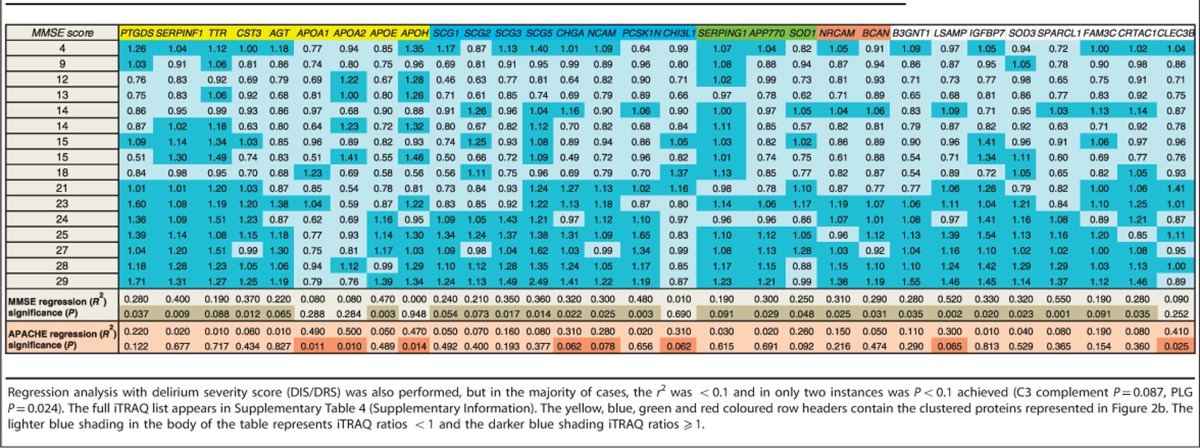
To determine whether there would be any relationship between the 89 proteins identified in all three iTRAQ experiments (both Sydney and Edinburgh study groups), and level of cognitive impairment or delirium severity, regression analysis of iTRAQ ratio vs MMSE, APACHE score and delirium index were performed (Table 3). Several proteins had significant associations with cognitive level as measured by the MMSE score and/or the APACHE III disease severity measure (31 proteins in total). When this subset was processed using the STRING v9.05 bioinformatics tool, the granin cluster increased in number of component proteins (Figure 2b), whereas other clusters either diminished considerably in size or disappeared altogether.
Table 3. Subset of proteins which have a significant regression (P<0.1, and in the majority of cases P<0.05) with iTRAQ ratio vs cognitive function (MMSE) and/or disease severity score (APACHE III, Sydney cohort only).
Discussion
Proteomics of delirium
Our current work is the first iTRAQ quantitative proteomics analysis of delirium CSF. More than 270 proteins were identified with a high level of confidence. Of these, about 10% had dysregulated protein expression levels in 50% or more of delirium subjects relative to the mild AD neurological control group. In total, 26 proteins were identified with dysregulated protein expression in at least six out of 12 delirium subjects from the Sydney Delirium Study (Supplementary Table 3). This relatively modest percentage of the CSF proteome dysregulated by disease is typical in proteomics studies with a case–control design, where 5–20% dysregulation is common. It is important to note that out of the dysregulated proteins subset, about 58% were identified in only one to three subjects, and only 12% were identified in eight or more delirium subjects (Figure 1). Several functional clusters of proteins were identified in the subset of proteins dysregulated in eight or more subjects. These included apolipoproteins and chromogranin/secretogranins (downregulated in most delirium subjects) and inflammation-related proteins (mostly upregulated in delirium). The Sydney Delirium Study also included a neurological comparison group (moderate AD), and interestingly, the majority of proteins which were dysregulated in delirium were unaffected in moderate AD relative to the mild AD control group. The inference we make from this observation is that even though the two disorders are related (that is, risk factors for each other), on a molecular level there are distinguishing features. However our cohort did not include severe AD, and it is possible that this distinction might disappear with increasing AD severity. To determine if the proteomic changes we observed would be replicated in an independent study group, from a different geographical region, a cohort of subjects from Edinburgh were assessed. In addition to delirium subjects, the Edinburgh study included control subjects who had also sustained a hip fracture but did not develop delirium. There was no outpatient AD group in the Edinburgh study. In the Edinburgh study, a total of seven proteins had a significantly dysregulated expression level in at least three out of five delirium subjects. If the low normal group is also included then 14 proteins were dysregulated in at least three out of six subjects. There was considerable overlap with the dysregulated proteins identified in the Sydney Delirium Study, including apolipoproteins, chromogranin/secretogranins and inflammation-related proteins.
Considering both Sydney and Edinburgh studies together, a total of 16 proteins were identified, in which significantly dysregulated protein expression was observed in at least eight out of 17 delirium subjects (Table 2). Of particular interest are several protein functional clusters which emerged, including proteins associated with inflammation, granins, apolipoproteins, clotting factors, protease inhibitors and regulatory proteins. Though members of these protein families have previously been explored in neurodegenerative diseases, none have been studied in the context of delirium.
Markers of inflammation
Significant upregulation of alpha-1-acid glycoprotein was observed in the majority of delirium subjects in both groups, and also in an additional batch of CSF samples from the Sydney study (n=12 delirium, n=18 AD), which were quantified by ELISA. Upregulation of alpha-1-acid glycoprotein appears to be an isoform-specific effect, as the iTRAQ study identified two isoforms, ORM1 and ORM2, but only ORM1 was upregulated in the majority of delirium subjects. Alpha-1-acid glycoprotein may be a specific biomarker for delirium, or at least the effects of systemic illness on the central nervous system, particularly as it does not appear to be dysregulated in dementia, even though inflammation is also a well-documented feature of AD. The ‘high normal' control group subjects of the Edinburgh study also do not have dysregulated expression of ORM1. However these observations will require confirmation with analyses on larger numbers of subjects and further determination of disease specificity by comparison with other neurodegenerative conditions. Inflammation is thought to be one of the main causes of delirium,37 consequently it is not surprising that in addition to alpha-1-acid glycoprotein, the expression of several other acute-phase proteins were observed to be dysregulated in the majority of delirium subjects of both Sydney and Edinburgh studies, including; alpha-2-macroglobulin, fibrinogen, alpha-1-antitrypsin, alpha-1-antichymotrypsin, transferrin, complement component 3 and haptoglobin. Alpha-1-acid glycoprotein is of particular interest as it is a steroid carrier, binds a variety of drugs, and could be a potential target for drug treatment of delirium. Many of the acute-phase markers have dysregulated expression in delirium (Table 2), however none of them are significantly associated with either cognition (MMSE) or disease severity (APACHE III; Table 3), which suggests that they may be indicators of inflammation rather than specific to delirium pathophysiology.
Chromogranins and secretogranins
The majority of delirium subjects in both studies had significantly lower expression levels of chromogranin A, chromogranin B (secretogranin I) and secretogranin III. The moderate AD-pooled group (Sydney Delirium Study) had either downregulated or unchanged granin expression levels whereas the normal controls (Edinburgh Delirium Study) had upregulated granin levels relative to the mild AD neurological control. The chromogranins and secretogranins are a family of neuroendocrine secretory granule proteins. Their normal physiological role is not well understood, however, they are repositiories of a variety of neuropeptides with hormone and neurotransmitter functions. They have not previously been identified in the context of delirium, however, their potential roles in the diagnosis of neurodegenerative diseases are attracting increased interest38, 39, 40 and more specific functions being identified, including binding to the Aβ peptides, well known for their role in AD neurodegeneration.41, 42 Lower expression levels of the granins observed in this study may relate to disturbance of neurotransmission in delirium, providing evidence for a long-standing delirium hypothesis.43 Three of the granins (chromogranin A, secretogranin I and secretogranin III), which were dysregulated (mostly downregulated) in at least 8 out of 17 delirium subjects (Table 2), were also positively associated with MMSE score using regression analysis (Table 3). Furthermore several additional granins and associated proteins (CHI3L1, SCG2, SCG5, NCAM, PCSK1N) were significantly associated with MMSE and/or APACHE III even though their expression was dysregulated in fewer than eight delirium subjects. Together these observations suggest that lower granin levels might be associated with greater delirium severity.
Apolipoproteins
Three apolipoproteins with dysregulated expression levels were identified in both studies; ApoA1, ApoJ (clusterin) and ApoE. These proteins were downregulated in the majority of delirium subjects. In addition, ApoH (beta-2-glycoprotein 1) expression was significantly dysregulated in the majority of Sydney Delirium Study subjects, however, expression levels varied considerably, with both up- and downregulation observed with roughly equal frequency. The apolipoprotein family are best known for their role in the transport of lipids and cholesterol, being the main protein components of HDL and LDL. They have been most extensively studied in the context of cardiovascular disease, but also more recently in neurodegenerative diseases. In particular ApoE has attracted considerable interest as the ApoEɛ4 allele is the main genetic risk factor for late-onset AD,44 and other neurodegenerative disorders.45 A recent meta-analysis suggested an association between delirium and ApoEɛ4 allele,46 however, the role of ApoEɛ4 allele in postoperative delirium is ambiguous, with reports of both significant association47 and absence of association.48, 49 Apart from the role of the different allelic forms, altered CSF levels of apolipoproteins may impact on brain metabolism, microvascular function and association with other proteins which impact on brain pathology, such as amyloid plaque in AD. The other apolipoproteins identified in this study have not previously been studied in delirium, however, a sizable and increasing literature is building on the role of ApoJ (clusterin) in AD. ApoJ is typically found in HDL along with ApoA1. This multifunctional protein may have roles in modulation of inflammation, complement inactivation, Aβ binding and modulation of neuronal plasticity and apoptosis.50 In view of its anti-inflammatory and anti-apoptotic roles, its downregulation in delirium CSF is likely to represent an adverse outcome. Apart from a few specific cases, the broad spectrum of apolipoproteins have not been extensively studied in neurodegenerative diseases in general, though a recent study on plasma apolipoprotein levels in mild cognitive impairment indicates that several apolipoproteins may have an impact on cognition and brain volumetrics in this AD prodrome condition, including ApoA1, ApoE, ApoJ, ApoH and ApoB/ApoA1 ratio.51
Although delirium is a multifactorial syndrome rather than a single disease process, uncovering similar changes in the proteome in delirium in two different clinical groups suggests that there may be some common pathways in delirium. This has implications for the possibility of discovering a diagnostic test and specific treatment(s) for delirium. For example, it is possible that some cases of delirium involve a predominantly inflammatory aetiology and identifying such cases might facilitate the use of anti-inflammatory drugs in that group.
Proteins significantly associated with cognition and delirium severity
Regression analysis of iTRAQ ratios vs MMSE or APACHE III scores identified significant associations of cognition and delirium severity in 31 proteins, of which only five also had significantly dysregulated expression levels in the majority of delirium subjects relative to the controls, these being; APOA1, APOE, SCG1, SCG3 and CHGA. The majority of proteins with significant associations to MMSE or APACHE III scores had dysregulated expression in fewer than eight subjects, suggesting that changes to functionally important proteins are subtle. Several of these may regulate; neuronal differentiation (SERPINF1, CHI3L1, SPARCL1, NCAM1, BCAN, NRCAM), hormone and small molecule binding (IGFBP7, TTR, PTGDS), neuroendocrine secretion (PCSK1N, SCG2, SCG5) and anti-oxidant defence (SOD1, SOD3). This outcome supports the possibility that some proteins with significantly altered expression levels in delirium may not correlate well with clinical indicators of cognition and disease severity. The increased size of the granin cluster (Figure 2b) and other proteins, which correlate with MMSE and/or APACHE scores, indicates that the converse may also be true. However the subject numbers are low, and these possibilities should be explored using larger group numbers and using targeted approaches to increase statistical power.
Pathophysiology of delirium and the relevance of proteomic changes
Delirium is a complex syndrome both in the variety of triggers or risk factors that may initiate onset, as well as the diversity of pathophysiological and cognitive features, which can include inflammation, oxidative stress, reduced blood flow, blood–brain barrier and vascular dysfunction, confusion, cognitive impairment and mood disturbance (Figure 2c). In this context, it is not surprising to find a diversity of proteomic changes representing multiple biological processes, molecular functions and cellular components. Less clear are how these changes orchestrate the delirium syndrome, which are primary or secondary events, the order in which changes occur or even if delirium can be defined by a single pathway.52 Furthermore, dysregulation in some of the proteins we have identified is also reported in other age-related neurodegenerative diseases, and may represent overlap of pathways and processes across different neurodegenerative conditions. However our data does suggest some hypotheses and allows us to make a number of predictions. One striking feature is the uniform upregulation of the inflammatory protein ORM1 and to a lesser extent other acute-phase proteins, while at the same time few correlate with cognition (MMSE) or health status (APACHE III). This suggests an important role for inflammation in delirium, but it is probably not directly related to the cognitive impairment aspect of the disorder. It may, however, be a trigger for other events, one of which could be upregulation of protease inhibitors, such as SERPINS A1 and A3 (Table 2). In fact upregulation of complement, coagulation, fibrinolysis and serpins is typical of the host defence/stress response and we observe upregulation of proteins in each of these groups (Table 2, Supplementary Tables 3 and 4). Although a full understanding of the role of protease inhibition in the brain remains to be elucidated, a neuroprotective role of serpins has been reported.53, 54 Upregulation of protease inhibitors may be a homeostatic response to inflammation, which if left unchecked could cause neuronal apoptosis.55, 56, 57 By contrast, several of the granin family proteins are downregulated in delirium subjects (Table 2), and all of these, as well as a few others are significantly correlated with MMSE and some with APACHE III also (Table 3). This does suggest a role for the granin family group in the cognitive aspects of delirium, and given their precursor role for a variety of neuropeptides, this is not too surprising.38 Some of these peptides, such as secretoneurin and secretogranin I (CHGB) proteolytic fragments, may regulate neurotransmitter levels. We suggest that the lower granin expression levels that we observe imply neurotransmitter deregulation, supporting one of the most popular theories of delirium aetiopathology. CHGA and CHGB both promote secretory granule biosynthesis, which are the main extracellular storage vesicles for catecholamines. Lower CHGB expression level in knockout mice results in fewer catecholamine storage vesicles and unregulated catecholamine release into plasma.58 Furthermore, expression of granin-related neuropeptides may be further compromised as a side effect of SERPIN protease inhibitor upregulation, potentially restricting release of neuropeptides from their granin precursors. In other words, protease inhibition may protect neurons from inflammation-related apoptosis, but as an unwanted side effect compromise granin processing. Interestingly, a number of proteases as well as protease inhibitor enzymes are correlated with MMSE scores (Table 3; SERPINs F1 and G1, CST3, PCSK1N, B3GNT1) suggesting a direct role in cognitive function. This hypothetical model predicts that the level of granin-derived peptides will be lower in the CSF of delirium patients. Altered neurotransmitter levels which have been reported in the delirium literature6 may then partly be due to lower levels of granins, granin-derived neuropeptides and upregulation of protease inhibitors. Lower levels of several apolipoproteins are also observed in the majority of delirium subjects (Table 2), and may occur in parallel with downregulation of the granins, as both protein groups are likely to be constituents of neurosecretory granules. However the apolipoproteins subserve diverse roles (Figure 2c and Supplementary Tables 5 and 6), and may also be contributing to vascular changes reported in delirium, or to neuronal plasticity/axonal regeneration.
Limitations
With 17 CSF samples from delirium subjects in total, ours is a relatively small study and there would be value in terms of statistical power in undertaking additional work involving higher subject numbers. In this study we have employed a neurological control group (mild AD), and an additional normal control group should be included in future work. Furthermore in this discovery proteomics study, we are reporting relative expression changes. Future work should include targeted analyses for absolute quantification such as ELISA or mass spectrometric multiple reaction monitoring approaches. Our two studies also use different methods to ascertain delirium. This can be considered both a limitation and a benefit; a limitation in that it presents a mismatch between the studies and a benefit in that the protein changes that are common to both studies, given their differences, are likely to be more robust biochemical indicators of delirium.
Summary
In this discovery proteomics project, we have quantified >270 proteins in the CSF of delirium and control subjects, and across two independent studies. Of these proteins, 16 were identified with significantly dysregulated protein expression level in the majority of delirium subjects. Within this group of 16 proteins, several family clusters were identified, including markers of inflammation, protease inhibitors, chromogranins/secretogranins and apolipoproteins. Furthermore some of these proteins and a number of others are significantly associated with cognition and delirium severity, as assessed by regression analysis with MMSE or APACHE III scores. These include the chromogranins/secretogranins, SERPINS and apolipoproteins, and also proteins whose functions include neuronal differentiation, hormone and small molecule binding, neuroendocrine secretion and anti-oxidant defence. This work offers some new protein targets that could be used to further explore mechanisms of delirium, and provides a list of potential biomarkers that could facilitate clinical work. Some possibilities which might be considered include; (i) minimally invasive surgical techniques to control inflammation and stress response,59 (ii) anti-apoptotic treatments to minimize neuronal cell damage60 and (iii) treatments to restore neurotransmitter levels.61 Furthermore, this study provides a good rationale for undertaking larger scale work, and targeted analysis of some of the specific proteins we have identified.
Acknowledgments
We thank all the patients who participated in these studies. We gratefully acknowledge the Julia Lowy Foundation for their generous financial support. RJH gratefully acknowledges funding from Research into Ageing and the British Geriatrics Society. The iTRAQ mass spectrometry was carried out in the Bioanalytical Mass Spectrometry Facility of UNSW, and subsidized access to this facility is acknowledged.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- MacLullich AM, Beaglehole A, Hall RJ, Meagher DJ. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21:30–42. doi: 10.1080/09540260802675031. [DOI] [PubMed] [Google Scholar]
- Davis DH, Muniz Terrera G, Keage H, Rahkonen T, Oinas M, Matthews FE, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135:2809–2816. doi: 10.1093/brain/aws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Jones RN, Habtemariam DA, Fong TG, Tommet D, Quach L, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172:1324–1331. doi: 10.1001/archinternmed.2012.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5:210–220. doi: 10.1038/nrneurol.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Ramos MI, Arroyo-Novoa CM, Lee KA, Padilla G, Puntillo KA. Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intensive Care Med. 2009;35:781–795. doi: 10.1007/s00134-009-1397-4. [DOI] [PubMed] [Google Scholar]
- Hall RJ, Shenkin SD, Maclullich AM. A systematic literature review of cerebrospinal fluid biomarkers in delirium. Dement Geriatr Cogn Disord. 2011;32:79–93. doi: 10.1159/000330757. [DOI] [PubMed] [Google Scholar]
- Lim YA, Rhein V, Baysang G, Meier F, Poljak A, Raftery MJ, et al. Abeta and human amylin share a common toxicity pathway via mitochondrial dysfunction. Proteomics. 2010;10:1621–1633. doi: 10.1002/pmic.200900651. [DOI] [PubMed] [Google Scholar]
- Poljak A, Sachdev P, Smythe GA. Causes and diagnosis of Alzheimer's disease: a proteomics approach. Curr Proteomics. 2006;3:81–112. [Google Scholar]
- Eckert A, Schulz KL, Rhein V, Gotz J. Convergence of amyloid-beta and tau pathologies on mitochondria in vivo. Mol Neurobiol. 2010;41:107–114. doi: 10.1007/s12035-010-8109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boogaard M, van Swelm RP, Russel FG, Heemskerk S, van der Hoeven JG, Masereeuw R, et al. Urinary protein profiling in hyperactive delirium and non-delirium cardiac surgery ICU patients. Proteome Sci. 2011;9:13. doi: 10.1186/1477-5956-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt EM, Marcantonio ER, Alsop DC, Jones RN, Rogers SO, Jr., Fong TG, et al. Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13:818 e1–10. doi: 10.1016/j.jamda.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan GA, Kvelde T, Lai C, Yap SL, Lin C, Hill MA. Cerebrospinal fluid in long-lasting delirium compared with Alzheimer's dementia. J Gerontol A Biol Sci Med Sci. 2010;65:1130–1136. doi: 10.1093/gerona/glq090. [DOI] [PubMed] [Google Scholar]
- Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- McCusker J, Cole MG, Dendukuri N, Belzile E. The delirium index, a measure of the severity of delirium: new findings on reliability, validity, and responsiveness. J Am Geriatr Soc. 2004;52:1744–1749. doi: 10.1111/j.1532-5415.2004.52471.x. [DOI] [PubMed] [Google Scholar]
- Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- Lawton MP, Moss M, Fulcomer M, Kleban MH. A research and service oriented multilevel assessment instrument. J Gerontol. 1982;37:91–99. doi: 10.1093/geronj/37.1.91. [DOI] [PubMed] [Google Scholar]
- Wagner DP, Draper EA, Abizanda Campos R, Nikki P, Le Gall JR, Loirat P, et al. Initial international use of APACHE. An acute severity of disease measure. Med Decis Making. 1984;4:297–313. doi: 10.1177/0272989X8400400305. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders; DSM-IV TR. Washington, USA; 2000. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Pearson A, de Vries A, Middleton SD, Gillies F, White TO, Armstrong IR, et al. Cerebrospinal fluid cortisol levels are higher in patients with delirium versus controls. BMC Res Notes. 2010;3:33. doi: 10.1186/1756-0500-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RJ, Ferguson KJ, Andrews M, Green AJ, White TO, Armstrong IR, et al. Delirium and cerebrospinal fluid S100B in hip fracture patients: a preliminary study. Am J Geriatr Psychiatry. 2013;21:1239–1243. doi: 10.1016/j.jagp.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Res. 1988;23:89–97. doi: 10.1016/0165-1781(88)90037-6. [DOI] [PubMed] [Google Scholar]
- Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Burg DW, Ertan H, Raftery MJ, Poljak A, Guilhaus M, et al. Global proteomic analysis of the insoluble, soluble, and supernatant fractions of the psychrophilic archaeon Methanococcoides burtonii. Part II: the effect of different methylated growth substrates. J Proteome Res. 2010;9:653–663. doi: 10.1021/pr9005102. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Burg DW, Raftery MJ, Poljak A, Guilhaus M, Pilak O, et al. Global proteomic analysis of the insoluble, soluble, and supernatant fractions of the psychrophilic archaeon Methanococcoides burtonii. Part I: the effect of growth temperature. J Proteome Res. 2010;9:640–652. doi: 10.1021/pr900509n. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Lauro FM, Ertan H, Burg DW, Poljak A, Raftery MJ, et al. Defining the response of a microorganism to temperatures that span its complete growth temperature range (−2 degrees C to 28 degrees C) using multiplex quantitative proteomics. Environ Microbiol. 2011;13:2186–2203. doi: 10.1111/j.1462-2920.2011.02467.x. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohee S, van Helden J. Evaluation of clustering algorithms for protein-protein interaction networks. BMC Bioinformatics. 2006;7:488. doi: 10.1186/1471-2105-7-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain HM, Bruford EA, Lovering RC, Lush MJ, Wright MW, Povey S. Guidelines for human gene nomenclature. Genomics. 2002;79:464–470. doi: 10.1006/geno.2002.6748. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Maclullich AM. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav Immun. 2013;28:1–13. doi: 10.1016/j.bbi.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh YP, Salton SR. The extended granin family: structure, function, and biomedical implications. Endocr Rev. 2011;32:755–797. doi: 10.1210/er.2010-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- Lechner T, Adlassnig C, Humpel C, Kaufmann WA, Maier H, Reinstadler-Kramer K, et al. Chromogranin peptides in Alzheimer's disease. Exp Gerontol. 2004;39:101–113. doi: 10.1016/j.exger.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Johansson P, Hansson O, Wallin A, Johansson JO, Andreasson U, et al. Converging pathways of chromogranin and amyloid metabolism in the brain. J Alzheimers Dis. 2010;20:1039–1049. doi: 10.3233/JAD-2010-091651. [DOI] [PubMed] [Google Scholar]
- Pla V, Paco S, Ghezali G, Ciria V, Pozas E, Ferrer I, et al. Secretory sorting receptors carboxypeptidase E and secretogranin III in amyloid beta-associated neural degeneration in Alzheimer's disease. Brain Pathol. 2012;23:274–284. doi: 10.1111/j.1750-3639.2012.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Aoki T, Ishizuka B. Postoperative delirium and plasma melatonin. Med Hypotheses. 1999;53:103–106. doi: 10.1054/mehy.1998.0724. [DOI] [PubMed] [Google Scholar]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Munster BC, Korevaar JC, Zwinderman AH, Leeflang MM, de Rooij SE. The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta-analysis. Am J Geriatr Psychiatry. 2009;17:856–862. doi: 10.1097/JGP.0b013e3181ab8c84. [DOI] [PubMed] [Google Scholar]
- Leung JM, Sands LP, Wang Y, Poon A, Kwok PY, Kane JP, et al. Apolipoprotein E e4 allele increases the risk of early postoperative delirium in older patients undergoing noncardiac surgery. Anesthesiology. 2007;107:406–411. doi: 10.1097/01.anes.0000278905.07899.df. [DOI] [PubMed] [Google Scholar]
- Abelha FJ, Fernandes V, Botelho M, Santos P, Santos A, Machado JC, et al. Apolipoprotein E e4 allele does not increase the risk of early postoperative delirium after major surgery. J Anesth. 2012;26:412–421. doi: 10.1007/s00540-012-1326-5. [DOI] [PubMed] [Google Scholar]
- Tagarakis GI, Tsolaki-Tagaraki F, Tsolaki M, Diegeler A, Tsilimingas NB, Papassotiropoulos A. The role of apolipoprotein E in cognitive decline and delirium after bypass heart operations. Am J Alzheimers Dis Other Demen. 2007;22:223–228. doi: 10.1177/1533317507299415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay Y, Imhof A, Vallet PG, Kovari E, Bouras C, Giannakopoulos P. Clusterin in neurological disorders: molecular perspectives and clinical relevance. Brain Res Bull. 2012;88:434–443. doi: 10.1016/j.brainresbull.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Song F, Poljak A, Crawford J, Kochan NA, Wen W, Cameron B, et al. Plasma apolipoprotein levels are associated with cognitive status and decline in a community cohort of older individuals. PLoS One. 2012;7:e34078. doi: 10.1371/journal.pone.0034078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21:1190–1222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Soeda S, Koyanagi S, Kuramoto Y, Kimura M, Oda M, Kozako T, et al. Anti-apoptotic roles of plasminogen activator inhibitor-1 as a neurotrophic factor in the central nervous system. Thromb Haemost. 2008;100:1014–1020. doi: 10.1160/th08-04-0259. [DOI] [PubMed] [Google Scholar]
- Miranda E, Lomas DA. Neuroserpin: a serpin to think about. Cell Mol Life Sci. 2006;63:709–722. doi: 10.1007/s00018-005-5077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection. Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Sonneville R, Verdonk F, Rauturier C, Klein IF, Wolff M, Annane D, et al. Understanding brain dysfunction in sepsis. Ann Intens Care. 2013;3:15. doi: 10.1186/2110-5820-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CG, Patel MB, Pandharipande PP. Pathophysiology of acute brain dysfunction: what's the cause of all this confusion. Curr Opin Crit Care. 2012;18:518–526. doi: 10.1097/MCC.0b013e328357effa. [DOI] [PubMed] [Google Scholar]
- Zhang K, Biswas N, Gayen JR, Miramontes-Gonzalez JP, Hightower CM, Mustapic M, et al. Chromogranin B: intra- and extra-cellular mechanisms to regulate catecholamine storage and release, in catecholaminergic cells and organisms. J Neurochem. 2014;129:48–59. doi: 10.1111/jnc.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl R, Bekker A, Babu R. Neuroendocrine and immune responses to surgery. Internet J Anesthesiol. 2012;30:3–15. [Google Scholar]
- Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- Nilsson A, Falth M, Zhang X, Kultima K, Skold K, Svenningsson P, et al. Striatal alterations of secretogranin-1, somatostatin, prodynorphin, and cholecystokinin peptides in an experimental mouse model of Parkinson disease. Mol Cell Proteomics. 2009;8:1094–1104. doi: 10.1074/mcp.M800454-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.