Abstract
Fluoxetine is the only psychopharmacological agent approved for depression by the US Food and Drug Administration for children and is commonly used therapeutically in a variety of neurodevelopmental disorders. Therapeutic response shows high individual variability, and severe side effects have been observed. In the current study we set out to identify biomarkers of response to fluoxetine as well as biomarkers that correlate with impulsivity, a measure of reward delay behavior and potential side effect of the drug, in juvenile male rhesus monkeys. The study group was also genotyped for polymorphisms of monoamine oxidase A (MAOA), a gene that has been associated with psychiatric disorders. We used peripheral metabolite profiling of blood and cerebrospinal fluid (CSF) from animals treated daily with fluoxetine or vehicle for one year. Fluoxetine response metabolite profiles and metabolite/reward delay behavior associations were evaluated using multivariate analysis. Our analyses identified a set of plasma and CSF metabolites that distinguish fluoxetine- from vehicle-treated animals and metabolites that correlate with impulsivity. Some metabolites displayed an interaction between fluoxetine and MAOA genotype. The identified metabolite biomarkers belong to pathways that have important functions in central nervous system physiology. Biomarkers of response to fluoxetine in the normally functioning brain of juvenile nonhuman primates may aid in finding predictors of response to treatment in young psychiatric populations and in progress toward the realization of a precision medicine approach in the area of neurodevelopmental disorders.
Introduction
Developmental childhood disorders such as attention deficit hyperactivity disorder, autism, mental retardation and cerebral palsy are frequently treated with antidepressant drugs of the selective serotonin reuptake inhibitor (SSRI) type to control behavioral symptoms.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 While short-term efficacy and toxicity have been extensively studied, little is known about long-term consequences of SSRI drug treatment in children and adolescents especially as they relate to brain development.
Rodent studies have demonstrated that while acute fluoxetine treatment has antidepressant effects,11 chronic treatment of the animals during early life increases depressive- and anxiety-like behaviors in adulthood.12, 13, 14, 15, 16, 17 It is therefore conceivable that antidepressant drugs can have profound effects on brain developmental events that become apparent later in adulthood.
Concerns about long-term consequences of SSRI treatment were initially raised in a subset of patients suffering from major depressive disorder where the drugs caused undesired and sometimes severe side effects including suicidality (suicidal ideas or behavior).18, 19, 20 Already in 1991 the US Food and Drug Administration was made aware of concerns that the SSRI fluoxetine, marketed as Prozac, was causing suicidal behaviors that occurred right at the onset of treatment. Similar observations were also obtained for other antidepressants including amitriptyline and paroxetine. Treatment-emergent suicidal ideation in response to SSRI treatment21, 22, 23, 24, 25, 26 was later confirmed by a meta-analysis,27 which prompted the US Food and Drug Administration to issue a black-box warning for several SSRIs including fluoxetine. This warning was particularly directed toward SSRI treatment of children and adolescents.28
The immediate pharmacological action of SSRIs is an increase of monoamine levels in the synaptic cleft. However, the emergence of therapeutic effects in patients takes 4–6 weeks of daily treatment.29 Before that many patients show decreased psychomotor retardation along with increased energy levels, but still suffer from the typical major depressive disorder symptoms of low self-esteem, worthlessness and guilt. This combination of symptoms can lead to a disinhibitory effect and an increased risk of suicidality. Other clinical symptoms that have been associated with suicidality in response to antidepressant treatment include insomnia, akathisia and panic attacks.18 Reports on the identification of reliable treatment-emergent suicidal ideation predictors or risk factors able to identify patient subgroups experiencing adverse side effects have been scarce. In one study patients with treatment-emergent suicidal ideation were compared with patients without increase in suicidal ideation and a subgroup that never reported suicidal ideation.30 Although the study was carried out with small cohort numbers, the results indicated that a combination of genetic markers may be able to classify treatment-emergent suicidal ideation patients.
As mentioned above, consequences for brain development are a major concern of long-term treatment with fluoxetine. This does not only apply to fetal exposure but also to children and adolescents subjected to antidepressant treatment.31 Adverse consequences that can affect behavior, cognitive abilities and emotion may result from chronic exposure to antidepressants and psychotropic drugs in early life. In addition, the drugs can potentially lead to structural central nervous system (CNS) alterations with unknown consequences on behavior in adulthood, a phenomenon known as neuronal imprinting. In light of the adverse effects that were observed in children taking antidepressants, it is critical to obtain improved clinical parameters that can help the physician with treatment. Biosignatures can be of great value not only for predicting therapeutic response to the drug but also in the delineation of pathways affected by the drug.
Similar to humans, rhesus monkeys have a prolonged stage of juvenile development between infancy and puberty and are therefore considered a good model to study long-term SSRI effects. The animals also have many polymorphisms in genes that have been associated with psychiatric disorders in humans.
Metabolites that reflect pathway activity, also referred to as ‘Authentic Biomarkers', provide a metric for predicting treatment response and undesired side effects.
Several recent studies have utilized metabolomics, a method to study a great number of metabolites, to interrogate psychiatric drug treatment effects. Atypical antipsychotic treatment influenced the metabolism of specific lipid classes in patients with schizophrenia.32 Metabolomic studies on treatment effects of traditional Chinese medicine in rats identified potential biomarker candidates.33, 34 In another study a pharmacometabolomic approach was used to guide targeted pharmacogenomic analyses in antidepressant responders versus nonresponders.35 A pilot study in depressed patients of old age revealed alterations in plasma metabolite levels of GABA, glycerol and several fatty acids compared with controls36 with many of the alterations normalized after remission.
Similar to fluoxetine the SSRI paroxetine inhibits presynaptic serotonin transporters leading to enhanced serotonergic synaptic transmission, which was previously shown to be essential for therapeutic efficiency.37 We have results from a metabolomics analysis in DBA/2Ola mice that enhanced serotonin availability resulting from chronic paroxetine treatment that leads to diverse downstream pathway alterations.38 Pathways affected by paroxetine treatment were directly or indirectly related to energy metabolism and included glycolysis, amino acid metabolism and hormone signaling. Mitochondrial dysfunction including energy metabolism has been repeatedly reported in neuropsychiatric disorders.39 Importantly, a comparison of CNS with blood plasma metabolite alterations identified several metabolites as biomarker candidates for the assessment of paroxetine treatment effects in the periphery.38
In the current study we have examined the long-term effects of fluoxetine in juvenile male rhesus monkeys, an animal with brain functions similar to humans that differ in two common gene polymorphisms for monoamine oxidase A (MAOA) activity. Animals were of the same age, housed under controlled conditions and fed an identical diet. Despite being an outbred population this improves the signal-to-noise ratio of the drug-elicited metabolite level changes as well as statistical analyses, thereby aiding the discovery of pathways affected by drug treatment.
We used metabolite profiling data obtained from plasma and cerebrospinal fluid (CSF) to identify biomarkers of pharmacological response and biomarkers that are associated with impulsivity behavior during childhood after 1 year of fluoxetine treatment. Brain-derived metabolites are more abundant and hence easier to detect in CSF compared with blood where metabolites from many other body organs are present. Still, brain-derived metabolites can be detected in blood, albeit using the more sensitive targeted metabolomics approach.
To compare fluoxetine's metabolic profile and behavioral effects, we used a reward delay test. The most common reward delay test used in children, reward devaluation, is associated with parent and teacher ratings of self-control and with the temperament trait conscientiousness,40 predicts later impulse control, achievement and competence into adolescence,41, 42 and is associated with location and complexity of neural networks.42, 43, 44 Poor performance on reward delay tests is commonly interpreted as impulsivity, or a deficit in response inhibition. Another potential interpretation has to do with reward valence, or the experienced value of the reward, with increased reward value leading to premature responses.40, 45
The identification of predictors/biosignatures of side effects is critical for pediatric psychiatry by enabling the physician to initiate preventive interventions in patients suffering from childhood disorders with behavioral components. Prediction of response to treatment will be valuable in childhood pharmacotherapy to optimize therapeutic response and minimize adverse side effects. Ultimately it is hoped that the metabolite biomarkers will aid in stratifying patients by correlating them with genetic and behavioral data. Affected pathway information will improve our understanding of adverse effects caused by fluoxetine in children.
Materials and methods
Assurance of compliance with animal codes
All procedures followed the Guide for the Care and Use of Laboratory Animals of the National Research Council. The CNPRC (California National Primate Research Center) is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Protocols for this project were approved before implementation by the UC Davis Institutional Animal Care and Use Committee.
Animals and animal care
Male rhesus monkeys (Macaca mulatta) approximately 1 year of age were selected from the colony at the CNPRC to form a cohort balanced for age, health history, infant stress responsiveness as evaluated routinely at 3 months of age, and genetic polymorphism of MAOA and 5HTTLPR genes, which affect serotonin-mediated brain functions. Genotyping for MAOA variable number tandem repeat (VNTR) polymorphisms was conducted by the UC Davis Veterinary Genetics Laboratory using PCR with forward and reverse primers. The male infants in our study were identified as hemizygous for low transcribing (7 VNTR) or high transcribing (4, 5 or 6 VNTR) polymorphisms.46 The animals were transferred to an indoor cage room and housed in double cages that allowed socialization in pairs. The cages were arranged to the right and left sides of a central area of the cage room and in two vertical tiers. On the basis of experience, MAOA genetic polymorphism was included in the design of the study and cage location variables were screened as potential covariates in data analyses.
All subjects received identical, standardized husbandry and enrichment according to CNPRC protocols. Routine husbandry included a 12 h light cycle, daily cleaning of cages and replacement with sterilized cages at 2 week intervals, feeding of commercial diet twice daily, enrichment with play objects inside the cage, mirrors on the outside of the cages and novel food items provided on a regular schedule.
The juvenile monkeys were gradually adapted to dosing and behavioral testing procedures. At the end of the first year of dosing, the impulsivity test and sampling for metabolomic analysis reported here were conducted. After 1 year of dosing, monkeys were assessed with the behavioral impulsivity test (reward delay) to evaluate the side effects of fluoxetine treatment on the behavior level. Blood and CSF specimens were collected for metabolomics analysis.
Animal health was evaluated daily. No conditions resulting in veterinary diagnosis were reported, with the exception of episodes of diarrhea treated with Tylosin. Linear and ponderal growth were measured at intervals throughout the study without indication of fluoxetine effects during the dosing period (Supplementary Table 1).
Study design
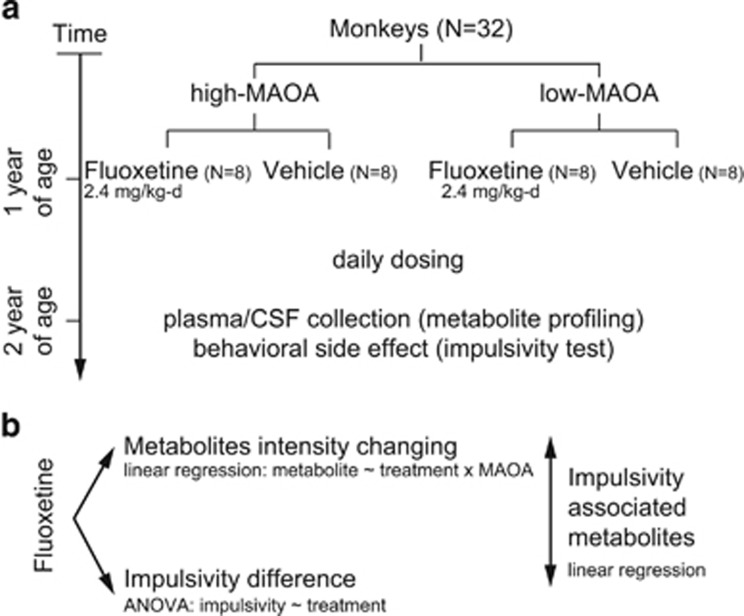
Juvenile male rhesus monkeys with high- and low-activity MAOA polymorphism genotypes (n=16/MAOA polymorphism group) were randomized within group to receive either fluoxetine or an equivalent volume of vehicle (n=16 per treatment group) as shown in Figure 1.
Figure 1.
Study design and analysis strategy. (a) Study design. Thirty-two juvenile male rhesus monkeys with high- and low-activity MAOA genotypes were used in our study. At 1 year of age, animals were randomized to receive either fluoxetine or an equivalent volume of vehicle per day (n=8 per group). After 1 year of dosing, monkeys were assessed with impulsivity test to evaluate the fluoxetine effect on the behavioral level and blood and cerebrospinal fluid (CSF) specimens were collected for metabolomics analysis. (b) Analysis strategy. To identify metabolite biomarkers for the response to fluoxetine, multivariate linear regression model was applied for plasma and CSF metabolite profiling data. Analysis of variance was used to examine the difference of impulsivity outcomes between fluoxetine-treated and control animals. To identify metabolites associated with impulsivity, we performed multivariate linear regression of the impulsivity outcomes (average screen intervals) on the intensity of each metabolite in plasma and CSF. MAOA, monoamine oxidase A.
Fluoxetine dosing
Fluoxetine dose selection was based on information in the human and nonhuman primate literature and on a preliminary pharmacokinetic/pharmacodynamic study to provide steady state circulating levels of fluoxetine/norfluoxetine in the range reported for therapeutic use of fluoxetine in children.47 Dosing was initiated at 1 year of age at 1.6 mg kg−1 per day and adjusted to 2.4 mg kg−1 per day after 11 months. Liquid fluoxetine (20 mg per 5 ml, Webster Veterinary) was prepared for oral dosing by dilution in flavored commercial flavoring syrup (Torani). Monkeys were trained to come to the front of the cage and receive the dose from the end of a 6 ml syringe, which was inserted into the cage. The vehicle administration consisted of fluid with the same taste and volume.
Impulsivity test
To compare fluoxetine's metabolic profile and behavioral effects, we used a reward delay test adapted for monkeys (Supplementary Figure 1) from similar tests in children to measure impulsivity.48, 49 For the impulsivity test, monkeys were relocated individually to a separate test room. The impulsivity test was hand administered in the Wisconsin General Test Apparatus in one session of 40 trials. For each trial, an opaque door was raised revealing a test board consisting of a small preferred food treat (raisin, miniature marshmallow) covered with a large transparent plastic box behind an opaque plastic screen. The screen was moved back 1 inch every 2 s for seven screen intervals until the box was fully disclosed, at which point the monkey could displace the box and retrieve the reward. If the monkey touched the screen or the box before the box was fully disclosed the trial was terminated. The apical end point for analysis was the number of screen intervals to complete the trial. Testing was blinded and randomized for group.
Blood and CSF sampling
Venous blood samples for plasma extraction were collected after an overnight fast under ketamine anesthesia (10 mg kg−1 intramuscular). The anesthesia was then supplemented with dexmedetomidine (0.0075-0.015 mg kg−1) for CSF collection, which was performed via the drip method from the cisterna magna under sterile conditions.
Metabolite extraction
Plasma and CSF samples were extracted with 1 ml of −20 °C isopropanol/acetonitrile/water (IPA/ACN/H2O) (3/3/2, v/v/v ). Samples were mixed for 5 min at 4 °C using an Eppendorf Orbital Mixing Chilling/Heating plate (Hauppauge, NY, USA) and 500 μl dried extracts were stored at −80 °C. See Supplementary Methods for details on metabolite measurements.
Statistical analysis
The design and analysis strategy are depicted in Figure 1. To identify metabolite biomarkers in response to fluoxetine treatment, multivariate linear regression model was applied and the metabolites with significant intensity difference between vehicle- and fluoxetine-treated groups were selected. We normalized the intensities of each metabolite to have a mean of zero and a s.d. of one. MAOA genotype was added in the regression analysis considering the treatment-by-genotype interaction. Regression model was formulated as Intensity of Metabolite~Treatment × Genotype. Owing to the batch bias in the plasma metabolite profiling, batch was added as a covariate. The significance level was set at P-value<0.05.
The apical end point of the impulsivity test was analyzed by two-way analysis of variance (GLM, JMP/SAS) using drug treatment and MAOA genotype as the independent variables. In addition, background variables were screened for association with this end point and included as covariates when appropriate. The significance level was P-value<0.05.
To identify metabolites that indicated the behavior-related side effect, we used multivariate linear regression and calculated the coefficients between the metabolite intensities and the impulsivity outcome (the average screen interval). As the covariate cage tier (‘top/bottom') introduced bias in the behavioral outcome, we controlled the cage location as a covariate variable in regression. In addition to the cage tier effect, the batch bias in the plasma metabolite profiling was also considered as a covariate in the regression for plasma metabolite profiling data. All calculations were performed under the R statistical environment (http://www.r-project.org/). Pathway analysis was conducted using MetaboAnalyst online pathway tool (http://www.metaboanalyst.ca).
Results
Plasma and CSF metabolite profiling in response to fluoxetine treatment
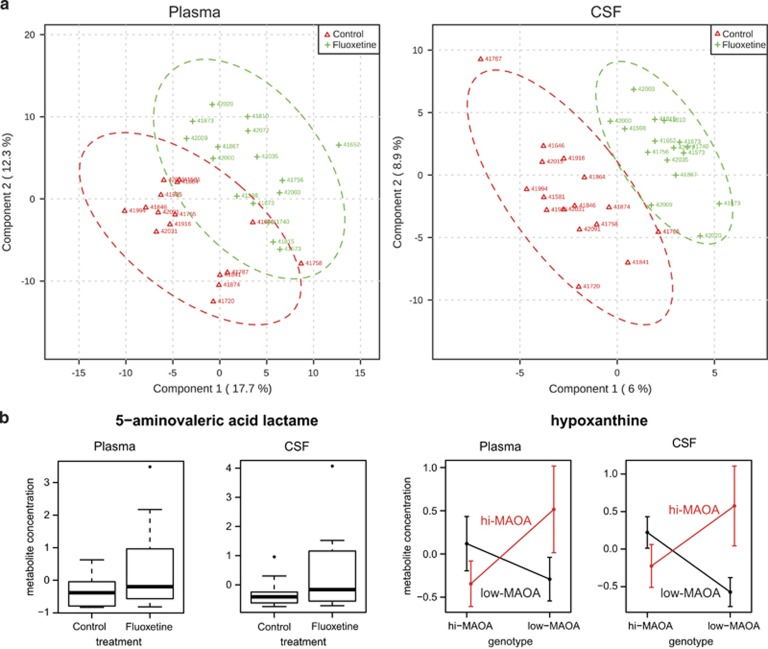
A total of 143 metabolites in plasma and 100 metabolites in CSF with known identities were covered in the analysis. To explore the global metabolite intensity differences among groups with different treatment and genotype, a partial least square discriminant analysis was performed on the plasma and CSF metabolite profiles. The partial least square discriminant analysis results demonstrated separation of metabolite profiles between fluoxetine- and vehicle-treated groups (Figure 2a) and separation between high- and low-MAOA genotypes. CSF displayed a better separation than plasma samples.
Figure 2.
Plasma and CSF metabolites in response to fluoxetine treatment. (a) Partial least square (PLS) analysis demonstrates separation of metabolite profiling between fluoxetine- (green) and vehicle-treated (red) monkeys in both plasma and CSF specimens. (b) Two metabolite candidates that respond to fluoxetine treatment in both plasma and CSF. 5-aminovaleric acid lactam (left) displayed increased metabolite level in fluoxetine-treated group. Hypoxanthine's levels (right) are dependent on treatment-by-genotype interaction. The upper and lower borders of the box represent the 25th and 75th percentile, respectively; the solid line represents the median; upper and lower whiskers are minimum and maximum without outliers, respectively; points represent outliers. CSF, cerebrospinal fluid; MAOA, monoamine oxidase A.
To identify metabolites that differ between fluoxetine and vehicle treatments, multivariate linear regression analyses were carried out for each metabolite. In plasma, we found 19 metabolites that showed significant intensity level differences (P-value <0.05) between control and fluoxetine groups. Odds ratios (ORs) and P-values are shown in Table 1. Among them, twelve metabolites, for example, 5-aminovaleric acid lactam (Figure 2b) and nicotinic acid presented higher intensity levels and seven metabolites, for example, arachidonic acid showed lower intensity levels in fluoxetine- compared with vehicle-treated animals. On the basis of the regression on treatment-by-genotype interaction, twenty-one metabolites displayed a significant interaction between fluoxetine and MAOA genotype, indicating that they depend on both factors. Ten metabolites, including arachidonic acid and hypoxanthine, showed lower intensity levels in high-MAOA compared with low-MAOA monkeys in the fluoxetine treatment group. An opposite trend was observed in the control group. Eleven metabolites, including dehydroascorbic acid, showed the opposite pattern, higher intensity levels in fluoxetine-treated high-MAOA monkeys versus low-MAOA monkeys, but with opposite trends in the control group.
Table 1. Plasma and CSF metabolite odds ratios and P-values in response to fluoxetine treatment.
| Metabolite |
Fluoxetine vs vehicle |
Fluoxetine-by-MAOA interaction |
||
|---|---|---|---|---|
| Odds ratio | P-value | Odds ratio | P-value | |
| Plasma | ||||
| Asparagine | 4.22 | 0.002* | 0.22 | 0.017* |
| Glyoxalureaa | 3.20 | 0.004* | 0.23 | 0.010* |
| Parabanic acida | 2.46 | 0.012* | 0.34 | 0.031* |
| Oxamic acid | 3.37 | 0.014* | 0.20 | 0.020* |
| Nicotinic acid | 2.08 | 0.016* | 0.43 | 0.044* |
| 2,3-Dihydroxypyridine | 2.42 | 0.030* | 0.25 | 0.017* |
| Shikimic acid | 2.52 | 0.030* | 0.08 | 0.000* |
| Dehydroascorbic acid | 2.81 | 0.036* | 0.19 | 0.018* |
| 5-Aminovaleric acid lactam | 3.09 | 0.024* | 0.43 | 0.213 |
| Glycolic acid | 2.49 | 0.029* | 0.39 | 0.107 |
| Urea | 2.87 | 0.039* | 0.40 | 0.194 |
| 2,3,5-Trihydroxypyrazinea | 2.50 | 0.049* | 1.00 | 0.997 |
| Indole-3-lactate | 0.31 | 0.014* | 5.98 | 0.009* |
| Arachidonic acid | 0.34 | 0.021* | 6.21 | 0.007* |
| Myristic acid | 0.34 | 0.031* | 4.65 | 0.029* |
| Hydroxylamine | 0.34 | 0.017* | 3.38 | 0.052 |
| N-acetyl-5-hydroxytryptamine | 0.34 | 0.035* | 3.22 | 0.103 |
| Palmitic acid | 0.35 | 0.038* | 4.07 | 0.052 |
| Fructose | 0.36 | 0.048* | 3.75 | 0.067 |
| 3-Phosphoglycerate | 2.17 | 0.098 | 0.13 | 0.003* |
| 2-Deoxyerythritola | 1.87 | 0.201 | 0.20 | 0.027* |
| Benzylalcohol | 1.77 | 0.253 | 0.20 | 0.029* |
| Allantoic acid | 0.46 | 0.104 | 6.29 | 0.009* |
| Cysteine | 0.42 | 0.078 | 5.78 | 0.015* |
| Fumaric acid | 0.71 | 0.475 | 5.27 | 0.019* |
| Linoleic acid | 0.41 | 0.070 | 5.02 | 0.022* |
| Isoheptadecanoic acid | 0.38 | 0.055 | 4.88 | 0.030* |
| 2-Ketoisocaproic acid | 0.64 | 0.366 | 4.24 | 0.045* |
| Hypoxanthine | 0.58 | 0.275 | 4.13 | 0.048* |
| CSF | ||||
| 5-Aminovaleric acid lactam | 2.83 | 0.035* | 0.44 | 0.230 |
| Isoheptadecanoic acid | 0.31 | 0.017* | 2.26 | 0.217 |
| Tryptophan | 0.32 | 0.025* | 3.75 | 0.060 |
| Ribitol | 0.32 | 0.026* | 3.27 | 0.092 |
| 2-Hydroxybutanoic acid | 0.37 | 0.041* | 3.16 | 0.094 |
| Hypoxanthine | 0.64 | 0.352 | 4.92 | 0.024* |
| Threitol | 2.39 | 0.072 | 0.19 | 0.018* |
| N-acetylmannosamine | 1.45 | 0.433 | 0.25 | 0.047* |
Abbreviations: CSF, cerebrospinal fluid; MAOA, monoamine oxidase A.
Identified by NIST mass spectral library. *P =0.04.
In CSF we found that tryptophan, ribitol, 2-hydroxybutanoic acid and isoheptadecanoic acid differed in intensity levels between fluoxetine- and vehicle-treated animals. Isoheptadecanoic acid also presented a similar trend in plasma (ORs=0.38 and P-value=0.055) and had a significantly lower intensity level in fluoxetine- versus vehicle-treated animals in CSF (ORs=0.31 and P-value=0.017). Moreover, 5-aminovaleric acid lactam displayed an increased level in the fluoxetine group (ORs=2.83 and P-value=0.035) and was also significantly different in plasma (ORs=3.09 and P-value=0.024, Figure 2b). Taking treatment-by-genotype interaction into consideration, three metabolites presented a significant interaction effect (Table 1). Two of them, threitol and N-acetylmannosamine, displayed higher intensity levels in high-MAOA monkeys compared with low-MAOA animals of the fluoxetine-treated group, with an opposite direction in the control group. For hypoxanthine for which we also found an interaction in plasma (ORs=4.13 and P-value=0.048, Figure 2b), we observed the same interaction trend in CSF (ORs=4.92 and P-value=0.024).
Fluoxetine increased juvenile rhesus monkey's impulsivity
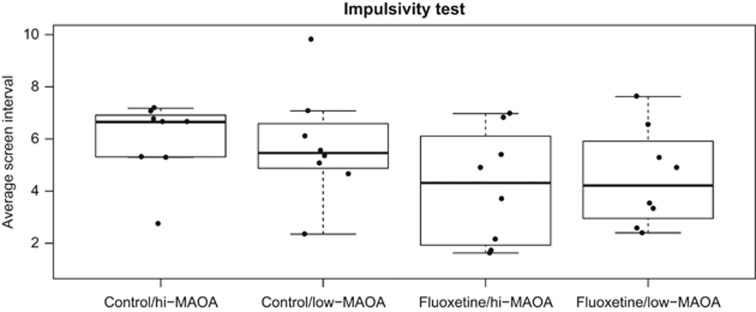
The impulsivity test measures how long the monkey can wait to receive the food reward by counting the screen intervals. Analysis of variance demonstrated significant effect of fluoxetine on screen interval with no significant effect of MAOA polymorphism and no interaction (Figure 3). Cage position (top or bottom tier) was identified as a covariate. The fluoxetine effect including cage position covariate was significant by analysis of variance (P=0.024). This test indicated that fluoxetine treatment could induce a high-impulsivity side effect in juvenile monkeys. However, no significant difference between high- and low-MAOA genotypes was observed.
Figure 3.
Fluoxetine increases juvenile rhesus monkey impulsivity. The number of screen intervals elapsing before the monkey responded were averaged across 40 trials of the test session. Fluoxetine, but not MAOA genotype, influenced performance (P=0.024). The upper and lower borders of the box represent the 25th and 75th percentile, respectively; the solid line represents the median; upper and lower whiskers are minimum and maximum without outliers, respectively. Points represent the average screen intervals across 40 trials of individual monkeys in each group. MAOA, monoamine oxidase A.
Metabolites associated with impulsivity
As we observed the impulsivity side effect on juvenile monkeys treated with fluoxetine for 1 year, we next investigated whether blood or CSF metabolite levels can reflect this behavior. Simple linear regression of the average screen interval on signal intensity of each metabolite provided a test of association between metabolite levels and impulsivity behavior. In plasma, we found three metabolites (xylitol, sucrose, guanosine) that displayed a negative association with the average screen interval (Table 2). These were independent of the batch effect bias of the plasma metabolite data and the cage tier.
Table 2. Plasma and CSF metabolites associated with impulsivity.
| Metabolite |
Average screen interval |
P-value |
|||
|---|---|---|---|---|---|
| Coefficients | 95% CI (2.5%, 97.5%) | P-value | Batch effect | Cage effect | |
| Sucrosea | −0.81 | (−1.45, −0.17) | 0.029* | 0.143 | 0.048* |
| Xylitola | −0.91 | (−1.65, −0.18) | 0.031* | 0.746 | 0.043* |
| Guanosinea | −1.37 | (−2.55, −0.18) | 0.043* | 0.128 | 0.111 |
| Valineb | −1.92 | (−3.12, −0.72) | 0.008* | — | 0.109 |
| Xylitolb | −1.23 | (−2.24, −0.23) | 0.032* | — | 0.061 |
| Alanine 2b | −1.38 | (−2.59, −0.17) | 0.044* | — | 0.266 |
| Pyruvic acidb | −1.23 | (−2.33, −0.13) | 0.047* | — | 0.768 |
| Alanine 1b | −1.36 | (−2.57, −0.14) | 0.048* | — | 0.273 |
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid.
Plasma.
CSF. *P = 0.04.
In CSF we had not observed a batch effect for metabolite profiling, so we regressed the average screen interval on the intensity of each metabolite by only adding cage location as a confounding variable. Five metabolites (xylitol, valine, pyruvic acid, alanine) were found negatively associated with the behavior outcome (Table 2) and the associations were independent of cage location. The negative coefficients of these metabolites from our models represent positive association between metabolite changes in blood and CSF specimens and impulsivity level during fluoxetine dosing.
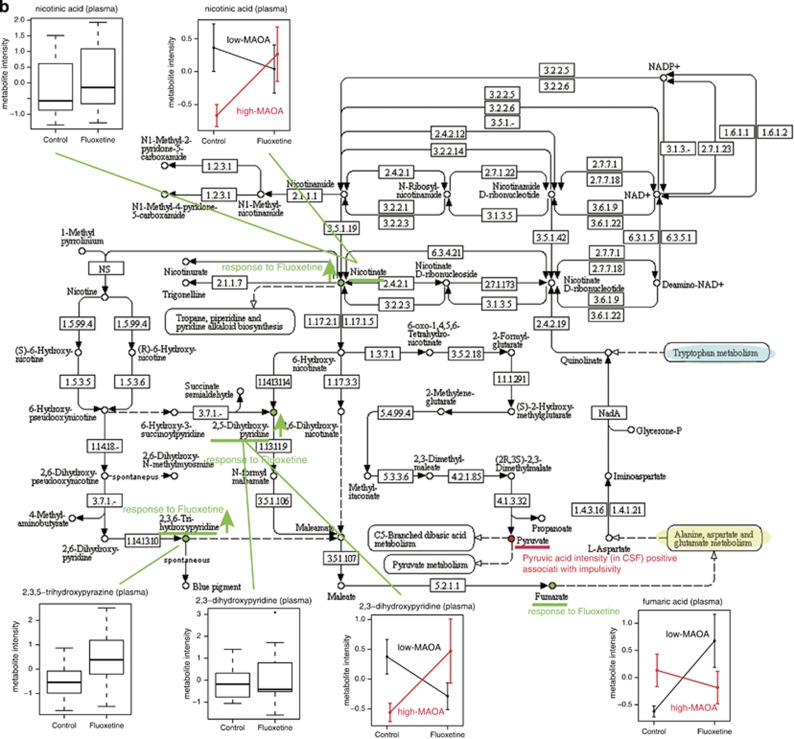
Pathways affected by fluoxetine
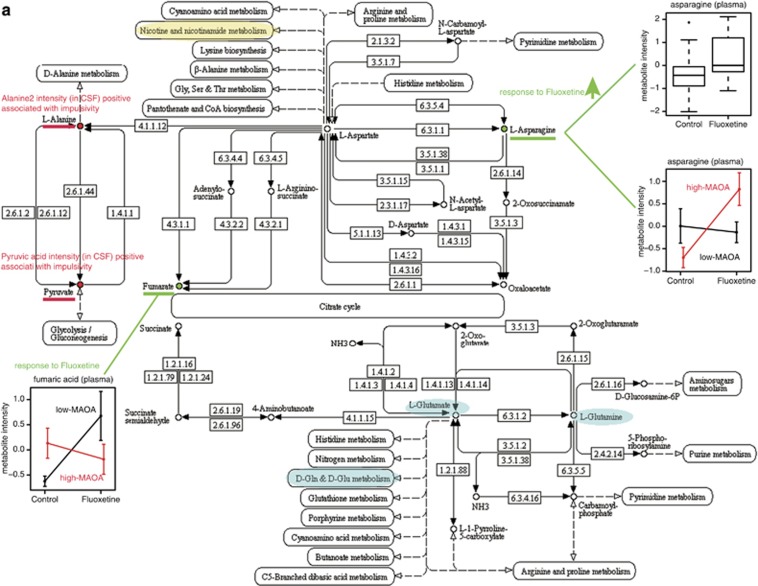
To explore the biological function and pathways that were affected by fluoxetine, we applied function annotation and enrichment analysis on the metabolite candidates that we found in plasma and CSF in response to fluoxetine treatment and indicating impulsivity (Figure 4). Two pathways that are linked, ‘Alanine, Aspartate, Glutamate' metabolism (Figure 4a) and ‘Nicotinate, Nicotinamide' metabolism (Figure 4b), were influenced by fluoxetine treatment. Two metabolites, asparagine and fumaric acid, responded to fluoxetine in plasma, and two impulsivity indicators, alanine and pyruvic acid, were mainly involved in ‘Alanine, Aspartate and Glutamate' metabolism. For ‘Nicotinate, Nicotinamide' metabolism, we found the impulsivity indicator pyruvic acid and four other metabolites (nicotinic acid, 2,3-dihydroxypyridine, 2,3,5-trihydroxypyrazine, fumaric acid) altered by fluoxetine as well as dependent on MAOA genotype. Both pathways are of relevance for psychiatric phenotypes, either containing metabolites with important functions in the CNS, such as nicotinate and glutamate, or being linked to other pertinent pathways that include neurotransmitters, such as serotonin in tryptophan metabolism.
Figure 4.
Molecular pathways. (a) ‘Alanine, Aspartate, Glutamate' metabolism and (b) ‘Nicotinate, Nicotinamide' metabolism pathways are implicated in fluoxetine treatment response, associated with impulsivity and dependent on MAOA genotype. Metabolites identified in response to fluoxetine treatment or associated with impulsivity are indicated. Connected pathways and metabolites relevant for psychiatric disorders are highlighted. CSF, cerebrospinal fluid; MAOA, monoamine oxidase A.
Monoamine metabolites were not assessed in the present biomarker screening study but pilot work demonstrated that 14 weeks of dosing at 2 mg kg−1 per day significantly increased CSF serotonin and showed a trend for decrease of the serotonin metabolite 5HIAA.47
Discussion
Drugs that increase monoamine levels in the synaptic cleft are used for the treatment of a variety of psychiatric diseases. Fluoxetine is approved for the treatment of childhood depression.4 Despite evidence that fluoxetine can have beneficial effects in children affected by attention deficit hyperactivity disorder, autism, mental retardation and cerebral palsy, a black-label warning for the drug was issued by the US Food and Drug Administration resulting from severe side effects in a subset of patients. Initially on the basis of case reports on patients suffering from major depressive disorder, these findings were later corroborated by clinical trials in greater patient populations.18, 19, 20
As fluoxetine continues to be widely prescribed for psychiatric diseases including children and adolescents, it is of utmost importance to enable physicians to stratify and exclude patients that may suffer from severe side effects. Biomarkers are critical to achieve the goal of precision medicine, in this case by excluding patients at high risk of developing side effects from fluoxetine treatment. As psychiatric diseases are considered to be neurodevelopmental disorders, antidepressant effects on brain development need to be studied and understood to identify affected pathways that in turn can reveal pertinent biomarker information.
Another study on the long-term effects of fluoxetine in juvenile rhesus monkeys that was published recently50 found no significant behavioral alterations following 1 year of treatment with the drug. Impulsivity was not assessed. However, the authors found an upregulation in the neocortex and hippocampus of the serotonin transporter, but no 5-HT1A receptor expression differences. Due to the limited sample size no conclusions could be drawn with regard to adverse effects of fluoxetine treatment. In an extension of their studies the authors also separated monkeys from their mothers at birth to simulate human childhood stress but did not find any behavioral differences between fluoxetine and vehicle-treated animals in adulthood.
We and others have used –omics technologies to identify molecular pathways and biomarker candidates in rodents that were treated chronically with an SSRI.38 In several of these studies, behavioral effects including anxiety- and affective-like symptoms in adult mice when treated with fluoxetine during adolescence were found.12, 13, 14, 15, 16, 17 Likewise, adult mice treated with fluoxetine during adolescence showed altered depression-like behavior in social interaction and forced-swim tests as well as anxiety-like responses in the elevated plus maze. In one case these effects mimicked behavior in 5-HTT knockout mice suggesting that serotonin has a critical role in brain development by modulating emotional behavior in the adult animals.51 In mice, SSRI treatment has been shown to upregulate the expression of several genes that are associated with neurogenesis, neuronal survival and neuronal differentiation.52 When several inbred mouse strains were compared, it was obvious that behavioral and neuronal effects of chronic fluoxetine treatment were linked. Cell proliferation in the hippocampus was only observed in those mouse strains that also showed a behavioral response to treatment.52
Although results from these studies have been very useful for the identification of metabolic and signaling pathways affected by SSRIs, rodents are arguably not the preferred species when it comes to studying drug effects on brain development. Here species closer in evolutionary development are more relevant for the assessment of behavioral alterations associated with human pathology.
In the present study we have used juvenile nonhuman primates to identify biomarkers and molecular pathways affected by long-term treatment with fluoxetine. This was achieved by a hypothesis-free approach with the help of metabolomics analyses. Metabolites can be considered a molecular correlate of the phenotype of an organism. They are dynamic and their levels are influenced by enzyme activities, protein–protein interactions and protein posttranslational modifications. Although brain metabolite profiles would be most relevant for assessing pathway alterations, peripheral fluids can be used as a proxy reflecting changes in the CNS. They are also the only source that can be assessed for eventual clinical translation.
In the current study we have used CSF and plasma from male rhesus monkeys that differ in two common gene polymorphisms for MAOA activity, treated with either fluoxetine or vehicle. Environmental factors were excluded as best as possible with animals receiving the same diet and housed under controlled conditions, thus minimizing the signal-to-noise ratio. Due to the fact that the macaques represent an outbred population, our study is similar to a clinical investigation with patients.
On the basis of the metabolomics analyses we were able to identify biomarkers for fluoxetine treatment response as well as biomarkers that correlate with impulsivity, a measure of reward delay behavior. Statistical analyses also took into account polymorphisms of MAOA.
Blood and CSF are the most common sample types for biomarker development for psychoactive agents. Though readily available, blood has the disadvantage of multiple sources of metabolites from different tissues and metabolic pathways. Lumbar puncture for CSF collection is more specific, but it is a rather invasive procedure and is not used in children for the evaluation of response to treatment on a routine basis. Although there is an exchange of blood and CSF, individual metabolite abundances vary. This is due to the fact that although the brain is the primary source for metabolites present in CSF, all body tissues contribute to the blood metabolome which therefore tends to be more complex. In addition to the qualitative metabolite differences between the two body fluids there are also quantitative differences for an individual metabolite which can originate from several body organs. Hence it is not surprising that our analyses did not result in the same metabolite signatures for blood and CSF (Tables 1 and 2). We only found two metabolites, 5-aminovaleric acid lactam and hypoxanthine, whose levels were significantly changed in both blood and CSF (Figure 2b). In future studies we plan to specifically target all the identified CSF metabolites in the blood which will allow us to identify and quantitate them in a more sensitive manner. Ultimately, blood will be the preferred body fluid that can be used as a source for a biosignature based assay in children.
Interrogation of the metabolites with regard to pathways revealed ‘Alanine, Aspartate, Glutamate' metabolism and ‘Nicotinate, Nicotinamide' metabolism to be affected by fluoxetine treatment. The pathways affected by fluoxetine treatment harbor several metabolites that have important functions in CNS physiology and have been implicated in psychiatric phenotypes. Both pathways are connected by L-aspartate oxidase, an enzyme that catalyzes the reaction of L-aspartate to iminoaspartate, the first step in the de novo biosynthesis of NAD+.
Although monoamine deficiency is the most frequently mentioned hypothesis for mood disorders, more recent evidence suggests that the glutamatergic system is also involved in the etiology.53, 54 The free acid form of 5-aminovaleric acid lactam, one of two metabolites whose levels were significantly affected in both plasma and CSF, is a gamma-aminobutyric acid (GABA) homolog and has been shown to be a weak GABA agonist,55 further implicating the glutamatergic system. We have preliminary data from mice that indicate that SSRI treatment results in altered glutamate levels in the hippocampus (Park et al., unpublished). Furthermore, ‘Alanine, Aspartate, Glutamate' metabolism, is an important part of the energy metabolism that we and others have found to be affected in several mouse models and upon SSRI treatment.38
Nicotinamide, a precursor of the pathway leading to NAD+, has been shown to have benzodiazepine-like anxiolytic activities and is an endogenous GABA receptor ligand.56 ‘Nicotinate, Nicotinamide' metabolism is also connected to tryptophan metabolism which produces serotonin, the neurotransmitter whose concentration in the synaptic cleft is most affected by the antidepressant drug used in the current study.
We used metabolomics analyses to identify biomarker candidates that are part of molecular pathways affected by fluoxetine treatment. However, due to the small sample size (n=16 per group) in comparison with the number of analyzed metabolites tested in our study (approximately 100) the biomarker candidates could not reach the Bonferroni level. In addition, because these studies were conducted in normally functioning brains of the juvenile monkeys, generalization of the significance of these pathways for neurodevelopmental disorders in children will require further studies. Time course analyses are needed to assess the metabolite signature with regard to its potential to serve as a treatment response biomarker. Although the biomarker candidates may not necessarily underlie neurodevelopmental etiology, they could provide diagnostic readouts for treatment of juvenile patients. If confirmed, the identified molecular pathways could also delineate new targets for manipulation of their activities as an option for treatment.
Acknowledgments
Supported by NIH grants HD065826, HD065826 (supplement) (MSG PI), OD011107 (Harris Lewin, PI), WCMC funding U24 DK097154 (OF PI) and the Max Planck Society (CWT PI).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Riddle MA, Scahill L, King RA, Hardin MT, Anderson GM, Ort SI, et al. Double-blind, crossover trial of fluoxetine and placebo in children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:1062–1069. doi: 10.1097/00004583-199211000-00011. [DOI] [PubMed] [Google Scholar]
- Ricketts RW, Goza AB, Ellis CR, Singh YN, Singh NN, Cooke JC, 3rd, et al. Fluoxetine treatment of severe self-injury in young adults with mental retardation. J Am Acad Child Adolesc Psychiatry. 1993;32:865–869. doi: 10.1097/00004583-199307000-00024. [DOI] [PubMed] [Google Scholar]
- Geller DA, Hoog SL, Heiligenstein JH, Ricardi RK, Tamura R, Kluszynski S, et al. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J Am Acad Child Adolesc Psychiatry. 2001;40:773–779. doi: 10.1097/00004583-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown E, et al. Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2002;41:1205–1215. doi: 10.1097/00004583-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Turner SM, Piacentini J, Beidel DC, Clarvit SR, Davies SO, et al. Fluoxetine in children and adolescents with OCD: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2002;41:1431–1438. doi: 10.1097/00004583-200212000-00014. [DOI] [PubMed] [Google Scholar]
- Walkup J, Labellarte M, Riddle MA, Pine DS, Greenhill L, Fairbanks J, et al. Treatment of pediatric anxiety disorders: an open-label extension of the research units on pediatric psychopharmacology anxiety study. J Child Adolesc Psychopharmacol. 2002;12:175–188. doi: 10.1089/104454602760386879. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Stigler KA, Posey DJ. Treatment of aggression in children and adolescents with autism and conduct disorder. J Clin Psychiatry. 2003;64 (Suppl 4:16–25. [PubMed] [Google Scholar]
- Posey DJ, Erickson CA, Stigler KA, McDougle CJ. The use of selective serotonin reuptake inhibitors in autism and related disorders. J Child Adolesc Psychopharmacol. 2006;16:181–186. doi: 10.1089/cap.2006.16.181. [DOI] [PubMed] [Google Scholar]
- Leskovec TJ, Rowles BM, Findling RL. Pharmacological treatment options for autism spectrum disorders in children and adolescents. Harv Rev Psychiatry. 2008;16:97–112. doi: 10.1080/10673220802075852. [DOI] [PubMed] [Google Scholar]
- Soorya L, Kiarashi J, Hollander E. Psychopharmacologic interventions for repetitive behaviors in autism spectrum disorders. Child Adolesc Psychiatr Clin N Am. 2008;17:753–771. doi: 10.1016/j.chc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Deupree JD, Reed AL, Bylund DB. Differential effects of the tricyclic antidepressant desipramine on the density of adrenergic receptors in juvenile and adult rats. J Pharmacol Exp Ther. 2007;321:770–776. doi: 10.1124/jpet.106.118935. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, van de Poll NE, Corner MA, van Oyen HG, Bour HL. Suppression of active sleep by chronic treatment with chlorimipramine during early postnatal development: effects upon adult sleep and behavior in the rat. Brain Res. 1981;204:129–146. doi: 10.1016/0006-8993(81)90657-0. [DOI] [PubMed] [Google Scholar]
- Vogel G, Neill D, Hagler M, Kors D. A new animal model of endogenous depression: a summary of present findings. Neurosci Biobehav Rev. 1990;14:85–91. doi: 10.1016/s0149-7634(05)80164-2. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova NN, Lindholm J, Pruunsild P, Timmusk T, Castrén E. Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur Neuropsychopharmacol. 2009;19:97–108. doi: 10.1016/j.euroneuro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Oh JE, Zupan B, Gross S, Toth M. Paradoxical anxiogenic response of juvenile mice to fluoxetine. Neuropsychopharmacology. 2009;34:2197–2207. doi: 10.1038/npp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Glod C, Cole JO. Emergence of intense suicidal preoccupation during fluoxetine treatment. Am J Psychiatry. 1990;147:207–210. doi: 10.1176/ajp.147.2.207. [DOI] [PubMed] [Google Scholar]
- Masand P, Gupta S, Dewan M. Suicidal ideation related to fluoxetine treatment. N Engl J Med. 1991;324:420. doi: 10.1056/nejm199102073240616. [DOI] [PubMed] [Google Scholar]
- Wirshing WC, Van Putten T, Rosenberg J, Marder S, Ames D, Hicks-Gray T, et al. Fluoxetine, akathisia, and suicidality: is there a causal connection. Arch Gen Psychiatry. 1992;49:580–581. doi: 10.1001/archpsyc.1992.01820070074012. [DOI] [PubMed] [Google Scholar]
- Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292:338–343. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- Licinio J, Wong ML. Depression, antidepressants and suicidality: a critical appraisal. Nat Rev Drug Discov. 2005;4:165–171. doi: 10.1038/nrd1634. [DOI] [PubMed] [Google Scholar]
- March JS, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, et al. The Treatment for Adolescents With Depression Study (TADS): long-term effectiveness and safety outcomes Arch Gen Psychiatry 2007641132–1143.Erratum in: Arch Gen Psychiatry 2008; 65: 101. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Beasley CM, Jr, Wines JD, Jr, Tamura RN, Cusin C, Shear D, et al. Treatment-associated suicidal ideation and adverse effects in an open, multicenter trial of fluoxetine for major depressive episodes. Psychother Psychosom. 2007;76:40–46. doi: 10.1159/000096363. [DOI] [PubMed] [Google Scholar]
- Mulder RT, Joyce PR, Frampton CM, Luty SE. Antidepressant treatment is associated with a reduction in suicidal ideation and suicide attempts. Acta Psychiatr Scand. 2008;118:116–122. doi: 10.1111/j.1600-0447.2008.01179.x. [DOI] [PubMed] [Google Scholar]
- Seemüller F, Riedel M, Obermeier M, Bauer M, Adli M, Mundt C, et al. The controversial link between antidepressants and suicidality risks in adults: data from a naturalistic study on a large sample of in-patients with a major depressive episode. Int J Neuropsychopharmacol. 2009;12:181–189. doi: 10.1017/S1461145708009139. [DOI] [PubMed] [Google Scholar]
- Carpenter DJ, Fong R, Kraus JE, Davies JT, Moore C, Thase ME, et al. Meta-analysis of efficacy and treatment-emergent suicidality in adults by psychiatric indication and age subgroup following initiation of paroxetine therapy: a complete set of randomized placebo-controlled trials. J Clin Psychiatry. 2011;72:1503–1514. doi: 10.4088/JCP.08m04927blu. [DOI] [PubMed] [Google Scholar]
- Marshall E. Antidepressants and children. Buried data can be hazardous to a company's health. Science. 2004;304:1576–1577. doi: 10.1126/science.304.5677.1576. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Menke A, Domschke K, Czamara D, Klengel T, Hennings J, Lucae S, et al. Genome-wide association study of antidepressant treatment-emergent suicidal ideation. Neuropsychopharmacology. 2012;37:797–807. doi: 10.1038/npp.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlstadt I, Vitiello B. Use of atypical antipsychotics in children: balancing safety and effectiveness. Am Family Physician. 2010;81:585–589. [PubMed] [Google Scholar]
- Kaddurah-Daouk R, McEvoy J, Baillie RA, Lee D, Yao JK, Doraiswamy PM, et al. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol Psychiatry. 2007;12:934–945. doi: 10.1038/sj.mp.4002000. [DOI] [PubMed] [Google Scholar]
- Dai Y, Li Z, Xue L, Dou C, Zhou Y, Zhang L, et al. Metabolomics study on the anti-depression effect of xiaoyaosan on rat model of chronic unpredictable mild stress. J Ethnopharmacol. 2010;128:482–489. doi: 10.1016/j.jep.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Su ZH, Li SQ, Zou GA, Yu CY, Sun YG, Zhang HW, et al. Urinary metabonomics study of anti-depressive effect of Chaihu-Shu-Gan-San on an experimental model of depression induced by chronic variable stress in rats. J Pharm Biomed Anal. 2011;55:533–539. doi: 10.1016/j.jpba.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Ji Y, Hebbring S, Zhu H, Jenkins GD, Biernacka J, Snyder K, et al. Glycine and a glycine dehydrogenase (GLDC) SNP as citalopram/escitalopram response biomarkers in depression: pharmacometabolomics-informed pharmacogenomics. Clin Pharmacol Ther. 2011;89:97–104. doi: 10.1038/clpt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige LA, Mitchell MW, Krishnan KR, Kaddurah-Daouk R, Steffens DC. A preliminary metabolomic analysis of older adults with and without depression. Int J Geriatr Psychiatry. 2007;22:418–423. doi: 10.1002/gps.1690. [DOI] [PubMed] [Google Scholar]
- Delgado PL. How antidepressants help depression: mechanisms of action and clinical response. J Clin Psychiatry. 2004;65 (Suppl 4:25–30. [PubMed] [Google Scholar]
- Webhofer C, Gormanns P, Tolstikov V, Zieglgänsberger W, Sillaber I, Holsboer F, et al. Metabolite profiling of antidepressant drug action reveals novel drug targets beyond monoamine elevation. Transl Psychiatry. 2011;1:e58. doi: 10.1038/tp.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A, Gorini A, Villa RF. Affective disorders, antidepressant drugs and brain metabolism. Mol Psychiatry. 2003;8:773–785. doi: 10.1038/sj.mp.4001353. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Tsukayama E, Kirby TA. Is it really self-control? Examining the predictive power of the delay of gratification task. Pers Soc Psychol Bull. 2013;39:843–855. doi: 10.1177/0146167213482589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Peake PK. The nature of adolescent competencies predicted by preschool delay of gratification. J Pers Soc Psychol. 1988;54:687–696. doi: 10.1037//0022-3514.54.4.687. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, et al. Behavioral and neural correlates of delay of gratification 40 years later. Proc Natl Acad Sci USA. 2011;108:14998–15003. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Yourganov G, Askren MK, Ayduk O, Casey BJ, Gotlib IH, et al. Dimensionality of brain networks linked to life-long individual differences in self-control. Nat Commun. 2013;4:1373. doi: 10.1038/ncomms2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Hakimi S, Rangel A. Activity in dlPFC and its effective connectivity to vmPFC are associated with temporal discounting. Front Neurosci. 2014;8:50. doi: 10.3389/fnins.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure J, Podos J, Richardson HN. Isolating the delay component of impulsive choice in adolescent rats. Front Integr Neurosci. 2014;8:3. doi: 10.3389/fnint.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, et al. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE. Fluoxetine: juvenile pharmacokinetics in a nonhuman primate model. Psychopharmacology (Berl) 2014;231:4041–4047. doi: 10.1007/s00213-014-3537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, et al. Neurobehavioral evaluation of rhesus monkey infants fed cow's milk formula, soy formula, or soy formula with added manganese. Neurotoxicol Teratol. 2005;27:615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL. Iron deprivation during fetal development changes the behavior of juvenile rhesus monkeys. J Nutr. 2007;137:979–984. doi: 10.1093/jn/137.4.979. [DOI] [PubMed] [Google Scholar]
- Shrestha SS, Nelson EE, Liow JS, Gladding R, Lyoo CH, Noble PL, et al. Fluoxetine administered to juvenile monkeys: effects on the serotonin transporter and behavior. Am J Psychiatry. 2014;171:323–331. doi: 10.1176/appi.ajp.2013.13020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Alcantara LF, Warren BL, Riggs LM, Parise EM, Vialou V, et al. Fluoxetine exposure during adolescence alters responses to aversive stimuli in adulthood. J Neurosci. 2014;34:1007–1021. doi: 10.1523/JNEUROSCI.5725-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Schultz LE, Gulati A, Cameron MD, Pletcher MT. Genetic Regulation of Behavioral and Neuronal Responses to Fluoxetine. Neuropsychopharmacology. 2008;33:1312–1322. doi: 10.1038/sj.npp.1301497. [DOI] [PubMed] [Google Scholar]
- Jun C, Choi Y, Lim SM, Bae S, Hong YS, Kim JE, et al. Disturbance of the glutamatergic system in mood disorders. Exp Neurobiol. 2014;23:28–35. doi: 10.5607/en.2014.23.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Callery PS, Geelhaar LA. 1-Piperideine as an in vivo precursor of the gamma-aminobutyric acid homologue 5-aminopentanoic acid. J Neurochem. 1985;45:946–948. doi: 10.1111/j.1471-4159.1985.tb04085.x. [DOI] [PubMed] [Google Scholar]
- Paul SM, Marangos PJ, Skolnick P, Goodwin FK. Biological substrates of anxiety: benzodiazepine receptors and endogenous ligands. Encephale. 1982;8:131–144. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.