Abstract
Oxytocin (OT) is a neuropeptide involved in mammalian social behavior. It is currently in clinical trials for the treatment of autism spectrum disorder (ASD). Previous studies in healthy rodents (prairie voles and C57BL/6J mice) have shown that there may be detrimental effects of long-term intranasal administration, raising the questions about safety and efficacy. To investigate the effects of OT on the aspects of ASD phenotype, we conducted the first study of chronic intranasal OT in a well-validated mouse model of autism, the BTBR T+ Itpr3tf/J inbred strain (BTBR), which displays low sociability and high repetitive behaviors. BTBR and C57BL/6J (B6) mice (N=94) were administered 0.8 IU/kg of OT intranasally, daily for 30 days, starting on day 21. We ran a well-characterized set of behavioral tasks relevant to diagnostic and associated symptoms of autism, including juvenile reciprocal social interactions, three-chambered social approach, open-field exploratory activity, repetitive self-grooming and fear-conditioned learning and memory, some during and some post treatment. Intranasal OT did not improve autism-relevant behaviors in BTBR, except for female sniffing in the three-chambered social interaction test. Male saline-treated BTBR mice showed increased interest in a novel mouse, both in chamber time and sniffing time, whereas OT-treated male BTBR mice showed a preference for the novel mouse in sniffing time only. No deleterious effects of OT were detected in either B6 or BTBR mice, except possibly for the lack of a preference for the novel mouse's chamber in OT-treated male BTBR mice. These results highlight the complexity inherent in understanding the effects of OT on behavior. Future investigations of chronic intranasal OT should include a wider dose range and early developmental time points in both healthy rodents and ASD models to affirm the efficacy and safety of OT.
Introduction
Oxytocin (OT) is a mammalian neuropeptide with well-conserved biological roles in labor, milk letdown and social bonding.1, 2, 3 In recent years, numerous single-dose studies have been conducted on the effects of OT on social cognition in healthy humans; see extensive reviews for more details.4, 5, 6, 7 Outcomes reported include increased trust,8 empathic accuracy,9, 10 time spent looking at eyes11 and face identity recognition memory.12, 13 Imaging studies have demonstrated attenuation of amygdala activity with a single dose of OT versus placebo.14, 15, 16 It is, however, worth noting that administration of OT has also been associated with increased competition towards out-group members,17 higher envy and gloating,18 and reduced trust in patients with borderline personality disorder.19 Effects may also be dose-dependent,20 and context can also be important. For example, OT may promote sociality when administered in a safe environment, and defensiveness when administered in a conflictual setting21, 22 or to persons with an adverse early history.23, 24
A growing number of single-dose infusion studies have shown positive effects of OT in individuals with autism spectrum disorder (ASD). In these studies, OT promoted retention of social information and reduced repetitive behaviors.25, 26 Trials of intranasal OT also have demonstrated positive effects on empathic accuracy27 and cooperation and trust during play with a partner.28 The several small (or single subject) multi-dose studies of intranasal OT administered to children and adolescents with ASD used over a 2–6-month period suggest that OT is well tolerated and improves social communication in these individuals.29, 30 Studies of maladaptive behavior, especially those with treatment regimes lasting days rather than months, have had more mixed results with some finding no effects.31 Finally, a recent relatively large randomized clinical trial in adults with ASD found that taking OT produced improvements in empathic accuracy, reduced repetitive behaviors and increased the quality of life.32 A recent meta-analysis of studies of intranasal OT treatments for ASD found an overall effect size of d=57 with Cohen's d, as well as a significant combined effect on outcome measures.33
One concern with the proposed use of intranasal OT for the treatment of developmental disorders is the potential for negative long-term effects of chronic exposure to OT, especially as autism typically is diagnosed and most intensively treated in children. Exposure could adversely affect endogenous OT production or receptor systems in the developing brain.34 Remarkably, very little animal data have been published on this topic. Our previous study in prairie voles showed that although intranasal OT had acute positive effects at a similar dosage to that being used in humans, later in life OT-treated males had deficits in the formation of a pair-bond.35 A subsequent study in highly social C57BL/6J (B6) male mice found similar results, with acute OT increasing male–female social interaction, whereas chronic OT decreasing male–female and male–male social interaction.36 This study attributed the long-term behavioral changes to a widespread downregulation of OT receptors.37 Finally, a study of chronic central infusion in mice found both an increase in anxiety-like behaviors and a downregulation of OT receptors in many limbic areas.38 Although it is difficult to directly compare the doses given, and it is still controversial as to how much of the intranasally administered OT crosses the blood–brain barrier, this central infusion study reinforces concerns regarding chronic administration.
In addition to social behavior, OT has a recognized role in anxiety39, 40, 41 and learning and memory, including the learning of fearful stimuli.42 Anxiety is often a comorbid feature of autism,43 as is dysfunction of other amygdala-dependent processes such as fear conditioning.44 In general, OT reduces fear expression and enhances fear extinction in rodents,45 an effect which appears to be specific with injection in the central amygdala before conditioning.46 Intranasal OT that is given following the acquisition phase of fear conditioning in humans enhanced fear-potentiated startle;47 however, there are considerable gaps in our knowledge of appropriate dosing or administration schedules of OT in humans, and there are few human studies that are comparable to those conducted in animals.
The effects of intranasal OT have not been previously studied in a valid rodent model of reduced social behavior. BTBR T+ Itpr3tf/J (BTBR) mice have been shown to display low levels of sociability in a three-chamber choice task,48, 49, 50, 51, 52, 53 learning impairments in complex but not simple learning,54, 55 low juvenile reciprocal social interactions51 and high levels of repetitive self-grooming,51, 53, 56 as well as altered OT systems.50, 57, 58 In addition to social behavior, these behaviors give this model a face validity with regard to multiple aspects of the ASD phenotype.59
In this first comprehensive study of the effects of chronic intranasal exposure to OT in BTBR mice, with B6 mice as a strain control, we administered OT once daily to BTBR mice at the daily dosage that is currently in use in human trials,32 as well as for which we previously found significant effects in prairie voles.35 Both strains completed a well-characterized set of behavioral tasks, some on- and some off-treatment, including social behavioral tasks (juvenile reciprocal social interactions and the three-chambered social approach task); anxiety/exploratory behavior (open field); repetitive behavior (repetitive self-grooming); and classical fear learning. We hypothesized that OT might prove to be beneficial to the impaired social interactions and learning displayed by BTBR mice; whereas high natural levels of social behaviors in B6 mice36 and prairie voles35 could have produced a ceiling effect for some specific behaviors (such as partner-preference behavior) with OT treatments in previous studies. We also predicted that exploratory behavior might be increased and self-grooming might be decreased in BTBR receiving OT. On the basis of a large literature detailing the sex differences of developmental exposure to OT,35, 60, 61 overall, we expected males to be more sensitive to exogenous OT and thus to see the effects in males but not necessarily in females.
Materials and methods
Subjects
Subjects were produced from the breeding pairs of B6 and BTBR mice originally purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and bred as harem trios in a conventional mouse vivarium at the University of California Davis School of Medicine in Sacramento, MIND Institute's Intellectual and Developmental Disabilities Research Center (IDDRC). They were weaned at 20 days of age and housed by sex and strain in Tecniplast cages in groups not exceeding two to four per cage. Cages were housed in ventilated racks in a temperature (68–72°F)- and humidity (~25%)-controlled colony room, on a 12-h circadian cycle, lights on from 0700 to 1900 h. Standard rodent chow and tap water were available ad libitum. In addition to the standard bedding, a Nestlet square, shredded brown paper and a cardboard tube (Jonesville Corporation, Jonesville, MI, USA) were provided in each cage. Animals were paw tattooed for identification. All the procedures were conducted in compliance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by UC Davis Institute Animal Care and Use Committee (Protocols #16839 and #16587).
Intranasal OT treatments
BTBR and B6 mice were administered 0.8 IU/kg OT or saline vehicle treatments once daily in the morning between 0700 and 1200 h. This dosage is similar to the total daily dosage being used currently in clinical trials31, 32, 62 and other studies with clinical populations.4, 20, 32, 62 Specifically, it would be equivalent to a 40-IU dosage given to a 110-lb subject. For most measures, group sizes were 11–12 mice. For the intranasal administration, a cannula needle (33 gauge, 2.8 mm length, Plastics One, Roanoke, VA, USA) was attached to the cannula tubing, flushed and filled with the compound. It was attached to an airtight Hamilton syringe. The animal was held still and 25 μl of compound was expelled slowly through the cannula needle and allowed to absorb into the nasal mucosa (divided between the two nostrils; the animal was not stuck by the needle, the blunt needle was used to aid in expelling very small amounts). Following administration, the animal was returned to its home-cage with its familiar companion. Administration was rapid (less than 30 s) and handling was consistent across treatment groups. This method of administration has been used before in prairie voles,35 as well as in B6 mice.36
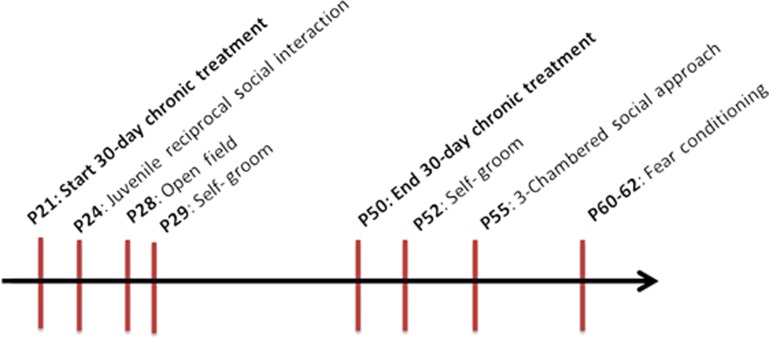
The timeline for treatments and behavioral testing is given in Figure 1. Acute behavioral effects of OT (on-treatment) were assessed 45 min post administration, as this has been shown to be an effective time point in many behavioral, neural and physiological studies.63, 64, 65 Furthermore, in a microdialysis study of intranasal OT application in rats and mice, OT peaked in the microdialysate samples (from the amygdala and hippocampus) at 30–60 min post administration in both the rodent species.66
Figure 1.
Timeline of study procedures. N=11–12 per sex per drug treatment group for juvenile reciprocal interactions, open-field activity and repetitive self-grooming.
Behavioral testing
The timeline for behavioral testing is detailed in Figure 1. Scoring from video was done using Noldus Observer 8.0XT software (Noldus Information Technology, Leesburg, VA, USA); all testers and scorers were blinded as to the treatment. All arenas were cleaned with 70% ethanol between the treatments.
Juvenile reciprocal social interactions
Juvenile reciprocal social interactions were tested in mice between postnatal days 24–26 in the Noldus PhenoTyper Observer 3000 chamber (25 cm × 25 cm × 35 cm), as previously described.51, 67, 68, 69 The floor of the arena was covered with a 0.5-cm layer of clean bedding. Subjects and stimulus partners were individually housed in a clean cage for 1 h before the test. An individual B6 or BTBR subject mouse was then placed in the arena, with an age- and sex-matched juvenile B6 partner. Stimulus mice did not receive intranasal treatment or procedures. Interactions were recorded for 10 min, the period during which majority of the social interactions occur. Parameters of juvenile mouse social behaviors were chosen from the established literature and from our previous studies48, 51, 53, 57, 68, 70 and are given in Table 1.
Table 1. Results from the juvenile reciprocal interaction test (means±s.e.m.).
| Behavior | B6 saline | B6 OT | BTBR saline | BTBR OT |
|---|---|---|---|---|
|
Males |
N=11 |
N=12 |
N=12 |
N=12 |
| Nose–nose sniff(s) | 39.091±3.370 | 45.667±6.459 | 13.167±1.403 | 16.333±2.407 |
| Body sniff(s) | 24.091±3.359 | 24.583±1.948 | 10.083±1.003 | 9.583±1.438 |
| Anogenital sniff(s) | 28.909±2.108 | 34.917±3.171 | 13.5±2.054 | 11.333±2.097 |
| Total sniff(s) | 92.091±6.646 | 104.5±9.202 | 36.750±3.160 | 37.250±3.266 |
| Front approach (freq) | 5.909±1.581 | 5.833±1.825 | 0.500±0.230 | 0.500±0.230 |
| Push–crawl (freq) | 1.636±0.411 | 2.083±0.434 | 0.667±0.256 | 0.667±0.396 |
| Push side-by-side (freq) | 2.000±0.809 | 2.333±0.333 | 1.583±0.358 | 2.250±0.617 |
| Follow(s) | 3.000±0.894 | 2.583±0.949 | 0.667±0.432 | 0.917±0.313 |
| Total social contact(s) | 108.766±14.966 | 131.581±13.578 | 98.011±10.369 | 76.561±9.709 |
| Self-groom (s) | 5.785±1.821 | 11.918±3.090 | 47.671±12.478 | 46.488±9.854 |
| Explore(s) | 490.508±17.154 | 468.101±14.413 | 463.148±11.618 | 482.754±13.414 |
| Digging(s) | 4.182±1.536 | 1.359±0.594 | 3.583±1.685 | 1.5±0.669 |
| Wall climbing (freq) | 23.091±2.722 | 27.333±3.532 | 13.167±2.174 | 11.500±3.056 |
|
Females |
N=12 |
N=11 |
N=12 |
N=12 |
| Nose–nose sniff(s) | 44.833±3.914 | 48.909±5.160 | 12.333±1.940 | 12.917±1.474 |
| Body sniff(s) | 28.167±4.106 | 31.091±3.607 | 11.417±2.076 | 8.917±1.998 |
| Anogenital sniff(s) | 40.833±4.559 | 40.818±5.131 | 12.333±2.438 | 12.667±3.018 |
| Total sniff(s) | 113.833±10.421 | 120.818±9.627 | 36.083±4.410 | 34.500±5.883 |
| Front approach (freq) | 7.167±1.014 | 8.818±1.571 | 0.750±0.250 | 0.750±0.250 |
| Push–crawl (freq) | 1.250±0.329 | 1.636±0.203 | 0.417±0.193 | 0.667±0.310 |
| Push side-by-side (freq) | 3.250±0.592 | 3.000±0.522 | 2.167±0.474 | 3.250±0.579 |
| Follow(s) | 15.417±7.332 | 11.909±5.606 | 0.167±0.112 | 0.667±0.256 |
| Total social contact(s) | 140.079±15.594 | 146.882±13.349 | 80.769±10.732 | 72.660±7.058 |
| Self-groom (s) | 9.261±2.501 | 9.378±2.767 | 36.110±5.663 | 22.054±6.375 |
| Explore(s) | 456.023±17.879 | 448.493±15.614 | 492.869±10.501 | 508.593±9.089 |
| Digging(s) | 2.75±1.122 | 3.255±0.983 | 1.667±0.987 | 3.667±1.534 |
| Wall climbing (freq) | 22.000±3.614 | 28.455±4.307 | 10.667±2.533 | 16.833±2.905 |
Total social contact included sniffing (nose–nose, anogenital, body), push–play behavior, following and huddling. Total social contact, approach and self-grooming were statistically analyzed and showed strain differences at P<0.001 in all the cases. No significant differences were detected for saline versus oxytocin (OT).
Open-field testing
General exploratory locomotion in a novel open-field environment was assayed as previously described.68 Open-field activity was considered an essential control for direct drug effects on physical activity, for example, sedation, which could confound the interpretation of results from the reciprocal interactions, self-grooming, fear conditioning and social approach tasks. Individual mice were placed in a VersaMax Animal Activity Monitoring System (AccuScan Instruments, Columbus, OH, USA) for a 30-min test session. The testing room was illuminated with dim lighting at ~40 lux.
Repetitive self-grooming
Spontaneous repetitive self-grooming behavior was scored as previously described.50 Each mouse was placed individually into a standard mouse cage, (46 cm length × 23.5 cm wide × 20 cm high). Cages were empty to eliminate digging in the bedding, which is a potentially competing behavior. The room was illuminated at ~40 lux. A front-mounted CCTV camera (Security Cameras Direct) was placed at ~1 m from the cages to record the sessions. Sessions were video-taped for 20 min. The first 10-min period was habituation and was unscored. Each subject was scored for cumulative time spent grooming all the body regions during the second 10 min of the test session.
Three-chambered social approach task
Social approach was tested in an automated three-chambered apparatus using methods similar to those previously described.50, 51, 53 Automated Ethovision XT videotracking software (Version 9.0, Noldus Information Technologies, Leesburg, VA, USA) and modified materials for the chambers were used to maximize throughput. The updated apparatus (40 cm × 60 cm × 23 cm) was a rectangular, three-chambered box made from matte white finish acrylic (P95 White, Tap Plastics, Sacramento, CA, USA). Opaque retractable doors (12 cm × 33 cm) were designed to create optimum entryways, encourage exploration across chamber openings (5 cm × 10 cm) and maintain manual division of the compartments. Three zones, defined using the EthoVision XT software, detected time in each chamber for each phase of the assay. Zones extending 2 cm from each novel object or novel mouse enclosure (inverted wire cup, Galaxy Cup, Kitchen Plus, http://www.kitchen-plus.com), and direction of the head, body and tail defined sniff time. A top-mounted infrared sensitive camera (Ikegami ICD-49, B&H Photo, New York, NY, USA) was positioned directly above every two units. Infrared lighting (Nightvisionexperts.com) provided uniform, low-level illumination.
The subject mouse was first contained in the center chamber for 10 min, then explored all three empty chambers for 10 min, then explored the three chambers containing a novel object in one side chamber and a novel mouse in the other side chamber. Novel stimulus mice were 129Sv/ImJ, a relatively inactive strain, aged 10–14 weeks old, and matched to the subject mice by sex. Stimulus mice were habituated as previously described.53, 71 Number of entries into the side chambers served as a within-task control for levels of general exploratory locomotion. Lack of innate side preference was confirmed during the initial 10 min of habituation to the entire arena (Table 2).
Table 2. Results from the habituation phase of the three-chambered social interaction test.
| Strain | Object | Center | Mouse |
|---|---|---|---|
| Male B6 saline | 183.601±10.612 | 209.982±8.704 | 201.237±9.047 |
| Male B6 OT | 211.042±11.465 | 180.796±7.146 | 204.494±8.421 |
| Male BTBR saline | 190.365±22.851 | 233.010±21.821 | 167.784±19.083 |
| Male BTBR OT | 156.332±20.982 | 264.658±23.236 | 171.453±18.888 |
| Female B6 saline | 201.987±13.262 | 190.153±7.651 | 202.448±14.077 |
| Female B6 OT | 187.383±13.593 | 183.262±8.843 | 222.737±12.096 |
| Female BTBR saline | 209.003±24.678 | 223.582±20.186 | 161.948±17.331 |
| Female BTBR OT | 221.261±23.210 | 196.349±16.720 | 171.625±23.591 |
Abbreviation: OT, oxytocin.
There were no significant differences due to strain or treatment.
Fear conditioning
Delay contextual and cued fear conditioning was conducted using an automated fear-conditioning chamber (Med Associates, St Albans, VT, USA) as previously described.72 The conditioning chamber (32 × 25 × 23 cm3, Med Associates) was interfaced to a PC installed with VideoFreeze software (version 1.12.0.0, Med Associates) and enclosed in a sound-attenuating cubicle. Training consisted of a 2-min acclimation period followed by three tone-shock (CS–US) pairings (80 dB tone, duration 30 s; 0.5 mA footshock, duration 1 s; intershock interval 90 s) and a 2.5-min period, during which no stimuli were presented. The environment was well lit (~100 lux), with a stainless steel grid floor and swabbed with vanilla odor cue (prepared from vanilla extract; McCormick; 1:100 dilution). A 5-min test of contextual fear conditioning was performed 24 h after training, in the absence of the tone and footshock, but in the presence of 100 lux overhead lighting, vanilla odor and chamber cues identical to those used on the training day. Cued fear conditioning, conducted 48 h after training, was assessed in a novel environment with distinct visual, tactile and olfactory cues. Overhead lighting was turned off. The cued test consisted of a 3-min acclimation period followed by a 3-min presentation of the tone CS and a 90-s exploration period. Cumulative time spent freezing in each condition was quantified by VideoFreeze software (Med Associates).
Data analysis
Data were analyzed in Statistica (Tulsa, OK, USA). Sexes were considered separately with treatment, strain and a treatment by strain interaction as the fixed factors. All significance levels were set at P<0.05 and all tests were two-tailed.
Because of the large number of behavioral variables that we measured in these tests, we focused on a limited number of the most salient behaviors to limit the possibility of type I error. We focused on the diagnostic criteria of ASD, including social behavior and repetitive behavior. For social behavior, we were most interested in social contact, both because OT is intimately involved in social bonds and ‘gentle touch' across many species.73, 74 We also focused on approach and directed sniffing behavior as reflecting the motivation to interact socially. Repetitive behavior is reflected in the repetitive self-grooming task, as well as in a social context during the juvenile reciprocal interactions. Other behavioral variables are presented in tables but not statistically analyzed.
Results
Juvenile reciprocal interactions
The effects of strain were significant for social contact for both males and females (males: F1,43=7.217, P=0.01; females: F1,43=30.607, P<0.001), as well as for approach bouts (males: F1,43=19.978, P<0.001; females: F1,43=62.444, P<0.001), and for self-grooming (males: F1,43=21.069, P<0.001; females: F1,43=16.574, P<0.001). BTBR displayed lower levels of social behavior and higher levels of self-grooming than B6. In all the cases, treatment effects were not significant, nor were the treatment by sex interactions (Table 1).
Open-field testing
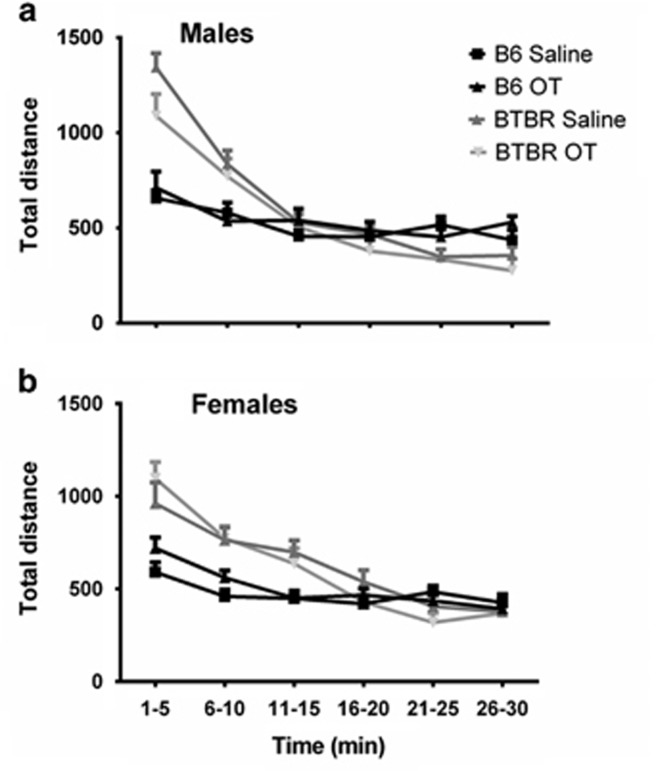
Total activity in the open field by males decreased significantly across time (F5,46=83.18, P<0.0001, Figure 2a), indicating the expected habituation to the novel environment. A significant time by strain interaction was detected (F5,46=32.86, P<0.0001), with a trend for lower exploratory activity in the BTBR group treated with OT during the first 10 min only (F1,46=3.17, P=0.082). There was no overall effect of treatment. In females (Figure 2b), total activity was significant for strain (F1,46=8.02, P=0.007), time (F5,46=55.26, P<0.0001), a strain by time interaction (F5,46=16.2, P<0.0001) and a strain by treatment interaction (F5,46=2.58, P=0.027). In female BTBR, OT-treated females displayed lower total activity than saline-treated females as the test went on, whereas in female B6, OT-treated females displayed very similar but slightly higher activity than saline-treated females (Figure 2b).
Figure 2.
Open-field activity. (a) Total activity in the open field by males declined across time intervals (P<0.0001), representing normal habituation to the novel open-field environment. A significant time by strain interaction was detected (P<0.0001), with a trend for a difference by strain (P=0.082). B6 saline, n=11; all other groups, n=12. In Figures 2–5 and Tables 1 and 2, data are presented as mean±s.e.m. (b) Total activity in the open field by females was significant for strain (P=0.007), time (P<0.0001), strain by time interaction (P<0.0001) and strain by treatment interaction (P=0.027). OT, oxytocin. B6 OT, n=11; all other groups, n=12.
Repetitive self-grooming
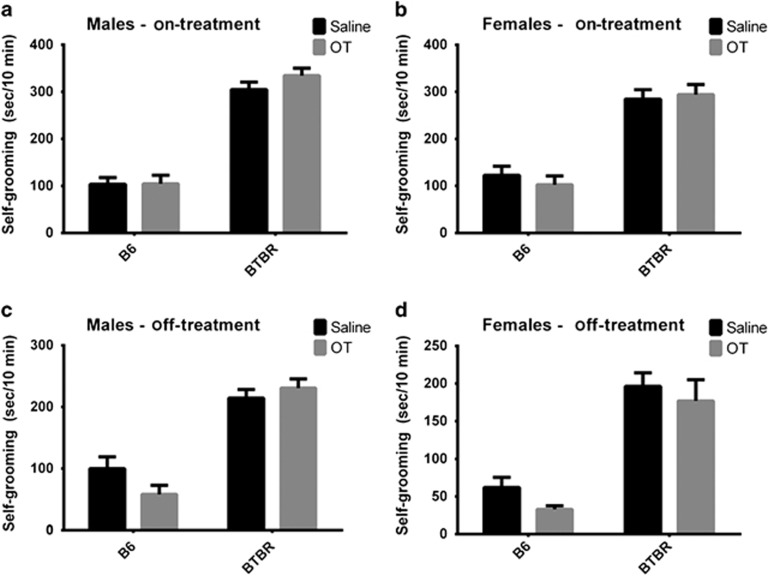
During the treatment period, strain differences were significant for self-grooming, with BTBR displaying the expected higher level of self-grooming as compared with B6 (males: F1,43=179.568, P<0.001; females: F1,43=78.169, P<0.001; Figure 3a). After cessation of treatment, BTBR again displayed more self-grooming than B6 (males: F1,38=79.422, P<0.001; females: F1,40=59.771, P<0.001; Figure 3b). OT did not significantly reduce self-grooming in either case. In males, while off-treatment, there was a trend for a strain by treatment interaction (F1,38=3.248, P=0.079).
Figure 3.
Repetitive self-grooming. (a and c) During and after the treatment, the effects of strain were significant for self-grooming in males (P<0.001 in both the cases). While off-treatment, males showed a trend for a strain by treatment interaction (P=0.079). During the treatment: B6 saline, n=11; all other groups, n=12. After treatment: B6 OT, n=8; B6 saline, n=11; BTBR OT, n=11; BTBR saline, n=12. (b and d) During (n=47) and after (n=44) the treatment, the effects of strain were significant for self-grooming in females (P<0.001 in both the cases). OT did not differ from saline vehicle. During the treatment, B6 OT, n=11; all other groups, n=12. After the treatment: B6 OT, n=10; B6 saline, n=12; BTBR OT, n=10; BTBR saline, n=12. OT, oxytocin.
Three-chambered social approach task
As expected, there were no differences in time spent in the three chambers during the habituation phase of the test (Table 2).
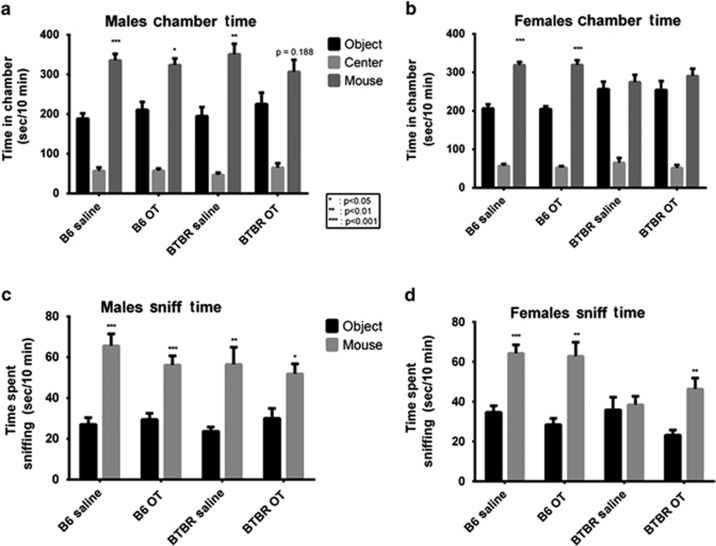
During the social approach phase, male B6 spent significantly more time in the chamber with the novel mouse as compared with time spent in the chamber with the novel object, in both the saline-treated group (t10=−5.118, P<0.001) and the OT-treated group (t7=−3.111, P=0.0171; Figure 4a), as expected from many previous publications. Unexpectedly, male BTBR treated with saline spent more time in the chamber with the novel mouse than in the chamber with the novel object (t11=−3.188, P=0.008). Male BTBR treated with OT spent approximately equal time in the two side chambers (t10=−1.414, P=0.188), although a trend appears for more time in the novel mouse chamber. It is possible that this unusual sociability in male BTBR mice dosed for 30 days with intranasal saline was because of the stress of repeated handling and treatments (see discussion).
Figure 4.
Three-chambered social interaction. (a) Male B6 mice treated with either saline (n=11) or OT (n=8) spent more time in the chamber with the novel mouse than in the chamber with the novel object (saline: P<0.001, OT: P=0.017), as did saline-treated male BTBR mice (n=12, P=0.008). OT-treated BTBR males (n=10) did not differ between the novel mouse over the novel object. (b) Female B6 mice treated with either saline (n=12) or OT (n=10) spent more time in the chamber with the novel mouse over the novel object (saline: P<0.0001; OT: P<0.001), whereas female BTBR mice which received either treatment (n=12 for saline and n=10 for OT) did not. (c) Male mice of both strains and all treatments spent significantly more time sniffing a novel mouse than a novel object (all P<0.05). (d) Both OT- and saline-treated B6 females spent more time sniffing a novel mouse than a novel object (P<0.01). However, OT-treated BTBR females spent significantly more time sniffing the novel mouse than the novel object (P=0.004), whereas saline-treated BTBR females did not. OT, oxytocin.
Female B6 treated with either saline (t11=−6.319, P<0.0001) or OT (t9=−6.089, P<0.001) spent more time in the chamber with the novel mouse than in the chamber with the novel object (Figure 4b), as expected. Female BTBR treated with either saline (t11=−0.496, P=0.629) or OT (t9=−0.878, P=0.403) failed to spend more time in the chamber with the novel mouse as compared with time in the chamber with the novel object, as expected.
Sniffing data recapitulated chamber time data in males. Male B6 spent more time sniffing the novel mouse than the novel object whether treated with saline (t10=−4.856, P<0.001) or OT (t7=−4.572, P=0.003; Figure 4c). Male BTBR spent more time sniffing the novel mouse when treated with either saline (t11=−3.262, P=0.004) or OT (t10=−2.497, P=0.031), consistent with the chamber time data, but again this was in contrast to the considerable literature from our laboratory and others that reported lack of sociability in BTBR on the three-chambered social approach task.
Female B6 spent more time sniffing the novel mouse than the novel object when treated with either saline (t11=−5.625, P<0.001) or OT (t9=−4.361, P=0.002; Figure 4d). Female BTBR did not spend more time sniffing the novel mouse than sniffing the novel object when treated with saline (t11=0.016, P=0.917), consistent with chamber time and previous literature. However, female BTBR treated with OT spent significantly more time sniffing the novel mouse than the novel object (t9=−3.849, P=0.004), which could indicate a beneficial effect of OT in female BTBR.
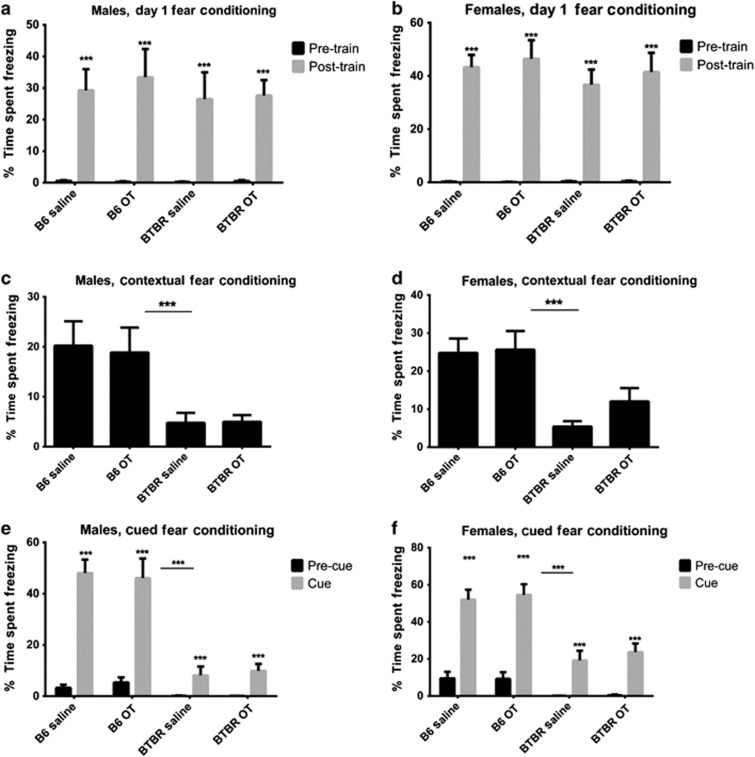
Fear conditioning
Mice of both strains showed significantly higher percent time freezing post training than before the onset of footshock (males, training effect: F1,37=61.671, P<0.0001; females, training effect: F1,40=189.832, P<0.0001; Figures 5a and b). Freezing during the contextual conditioning session differed significantly by strain (males: F1,37=18.097, P<0.001; females: F1,40=22.081, P<0.0001; Figures 5c and d), consistent with previous reports of lower fear conditioning in BTBR than B6.53, 75, 76 During the cued conditioning trial, freezing increased significantly post cue (males: F1,37=142.360, P<0.0001; females: F1,40=166.059, P<0.0001), with significant differences by strain (males: F1,37=62.305, P<0.0001; females: F1,40=38.134, P<0.0001) and a significant cue by strain interaction (males: F1,37=61.121, P<0.0001; females: F1,40=20.245, P<0.0001; Figures 5e and f). There were no effects of treatment, or strain by treatment interactions, on any measure.
Figure 5.
Contextual and cued fear conditioning. Males: B6 OT, n=8; B6 saline, n=11; BTBR OT, n=11; BTBR saline, n=12. Females: B6 OT, n=10; B6 saline, n=12; BTBR OT, n=10; BTBR saline, n=12. (a and b) Both strains froze similarly in response to the unconditioned stimulus (all P<0.0001). (c and d) BTBR mice froze significantly less during the contextual cues session (all P<0.0001). (e and f) Although both strains displayed freezing to the auditory cue (all P<0.0001), BTBR froze less than B6, showing a significant strain and strain by cue interaction (P<0.0001). OT, oxytocin.
Discussion
This, to our knowledge, was the first study to examine the effects of intranasally administered OT in BTBR mice, or any other rodent model of autism. As a whole, the findings of this study do not indicate any major therapeutic advantage or disadvantage to the use of intranasal OT, in the measures of juvenile and adult sociability, repetitive and cognitive behaviors. These findings are interesting from several different perspectives, both in relation to the current human clinical data and to the literature on OT administration in other rodent models; they are also notable as including both sexes and a developmental, rather than adult, administration.
This study is perhaps best considered in the context of the other papers to examine the effects of intranasal OT administration in B6 mice by Huang et al.36 and in prairie voles by our laboratory,35 both of which found acute facilitation but chronic decreases in social behavior. The dosages used in the previous B6 study were given in smaller volume and were similar to the highest dosages in the prairie vole study, and were an order of magnitude higher than that currently being used in some clinical autism trials. In the present study, dosages were based on prairie vole and human data. The ages and length of administration also differed between the Huang study (administration starting at week 12–20, continuing for 7–21 days) and the current study (20–50 days). The recapitulation of well-known strain differences between B6 and BTBR mice,49 as well as the very similar treatment methodology between the present study and the Huang study (which did find treatment effects), lend additional weight to the present negative findings.
There is a long history of studying the effects of acute or short-term exposure to intraperitoneal, subcutaneous and intracerebroventricular OT on the social behavior in adult rodents.77, 78, 79, 80, 81, 82 There is one previous study by Teng et al.,83 in which OT was administered intraperitoneally to two other strains of mice with either social deficits (BALB/cByJ) or repetitive behavior (C58/J). OT was found to increase the sociability in both strains when administered subchronically (four doses separated by two days in between). This study differed from the current study, as well as from the previous prairie vole35 and mouse studies36 in species/strain, age and mode of administration, and the frequency of administration (intermittent versus chronic), thus making direct comparisons difficult. However, the study by Teng et al.83 raises the possibility that intermittently administered OT may be able to ameliorate social deficits, whereas chronic OT has either failed or worsened social behavior in other rodent studies. It is possible that pulses of exogenous OT could lead to upregulation rather than downregulation of the OT receptor caused by flooding of the system in chronic exposure.34 For example, in developmental studies in which prairie voles were raised biparentally (versus by a single mother)84 and in which mice were raised communally (versus by a single mother),85 subjects showed higher levels of OT receptors. Intermittent injections of OT may better mimic pulses of endogenous OT release during the interaction with caregivers.86
The only significant positive effect of OT in BTBR mice was an increase in sniffing of a novel mouse during the social interaction test, in females only, and not on the chamber time parameter. Although sniff time is the more sensitive measure, these two parameters are usually corroborative within the same test session. The OT effect on sniff time alone, which was detected only in females, may be indicative but does not represent a robust treatment effect.
One of the most interesting findings from this study was that both saline-treated and OT-treated male BTBR mice showed significant sociability in the three-chambered social approach test on the sniffing parameter, and BTBR treated with saline also displayed sociability on the chamber time parameter. A large literature reports lack of sociability in BTBR on both parameters of the three-chambered social approach test.48, 49, 50, 51, 52, 87 However, these previous publications used BTBR that were either untreated or given only a single acute dose of saline or drug. One strong possibility which could explain this unpredicted finding is that the long-term handling needed to administer the daily intranasal treatments was stressful, and that male BTBR responded to the effects of long-term stress with an increase in sociability. Following this logic, the absence of sociability on chamber time in BTBR treated with OT could be viewed as a treatment-induced deficit. However, since this deficit was not seen in OT-treated BTBR on the sniffing parameter, this interpretation would require further investigation. To our knowledge, the effects of long-term handling stress on sociability in the three-chambered assay has not previously been tested. It is interesting to note that across many different species and strains of rodents, it is more common for stressors to lead to a decrease rather than an increase in social behavior.88, 89, 90, 91 Although BTBR mice have high basal levels of corticosterone,92 in other ways their responses to stress have been shown to be normal.71 In one study of an acute anxiolytic treatment in BTBR, diazepam increased the time spent in the social chamber,87 which would be the opposite of the purported stress effect seen here. Baseline levels of stress, due to other testing or husbandry conditions in different laboratories, could conceivably have long-term effects on development,93, 94 and produce different responses to stress or anxiety. Further research, focused specifically on sexually dimorphic effects of chronic handling stress on social behavior in mice, seems warranted.
When comparing these results with human data, it is important to note that the initial published results of clinical trials are not uniformly positive, even given their relatively short-term nature and varying outcome measures. Recent meta-analyses suggested a small-to-medium effect size of intranasal OT in autism;62, 95 however, in addition to new studies with negative findings,31 to date most studies on autism or associated syndromes have either had a relatively small number of participants,96, 97 or were not double-blinded.29 Although the current results from a mouse model of autism were not promising in terms of long-term benefits of intranasal OT therapy, they also did not reproduce negative effects of chronic treatment seen in previous studies. We would argue not only for the need for larger clinical trials, which are already in progress, but for refinement in the dose, frequency of administration, context of administration and for attention to individual difference factors, which might help to optimize the chance of positive benefits without long-term negative effects of OT treatment. As OT treatment regimens continue to be extensively explored in clinical trials, our preclinical findings indicate that intranasal OT treatment daily for 30 days does not produce deleterious behavioral effects in mice.
Acknowledgments
This research was funded by HD071998 to KLB, MS, SJ and SPM; OD P51OD01107 to the California National Primate Research Center; HD079125-01 MIND Institute IDDRC Core E to JLS, KRP and JNC.
SJ reports her involvement as the Site PI on a Department of Defense–funded, investigator-initiated clinical trial of intranasal oxytocin. The remaining authors declare no conflict of interest.
References
- Gimpl G, Fahrenholz F. The Oxytocin Receptor System: Structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Carter CS. Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol. 2014;65:17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- Zingg HH.OxytocinIn: Pfaff D, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT (eds). Hormones, Brain, and Behaviorvol. 3. Academic Press: New York, NY, USA; 2002779–802. [Google Scholar]
- Macdonald K, Feifel D. Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front Neurosci. 2013;7:35. doi: 10.3389/fnins.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav. 2012;61:410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Heinrichs M. Oxytocin, stress and social behavior: neurogenetics of the human oxytocin system. Curr Opin Neurobiol. 2013;23:11–16. doi: 10.1016/j.conb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci. 2012;15:681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Carson DS, Dadds MR, Mitchell PB, Cox RE. Does oxytocin influence the early detection of angry and happy faces. Psychoneuroendocrinology. 2009;34:220–225. doi: 10.1016/j.psyneuen.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves "mind-reading" in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddanthi S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Zink CF, Meyer-Lindenberg A. Human neuroimaging of oxytocin and vasopressin in social cognition. Horm Behav. 2012;61:400–409. doi: 10.1016/j.yhbeh.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJ. Oxytocin promotes human ethnocentrism. Proc Natl Acad Sci. 2011;108:1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biol Psychiatry. 2009;66:864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, et al. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci. 2010;6:556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB, Gomes AM, Carter CS, Lee R. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology. 2011;216:101–110. doi: 10.1007/s00213-011-2193-8. [DOI] [PubMed] [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, et al. The role of oxytocin in social bonding, stress regulation, and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, et al. Effects of oxytocin on recollections of maternal care and closeness. Proc Natl Acad Sci. 2010;107:21371–21375. doi: 10.1073/pnas.1012669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari R, van der Veen R, Parsons CE, Young KS, Voorthuis A, Bakermans-Kranenburg MJ, et al. Effects of intranasal oxytocin administration on memory for infant cues: Moderation by childhood emotional maltreatment. Soc Neurosci. 2014;9:536–547. doi: 10.1080/17470919.2014.932307. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology. 2003;1:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, et al. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2006;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Andari E, Duhamel J-R, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Kagitani-Shimono K, Mohri I, Yamamoto T, Sanefuji W, Nakimura A, et al. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2013;23:123–127. doi: 10.1089/cap.2012.0048. [DOI] [PubMed] [Google Scholar]
- Kosaka H, Munesue T, Ishitobi M, Asano M, Omori M, Sato M, et al. Long-term oxytocin administration improves social behaviors in a girl with autistic disorder. BMC Psychiatry. 2012;12:110. doi: 10.1186/1471-244X-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds MR, MacDonald E, Cauchi AJ, Williams K, Levy F, Brennan J. Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J Autism Dev Disord. 2014;44:521–531. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- Anagnostou E, Soorya L, Brian J, Dupuis A, Mankad D, Smile S, et al. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Res. 2014;1580:188–198. doi: 10.1016/j.brainres.2014.01.049. [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn MH, Bakersmans-Kranenburg MJ. A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2012;37:438–443. doi: 10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM. Developmental experiences and the oxytocin receptor system. Horm Behav. 2012;61:313–319. doi: 10.1016/j.yhbeh.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, et al. Chronic intranasal oxytocin causes long-term impairment in partner preference formation in male prairie voles. Biol Psychiatry. 2013;74:180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Michetti C, Busnelli M, Manago F, Sannino S, Scheggia E, et al. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology. 2014;39:1102–1114. doi: 10.1038/npp.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW, Houchard KR, Zhuang X. Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: a model of obsessive-compulsive disorder and Tourette's. BMC Biol. 2005;3:4. doi: 10.1186/1741-7007-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology. 2014;42:225–236. doi: 10.1016/j.psyneuen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Altemus M, Redwine LS, Leong YM, Frye CA, Porges SW, Carter CS. Responses to laboratory psychosocial stress in postpartum women. Psychosom Med. 2001;63:814–821. doi: 10.1097/00006842-200109000-00015. [DOI] [PubMed] [Google Scholar]
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol. 2004;78:333–339. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Leonzino M, Braida D, Sala M. Learning about oxytocin: pharmacologic and behavioral issues. Biol Psychiatry. 2013;76:360–366. doi: 10.1016/j.biopsych.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Vannucchi G, Masi G, Dell'Osso L, Marazziti D, Perugi G. Clinical features, developmental course, and psychiatric comorbidity of adult autism spectrum disorders. CNS Spectr. 2014;19:157–164. doi: 10.1017/S1092852913000941. [DOI] [PubMed] [Google Scholar]
- South M, Larson MJ, White SE, Dana J, Crowley MJ. Better fear conditioning is associated with reduced symptom severity in autism spectrum disorders. Autism Res. 2011;4:412–421. doi: 10.1002/aur.221. [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID, Slattery DA. Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacology. 2012;223:149–158. doi: 10.1007/s00213-012-2702-4. [DOI] [PubMed] [Google Scholar]
- Lahoud N, Maroun M. Oxytocinergic manipulations in corticolimbic circuit differentially affect fear acquistion and extinction. Psychoneuroendocrinology. 2013;38:2184–2195. doi: 10.1016/j.psyneuen.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Acheson D, Feifel D, de Wilde S, Mckinney R, Lohr J, Risbrough V. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology. 2013;229:199–208. doi: 10.1007/s00213-013-3099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Smith DG, Sukoff Rizzo SJ, Karras MN, Turner SM, Tolu SS, et al. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci Transl Med. 2012;4:131ra151. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Yang M, Abrams DN, Zhang JY, Weber MD, Katz AM, Clarke AM, et al. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol Behav. 2012;107:649–662. doi: 10.1016/j.physbeh.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz HL, Rothblat LA. Intact and impaired executive abilities in the BTBR mouse model of autism. Behav Brain Res. 2012;234:33–37. doi: 10.1016/j.bbr.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Gastrell PT, Karras MN, Solomon M, Crawley JN. Cognitive abilities on transitive inference using a novel touchscreen technology for mice. Cereb Cortex. 2013 (e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Pearson BL, Pobbe RLH, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, et al. Motor and cognitive stereotypics in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:228–235. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443–449. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SM, Sagar A, Levin-Decanini T, Liu W, Carter CS, Jacob S. Oxytocin and vasopressin systems in genetic syndromes and neurodevelopmental disorders. Brain Res. 2014;1580:199–218. doi: 10.1016/j.brainres.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Developmental consequences of oxytocin. Physiol Behav. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders. Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry. 2013;74:164–171. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Prehn K, Kazzer P, Lischke A, Heinrichs M, Herpertz SC, Domes G. Effects of intranasal oxytocin on pupil dilation indicate increased salience of socioaffective stimuli. Psychophysiology. 2013;50:528–537. doi: 10.1111/psyp.12042. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wohr M, Roullet FI, Katz AM, et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32:6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, et al. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;Chapter 8:Unit 8.24. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, et al. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010;171:1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier J, Senerth JM, Silverman JL, Matteson PG, Millinog JH, DiCicco-Bloom E, et al. Chronic desipramine treatment rescues depression-related, social and cognitive deficits in Engrailed-2 knockout mice. Genes Brain Behav. 2014;13:286–298. doi: 10.1111/gbb.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interactions and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neurosci Biobehav Rev. 2010;34:260–268. doi: 10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Stapley NW, Guariglia SR, Chadman KK. Cued and contextual fear conditioning in BTBR mice is improved with training or atomoxetine. Neurosci Lett. 2013;549:120–124. doi: 10.1016/j.neulet.2013.06.032. [DOI] [PubMed] [Google Scholar]
- MacPherson P, McGaffigan R, Wahlsten D, Nguyen PV. Impaired fear memory, altered object memory and modified hippocampal synaptic plasticity in split-brain mice. Brain Res. 2008;1210:179–188. doi: 10.1016/j.brainres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin centrally administered facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kim AJ, Lewis-Reese AD, Carter CS. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav. 2004;45:354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, et al. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology. 2013;72:187–196. doi: 10.1016/j.neuropharm.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Behav Neurosci. 2009;3:1–14. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Oxytocin adminsitration to parent enhances infant physiological and behavioral readiness for social engagement. Biol Psychiatry. 2012;72:982–989. doi: 10.1016/j.biopsych.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Pobbe RLH, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+tf/J mouse strain. Behav Brain Res. 2011;216:446–451. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier GL, Imamura N, Zanoletti O, Sandi C. Social deficits induced by peripubertal stress in rats are reversed by resveratrol. J Psychiatr Res. 2014;57:157–164. doi: 10.1016/j.jpsychires.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Tsuda MC, Ogawa S. Long-lasting consequences of neonatal maternal separation on social behaviors in ovariectomized female mice. PLoS One. 2012;7:e33028. doi: 10.1371/journal.pone.0033028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, et al. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PLoS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodriguez L, Feig LA. Chronic social instability induces anxiety and defective social interactions across generations. Biol Psychiatry. 2013;73:44–53. doi: 10.1016/j.biopsych.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res. 2009;197:462–465. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Bales KL, Lewis-Reese AD, Pfeifer LA, Kramer KM, Carter CS. Early experience affects the traits of monogamy in a sexually dimorphic manner. Dev Psychobiol. 2007;49:335–342. doi: 10.1002/dev.20216. [DOI] [PubMed] [Google Scholar]
- Bales KL, Boone E, Epperson P, Hoffman G, Carter CS. Are behavioral effects of early experience mediated by oxytocin. Front Psychiatry. 2011;2:24. doi: 10.3389/fpsyt.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti A, Melis M, Siddi S, Vellante M, Doneddu G, Fadda R. Oxytocin and autism: a systematic review of randomized controlled trials. J Child Adolesc Psychopharmacol. 2014;24:54–68. doi: 10.1089/cap.2013.0040. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34:917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Einfeld SL, Smith E, McGregor IS, Steinbeck K, Taffe J, Rice LJ, et al. A double-blind randomized control trial of oxytocin nasal spray in Prader Willi syndrome. Am J Med Genet A. 2014;164A:2232–2239. doi: 10.1002/ajmg.a.36653. [DOI] [PubMed] [Google Scholar]