Abstract
Background:
Epidemiological evidence suggests that use of aspirin after the diagnosis of colorectal cancer can lengthen survival. However, the supporting data vary between studies, and this hypothesis remains controversial. We conducted a meta-analysis to provide a quantitative assessment of the association between use of aspirin after diagnosis of colorectal cancer and patient survival.
Methods:
We searched the Medline and Embase databases up to April 2014 to identify studies related to aspirin use after diagnosis and all-cause mortality or colorectal cancer-specific mortality. Summary effect estimates with 95% confidence intervals (CIs) were derived using a fixed or random effects model, depending on the heterogeneity between the included studies.
Results:
Seven epidemiologic studies that consisted of six cohort studies and one nested case–control study were included in this meta-analysis. The hazard ratio (HR) of the association between aspirin use after colorectal cancer diagnosis and overall mortality, which was reported in five studies, was 0.74 (95% CI, 0.62–0.89) using a random model (heterogeneity test P=0.003, I2=75.3%), and for colorectal cancer-specific mortality (four studies), it was 0.75 (95% CI, 0.51–1.10) using a random model (heterogeneity test P=0.001, I2=84.1%). In addition, we analysed postdiagnosis aspirin use according to whether aspirin was also used before diagnosis. The HR for the overall mortality of patients who did not use aspirin before diagnosis, which was reported in four studies, was 0.84 (95% CI, 0.70–1.00), and for colorectal cancer-specific mortality (three studies), it was 0.79 (95% CI, 0.61–1.02). For those who did use aspirin before diagnosis, the HR for overall mortality (four studies) was 0.88 (95% CI, 0.83–0.93), and for colorectal cancer-specific mortality (three studies), it was 0.80 (95% CI, 0.59–1.09). Subgroup analysis showed that use of aspirin after diagnosis was associated with longer overall survival among patients with the variant PIK3CA gene but not for those with wild-type PIK3CA.
Conclusions:
Based on current evidence, the use of aspirin after diagnosis does not reduce colorectal cancer-specific mortality, but it does reduce all-cause mortality for colorectal cancer patients.
Keywords: aspirin, postdiagnosis, colorectal cancer, survival, PIK3CA, meta-analysis
Colorectal cancer originates in the colon or the rectum, and its incidence is currently increasing. It has been reported to be the third most common cancer and the fourth leading cause of cancer-related death worldwide (Ferlay et al, 2010). Several factors, including lifestyle and age, are risk factors for colorectal cancer. However, the prognosis of individuals with colorectal cancer has improved since the introduction of adjuvant chemotherapy. Recent studies have revealed that regular aspirin use after the diagnosis of colorectal cancer is associated with a lower risk of both colorectal cancer-specific and overall mortality (Chan et al, 2009; Bastiaannet et al, 2012).
Aspirin, a non-steroidal anti-inflammatory drug, is usually indicated for the treatment of pain and inflammation and for the prevention of stroke at low dosage. In addition to its intrinsic clinical effect, it has also been shown to reduce the risk of cardiovascular disease and cancer (Baigent et al, 2009; Rothwell et al, 2011, 2012a, 2012b; Algra & Rothwell, 2012), including colorectal cancer (Hollestein et al, 2014). Aspirin has also been reported to reduce both the long-term incidence of colorectal cancer and mortality owing to this malignancy (Din et al, 2010; Rothwell et al, 2010). However, most of these studies only considered the use of aspirin before the diagnosis of colorectal cancer.
One of the main mechanisms of action of aspirin is the inhibition of cyclo-oxygenase (COX), the enzyme responsible for biosynthesing the prostaglandins, which increase cellular proliferation, migration, and invasiveness, and promotes angiogenesis. Aspirin inhibits both COX-1 and COX-2. COX-2 is expressed in 70% of colorectal tumours and has an important role in colorectal carcinogenesis, invasion, and metastasis (Chan et al, 2009, 2012; Midgley et al, 2010; Bacchi et al, 2012; Wang & DuBois, 2013).
Recently, a number of studies have been conducted to assess the use of aspirin after diagnosis and the survival of colorectal cancer patients, with varying results. Although some of these studies suggested that postdiagnosis use of aspirin could lengthen colorectal cancer survival, others found no effect, resulting in uncertainty and controversy regarding these findings. McCowan et al (2012) reported postdiagnosis aspirin use was associated with a 30% reduction in all-cause mortality. Walker et al (2012) reported a 10% reduction in all-cause mortality in patients treated with postdiagnosis aspirin. Moreover, to date, no meta-analysis has been performed to analyse and summarise the evidence for survival benefit of postdiagnosis use of aspirin. Hence, we performed a meta-analysis of relevant epidemiological studies to obtain an overview of the association between the use of aspirin after diagnosis and the survival of colorectal cancer patients, for both all-cause mortality and colorectal cancer-specific mortality.
Materials and Methods
This review was conducted and reported following the Meta-analysis Of Observational Studies in Epidemiology guidelines (Stroup et al, 2000).
Search strategy
Any observational study that examined the relationship between the use of aspirin after colorectal cancer diagnosis and patient survival was eligible for inclusion in our study. We searched PubMed and Embase for articles published until April 2014 and used ‘aspirin' and ‘colorectal cancer' or ‘rectal cancer' or ‘colon cancer' as the search terms. We also conducted manual searches of reference lists from all the relevant original and review articles to identify any additional eligible studies. The medical subject heading, methods, patient population, design, exposure, and outcome variables of these articles were used to identify the relevant studies.
The literature search was independently undertaken by two authors (SWT and WJ) with a standardised approach. Any inconsistencies between the findings of these two authors were reviewed by the primary author (HJ), and a consensus was reached.
Criteria for inclusion
The study was eligible for inclusion if the following criteria were met: (1) the study was a prospective cohort or a prospective nested case–control study; (2) the study investigated the association between the postdiagnosis use of aspirin among colorectal cancer patients and their survival; and (3) effect estimates (hazard ratio (HR)) and 95% confidence intervals (CIs) were used. All English-language literature was eligible for inclusion in our analysis. Studies investigating only the use of aspirin before the diagnosis of colorectal cancer and survival were excluded.
Data extraction
The data were collected using a standardised data collection form and included the first author's name, publication year, country, database, study design, sample size, age at baseline, follow-up duration, dose, duration, effect estimates and 95% CIs, and covariates in the fully adjusted model.
Quality assessment
The Newcastle–Ottawa scale (NOS), which is widely used and has been partially validated for evaluating the quality of observational studies in a meta-analysis, was used to evaluate the methodological quality (Wells et al, 2009).The NOS consists of three aspects: selection, comparability, and exposure. Each satisfactory answer scores one point. A ‘star system' (range, 0–9) is used for assessment.
The quality assessment was independently conducted by two authors (YXF and WJ). Information was examined and adjudicated independently by an additional author (HJ) after referring to the original studies.
Statistical analysis
The study-specific adjusted HRs and corresponding 95% CIs were used as the common measure of association across studies. Heterogeneity was assessed using the Cochran Q and I2 statistics (Higgins et al, 2003). For the Q statistic, a P-value >0.10 for the χ2-test, and for the I2 statistic, an I2 value <25% was interpreted as low-level heterogeneity. A pooled effect was calculated with a fixed-effect model if there was no statistically significant heterogeneity; otherwise, a random effect model was employed.
Publication bias was assessed using the Begg and Egger regression asymmetry test, together with the funnel plot. All reported P-values are two-sided, and P-values <0.05 were considered statistically significant for all the included studies. Statistical analyses were performed using the STATA software (version 12.0; Stata Corporation, College Station, TX, USA).
Results
Description of the selected studies
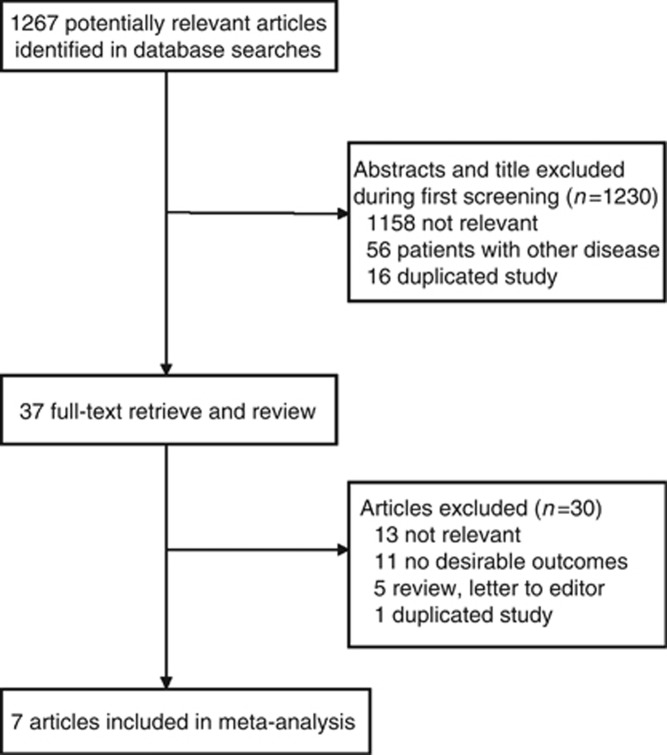
A total of 1267 potentially relevant articles were retrieved using our search strategy, of which 1230 were excluded because they were irrelevant (1158 articles), duplications (16 articles), or concerned diseases other than colorectal cancer (56 articles). This left 37 studies, all of which were evaluated based on the full text. Thirteen irrelevant articles, 11 articles reporting no desirable outcomes, 5 articles (review or letter to editor), and 1 duplicate article were excluded. Finally, seven studies were included in this meta-analysis, including one nested case–control study and six cohort studies (Chan et al, 2009; Bastiaannet et al, 2012; Liao et al, 2012; McCowan et al, 2012; Walker et al, 2012; Cardwell et al, 2014; Reimers et al, 2014). The detailed literature screening process is shown in Figure 1, and the exclusion reasons for duplicate and irrelevant are listed in Supplementary Table S1. All of these studies were published after 2009. Two studies were conducted in the United States, two in the Netherlands, and three in the United Kingdom. In prospective studies, participants entered the studies after they were diagnosed with colorectal cancers in different studies. Then those who were prescribed aspirin were classified as aspirin users and others were classified as aspirin non-users. In the nested case–control study, cases were ascertained by cancer registries, and each case was matched to five controls.
Figure 1.
Flow diagram of the search and selection process of the studies.
Among the included studies, six studies reported the association between postdiagnosis use of aspirin and all-cause mortality, and four studies reported the colorectal cancer-specific mortality. Four studies analysed the use of aspirin prior to colorectal cancer diagnosis with respect to all-cause mortality, and three studies reported colorectal cancer-specific mortality. According to the nine-point NOS, three studies had NOS scores of eight; two studies had NOS scores of seven, whereas two studies had scores of six. The general characteristics of each study are listed in Table 1.
Table 1. Characteristic of the included studies.
| Author (year) | Country/region | Database | Median follow-up | Study type | Sample size | No. of aspirin users | No. of aspirin non-users | Median age | Adjusted variable | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
|
Cardwell et al, 2014 |
Northern Ireland |
National
Cancer Data Repository |
NR |
Nested case–control study |
9089 |
2387 |
6711 |
NR |
Including surgery, chemotherapy, radiotherapy, statin use, metformin use, and comorbidities, including myocardial infarction, cerebrovascular disease, congestive heart disease, chronic pulmonary disease, peripheral vascular disease, peptic ulcer disease, renal disease and rheumatological disease, and diabetes |
7 |
|
Liao et al, 2012 |
US |
Nurses'
Health Study and the Health Professionals Follow-up Study |
14.75 |
Cohort study |
964 |
561 |
403 |
68 |
Disease stage, initially included age, sex, year of diagnosis, aspirin use after diagnosis, tumour location, tumour differentiation, body mass index, microsatellite instability status, sex |
8 |
|
Reimers et al, 2014 |
Netherlands |
PHARMO |
NR |
Cohort study |
999 |
180 |
794 |
NR |
Sex, age, comorbidity, year of incidence, histological grade, stage, and chemotherapy |
7 |
|
McCowan et al, 2012 |
UK |
The Health Informatics Centre |
4.22 |
Cohort study |
2990 |
1340 |
1650 |
73 |
Age, sex, social class, stage of disease diagnosis |
8 |
|
Bastiaannet et al, 2012 |
Netherlands |
PHARMO |
3.5 |
Cohort study |
4481 |
3305 |
1176 |
69 |
Sex, age, comorbidity, year of incidence, grade, stage and treatment |
6 |
|
Walker et al, 2012 |
UK |
General Practice Research
Database |
NR |
Cohort study |
13 994 |
2619 |
1365 |
NR |
Age, gender, smoking, BMI, alcohol use and comorbidity |
8 |
| Chan et al, 2009 | US | Mailed questionnaire | 11.8 | Cohort study | 1279 | 549 | 730 | 60.7 | Age, sex, date of diagnosis, stage of cancer, site of primary cancer, histological grade of cancer, aspirin use, smoking, BMI, cancer in a parent or sibling | 6 |
Abbreviations: BMI=body mass index; NR=not reported.
Aspirin use after colorectal cancer diagnosis and survival
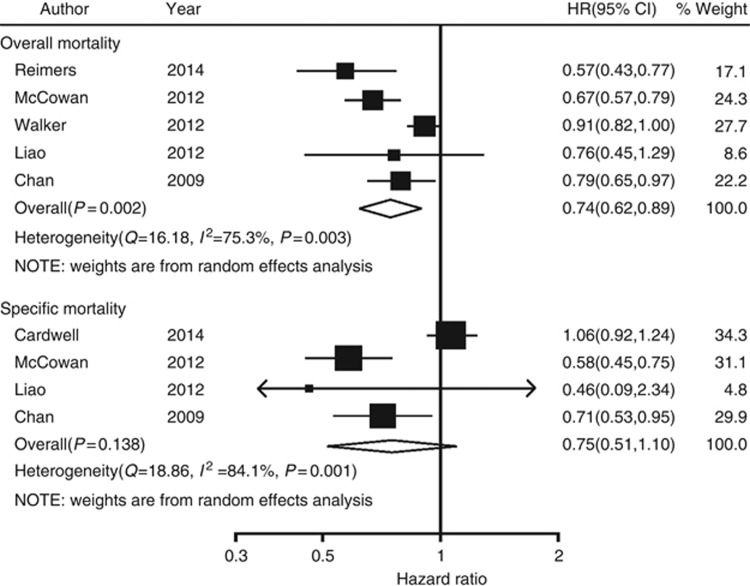
The estimated pooled HRs showed a statistical significantly association between the use of aspirin after diagnosis and all-cause mortality, compared with aspirin non-users (pooled HR=0.74; 95% CI, 0.62–0.89; Figure 2) using a random model (heterogeneity test P=0.003, I2=75.3%) without publication bias (Egger test P=0.091). However, no association was found between the use of aspirin after diagnosis and colorectal cancer-specific mortality, compared with aspirin non-users (pooled HR=0.75; 95% CI, 0.51–1.10; Figure 2) using a random model (heterogeneity test P=0.001, I2=84.1%) without publication bias (Egger test P=0.397). Funnel plot for publication bias assessment is illustrated in Supplementary Figure S1.
Figure 2.
Forest plot of aspirin use after diagnosis of colorectal cancer and patient survival.
In order to compare the outcome of patients who regularly used aspirin before diagnosis but then discontinued after diagnosis, we analysed postdiagnosis aspirin use according to whether aspirin was used before diagnosis.
Prediagnosis aspirin use
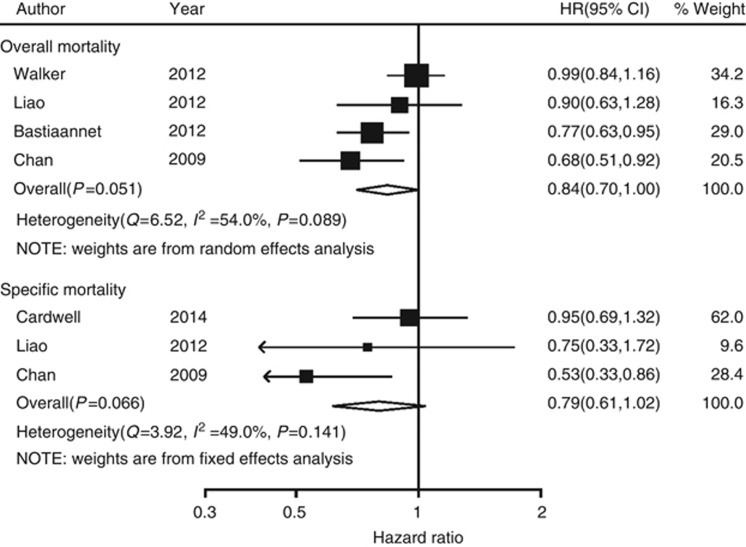
Prediagnosis aspirin non-users were defined as those who did not use aspirin prior to diagnosis of colorectal cancer but started to use aspirin after diagnosis. There was a statistical significantly association between aspirin use and all-cause mortality, compared with aspirin non-users (pooled HR=0.84; 95% CI, 0.70–1.00; Figure 3), but not colorectal cancer-specific mortality (pooled HR=0.79; 95% CI, 0.61–1.02; Figure 3) in this group. Prediagnosis aspirin users were defined as those who regularly used aspirin most weeks prior to the diagnosis of colorectal cancer and continued to use aspirin after diagnosis. For this group, there was also a statistically significant association between aspirin use and all-cause mortality, compared with aspirin non-users (pooled HR=0.88; 95% CI, 0.83–0.93; Figure 4), but not for colorectal cancer-specific mortality (pooled HR=0.80; 95% CI, 0.59–1.09; Figure 4). Funnel plot for publication bias assessment is illustrated in Supplementary Figure S1.
Figure 3.
Forest plot of aspirin use after diagnosis of colorectal cancer (prediagnosis aspirin non-users) and patient survival.
Figure 4.
Forest plot of aspirin use after diagnosis of colorectal cancer (prediagnosis aspirin users) and patient survival.
We found no evidence of an interaction between prediagnosis aspirin non-users and prediagnosis aspirin users. The interaction P for all-cause mortality and colorectal cancer specific mortality was 0.6262 and 0.9509, respectively.
Subgroup analysis
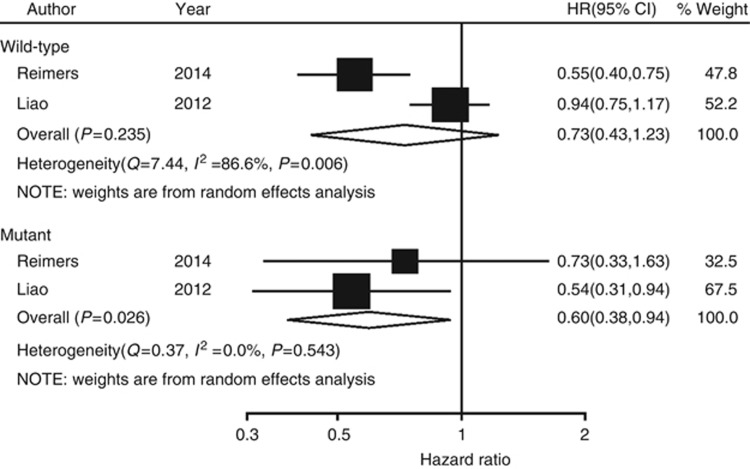
A subgroup analysis was conducted according to the status of the PIK3CA gene. The results suggest that, among patients with a mutated PIK3CA gene, use of aspirin after diagnosis was associated with longer overall survival, compared with aspirin non-users (pooled HR=0.60; 95% CI, 0.38–0.94; Figure 5). However, among patients with the wild-type PIK3CA gene, use of aspirin after diagnosis was not associated with longer overall survival, compared with aspirin non-users (pooled HR=0.73; 95% CI, 0.43–1.23; Figure 5). No significant interactions were found between mutated PIK3CA gene and wild-type PIK3CA gene (interaction P=0.5795).
Figure 5.
Forest plot of the risk of aspirin use and overall mortality after diagnosis of colorectal cancer according to tumour PIK3CA mutation status.
Sensitivity analysis
Sensitivity analysis was performed to test the stability of the results by excluding each study iteratively. This showed that the results were not affected by sequential exclusion of any particular trial except for one study (Cardwell et al, 2014). The detailed sensitivity analysis results are listed in Table 2.
Table 2. Sensitivity analysis.
| Design/effect | Study (year) | HR | LL | UL |
|---|---|---|---|---|
|
Aspirin postdiagnosis | ||||
| All-cause mortality | 0.74 | 0.62 | 0.89 | |
| Reimers et al, 2014 | 0.79 | 0.66 | 0.94 | |
| McCowan et al, 2012 | 0.77 | 0.63 | 0.95 | |
| Walker et al, 2012 | 0.69 | 0.61 | 0.79 | |
| Liao et al, 2012 | 0.74 | 0.61 | 0.91 | |
| Chan et al, 2009 | 0.73 | 0.57 | 0.93 | |
| Colorectal cancer-specific mortality | 0.75 | 0.51 | 1.10 | |
| Cardwell et al, 2014 | 0.63 | 0.52 | 0.76 | |
| McCowan et al, 2012 | 0.86 | 0.59 | 1.24 | |
| Liao et al, 2012 | 0.77 | 0.52 | 1.14 | |
| |
Chan et al, 2009 |
0.75 |
0.43 |
1.31 |
|
Users prediagnosis | ||||
| All-cause mortality | 0.88 | 0.83 | 0.93 | |
| Walker et al, 2012 | 0.88 | 0.83 | 0.93 | |
| Liao et al, 2012 | 0.88 | 0.83 | 0.93 | |
| Chan et al, 2009 | 0.87 | 0.83 | 0.92 | |
| Bastiaannet et al, 2012 | 0.87 | 0.78 | 0.97 | |
| Colorectal cancer-specific mortality | 0.80 | 0.59 | 1.09 | |
| Cardwell et al, 2014 | 0.86 | 0.57 | 1.28 | |
| Liao et al, 2012 | 0.82 | 0.59 | 1.12 | |
| |
Chan et al, 2009 |
0.70 |
0.43 |
1.11 |
|
Nonusers prediagnosis | ||||
| All-cause mortality | 0.84 | 0.70 | 1.00 | |
| Walker et al, 2012 | 0.77 | 0.66 | 0.89 | |
| Liao et al, 2012 | 0.82 | 0.66 | 1.03 | |
| Chan et al, 2009 | 0.89 | 0.75 | 1.05 | |
| Bastiaannet et al, 2012 | 0.86 | 0.68 | 1.09 | |
| Colorectal cancer-specific mortality | 0.79 | 0.61 | 1.02 | |
| Cardwell et al, 2014 | 0.58 | 0.38 | 0.88 | |
| Liao et al, 2012 | 0.79 | 0.60 | 1.03 | |
| Chan et al, 2009 | 0.92 | 0.68 | 1.24 | |
Abbreviations: HR=hazard ratio; LL=lower limit; UL=upper limit.
Discussion
There was uncertainty and controversy regarding the association between postdiagnosis use of aspirin and survival of colorectal cancer patients in the previous studies. In addition,whether patients with a mutant form of the PIK3CA gene might benefit from the prescription of aspirin was reported with different results. In this study, we investigated the association between the postdiagnosis use of aspirin and survival of colorectal cancer patients. We carried a broad search of manually reviewed databases, which yielded seven studies that met out inclusion criteria. Statistical analysis of these studies showed that postdiagnosis use of aspirin did not improve colorectal cancer-specific mortality but improved all-cause mortality for colorectal cancer patients. In addition, subgroup analysis suggested that the use of aspirin after diagnosis was associated with longer overall survival among patients with a mutated form of the PIK3CA gene but not for those with the wild-type PIK3CA gene.
The results suggest that the use of aspirin after diagnosis does not reduce colorectal cancer-specific mortality, but it does reduce all-cause mortality for colorectal cancer patients. The mechanism by which the use of aspirin prior to colorectal cancer diagnosis reduced all-cause mortality remains unknown. However, high levels of prostaglandin endoperoxide synthase 2, which is inhibited by aspirin, are associated with a poor prognosis in colorectal cancer. In addition, aspirin has been reported to prevent a number of diseases, including cardiovascular disease. This mechanism might also contribute to the longer survival of colorectal patients.
The analysis of prediagnosis aspirin might be a confounder for the relationship between aspirin use after diagnosis of colorectal cancer and patient survival. But, in the clinical settings, some colorectal patients were prescribed aspirin to prevent cardiovascular diseases and so on and continued to use aspirin after colorectal diagnosis. The result could give some information for those patients.
The included studies have different characteristics. Both of the study by Walker et al (2012) and Cardwell et al (2014) used the CPRD database in United Kingdom. We included both studies because they reported different outcomes. Walker et al (2012) conducted a cohort study and compared aspirin users with non-users based upon use in the first year after diagnosis in individuals surviving 1 year. They adjusted gender, age, smoking status, alcohol use, body mass index, and comorbidity as potential confounders. However, they did not adjust tumour stage and cancer treatments as Cardwell et al (2014) did. Cardwell et al (2014) conducted a nested case–control analysis and included 1559 colorectal cancer-specific deaths. One case was matched to five controls, and conditional logistic regression was performed. Cardwell et al (2014) focussed solely on low-dose aspirin usage while Walker et al (2012) analysed both the low-dose aspirin usage and high-dose aspirin usage. In addition, the analysis in the studies by Walker et al (2012) and Cardwell et al (2014) was all restricted to individuals with at least 1 year of follow-up (Walker et al, 2012; Cardwell et al, 2014). Bastiaannet et al (2012) and McCowan et al (2012) used a time-varying covariate model to compare aspirin users with non-users. In the study by Bastiaannet et al (2012), they excluded patients who died within 30 days from diagnosis. In all, 45% of the populations in the study by McCowan et al (2012) were prescribed aspirin, which was higher than other studies. The study by McCowan et al (2012) did not adjust some important variables, such as comorbidity, smoking status, body mass index, and so on. The study by Chan et al (2009) excluded stage IVcolorectal cancer patients. In the analysis, they excluded deaths that occurred within 12 months of completing aspirin assessment. Both of the study by Liao et al (2012) and Reimers et al (2014) focussed on the effect of aspirin on survival based on the mutation of some biomarkers, such as PIK3CA. Studies included were conducted under different settings as discussed, such as different aspirin dosage, patients characteristic, statistical analysis method, and so onn, which would lead to the heterogeneity between studies.
Liao et al (2012) and Reimers et al (2014) conducted cohort studies to explore the relationship between aspirin use after diagnosis of colorectal cancer and patient survival according to the status of the PIK3CA gene. The two studies reported completely opposite results. Liao et al (2012) reported that use of aspirin after diagnosis was associated with longer overall survival among patients with the mutant PIK3CA gene but not for those with wild-type PIK3CA. Although Reimers et al (2014) reported a benefit for those with wild-type PIK3CA, Liao et al (2012) postulated that by blocking the PIK3CA pathway PTGS2 activity decreases, which leads to apoptosis of colon cancer cells. The result echoes with a previous randomised clinical trial by Domingo et al (2013). In addition, one drawback of both the two studies is the limited sample sizes, especially in the mutant PIK3CA gene group, with 100 and 161 patients, respectively. The small sample size could not have had an enough power to detect the difference between aspirin users and non-users. After combining the two separate studies together, a subgroup analysis showed that use of aspirin after diagnosis was associated with longer overall survival among patients with the variant PIK3CA gene but not for those with wild-type PIK3CA.
The strength of our study lies in the inclusion of all the observational studies concerning postdiagnosis use of aspirin and the survival of colorectal cancer patients. We present all available evidence in a systematic, quantitative, and unbiased fashion. However, our study also has some limitations that deserve further discussion. First, and most importantly, this meta-analysis used data from epidemiological studies, which were unable to control the same important potential confounders. The demographics of a study population may lead to valid differences in the magnitude and direction of association. Second, we did not analyse the effect of drug dosage and duration in the subgroup analysis. Dosage and duration are important factors to support the relationship between postdiagnosis use of aspirin and the survival of colorectal cancer patients. Few of the included studies provided information on the effect of dosage and duration. Third, we only included English language studies, which could lead to an incomplete search of relevant studies. Fourth, some bias lying in the included observational studies could not be ignored, such as immortal time bias and confounding by indication. Immortal time in epidemiology refers to a period of cohort follow-up time during which death (or an outcome that determines end of follow-up) cannot occur (Shariff et al, 2008). Confounding by indication refers to an extraneous determinant of the outcome parameter that is present if a perceived high risk or poor prognosis is an indication for intervention (Grobbee & Hoes, 1997). Becuse of the limitation of this meta-analysis, further studies are required to determine the clinical importance of our findings.
In summary, this comprehensive meta-analysis reveals that postdiagnosis use of aspirin does not reduce colorectal cancer-specific mortality but does reduce all-cause mortality among colorectal cancer patients. In addition, patients with a mutant form of the PIK3CA gene might benefit from the prescription of aspirin.
Acknowledgments
This work was supported by grants from the leading talents of science in Shanghai 2010(022), the key discipline construction of evidence-based public health in Shanghai (12GWZX0602), the National Natural Science Foundation of China (No. 81072388, No. 81202285, No. 81373105) and Natural Science Foundation of Shanghai (12ZR1453700).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem. 2012;11:52–64. doi: 10.2174/187152312803476255. [DOI] [PubMed] [Google Scholar]
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaannet E, Sampieri K, Dekkers OM, de Craen AJ, van Herk-Sukel MP, Lemmens V, van den Broek CB, Coebergh JW, Herings RM, van de Velde CJ, Fodde R, Liefers GJ. Use of aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer. 2012;106:1564–1570. doi: 10.1038/bjc.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell CR, Kunzmann AT, Cantwell MM, Hughes C, Baron JA, Powe DG, Murray LJ. Low-dose aspirin use after diagnosis of colorectal cancer does not increase survival: a case-control analysis of a population-based cohort. Gastroenterology. 2014;146 (700-708:e2. doi: 10.1053/j.gastro.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Chan AT, Arber N, Burn J, Chia WK, Elwood P, Hull MA, Logan RF, Rothwell PM, Schror K, Baron JA. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev Res (Phila) 2012;5:164–178. doi: 10.1158/1940-6207.CAPR-11-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din FV, Theodoratou E, Farrington SM, Tenesa A, Barnetson RA, Cetnarskyj R, Stark L, Porteous ME, Campbell H, Dunlop MG. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut. 2010;59:1670–1679. doi: 10.1136/gut.2009.203000. [DOI] [PubMed] [Google Scholar]
- Domingo E, Church DN, Sieber O, Ramamoorthy R, Yanagisawa Y, Johnstone E, Davidson B, Kerr DJ, Tomlinson IP, Midgley R. Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J Clin Oncol. 2013;31:4297–4305. doi: 10.1200/JCO.2013.50.0322. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Grobbee DE, Hoes AW. Confounding and indication for treatment in evaluation of drug treatment for hypertension. BMJ. 1997;315:1151–1154. doi: 10.1136/bmj.315.7116.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollestein LM, van Herk-Sukel MP, Ruiter R, de Vries E, Mathijssen RH, Wiemer EA, Stijnen T, Coebergh JW, Lemmens VE, Herings RM, Stricker BH, Nijsten T. Incident cancer risk after the start of aspirin use: results from a Dutch population-based cohort study of low dose aspirin users. Int J Cancer. 2014;135:157–165. doi: 10.1002/ijc.28634. [DOI] [PubMed] [Google Scholar]
- Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, Sun R, Nosho K, Meyerhardt JA, Giovannucci E, Fuchs CS, Chan AT, Ogino S. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan C, Munro AJ, Donnan PT, Steele RJ. Use of aspirin post-diagnosis in a cohort of patients with colorectal cancer and its association with all-cause and colorectal cancer specific mortality. Eur J Cancer. 2012;49:1049–1057. doi: 10.1016/j.ejca.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Midgley RS, McConkey CC, Johnstone EC, Dunn JA, Smith JL, Grumett SA, Julier P, Iveson C, Yanagisawa Y, Warren B, Langman MJ, Kerr DJ. Phase III randomized trial assessing rofecoxib in the adjuvant setting of colorectal cancer: final results of the VICTOR trial. J Clin Oncol. 2010;28:4575–4580. doi: 10.1200/JCO.2010.29.6244. [DOI] [PubMed] [Google Scholar]
- Reimers MS, Bastiaannet E, Langley RE, van Eijk R, van Vlierberghe RL, Lemmens VE, van Herk-Sukel MP, van Wezel T, Fodde R, Kuppen PJ, Morreau H, van de Velde CJ, Liefers GJ. Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA Intern Med. 2014;174 (5:732–739. doi: 10.1001/jamainternmed.2014.511. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol. 2008;19:841–843. doi: 10.1681/ASN.2007121354. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Walker AJ, Grainge MJ, Card TR. Aspirin and other non-steroidal anti-inflammatory drug use and colorectal cancer survival: a cohort study. Br J Cancer. 2012;107:1602–1607. doi: 10.1038/bjc.2012.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, DuBois RN. The role of anti-inflammatory drugs in colorectal cancer. Annu Rev Med. 2013;64:131–144. doi: 10.1146/annurev-med-112211-154330. [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute: Ottawa, Canada; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.