Abstract
Background:
The randomised phase III TURANDOT trial compared first-line bevacizumab–paclitaxel (BEV–PAC) vs bevacizumab–capecitabine (BEV–CAP) in HER2-negative locally recurrent/metastatic breast cancer (LR/mBC). The interim analysis revealed no difference in overall survival (OS; primary end point) between treatment arms; however, progression-free survival (PFS) and objective response rate were significantly superior with BEV–PAC. We sought to identify patient populations that may be most appropriately treated with one or other regimen.
Methods:
Patients with HER2-negative LR/mBC who had received no prior chemotherapy for advanced disease were randomised to either BEV–PAC (bevacizumab 10 mg kg−1 days 1 and 15 plus paclitaxel 90 mg m−2 days 1, 8 and 15 q4w) or BEV–CAP (bevacizumab 15 mg kg−1 day 1 plus capecitabine 1000 mg m−2 bid days 1–14 q3w). The study population was categorised into three cohorts: triple-negative breast cancer (TNBC), high-risk hormone receptor-positive (HR+) and low-risk HR+. High- and low-risk HR+ were defined, respectively, as having ⩾2 vs ⩽1 of the following four risk factors: disease-free interval ⩽24 months; visceral metastases; prior (neo)adjuvant anthracycline and/or taxane; and metastases in ⩾3 organs.
Results:
The treatment effect on OS differed between cohorts. Non-significant OS trends favoured BEV–PAC in the TNBC cohort and BEV–CAP in the low-risk HR+ cohort. In all three cohorts, there was a non-significant PFS trend favouring BEV–PAC. Grade ⩾3 adverse events were consistently less common with BEV–CAP.
Conclusions:
A simple risk factor index may help in selecting bevacizumab-containing regimens, balancing outcome, safety profile and patient preference. Final OS results are expected in 2015 (ClinicalTrials.gov NCT00600340).
Keywords: bevacizumab, metastatic breast cancer, triple-negative breast cancer, risk factor, prognostic index
Three randomised phase III trials (E2100 (Miller et al, 2007) AVADO (Miles et al, 2010) and RIBBON-1 (Robert et al, 2011)) demonstrated that adding bevacizumab to first-line chemotherapy for locally recurrent or metastatic breast cancer (LR/mBC) significantly improves progression-free survival (PFS; primary end point) and objective response rate (ORR), but not overall survival (OS). Two of the regimens evaluated in these trials (bevacizumab plus weekly paclitaxel (BEV–PAC) in E2100 (Miller et al, 2007) and bevacizumab plus capecitabine (BEV–CAP) in RIBBON-1 (Robert et al, 2011)) are approved by health authorities in many countries worldwide but not in the USA. Two subsequent trials in the first-line setting, CALGB 40502 (Rugo et al, 2012) and TURANDOT (Lang et al, 2013), compared different bevacizumab-containing regimens. CALGB 40502 compared BEV–PAC vs bevacizumab combined with either nanoparticle albumin-bound (nab)-paclitaxel or ixabepilone. TURANDOT compared the two approved bevacizumab-containing regimens, that is, BEV–PAC vs BEV–CAP. Importantly, despite the inherent limitations of cross-trial comparisons, both trials essentially replicated the efficacy of the ‘standard' bevacizumab regimens observed in E2100 and RIBBON-1.
At the prespecified interim analysis of the TURANDOT trial, there was no detectable difference in OS (primary end point) between treatment groups (Lang et al, 2013). However, BEV–PAC was significantly superior to BEV–CAP for both PFS and ORR (secondary end points). We conducted subgroup analyses of TURANDOT to explore potential differences in the magnitude of treatment effect according to clinical characteristics. Our aim was to identify patient populations defined by risk factors that may be most appropriately treated with one or other regimen. The risk factors were based largely on an analysis of prognostic factors in the ATHENA study, in which >2000 patients received first-line bevacizumab-containing therapy for HER2-negative LR/mBC (Llombart-Cussac et al, 2013). In ATHENA, the prognostic factors most closely and robustly associated with worse OS were: liver metastases or ⩾3 metastatic organs; disease-free interval (DFI) ⩽24 months; prior anthracycline and/or taxane therapy; and triple-negative breast cancer (TNBC). The prognostic factor index enabled identification of patients with a poorer prognosis for whom particular systemic treatment regimens may be of interest.
Materials and Methods
Study design
The design of TURANDOT (ClinicalTrials.gov NCT00600340) has been published previously (Lang et al, 2013). Briefly, patients with HER2-negative LR/mBC who had received no prior chemotherapy for their disease were randomised to either BEV–PAC (bevacizumab 10 mg kg−1, days 1 and 15, plus paclitaxel 90 mg m−2, days 1, 8 and 15, every 4 weeks) or BEV–CAP (bevacizumab 15 mg kg−1 day 1 plus capecitabine 1000 mg m−2 twice daily days 1–14, every 3 weeks). Concomitant endocrine therapy was not permitted. Stratification factors were oestrogen receptor (ER)/progesterone receptor (PgR) status, country and menopausal status.
Statistical methods
All analyses were based on the dataset from the prespecified interim analysis (data cut-off: 1 September 2011). Efficacy was analysed in the intent-to-treat population (all randomised patients); safety was analysed in the safety population (all patients who received at least one dose of study therapy). Post hoc subgroup analyses were performed according to hormone receptor status (negative ER, PgR and HER2 (TNBC) vs positive ER and/or PgR (hormone receptor-positive (HR+))).
In additional exploratory analyses, the HR+ population was further categorised into high-risk and low-risk subgroups, defined, respectively, as having two or more (high risk) vs one or none (low risk) of the following four risk factors: DFI ⩽24 months; visceral metastases; prior (neo)adjuvant anthracycline and/or taxane; or ⩾3 metastatic organs. These risk factors differ from those identified in ATHENA. First, as we had already analysed the subgroup of patients with TNBC (essentially a stratification factor in TURANDOT (Inbar et al, 2013)) and considered these to be biologically quite different from the HR+ subgroup, with different post-study treatment options available, we classified TNBC as one subgroup and then used the risk factors above as a guide to subdivide the HR+ subgroup according to the remaining risk factors. Second, we used visceral metastases rather than liver-only metastases because lung metastases also showed strong prognostic value in the ATHENA univariate analysis (P=0.004).
Overall survival and PFS were compared between treatment arms within the risk groups using Cox proportional hazard methods and Kaplan–Meier estimates. In multivariate Cox models for OS, the effect between treatment arms was adjusted for significant baseline characteristics identified using a stepwise selection process (level to enter: P<0.1; level to stay: P⩽0.05). Factors were considered for the multivariate selection process only if P⩽0.25 in the univariate Cox analysis. For hazard ratios, 1-year OS rates and median PFS, 95% confidence intervals (CIs) were calculated. Cox proportional hazard methods were also used to analyse the impact of the selected risk groups on OS and PFS in models including the treatment arms and corresponding interactions. All statistical tests were considered exploratory and no alpha adjustment for multiple testing was applied.
Results
Patient population and treatment exposure
The intent-to-treat population included 130 patients (23%) with TNBC and 433 (77%) with HR+ disease. One patient with PgR-negative disease but unknown ER status was excluded from the present analysis. Within the HR+ population, 308 patients (55% of all patients) were classified as high risk and 125 (22% of all patients) as low risk.
As might be expected in retrospective exploratory subgroup analyses, there were some imbalances in baseline characteristics between the treatment arms (Table 1). For example, within the TNBC cohort, the BEV–PAC arm included fewer patients with Eastern Cooperative Oncology Group (ECOG) performance status ⩾1, positive lymph node status, metastatic disease at first diagnosis or liver metastases compared with the BEV–CAP arm, but more patients with lung metastases. In the high-risk HR+ group, the BEV–PAC arm included fewer patients with a DFI >12 months. There were also differences between the cohorts, as expected when defining cohorts according to risk factors.
Table 1. Baseline characteristics according to risk group.
| |
TNBC cohort |
High-risk HR+ |
Low-risk HR+ |
|||
|---|---|---|---|---|---|---|
| Characteristic | BEV–PAC (n=63) | BEV–CAP (n=67) | BEV–PAC (n=146) | BEV–CAP (n=162) | BEV–PAC (n=75) | BEV–CAP (n=50) |
| Median age, years (range) |
54 (29–84) |
56 (28–77) |
58 (29–86) |
57 (27–79) |
61 (33–77) |
61 (31–86) |
| Premenopausal, n (%) |
17 (27) |
18 (27) |
27 (18) |
26 (16) |
8 (11) |
7 (14) |
|
ECOG performance status,
n
(%) | ||||||
| 0 | 47 (75) | 40 (60) | 99 (68) | 106 (65) | 47 (63) | 33 (66) |
| 1 | 13 (21) | 24 (36) | 39 (27) | 51 (31) | 23 (31) | 16 (32) |
| 2 |
3 (5) |
3 (4) |
7 (5) |
3 (2) |
5 (7) |
1 (2) |
|
Disease-free interval,
n
(%) | ||||||
| ⩽12 months | 9 (14) | 2 (3) | 6 (4) | 8 (5) | 0 | 0 |
| >12 to ⩽24 months | 14 (22) | 14 (21) | 33 (23) | 20 (12) | 3 (4) | 0 |
| >24 months | 28 (44) | 31 (46) | 62 (42) | 101 (62) | 57 (76) | 39 (78) |
| Nonea |
12 (19) |
20 (30) |
45 (31) |
33 (20) |
15 (20) |
11 (22) |
| Metastatic at first diagnosis |
12 (19) |
20 (30) |
32 (22) |
29 (18) |
15 (20) |
10 (20) |
|
Metastatic organs,
n
(%) | ||||||
| ⩾3 | 26 (41) | 28 (42) | 76 (52) | 93 (57) | 2 (3) | 3 (6) |
| Visceral | 41 (65) | 46 (69) | 130 (89) | 145 (90) | 14 (19) | 12 (24) |
| Liver | 17 (27) | 29 (43) | 89 (61) | 91 (56) | 7 (9) | 6 (12) |
| Lung | 35 (56) | 28 (42) | 69 (47) | 87 (54) | 8 (11) | 7 (14) |
| Bone | 22 (35) | 25 (37) | 86 (59) | 95 (59) | 49 (65) | 30 (60) |
| Lymph nodes |
35 (56) |
48 (72) |
77 (53) |
97 (60) |
32 (43) |
26 (52) |
| Prior (neo)adjuvant chemotherapy, n (%) |
45 (71) |
42 (63) |
97 (66) |
107 (66) |
37 (49) |
27 (54) |
| Taxane | 19 (30) | 14 (21) | 32 (22) | 32 (20) | 6 (8) | 4 (8) |
| Anthracycline | 39 (62) | 33 (49) | 82 (56) | 93 (57) | 23 (31) | 18 (36) |
| Neither anthracycline nor taxane | 4 (6) | 6 (9) | 9 (6) | 11 (7) | 13 (17) | 9 (18) |
Abbreviations: BEV=bevacizumab; CAP=capecitabine; ECOG=Eastern Cooperative Oncology Group; HR+=hormone receptor-positive; PAC=paclitaxel; TNBC=triple-negative breast cancer.
Either no primary therapy or no response to primary therapy.
Imbalances in the extent of bevacizumab and chemotherapy exposure were also apparent within and between the treatment cohorts (Supplementary Appendix Table 1). In the TNBC and high-risk HR+ cohorts, the median duration of bevacizumab exposure was longer in the BEV–PAC than the BEV–CAP treatment arm, whereas chemotherapy exposure was similar in the two treatment arms. However, in the low-risk HR+ cohort, the median duration of treatment for both bevacizumab and chemotherapy was longer in the BEV–CAP treatment arm.
Efficacy
The median duration of follow-up in the TNBC cohort was 21.4 months in the BEV–PAC arm vs 19.2 months in the BEV–CAP arm. Corresponding values were 19.0 vs 18.0 months, respectively, in the high-risk HR+ cohort and 17.3 vs 19.6 months, respectively, in the low-risk HR+ subgroup.
Analysis of the primary end point OS using a Cox model with the three-level risk factor and the treatment group showed that the three risk groups (TNBC, high-risk HR+ and low-risk HR+) had strong prognostic value (P=0.0004) but revealed no significant treatment effect (P=0.7847). The OS hazard ratio compared with TNBC was 0.42 (95% CI 0.27–0.66) for low-risk HR+ and 0.65 (95% CI 0.47–0.89) for high-risk HR+. No significant interaction between the three-level risk factor and treatment arms was observed (P=0.4277) in a corresponding Cox model of OS.
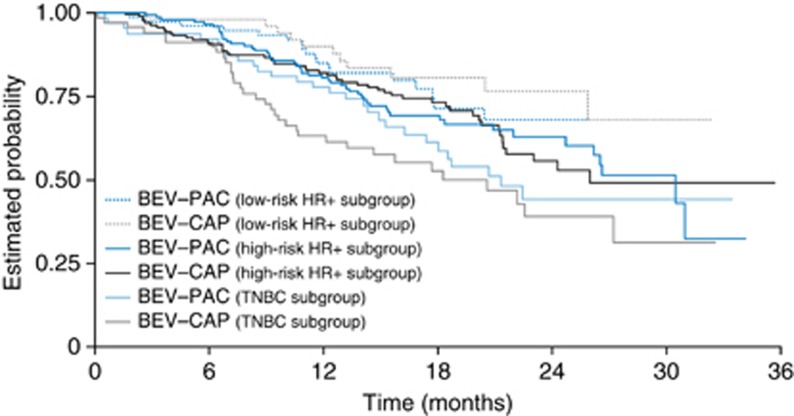
Comparison of OS between the two treatment arms showed differing treatment effects in the three cohorts, although none of the treatment effects was statistically significant. In the TNBC cohort, interim OS results after events in 48% of patients showed a non-significant trend favouring BEV–PAC (hazard ratio 1.33; Figure 1 and Table 2). The 1-year OS rate also favoured the BEV–PAC arm (78% vs 63%). In the multivariate Cox proportional hazard model adjusted for the significant baseline characteristics bone metastases (yes vs no) and menopausal status (postmenopausal vs premenopausal), the OS hazard ratio for BEV–CAP vs BEV–PAC was 1.30 (95% CI 0.77–2.17), supporting the non-significant trend favouring BEV–PAC observed in the univariate analysis.
Figure 1.
Overall survival (primary end point) according to risk category and treatment arm. Abbreviations: BEV–CAP=bevacizumab–capecitabine; BEV–PAC=bevacizumab–paclitaxel; HR+=hormone-receptor positive; TNBC=triple-negative breast cancer.
Table 2. Efficacy outcomes according to risk group.
|
TNBC |
High-risk HR+ |
Low-risk HR+ |
||||
|---|---|---|---|---|---|---|
| BEV–PAC (n=63) | BEV–CAP (n=67) | BEV–PAC (n=146) | BEV–CAP (n=162) | BEV–PAC (n=75) | BEV–CAP (n=50) | |
|
Overall survival | ||||||
| Events, n (%) | 28 (44) | 34 (51) | 50 (34) | 52 (32) | 18 (24) | 11 (22) |
| 1-Year overall survival rate, % (95% CI) | 78 (68–88) | 63 (51–75) | 80 (74–87) | 82 (76–88) | 85 (77–93) | 90 (81–98) |
| Hazard ratio (95% CI)a |
1.33 (0.80–2.19) |
0.97 (0.66–1.43) |
0.80 (0.38–1.69) |
|||
|
Progression-free survival | ||||||
| Events, n (%) | 50 (79) | 54 (81) | 93 (64) | 125 (77) | 33 (44) | 35 (70) |
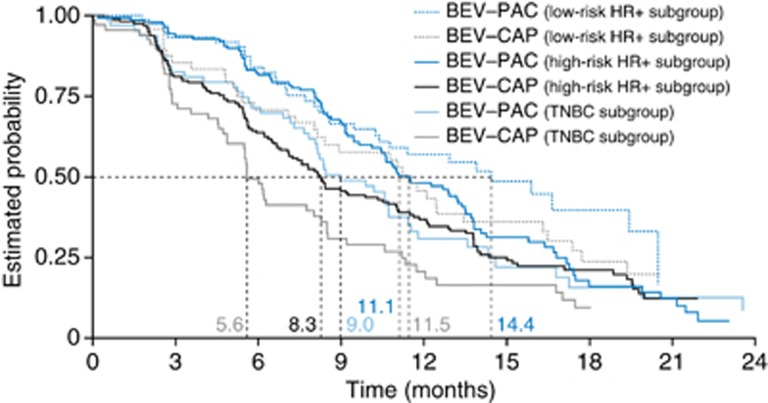
| Median, months (95% CI) | 9.0 (7.8–10.7) | 5.6 (4.9–8.0) | 11.1 (10.4–13.4) | 8.3 (7.1–10.7) | 14.4 (10.4–20.5) | 11.5 (8.1–16.3) |
| Hazard ratio (95% CI)a |
1.37 (0.93–2.02) |
1.29 (0.98–1.69) |
1.39 (0.86–2.25) |
|||
|
Objective response | ||||||
| Response rate, % (95% CI) | 49 (36–62) | 19 (11–31) | 46 (38–54) | 30 (23–38) | 35 (24–47) | 28 (16–42) |
| Difference (95% CI) | 30 (14–45) | 16 (5–26) | 7 (−10–23) | |||
| PD as best response, % | 6 | 25 | 7 | 15 | 5 | 10 |
Abbreviations: BEV=bevacizumab; CAP=capecitabine; CI=confidence interval; HR+=hormone receptor-positive; PAC=paclitaxel; PD=progressive disease; TNBC=triple-negative breast cancer.
BEV–CAP vs BEV–PAC, univariate unstratified Cox proportional hazards model.
In the HR+ cohort (irrespective of risk status), the OS hazard ratio for BEV–CAP vs BEV–PAC was 0.95 (95% CI 0.67–1.34) after events in 30% of patients. In multivariate Cox proportional hazards analyses of this subgroup, ECOG performance status and the presence of liver metastases were identified as significant prognostic factors for OS. The OS hazard ratio was 0.91 (95% CI 0.64–1.31), again supporting the lack of marked OS benefit with one regimen over another seen in the univariate analysis (Supplementary Appendix Figure 1). However, when the HR+ cohort was divided into high- and low-risk subgroups, OS appeared more favourable with BEV–CAP in the low-risk HR+ cohort (Figure 1 and Table 2). The OS hazard ratio for BEV–CAP vs BEV–PAC was 0.80 and 1-year OS rates were 85% with BEV–PAC vs 90% with BEV–CAP. In contrast, OS outcomes in the high-risk HR+ cohort were almost identical in the two treatment arms. These findings were supported by a multivariate Cox analysis adjusted for significant baseline characteristics (data not shown).
Analysis of PFS, a secondary end point, showed a similar significant impact for the three risk groups (TNBC, high-risk HR+ and low-risk HR+ Wald chi-squared test, P=0.0002) and a significant treatment effect favouring BEV–PAC (P=0.0042). Again, no significant interaction between the risk factors and the treatment arms was observed (P=0.9665). This finding was supported in analyses of treatment effect within each risk factor cohort. Although none of the subgroup differences reached statistical significance, the trend was in the same direction as the significant PFS benefit observed in the overall population (Figure 2 and Table 2). Objective response rate was also more favourable with BEV–PAC in the TNBC and high-risk HR+ cohorts, but did not differ significantly between treatment arms in the low-risk HR+ cohort (Table 2).
Figure 2.
Progression-free survival (secondary end point) according to risk category and treatment arm. Abbreviations: BEV–CAP=bevacizumab–capecitabine; BEV–PAC=bevacizumab–paclitaxel; HR+=hormone-receptor positive; TNBC=triple-negative breast cancer.
Post-study therapy
In all three cohorts, as in the overall study population, slightly more patients receiving BEV–CAP than BEV–PAC received chemotherapy after discontinuing study therapy (Table 3), consistent with the higher proportion of patients with disease progression at the time of data cut-off. The main qualitative difference between treatment arms was the use of post-progression taxane and capecitabine. Also consistent with the proportion of patients with disease progression at the time of data cut-off, the proportion receiving further chemotherapy was highest in the TNBC cohort, but similar in the two HR+ cohorts.
Table 3. Summary of anti-cancer therapy after discontinuation of study treatment according to risk group.
| |
TNBC |
High-risk HR+ |
Low-risk HR+ |
|||
|---|---|---|---|---|---|---|
| Patients, n (%) | BEV–PAC (n=63) | BEV–CAP (n=67) | BEV–PAC (n=146) | BEV–CAP (n=162) | BEV–PAC (n=75) | BEV–CAP (n=50) |
| Bevacizumab |
2 (3) |
4 (6) |
4 (3) |
5 (3) |
3 (4) |
5 (10) |
| Chemotherapy |
32 (51) |
40 (60) |
57 (39) |
79 (49) |
30 (40) |
25 (50) |
| Second line | 30 (48) | 37 (55) | 50 (34) | 71 (44) | 20 (27) | 21 (42) |
| Taxane | 3 (5) | 16 (24) | 2 (1) | 45 (28) | 2 (3) | 12 (24) |
| Capecitabine | 14 (22) | 2 (3) | 33 (23) | 7 (4) | 12 (16) | 2 (4) |
| Anthracycline | 4 (6) | 9 (13) | 12 (8) | 15 (9) | 7 (9) | 2 (4) |
| Platinum | 9 (14) | 9 (13) | 1 (1) | 16 (10) | 0 | 1 (2) |
| Gemcitabine | 7 (11) | 7 (10) | 3 (2) | 4 (2) | 0 | 1 (2) |
| Vinorelbine | 3 (5) | 7 (10) | 3 (2) | 10 (6) | 0 | 3 (6) |
| Other |
3 (5) |
2 (3) |
1 (1) |
3 (2) |
0 |
3 (6) |
| Third line | 16 (25) | 18 (27) | 24 (16) | 32 (20) | 14 (19) | 11 (22) |
Abbreviations: BEV=bevacizumab; CAP=capecitabine; HR+=hormone receptor-positive; PAC=paclitaxel; TNBC=triple-negative breast cancer.
Safety
Overall, grade ⩾3 adverse events were less common with BEV–CAP than with BEV–PAC in all three cohorts (Table 4). Differences between the treatment arms were driven by the known safety profiles of the chemotherapy partners, whereas differences within the treatment arms and between cohorts reflected the duration of treatment exposure. For example, hand-foot syndrome was the most common grade ⩾3 adverse event with BEV–CAP in all three cohorts, and was most frequent in the low-risk HR+ cohort. Grade ⩾3 cognitive and neurologic adverse events, of particular relevance in TNBC, were uncommon in both treatment arms: the incidence of grade ⩾3 dizziness was 2% with BEV–PAC vs 0% with BEV–CAP. Grade ⩾3 epilepsy was reported in 0% vs 1% with BEV–PAC vs BEV–CAP, respectively, and grade ⩾3 speech disorder in 2% vs 0%, respectively.
Table 4. Summary of grade ⩾3 AEs in >2% of patients in any treatment group in any cohort.
| |
TNBC |
High-risk HR+ |
Low-risk HR+ |
|||
|---|---|---|---|---|---|---|
| Patients, % | BEV–PAC (n=63) | BEV–CAP (n=67) | BEV–PAC (n=145) | BEV–CAP (n=160) | BEV–PAC (n=75) | BEV–CAP (n=50) |
| Any grade ⩾3 AE |
63.5 |
41.8 |
61.4 |
50.6 |
61.3 |
48.0 |
| Hand-foot syndrome |
0 |
11.9 |
0.7 |
13.8 |
0 |
28.0 |
| Neutropenia |
20.6 |
0 |
17.9 |
3.1 |
16.0 |
0 |
| Peripheral neuropathy |
9.5 |
0 |
15.2 |
0 |
14.7 |
2.0 |
| Hypertension |
1.6 |
3.0 |
4.8 |
8.1 |
5.3 |
2.0 |
| Leucopenia |
7.9 |
0 |
6.9 |
0.6 |
6.7 |
0 |
| Diarrhoea |
1.6 |
7.5 |
2.8 |
3.8 |
4.0 |
8.0 |
| Polyneuropathy |
4.8 |
0 |
3.4 |
0 |
5.3 |
0 |
| Fatigue |
1.6 |
1.5 |
4.8 |
1.9 |
2.7 |
0 |
| Bone pain |
0 |
1.5 |
1.4 |
4.4 |
2.7 |
2.0 |
| Syncope |
0 |
0 |
0 |
0 |
1.3 |
4.0 |
| Pulmonary embolism |
1.6 |
1.5 |
0.7 |
1.3 |
0 |
4.0 |
| Vomiting |
0 |
1.5 |
0.7 |
0.6 |
4.0 |
0 |
| GGT increased |
0 |
0 |
3.4 |
1.9 |
0 |
2.0 |
| Asthenia |
1.6 |
1.5 |
3.4 |
1.9 |
1.3 |
2.0 |
| Peripheral sensory neuropathy |
3.2 |
0 |
2.1 |
0 |
2.7 |
0 |
| Nail disorder |
3.2 |
0 |
2.8 |
0 |
2.7 |
0 |
| Myalgia |
3.2 |
0 |
0 |
0 |
1.3 |
0 |
| Thrombocytopenia |
3.2 |
3.0 |
0 |
1.9 |
1.3 |
0 |
| Epistaxis |
3.2 |
0 |
0 |
0.6 |
1.3 |
0 |
| Pain in extremity |
3.2 |
0 |
0.7 |
1.3 |
1.3 |
2.0 |
| Anaemia |
1.6 |
3.0 |
1.4 |
3.1 |
0 |
2.0 |
| Pathological fracture |
0 |
3.0 |
0 |
0.6 |
0 |
0 |
| Dyspnoea |
1.6 |
3.0 |
2.8 |
2.5 |
0 |
2.0 |
| Neurotoxicity |
1.6 |
0 |
0 |
0 |
2.7 |
0 |
| Deep vein thrombosis |
0 |
0 |
0 |
2.5 |
1.3 |
0 |
| Febrile neutropenia |
0 |
0 |
2.1 |
0.6 |
0 |
0 |
| Alopecia |
1.6 |
0 |
2.1 |
0 |
0 |
0 |
| Hypocalcaemia |
0 |
0 |
2.1 |
0 |
0 |
0 |
| Hypersensitivity | 1.6 | 0 | 2.1 | 0 | 0 | 0 |
Abbreviations: AE=adverse event; BEV=bevacizumab; CAP=capecitabine; GGT=gamma-glutamyltransferase; HR+=hormone receptor-positive; PAC=paclitaxel; TNBC=triple-negative breast cancer.
Discussion
Numerous subgroup analyses of trials evaluating bevacizumab in LR/mBC have been undertaken in an attempt to identify patients who benefit most from this treatment option (O'Shaughnessy et al, 2009; Miles et al, 2013).To date, significant OS differences between bevacizumab vs non-bevacizumab regimens have been elusive, although it is perhaps unsurprising that exploratory analyses of small subgroups within trials that were not powered to detect OS differences even in the overall population yield no significant differences. The exception was the subgroup of patients previously exposed to taxanes (Miles et al, 2013), but this finding must be interpreted with caution given the small sample size, exploratory nature of these post hoc retrospective analyses and lack of adjustment for multiple testing.
Rather than adding to the puzzle of determining which patients should receive bevacizumab, the TURANDOT trial provides an opportunity to explore which specific bevacizumab-containing regimen may be most appropriate for a given population. Bevacizumab-containing therapy is often preferred to chemotherapy alone in TNBC (Dawood et al, 2012) because of the generally poor prognosis, lack of effective treatments and high efficacy of bevacizumab-containing therapies shown in TNBC subgroup analyses in various treatment settings (Brufsky et al, 2012; von Minckwitz et al, 2012; Miles et al, 2013). The present analysis suggests that in these patients at high risk of progression, BEV–PAC may be a more appropriate option than BEV–CAP, showing a non-significant trend towards more favourable OS and PFS compared with BEV–CAP, and a substantially higher ORR (49% vs 19%). The 1-year OS rate of 78% with BEV–PAC in the TNBC cohort is among the highest reported in this setting: 1-year OS rates with non-bevacizumab-containing regimens have not exceeded 65% in subgroup analyses (O'Shaughnessy et al, 2009, 2011a, 2011b; Baselga et al, 2013). Thus BEV–PAC is a valid, effective treatment choice, particularly in this setting with limited active therapies available.
Patients with HER2-negative HR+ disease generally have a better prognosis. For example, in the E2100 trial median PFS with paclitaxel alone was 7.7 months in patients with HR+ disease compared with 5.8 months in the overall population. The addition of bevacizumab to paclitaxel yielded a median PFS of 11.9 months and a 54% ORR in the HR+ subgroup of E2100 (Roche data on file 2007). Furthermore, unlike TNBC, sequential endocrine therapy is an option in HR+ disease. Nevertheless, this group represents a highly heterogeneous population and includes patients with rapidly progressing aggressive disease. Among patients with high-risk HR+ disease in TURANDOT there was no apparent difference in OS between the two regimens, but both PFS and ORR showed non-significant trends favouring BEV–PAC. Patients' treatment goals may be one of the most important driving factors in treatment selection, as potential efficacy differences are subtle and must be balanced against differing safety profiles and patient preferences. For some patients, the possibility of avoiding taxane therapy as their first treatment for LR/mBC may represent the key factor influencing treatment decisions. Although we were unable to identify any significant difference in quality of life between the regimens (Lang et al, 2012), clinical experience suggests that patients may prefer BEV–CAP to BEV–PAC because of the more acceptable side-effect profile. Moreover, in patients with low-risk HR+ disease, a trend for better survival was observed with first-line BEV–CAP.
The failure to detect a difference in quality of life between regimens in TURANDOT may be due to a true lack of difference, the analysis approach adopted for TURANDOT, or insufficient sensitivity of available quality-of-life instruments to detect subtle differences in predominantly asymptomatic patients. Nonetheless, the superior safety profile with BEV–CAP and the trend towards better OS in a well-defined subset may represent important considerations when discussing treatment options with such patients.
This conclusion has three major caveats. First, the post hoc exploratory nature of the analyses means that findings should be interpreted with caution and regarded as hypothesis-generating. Nevertheless, the risk factors used to define the low- and high-risk HR+ subgroups in this analysis are very similar to those previously identified in ATHENA (Llombart-Cussac et al, 2013). Second, in these interim analyses the numbers of deaths in each subgroup were relatively small, particularly in the low-risk group. Third, differences in post-study therapy between treatment arms and cohorts may dilute the effect on OS of first-line treatment, particularly in the low-risk HR+ subgroup in whom long post-progression survival is expected. It will be interesting to see if these patterns remain evident or are perhaps strengthened with the final OS analysis anticipated in 2015.
Notwithstanding these limitations, the simple risk factor index explored in these analyses of TURANDOT is prognostic for both PFS and OS. Our findings suggest that such an index may be used to guide treatment choice when selecting bevacizumab-containing therapy, balancing outcome with safety and patient preference.
Acknowledgments
We thank the investigators and staff at all participating centres. The TURANDOT trial was sponsored by the Central European Cooperative Oncology Group (CECOG) and supported by F Hoffmann-La Roche Ltd, Basel, Switzerland. F Hoffmann-La Roche had no role in the conduct or analysis of the trial, or in the interpretation of the results or final content and decision to submit the manuscript for publication. Support for third-party writing assistance for this manuscript was provided by F Hoffmann-La Roche Ltd.
Speaker honoraria: TB (Roche), RG (Roche), AE (Roche), KK (AstraZeneca, Roche, Merck Serono, Eli Lilly, sanofi-aventis, GlaxoSmithKline and Novartis Research), BM (Roche, Eli Lilly, Novartis, AstraZeneca and GlaxoSmithKline), CZ (Roche, Eli Lilly and Pfizer); Research support: RG (Roche); Advisor: KK (Boehringer Ingelheim, Roche and Eli Lily), BM (Roche and Merck Serono); Employment: SA (IST GmbH), DM (IST GmbH; IST GmbH is the clinical research organisation providing consultancy and various other services to Roche and the Central European Cooperative Oncology Group). All other authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Baselga J, Gómez P, Greil R, Braga S, Climent MA, Wardley AM, Kaufman B, Stemmer SM, Pêgo A, Chan A, Goeminne JC, Graas MP, Kennedy MJ, Ciruelos Gil EM, Schneeweiss A, Zubel A, Groos J, Melezínková H, Awada A. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31:2586–2592. doi: 10.1200/JCO.2012.46.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brufsky A, Valero V, Tiangco B, Dakhil S, Brize A, Rugo HS, Rivera R, Duenne A, Bousfoul N, Yardley DA. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: subgroup analysis of the RIBBON-2 trial. Breast Cancer Res Treat. 2012;133:1067–1075. doi: 10.1007/s10549-012-2008-6. [DOI] [PubMed] [Google Scholar]
- Dawood S, Shaikh AJ, Buchholz TA, Cortes J, Cristofanilli M, Gupta S, Gonzalez-Angulo AM. The use of bevacizumab among women with metastatic breast cancer: a survey on clinical practice and the ongoing controversy. Cancer. 2012;118:2780–2786. doi: 10.1002/cncr.26579. [DOI] [PubMed] [Google Scholar]
- Inbar MJ, Lang I, Kahan Z, Greil R, Beslija S, Stemmer SM, Kaufman B, Ahlers S, Brodowicz T, Zielinski C, Central European Cooperative Oncology Group Efficacy of first-line bevacizumab (BEV)-based therapy for metastatic triple-negative breast cancer (TNBC): subgroup analysis of TURANDOT. J Clin Oncol. 2013;31 (suppl:abstract 1040. [Google Scholar]
- Lang I, Brodowicz T, Ryvo L, Kahan Z, Greil R, Beslija S, Stemmer SM, Kaufman B, Zvirbule Z, Steger GG, Melichar B, Pienkowski T, Sirbu D, Messinger D, Zielinski C, Central European Cooperative Oncology Group Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer: interim efficacy results of the randomised, open-label, non-inferiority, phase 3 TURANDOT trial. Lancet Oncol. 2013;14:125–133. doi: 10.1016/S1470-2045(12)70566-1. [DOI] [PubMed] [Google Scholar]
- Lang I, Inbar MJ, Kahan Z, Greil R, Beslija S, Stemmer SM, Kaufman B, Ahlers S, Brodowicz T, Zielinski C.2012Quality of life results from the TURANDOT trial comparing two bevacizumab-containing regimens as first-line treatment for HER2-negative metastatic breast cancer Cancer Res 72(24 suppl462s(poster P5-17-03). [Google Scholar]
- Llombart-Cussac A, Pivot XB, Biganzoli L, Cortes-Funes H, Pritchard KI, Pierga J-Y, Smith IE, Thomssen C, Palacios G, Srock S, Sampayo M, Cortes J. A prognostic factor (PF) index for overall survival in a HER2-negative endocrine-resistant metastatic breast cancer (MBC) population: analysis from the ATHENA trial. J Clin Oncol. 2013;31 (suppl:abstract 555. [Google Scholar]
- Miles DW, Chan A, Dirix LY, Cortés J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- Miles D, Diéras V, Cortés JA, Duenne AA, Yi J, O'Shaughnessy J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol. 2013;24:2773–2780. doi: 10.1093/annonc/mdt276. [DOI] [PubMed] [Google Scholar]
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy J, Dieras V, Glaspy J, Brufsky A, Miller K, Miles D, Koralewski D, Phan S, Bhattacharya S.2009Comparison of subgroup analyses of PFS from three phase III studies of bevacizumab in combination with chemotherapy in patients with HER2-negative metastatic breast cancer (MBC) Cancer Res 69(suppl512s(abstract 207). [Google Scholar]
- O'Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy J, Schwartzberg LS, Danso MA, Rugo HS, Miller K, Yardley DA, Carlson RW, Finn RS, Charpentier E, Freese M, Gupta S, Blackwood-Chirchir A, Winer EP. A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC) J Clin Oncol. 2011;29 (suppl:abstract 1007. [Google Scholar]
- Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O'Shaughnessy J. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- Roche data on file 2007A randomized phase III trial of paclitaxel versus paclitaxel plus bevacizumab (rhuMAb VEGF) as first-line therapy for locally recurrent or metastatic breast cancerClinical Study Report E2100.
- Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, Mayer EL, Naughton M, Layman RM, Carey LA, Somer RA, Perez EA, Hudis C, Winer EP. CALGB 40502/NCCTG N063H: randomized phase III trial of weekly paclitaxel (P) compared to weekly nanoparticle albumin bound nab-paclitaxel (NP) or ixabepilone (Ix) with or without bevacizumab (B) as first-line therapy for locally recurrent or metastatic breast cancer (MBC) J Clin Oncol. 2012;30 (suppl:abstract CRA1002. doi: 10.1200/JCO.2014.59.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, Schrader I, Kittel K, Hanusch C, Kreienberg R, Solbach C, Gerber B, Jackisch C, Kunz G, Blohmer JU, Huober J, Hauschild M, Fehm T, Müller BM, Denkert C, Loibl S, Nekljudova V, Untch M, German Breast Group; Arbeitsgemeinschaft Gynäkologische Onkologie–Breast Study Groups Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366:299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.